On the Question of the Regio-Orientation, Stereo-Orientation and Molecular Mechanism in the Cascade Cycloaddition/Rearrangement/Elimination Processes Leading to Nitro-Substituted Thiopyran Analogs: DFT Computational Study
Abstract
1. Introduction
- -
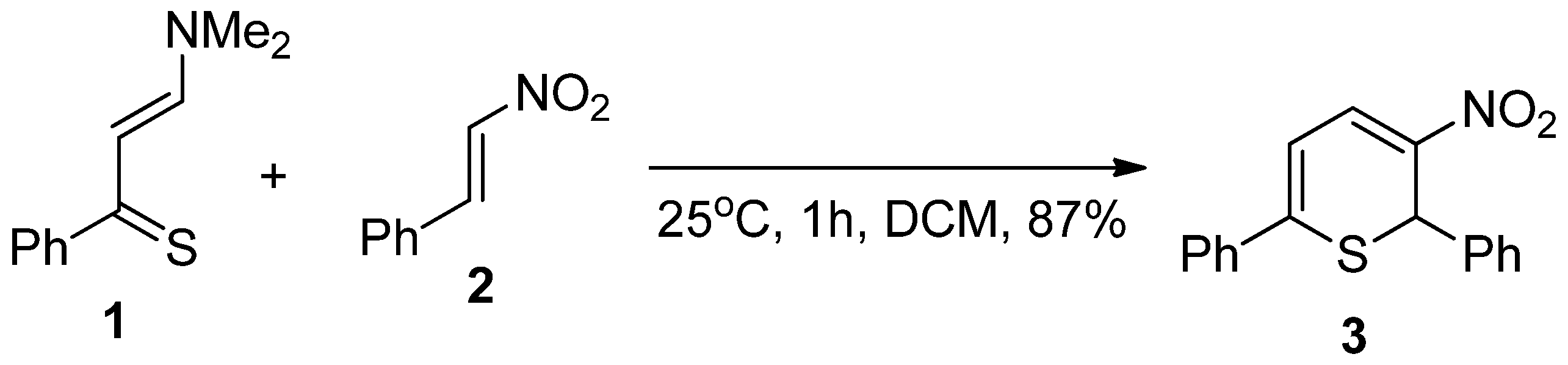
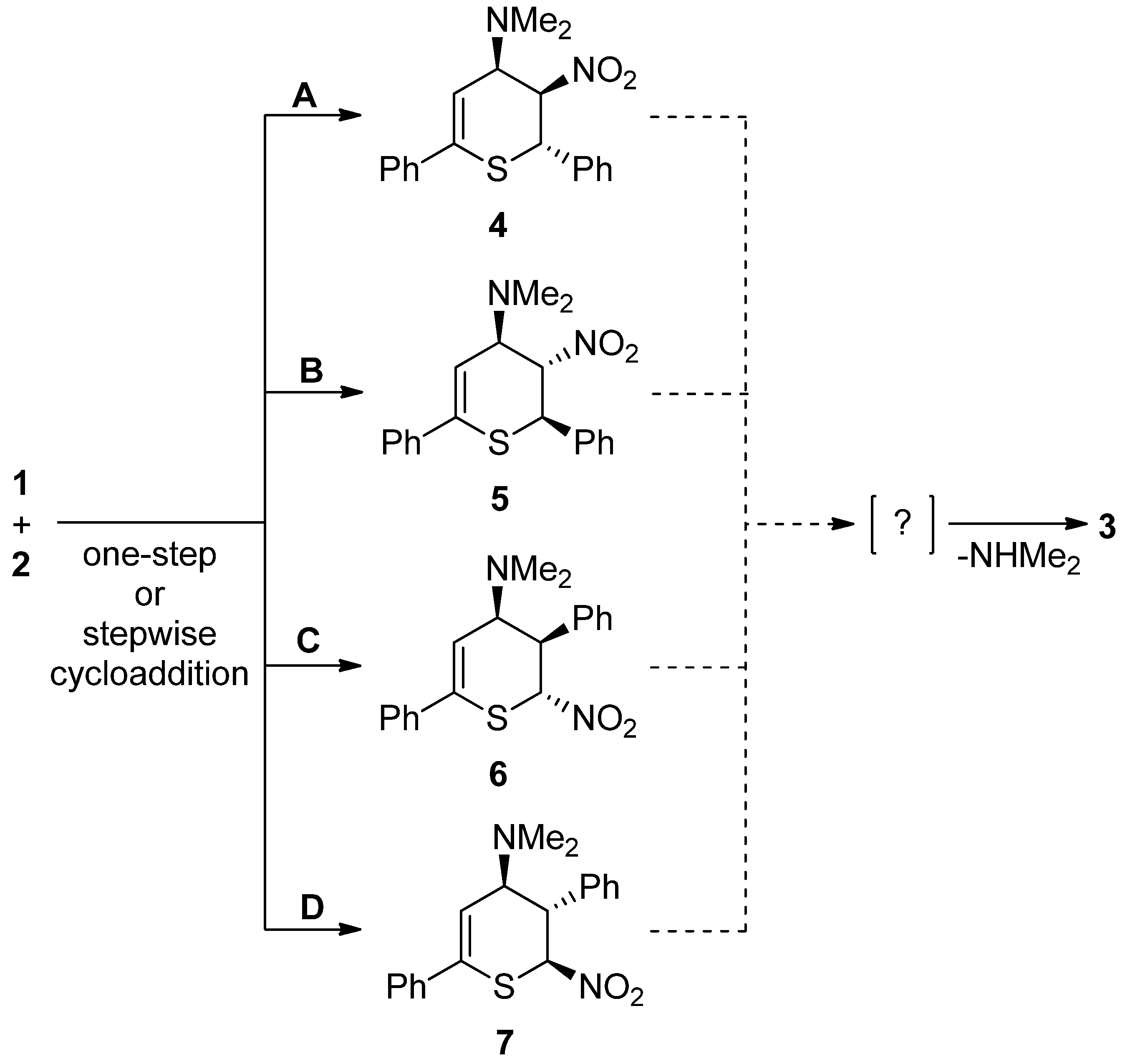
- The question of the regioselectivity of the cycloaddition reaction (Scheme 1). The regio-orientation of the detected final products suggests that the 1,5-diphenyl-3-dimethylamino-4-nitrohexane-6-tio-cyclohexa-1-ene (3 or 4) should be considered the primary cycloaddition product. It is, however, possible that this product is formed as a result of the cycloreversion of less thermodynamically stable 1,4-diphenyl-3-dimethylamino-5-nitrohexane-6-tio-cyclohexa-1-ene (5 or 6) that forms more easily from the kinetic point of view. This type of balance between thermodynamic and kinetic factors regarding the problem of cycloaddition regioselectivity was observed in the case of reactions with participation of conjugated nitroalkenes [22,23,24,25].
- -
- The problem of the stereoselectivity of the cycloaddition reaction (Scheme 1). In the framework of both regioisomeric approaches, two stereoisomeric reaction channels are possible due to the tendency to form 3,4-cis- and 3,4-trans cycloadducts. Assuming the syn-mechanism of the deamination stage, the last one should be more probable. However, this mechanism was not analyzed in any way. In the case of the stepwise elimination mechanism, the final product can be formed from both stereoisomeric 1,5-diphenyl-3-dimethylamino-4-nitrohexane-6-tio-cyclohexa-1-enes 4 and 5. Recently, different types of stepwise mechanisms were documented for elimination reactions earlier defined as a single-step [26,27,28].
- -
- Mechanistic aspect of the cycloaddition process. Three decades ago, the single-step mechanism with the pericyclic reorganization of the electron density was widely accepted [29]. However, recent discoveries undermine this point of view [30]. At this moment it is known that in many cases, the single-step mechanism is not pericyclic [31,32,33]. Moreover, the single-step scheme can compete with stepwise mechanism with biradical [34] or zwitterionic intermediates [35,36]. These issues are not clear regarding this title process.
- -
- The deamination reaction is generally considered a difficult process in comparison to other 1,2-elimination reactions. The theoretically possible, competitive channel of the extrusion of the nitrous acid cannot be, however, achieved realistically. These types of extrusion are generally realized as single-step, pseudocyclic processes [37,38] under mild reaction conditions.
- -
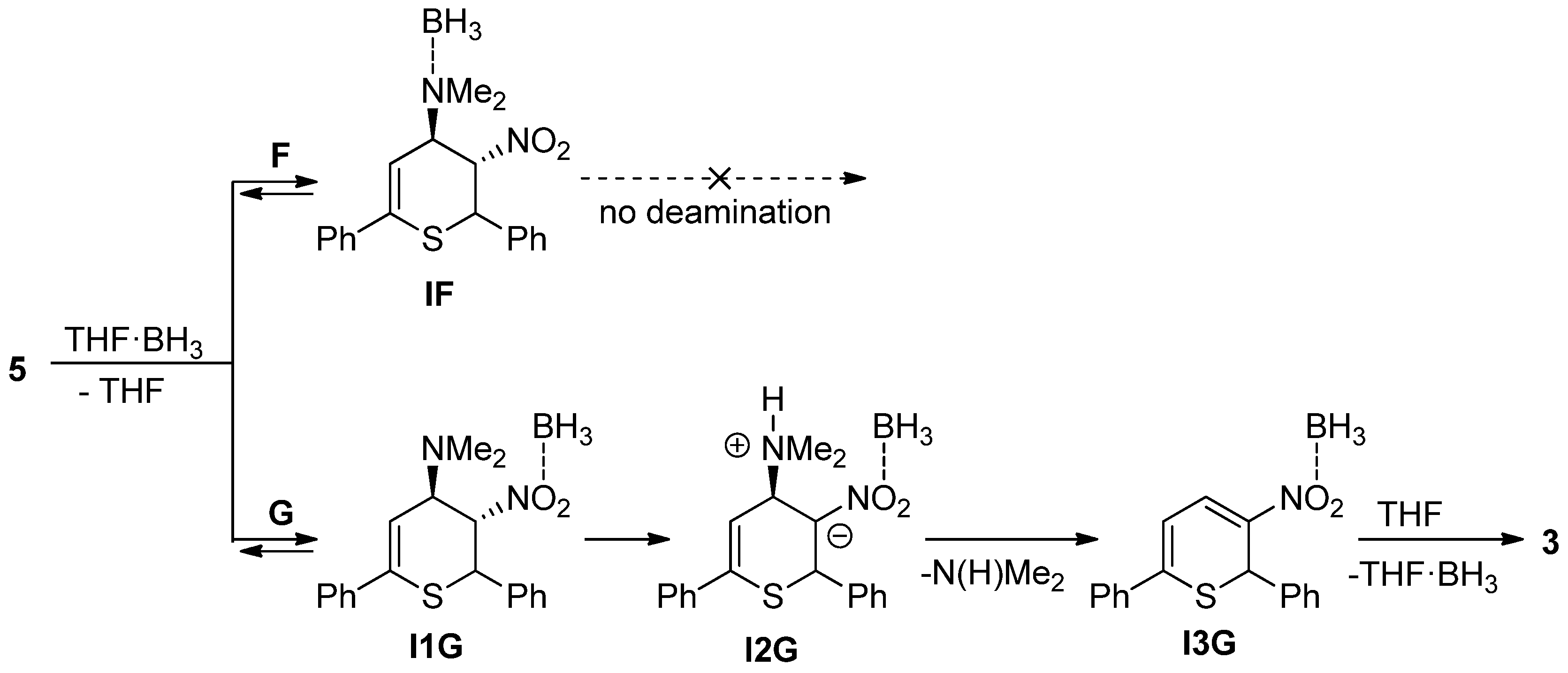
- The deamination process of 2-amino-1-nitroethyl molecular segments is generally significantly accelerated by the presence of the LA-catalysts such as boron hydride or boron trifluoride [39,40,41]. Therefore, in the last part of our research we decided to shed light on the kinetic aspects and the molecular mechanism of the transfer of respective LA to the cycloaddition product and the further decomposition via elimination stage.
2. Results and Discussion
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wei, J.; Chen, L.; Zhu, K.; Liu, Y.; Li, H.; Zhao, M.; Wu, Y. Design, Synthesis, and Fungicidal Activity Evaluation of 2-Methyl-5-Phenylthiazole-4-Carboxamides Bearing Morpholine, Thiomorpholine, or Thiomorpholine 1,1-Dioxide Moiety. Chem. Heterocycl. Compd. 2024, 60, 536–543. [Google Scholar] [CrossRef]
- Lelyukh, M.I.; Komarenska, Z.M.; Chaban, T.I.; Chaban, I.H. An Overview of the Synthetic Routes toward [1,2,4]Triazolo[3,4-b][1,3,4]Thiadiazoles (Microreview). Chem. Heterocycl. Compd. 2024, 60, 342–344. [Google Scholar] [CrossRef]
- Longo, L.S., Jr.; Siqueira, F.A. Synthesis of Imidazo[2,1-b]thiazol-5-amines Using Groebke–Blackburn–Bienaymé Multicomponent Reaction (Microreview). Chem. Heterocycl. Compd. 2024, 60, 339–341. [Google Scholar] [CrossRef]
- Chaban, T.I.; Klenina, O.V.; Chaban, I.H.; Lelyukh, M.I. Recent Advances in the Synthesis of Thiazolo[4,5-b]Pyridines. Part 2: Focus on Thiazole Annulation to Pyridine Ring. Chem. Heterocycl. Compd. 2024, 60, 130–132. [Google Scholar] [CrossRef]
- Chaban, T.I.; Lelyukh, M.I.; Chaban, I.H.; Kasyanchuk, O.Y. Approaches to the Synthesis of Thiazolo[3,2-a]Pyridines (Microreview). Chem. Heterocycl. Compd. 2024, 60, 124–126. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Zawadzinska-Wrochniak, K.; Wroblewska, A.; Jasinski, R. Syn-Propanethial S-Oxide as the Natural Available Building Block for the Preparation of Nitrofunctionalized Sulfur-Containing Five-Membered Heterocycles: MEDT Study. Molecules 2024, 29, 4892. [Google Scholar] [CrossRef]
- Batenko, N.; Gaile, A. 2-Substituted Benzothiazole-Based Sensors for Optical Detection of Biogenic Amines and Ammonia (Microreview). Chem. Heterocycl. Compd. 2025, 61, 51–53. [Google Scholar] [CrossRef]
- Alobaide, Z.N.; Habel, A.; Awin, T.; Buzgaia, N.; Elamari, A.; Elteera, F.; Elabbar, F. Arum cyrenaicum: A Comprehensive Review of Phytochemistry and Bioactivity. Sci. Rad. 2024, 3, 218–227. [Google Scholar] [CrossRef]
- Gassim, H.B.M.; Hassan, A.M.; Abadi, R.S.M.; Mustafa, Y.A.A. Phytochemical Constituents and Antioxidant Activity of Ricinus communis Linn Leaf and Seeds Extracts. Sci. Rad. 2024, 3, 74–88. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kącka-Zych, A.; Kula, K. Recent Progress in the Field of Cycloaddition Reactions Involving Conjugated Nitroalkenes. Curr. Chem. Lett. 2019, 8, 13–38. [Google Scholar] [CrossRef]
- Łapczuk, A. The [3+2] Cycloaddition Reaction as an Attractive Way for the Preparation of Nicotine Analogs (Microreview). Chem. Heterocycl. Compd. 2023, 59, 109–111. [Google Scholar] [CrossRef]
- Hou, S.-Y.; Yan, B.-C.; Sun, H.-D.; Puno, P.-T. Recent Advances in the Application of [2+2] Cycloaddition in the Chemical Synthesis of Cyclobutane-Containing Natural Products. Nat. Prod. Bioprospect. 2024, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Bera, N.; Ghosh, S. [2+2] Photochemical Cycloaddition in Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 1310–1326. [Google Scholar] [CrossRef]
- Eschenbrenner-Lux, V.; Kumar, K.; Waldmann, H. The Asymmetric Hetero-Diels–Alder Reaction in the Syntheses of Biologically Relevant Compounds. Angew. Chem. Int. Ed. 2014, 53, 11146–11157. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.A. Catalytic Asymmetric Hetero-Diels–Alder Reactions of Carbonyl Compounds and Imines. Angew. Chem. Int. Ed. 2000, 39, 3558–3588. [Google Scholar] [CrossRef]
- Schmidt, R.R. Hetero-Diels–Alder Reaction in Highly Functionalized Natural Product Synthesis. Acc. Chem. Res. 1986, 19, 250–259. [Google Scholar] [CrossRef]
- Jørgensen, K.A. Hetero-Diels–Alder Reactions of Ketones—A Challenge for Chemists. Eur. J. Org. Chem. 2004, 2004, 2093–2102. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Wróblewska, A.; Wielgus, E.; Dolot, R.; Jasiński, R. Fully Selective Synthesis of Spirocyclic-1,2-Oxazine N-Oxides via Non-Catalysed Hetero Diels–Alder Reactions with the Participation of Cyanofunctionalized Conjugated Nitroalkenes. Molecules 2023, 28, 4586. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Mirosław, B.; Wielgus, E.; Olszewska, A.; Jasiński, R. Green, One-Pot Synthesis of 1,2-Oxazine-Type Herbicides via Non-Catalyzed Hetero Diels–Alder Reactions Comprising (2E)-3-Aryl-2-Nitroprop-2-Enenitriles. J. Clean. Prod. 2022, 356, 131878. [Google Scholar] [CrossRef]
- Karpov, I.D.; Kolobov, A.V.; Filippov, I.P.; Rostovskii, N.V.; Ovchinnikov, K.L. A One-Pot Synthesis of 3-Nitro-2H-Thiopyrans and Their Selective Reduction to 3-Nitro-3,4-Dihydro-2H-Thiopyrans. Chem. Heterocycl. Compd. 2024, 60, 251–256. [Google Scholar] [CrossRef]
- Kras, J.; Wróblewska, A.; Kącka-Zych, A. Unusual Regioselectivity in [3+2] Cycloaddition Reactions between (E)-3-Nitroacrylic Acid Derivatives and (Z)-C,N-Diphenylimine N-Oxide. Sci. Rad. 2023, 2, 112–117. [Google Scholar] [CrossRef]
- Stecko, S.; Paśniczek, K.; Jurczak, M.; Urbańczyk-Lipkowska, Z.; Chmielewski, M. Kinetic and Thermodynamic Aspects in the 1,3-Dipolar Cycloaddition of Five-Membered Cyclic Nitrones to α,β-Unsaturated γ- and δ-Lactones. Tetrahedron Asymmetry 2007, 18, 1085–1093. [Google Scholar] [CrossRef]
- Sudibyo, H.; Budhijanto, B.; Marbelia, L.; Güleç, F.; Budiman, A. Kinetic and Thermodynamic Evidences of the Diels–Alder Cycloaddition and Pechmann Condensation as Key Mechanisms of Hydrochar Formation during Hydrothermal Conversion of Lignin-Cellulose. Chem. Eng. J. 2024, 480, 148116. [Google Scholar] [CrossRef]
- Karaś, A.; Łapczuk, A. Computational Model of the Formation of Novel Nitronorbornene Analogs via Diels–Alder Process. React. Kinet. Mech. Catal. 2025, 138, 2671–2689. [Google Scholar] [CrossRef]
- Kącka, A.; Jasiński, R. A Dramatic Change of Kinetic Conditions and Molecular Mechanism of Decomposition Processes of Nitroalkyl Carboxylates Catalyzed by Ethylammonium Cations. Comput. Theor. Chem. 2017, 1104, 37–42. [Google Scholar] [CrossRef]
- Kącka, A.; Domingo, L.R.; Jasiński, R. Does a Fluorinated Lewis Acid Catalyst Change the Molecular Mechanism of the Decomposition Process of Nitroethyl Carboxylates? Res. Chem. Intermed. 2018, 44, 325–337. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Ríos-Gutiérrez, M.; Domingo, L.R. A Molecular Electron Density Theory Study of the Lewis Acid–Catalyzed Decomposition Reaction of Nitroethyl Benzoate Using Aluminum Derivatives. J. Phys. Org. Chem. 2019, 32, e3938. [Google Scholar] [CrossRef]
- Houk, K.N.; Lin, Y.T.; Brown, F.K. For the Concerted Mechanism of the Diels–Alder Reaction of Butadiene with Ethylene. J. Am. Chem. Soc. 1986, 108, 554–556. [Google Scholar] [CrossRef]
- Jasiński, R. On the Question of Stepwise [4+2] Cycloaddition Reactions and Their Stereochemical Aspects. Symmetry 2021, 13, 1911. [Google Scholar] [CrossRef]
- Yepes, D.; Murray, J.S.; Pérez, P.; Domingo, L.R.; Politzer, P.; Jaque, P. Complementarity of Reaction Force and Electron Localization Function Analyses of Asynchronicity in Bond Formation in Diels–Alder Reactions. Phys. Chem. Chem. Phys. 2014, 16, 6726–6734. [Google Scholar] [CrossRef]
- Pedrón, M.; Delso, I.; Tejero, T.; Merino, P. Concerted Albeit Not Pericyclic Cycloadditions: Understanding the Mechanism of the (4+3) Cycloaddition between Nitrones and 1,2-Diaza-1,3-dienes. Eur. J. Org. Chem. 2019, 2019, 391–400. [Google Scholar] [CrossRef]
- Domingo, L.R. State of the Art of the Bonding Changes along the Diels–Alder Reaction between Butadiene and Ethylene: Refuting the Pericyclic Mechanism. Org. Chem. Curr. Res. 2013, 2, 120. [Google Scholar] [CrossRef]
- Jasiński, R. A Reexamination of the Molecular Mechanism of the Diels–Alder Reaction between Tetrafluoroethene and Cyclopentadiene. React. Kinet. Mech. Catal. 2016, 119, 49–57. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Wróblewska, A.; Jasiński, R. A New Insight on the Molecular Mechanism of the Reaction between 2-Methoxyfuran and Ethyl (Z)-3-Phenyl-2-Nitroprop-2-enoate: MEDT Computational Study. Molecules 2024, 29, 4876. [Google Scholar] [CrossRef] [PubMed]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Molecular Mechanism of Thermal and LA-Catalysed Diels–Alder Reaction between Cyclopentadiene and Isopropyl 3-Nitroprop-2-Enate. Molecules 2023, 28, 5289. [Google Scholar] [CrossRef]
- Sadowski, M.; Kula, K. Unexpected Course of Reaction Between (1E,3E)-1,4-Dinitro-1,3-butadiene and N-Methyl Azomethine Ylide—A Comprehensive Experimental and Quantum-Chemical Study. Molecules 2024, 29, 5066. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Lv, X.-J.; Liu, C.; Liu, Y.-K. Triethylamine-Promoted Henry Reaction/Elimination of HNO2/Cyclization Sequence of Functionalized Nitroalkanes and 2-Oxoaldehydes: Diversity-Oriented Synthesis of Oxacycles. Org. Lett. 2023, 25, 4033–4037. [Google Scholar] [CrossRef]
- Blomquist, A.T.; Shelley, T.H., Jr. Preparation of 2-Nitro-1-Alkenes from Nitro Amines. J. Am. Chem. Soc. 1948, 70, 147–149. [Google Scholar] [CrossRef]
- Kametani, T.; Nemoto, H. A New Stereocontrolled Synthesis of D-Ring Aromatic Steroid. Tetrahedron Lett. 1979, 20, 3309–3310. [Google Scholar] [CrossRef]
- Emmons, W.D.; Cannon, W.N.; Dawson, J.W.; Ross, R.M. Low Temperature Pyrolysis of Boron Trifluoride—Mannich Base Complexes. 2-Nitro-1-Alkenes. J. Am. Chem. Soc. 1953, 75, 1993–1994. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Electrophilicity w and Nucleophilicity N Scales for Cationic and Anionic Species. Sci. Rad. 2025, 4, 1–17. [Google Scholar] [CrossRef]
- Aitouna, A.O.; Rossafi, B.; El Alaoui, H.E.A.; Zeroual, A. Molecular Electron Density Study on the [3+2] Cycloaddition Reaction between Diphenylnitrylimine and Cinnamaldehyde. Sci. Rad. 2025, 4, 18–28. [Google Scholar] [CrossRef]
- Abdoul-Hakim, M.; Kenzy, C.; Subramaniam, M.; Zeroual, A.; Syed, A.; Bahkali, A.H.; Verma, M.; Wang, S.; Garmes, H. Elucidating Chemoselectivity and Unraveling the Mechanism of 1,3-Dipolar Cycloaddition between Diphenyl Nitrilimine and (Isoxazol-3-yl)methylbenzimidazole through Molecular Electron Density Theory. Chem. Heterocycl. Compd. 2024, 60, 617–626. [Google Scholar] [CrossRef]
- Aitouna, A.O.; Syed, A.; Alfagham, A.T.; Mazoir, N.; de Julián-Ortiz, J.V.; Elgorban, A.M.; El Idrissi, M.; Wong, L.S.; Zeroual, A. Investigating the Chemical Reactivity and Molecular Docking of 2-Diazo-3,3,3-Trifluoro-1-Nitropropane with Phenyl Methacrylate Using Computational Methods. Chem. Heterocycl. Compd. 2024, 60, 592–599. [Google Scholar] [CrossRef]
- Fałowska, A.; Grzybowski, S.; Kapuściński, D.; Sambora, K.; Łapczuk, A. Modeling of the General Trends of Reactivity and Regioselectivity in Cyclopentadiene–Nitroalkene Diels–Alder Reactions. Molecules 2025, 30, 2467. [Google Scholar] [CrossRef]
- Kula, K.; Zawadzińska, K. Local Nucleophile-Electrophile Interactions in [3+2] Cycloaddition Reactions between Benzonitrile N-Oxide and Selected Conjugated Nitroalkenes in the Light of MEDT Computational Study. Curr. Chem. Lett. 2021, 9, 9–16. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the Mechanism of Polar Diels–Alder Reactions. Org. Biomol. Chem. 2009, 7, 3576–3583. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Useful Classification of Organic Reactions Based on the Flux of the Electron Density. Sci. Rad. 2023, 2, 1–24. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk, A.; Sadowski, M.; Kras, J.; Zawadzińska, K.; Demchuk, O.M.; Gaurav, G.K.; Wróblewska, A.; Jasiński, R. On the Question of the Formation of Nitro-Functionalized 2,4-Pyrazole Analogs on the Basis of Nitrylimine Molecular Systems and 3,3,3-Trichloro-1-Nitroprop-1-ene. Molecules 2022, 27, 8409. [Google Scholar] [CrossRef] [PubMed]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Regioselectivity and the Molecular Mechanism of [3+2] Cycloaddition Reactions between Nitrous Oxide and Conjugated Nitroalkenes: DFT Computational Study. Molecules 2022, 27, 8441. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, K.; Gadocha, Z.; Pabian, K.; Wróblewska, A.; Wielgus, E.; Jasiński, R. First Examples of [3+2] Cycloadditions with the Participation of the (E)-3,3,3-Tribromo-1-Nitroprop-1-ene. Materials 2022, 15, 7584. [Google Scholar] [CrossRef] [PubMed]
- Fryźlewicz, A.; Olszewska, A.; Zawadzińska, K.; Woliński, P.; Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3+2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems. Organics 2022, 3, 59–76. [Google Scholar] [CrossRef]
- Wroblewska, A.; Sadowski, M.; Jasiński, R. Selectivity and the Molecular Mechanism of the Au(III)-Catalysed [3+2]-Cycloaddition Reaction between (Z)-C,N-Diphenylnitrone and Nitroethene in the Light of the Molecular Electron Density Theory Computational Study. Chem. Heterocycl. Compd. 2024, 60, 639–645. [Google Scholar] [CrossRef]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Energetic Aspects and Molecular Mechanism of 3-Nitro-substituted 2-Isoxazolines Formation via Nitrile N-Oxide [3+2] Cycloaddition: An MEDT Computational Study. Molecules 2024, 29, 3042. [Google Scholar] [CrossRef]
- Dresler, E.; Woliński, P.; Wróblewska, A.; Jasiński, R. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions between Aryl Azides and Ethyl Propiolate. Molecules 2023, 28, 8152. [Google Scholar] [CrossRef]
- Kącka-Zych, A. Understanding the molecular mechanism rearrangement of internal nitronic ester into nitronorbornene in the light of MEDT study. Molecules 2019, 24, 462. [Google Scholar] [CrossRef]
- Borodkin, G.I. Fluorination of Heterocyclic Compounds Accompanied by Molecular Rearrangements. Chem. Heterocycl. Compd. 2024, 60, 323–335. [Google Scholar] [CrossRef]
- Jasiński, R. On the Question of the Molecular Mechanism of N-Nitropyrazole Rearrangement. Chem. Heterocycl. Compd. 2020, 56, 1210–1212. [Google Scholar] [CrossRef]
- Klein, J.; Becker, J.Y. Metalation Reactions—XIV: The Generality of the 1,3-Sigmatropic Shift of Hydrogen in Allenyllithium Compounds. Tetrahedron 1972, 28, 5385–5392. [Google Scholar] [CrossRef]
- Lapczuk-Krygier, A.; Jaskowska, J.; Jasinski, R. The influence of Lewis acid catalyst on the kinetic and molecular mechanism of nitrous acid elimination from 5-nitro-3-phenyl-4,5-dihydroisoxazole: DFT computational study. Chem. Heterocycl. Compd. 2018, 54, 1172–1774. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Analysis of the possibility and molecular mechanism of carbon dioxide consumption in the Diels-Alder processes. Pure Appl. Chem. 2021, 93, 427–446. [Google Scholar] [CrossRef]
- Chit, T.; Morimoto, H.; Andres, H. Preparation, NMR characterization, and labeling reactions of tritiated borane-THF complex and high specific radioactivity. J. Org. Chem. 1995, 60, 7503–7507. [Google Scholar] [CrossRef]
- Wrackmeyer, B.; Schwarze, B. Reaction of borane in tetrahydrofuran with 1,1,3,3-tetramethyldisilazane and cyclic disilazanes—Competition between N–Si and N–H bond cleavage. Z. Naturforsch. B 1996, 51, 1707–1715. [Google Scholar] [CrossRef]
- Todd, R.C.; Hossain, M.; Josyula, K.V.; Gao, P.; Kuo, J.; Tan, C.T. New amine-stabilized deuterated borane–tetrahydrofuran complex (BD3–THF): Convenient reagent for deuterium incorporations. Tetrahedron Lett. 2007, 48, 2335–2337. [Google Scholar] [CrossRef]
- Yoon, N.M.; Pak, C.S.; Krishnamurthy, S.; Stocky, T.P. Selective reductions. XIX. Rapid reaction of carboxylic acids with borane-tetrahydrofuran. Remarkably convenient procedure for the selective conversion of carboxylic acids to the corresponding alcohols in the presence of other functional groups. J. Org. Chem. 1973, 38, 2786–2792. [Google Scholar] [CrossRef]
- Gonzales, J.M.; Cox, R.S.; Brown, S.T.; Allen, W.D.; Schaefer, H.F. Assessment of Density Functional Theory for Model SN2 Reactions: CH3X + F− (X = F, Cl, CN, OH, SH, NH2, PH2). J. Phys. Chem. A 2001, 105, 11327–11346. [Google Scholar] [CrossRef]
- Capurso, M.; Gette, R.; Radivoy, G.; Dorn, V. The Sn2 Reaction: A Theoretical-Computational Analysis of a Simple and Very Interesting Mechanism. Proceedings 2019, 41, 81. [Google Scholar] [CrossRef]
- Morán-González, L.; Besora, M.; Maseras, F. Seeking the Optimal Descriptor for SN2 Reactions through Statistical Analysis of Density Functional Theory Results. J. Org. Chem. 2022, 87, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Demchuk, O.M.; Jasinski, R.; Strzelecka, D.; Dziuba, K.; Kula, K.; Chrzanowski, J. A Clean and Simple Method for Deprotection of Phosphines from Borane Complexes. Pure Appl. Chem. 2018, 90, 49–62. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Fox, Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. A New Basis Set Exchange: An Open, Up-to-date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Łapczuk, A.; Rios-Gutierrez, M. Mechanistic aspects of the [3+2] cycloaddition reaction of the trifluroacetonitrile with diarylnitrilimines in the light of the MEDT quantum chemical study. Molecules 2025, 30, 2410. [Google Scholar] [CrossRef]
- Woliński, P.; Zawadzińska-Wrochniak, K.; Dresler, E.; Jasiński, R. On the Question of the Course of the Hetero Diels–Alder Reactions Between N-(2,2,2-Trichloroethylidene)Carboxamides and Dicyclohexylcarbodiimide: A New Case of the Stepwise Zwitterionic Cycloaddition Process. Molecules 2025, 30, 2692. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative Characterization of the Global Electrophilicity Power of Common Diene/Dienophile Pairs in Diels–Alder Reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R. 1999–2024, a Quarter Century of the Parr’s Electrophilicity ω Index. Sci. Rad. 2024, 3, 157–186. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions: A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Perez, P.; Saez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]

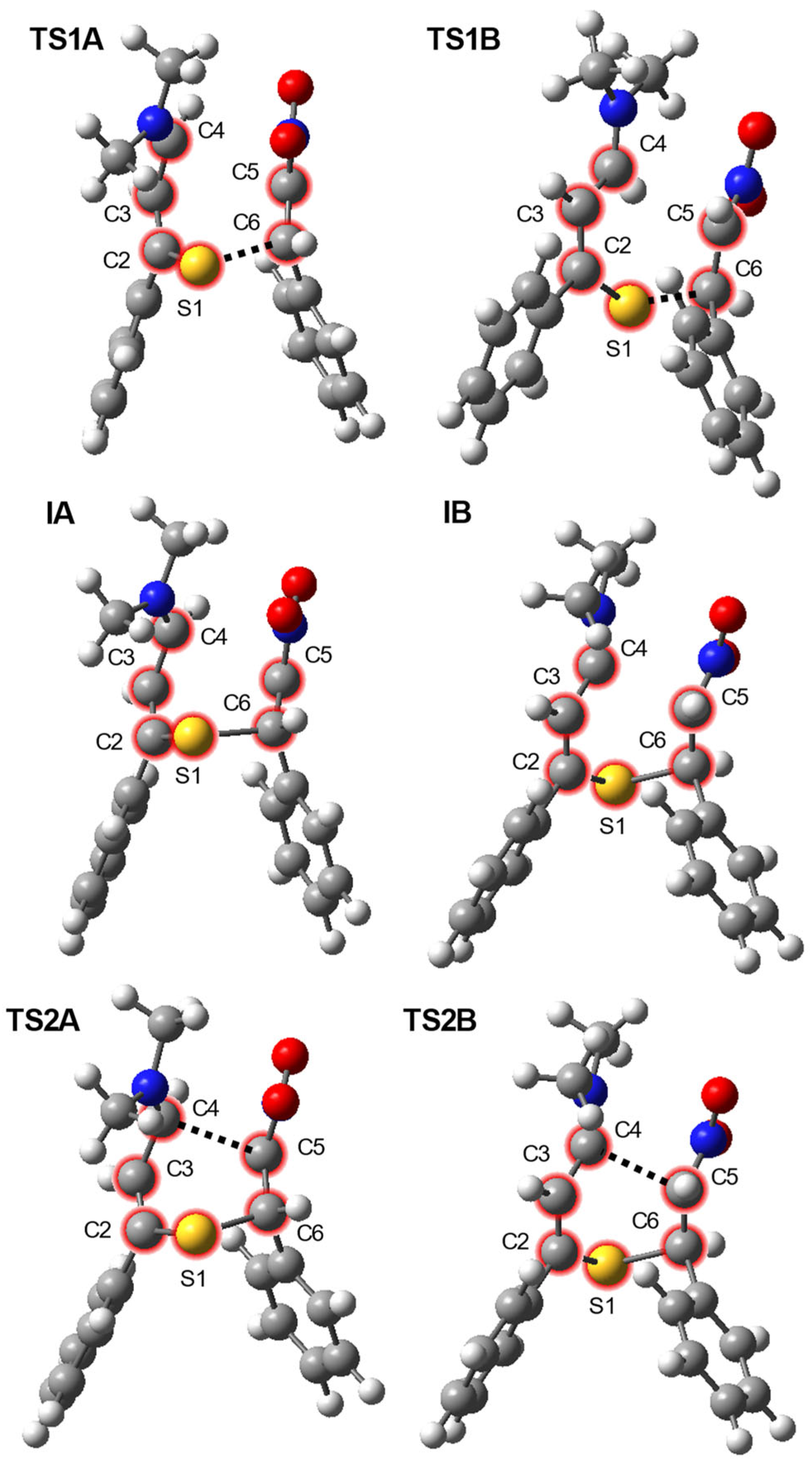
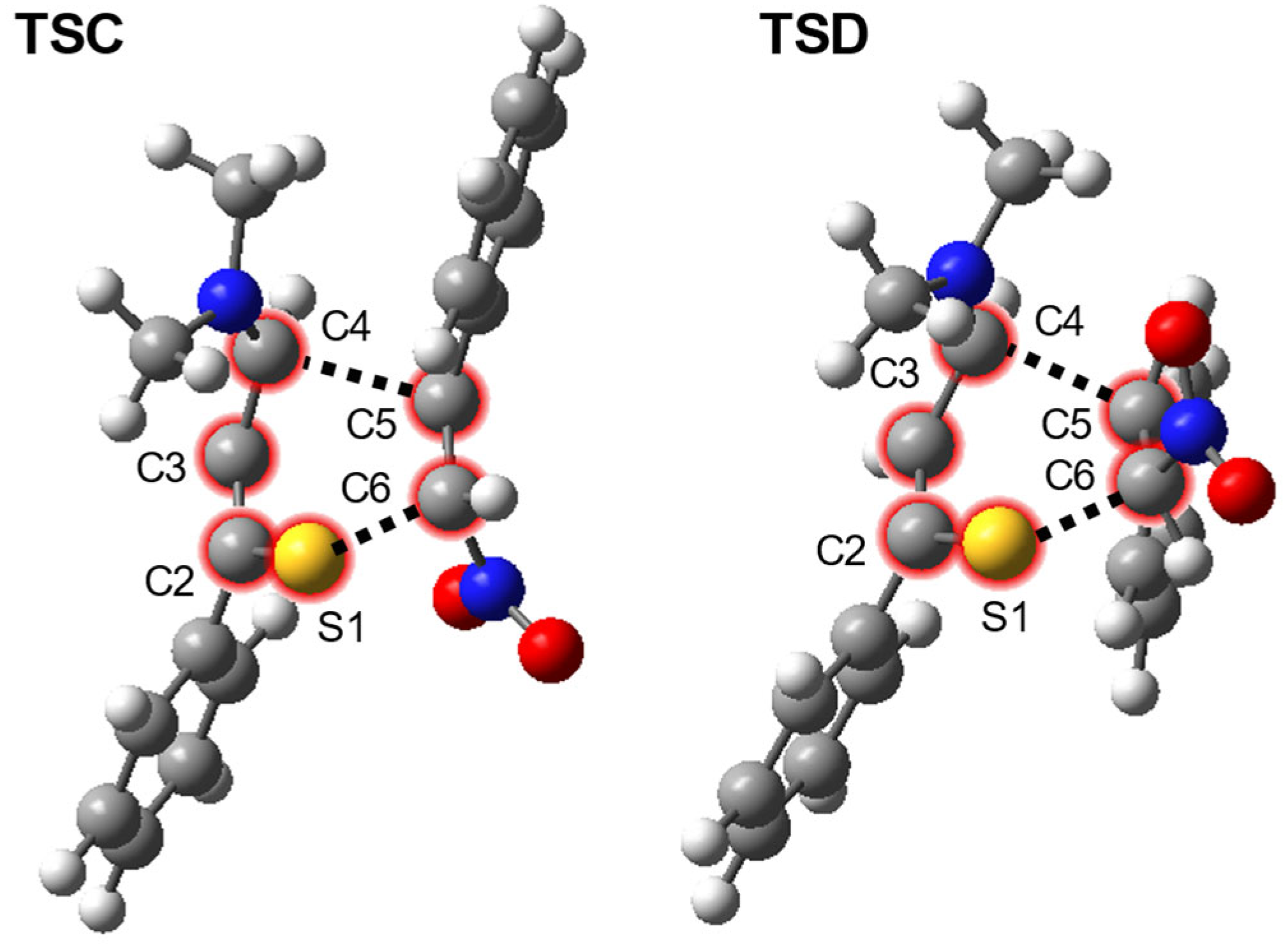
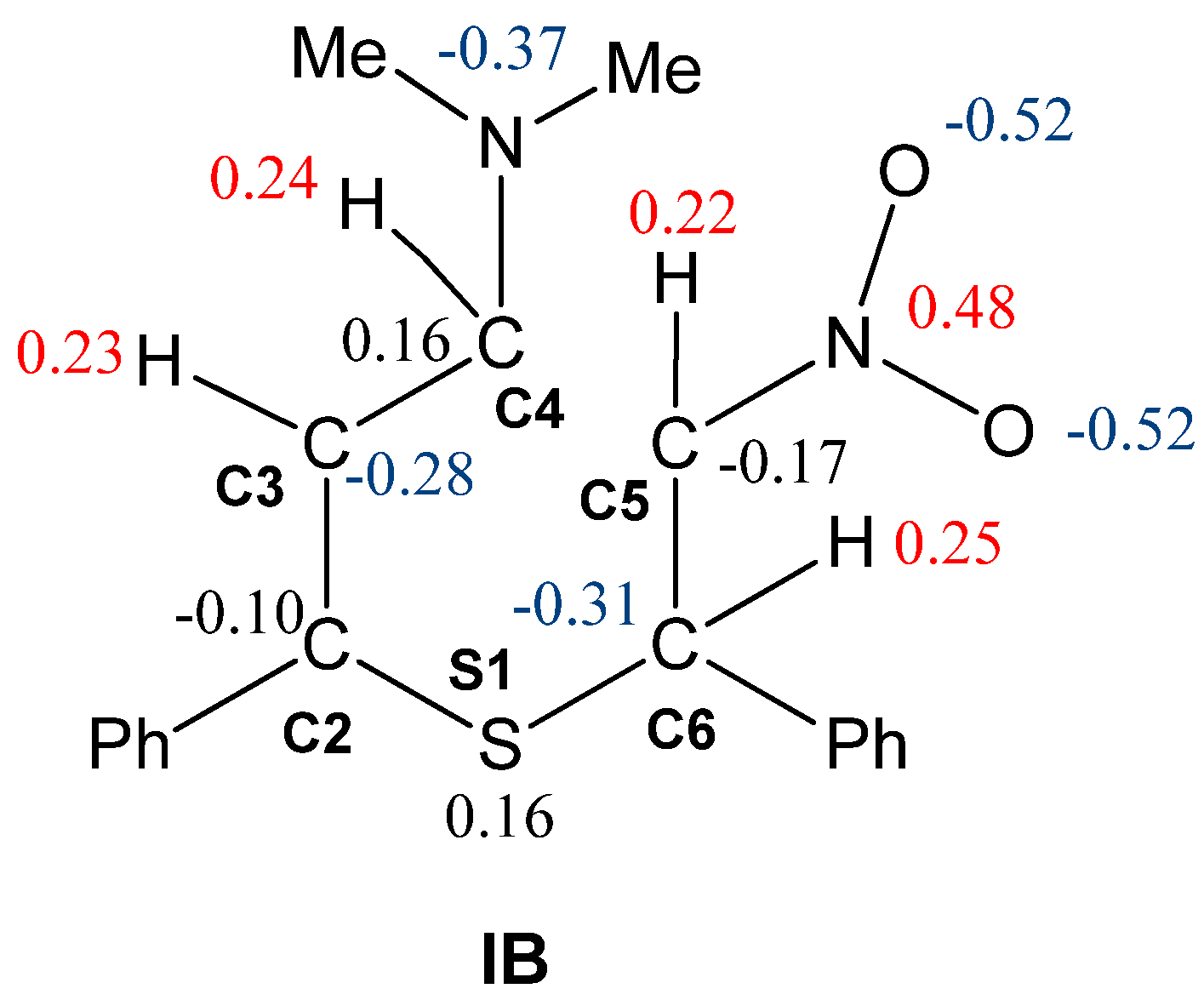
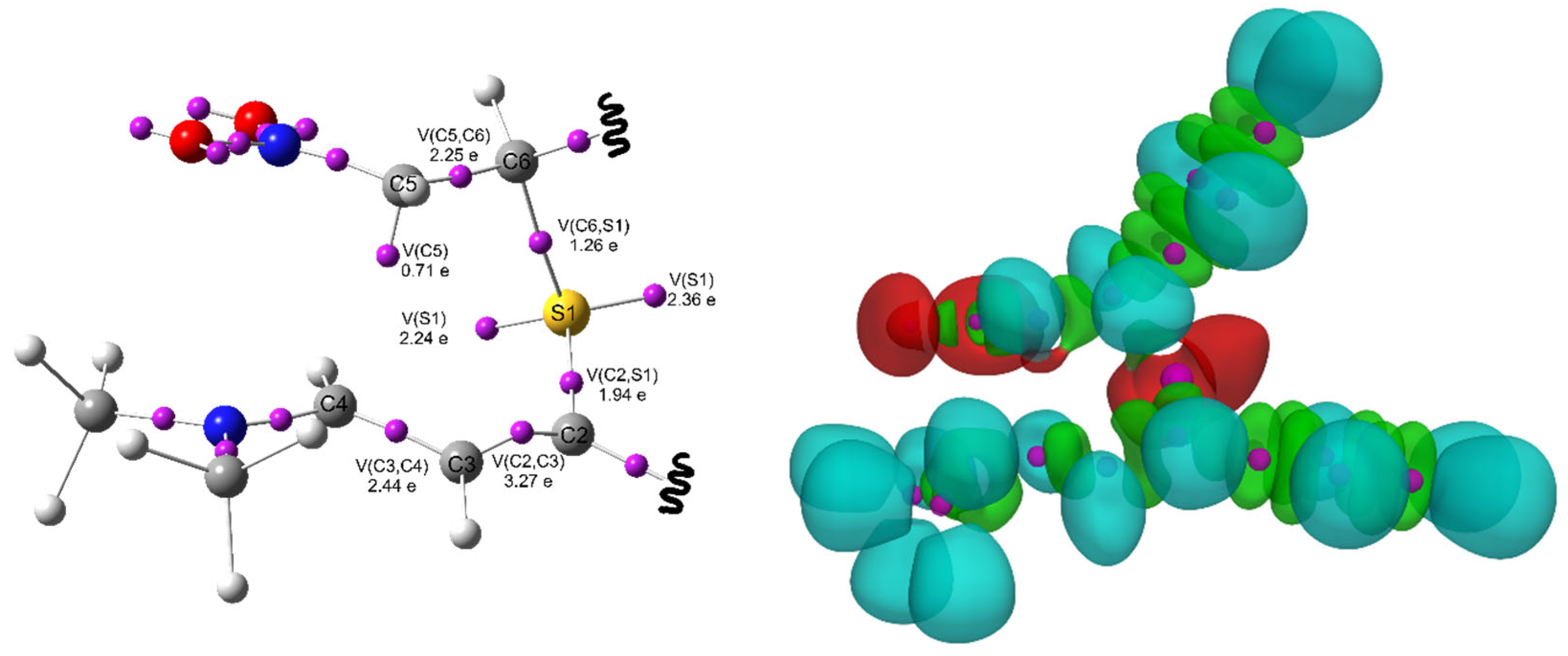


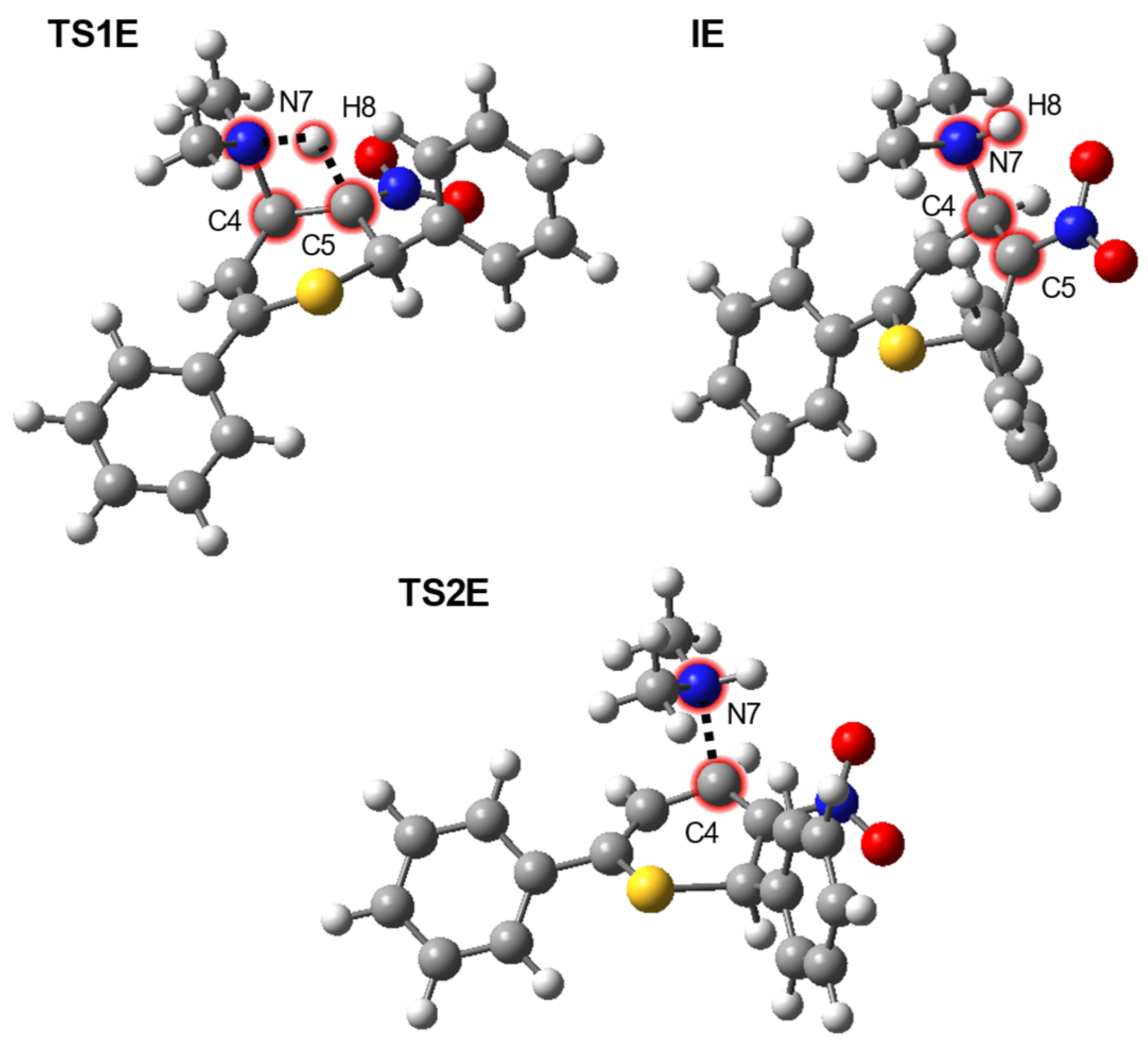
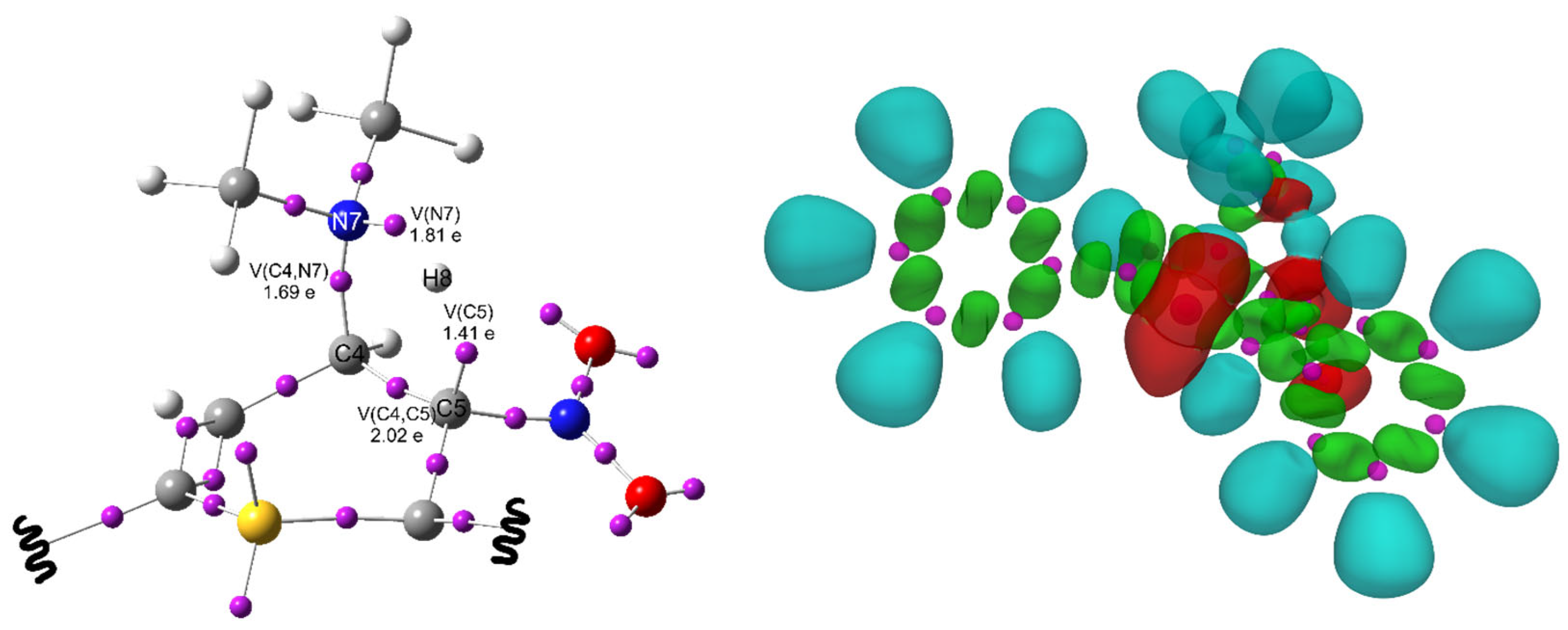

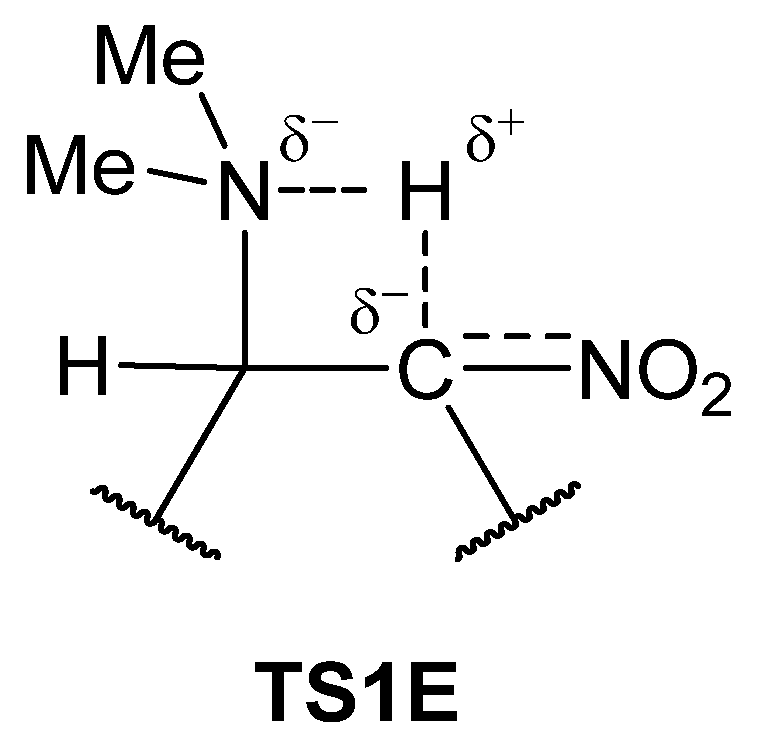
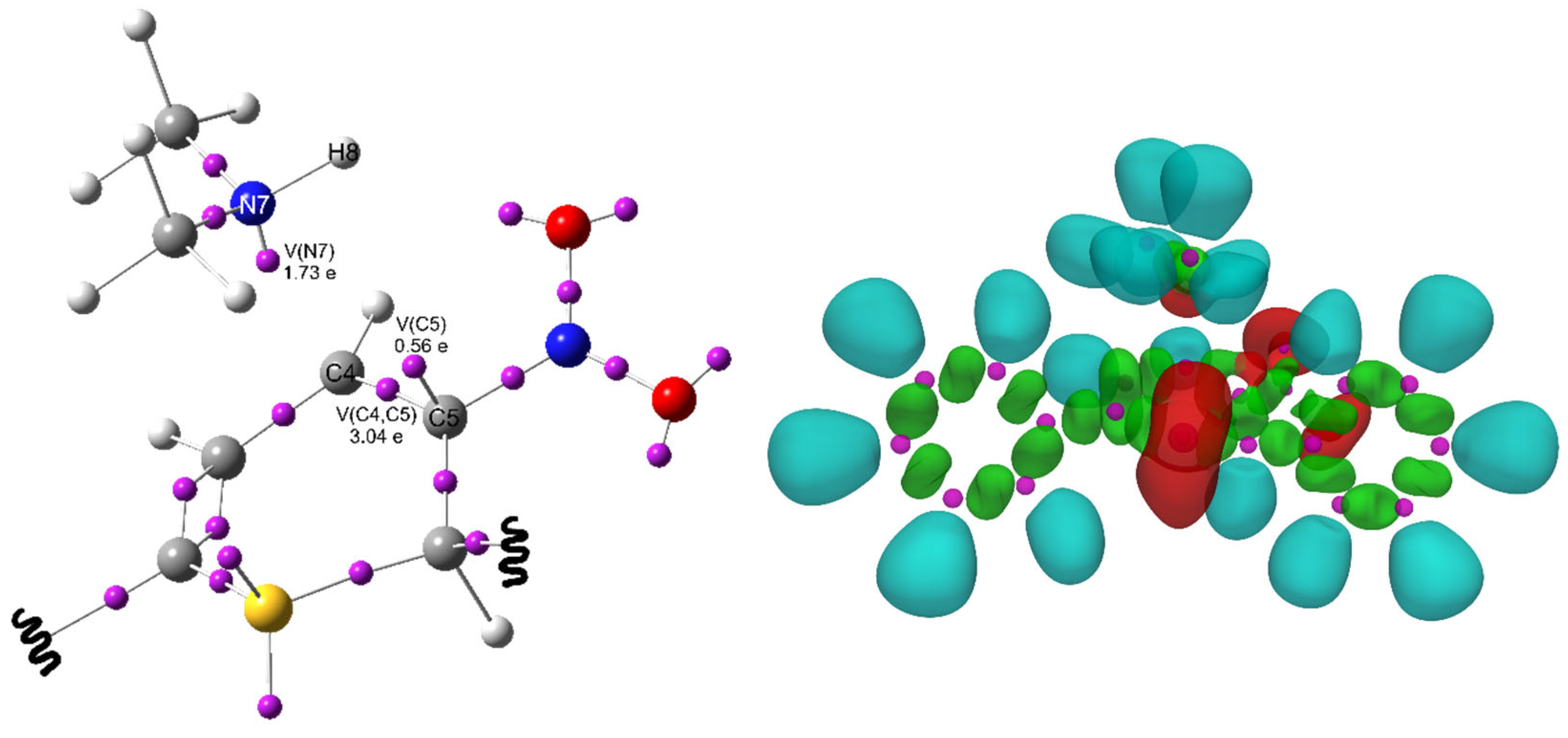

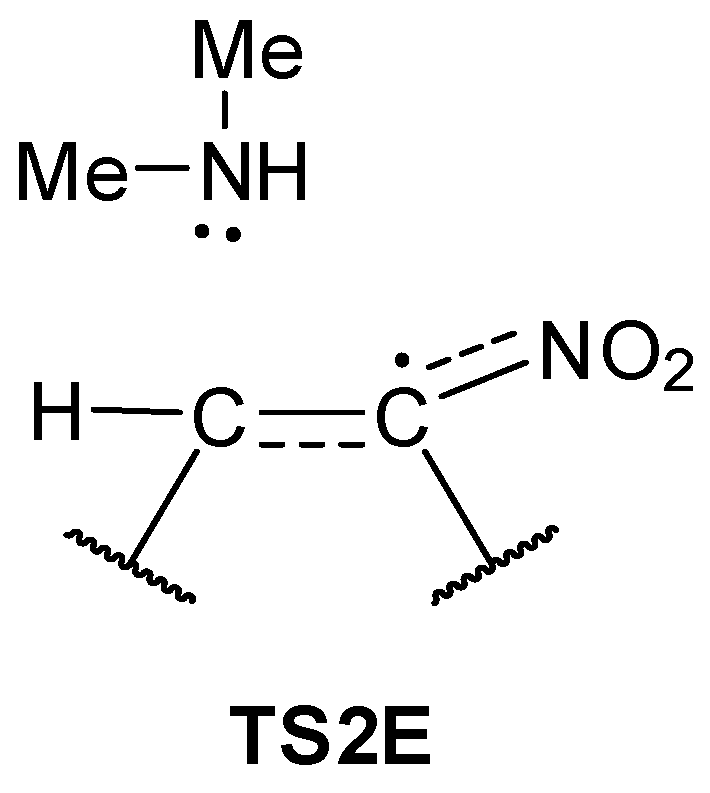
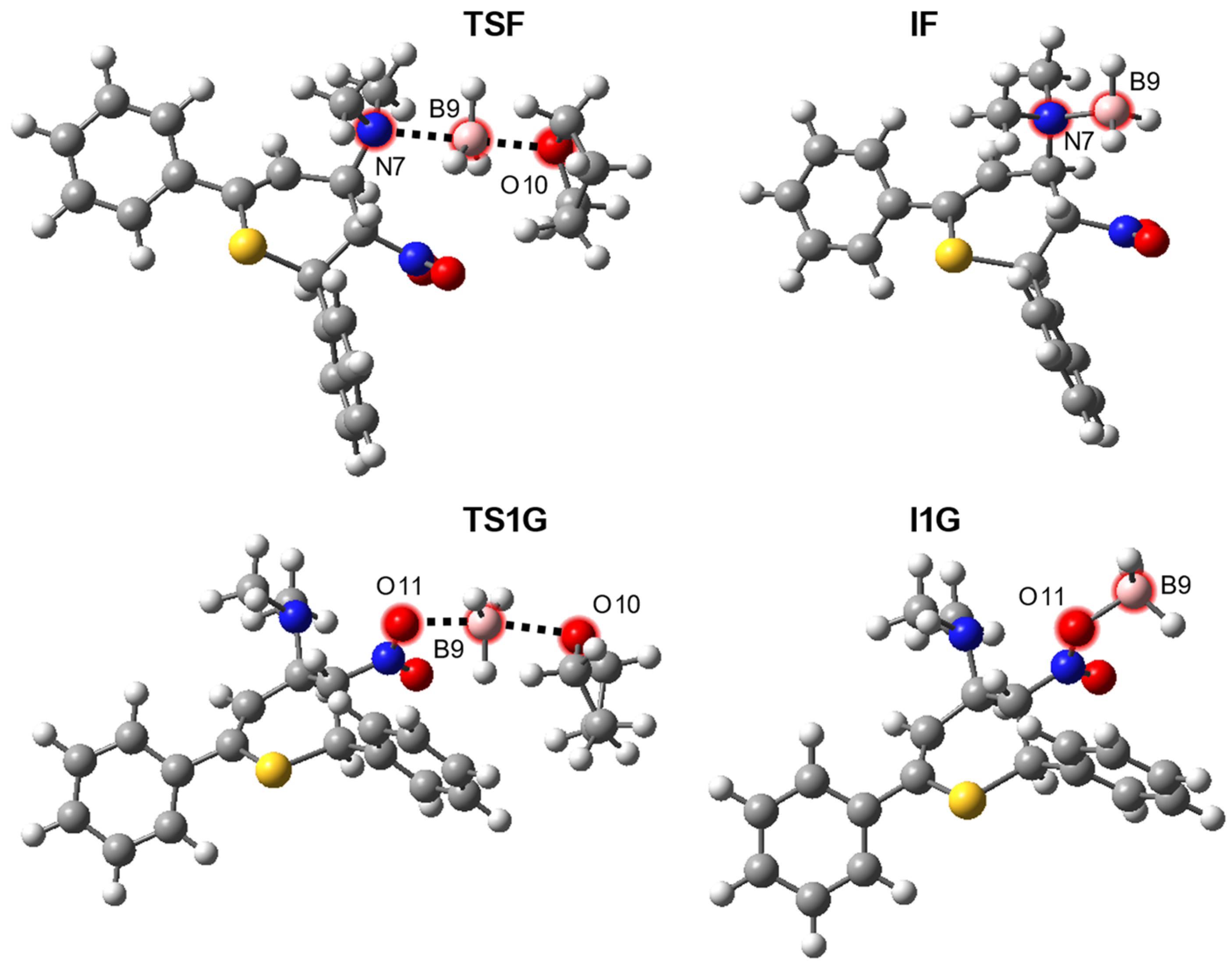
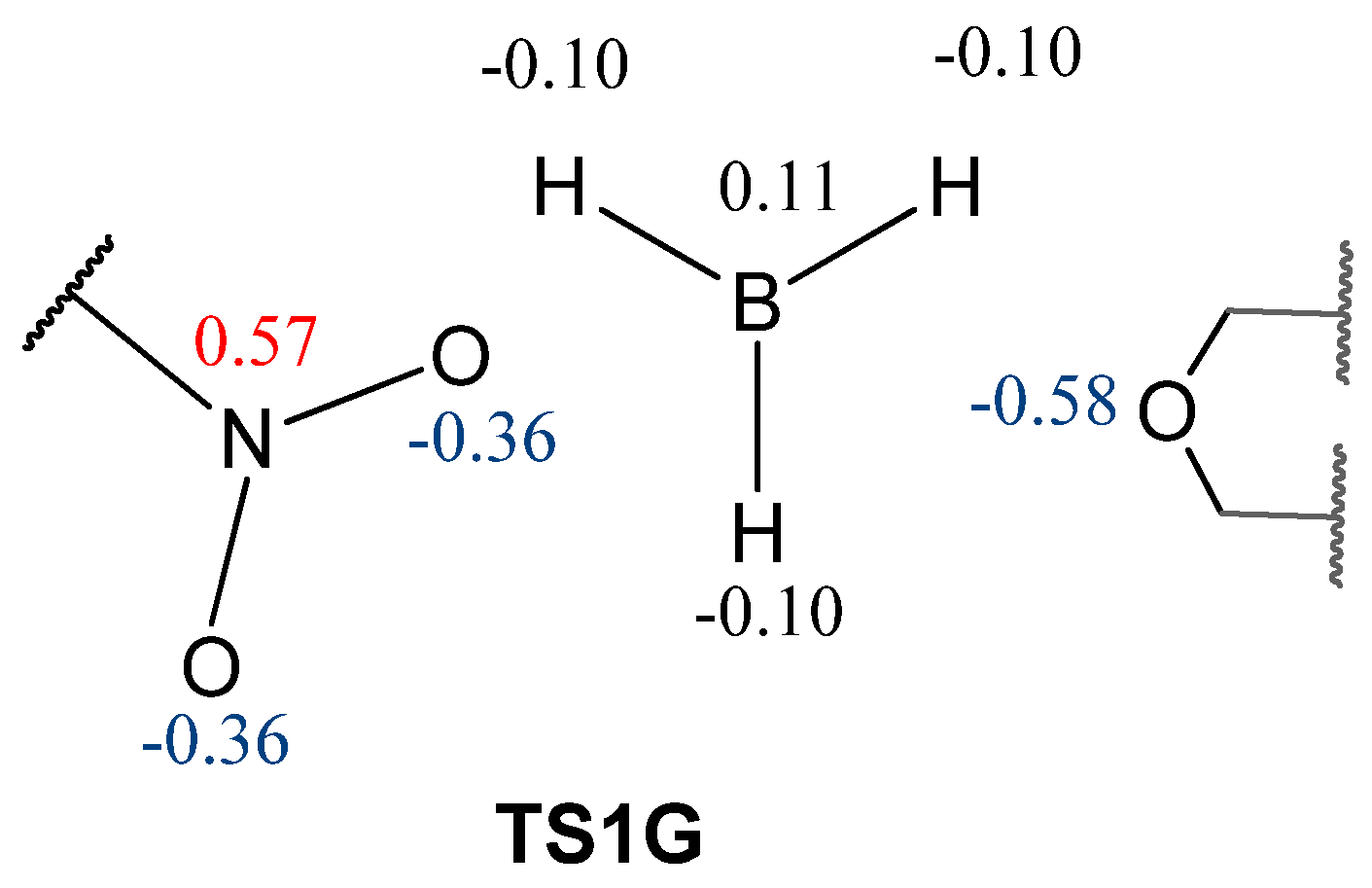

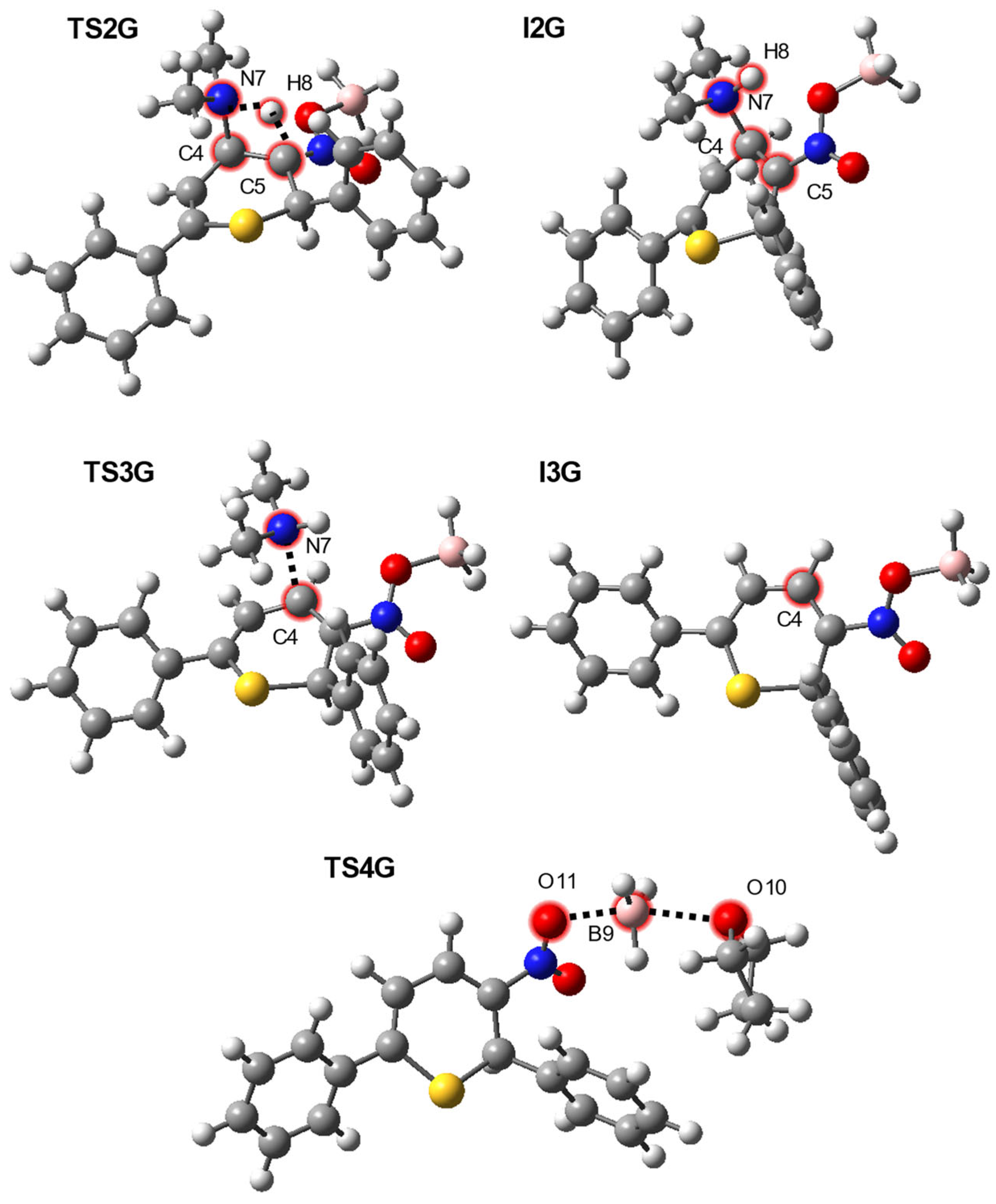
| Path | Transition | ∆H | ∆S | ∆G |
|---|---|---|---|---|
| A | 1 + 2→MCA | −10.0 | −39.4 | 1.7 |
| 1 + 2→TS1A | 2.2 | −52.5 | 17.9 | |
| 1 + 2→IA | 2.6 | −50.9 | 17.8 | |
| 1 + 2→TS2A | 2.7 | −52.5 | 18.3 | |
| 1 + 2→4 | −24.3 | −53.1 | −8.5 | |
| B | 1 + 2→MCB | −20.3 | −41.6 | −7.9 |
| 1 + 2→TS1B | −5.1 | −53.1 | 10.7 | |
| 1 + 2→IB | −3.6 | −52.6 | 12.0 | |
| 1 + 2→TS2B | −3.6 | −52.6 | 12.1 | |
| 1 + 2→5 | −27.9 | −50.0 | −13.0 | |
| C | 1 + 2→MCC | −9.6 | −40.8 | 2.5 |
| 1 + 2→TSC | 13.9 | −52.9 | 29.6 | |
| 1 + 2→6 | −19.6 | −53.2 | −3.8 | |
| D | 1 + 2→MCD | −10.6 | −44.9 | 2.8 |
| 1 + 2→TSD | 12.6 | −52.3 | 28.2 | |
| 1 + 2→7 | −22.6 | −54.4 | −6.4 |
| Path | Structure | Interatomic Distances [Å] | GEDT [e] | |||||
|---|---|---|---|---|---|---|---|---|
| S1–C2 | C2–C3 | C3–C4 | C4–C5 | C5–C6 | C6–S1 | |||
| 1 | 1.691 | 1.403 | 1.396 | |||||
| 2 | 1.332 | |||||||
| A | MCA | 1.692 | 1.402 | 1.397 | 3.684 | 1.333 | 3.665 | |
| TS1A | 1.740 | 1.363 | 1.436 | 2.913 | 1.412 | 2.167 | −0.50 | |
| IA | 1.763 | 1.352 | 1.449 | 2.801 | 1.448 | 1.985 | −0.66 | |
| TS2A | 1.767 | 1.346 | 1.460 | 2.399 | 1.471 | 1.924 | −0.60 | |
| 4 | 1.773 | 1.335 | 1.505 | 1.562 | 1.528 | 1.832 | ||
| B | MCB | 1.693 | 1.409 | 1.385 | 3.762 | 1.334 | 3.901 | |
| TS1B | 1.754 | 1.364 | 1.424 | 2.802 | 1.445 | 1.987 | −0.48 | |
| IB | 1.756 | 1.357 | 1.437 | 2.362 | 1.460 | 1.958 | −0.55 | |
| TS2B | 1.756 | 1.357 | 1.437 | 2.362 | 1.460 | 1.958 | −0.55 | |
| 5 | 1.776 | 1.335 | 1.508 | 1.530 | 1.532 | 1.830 | ||
| C | MCC | 1.689 | 1.403 | 1.396 | 3.241 | 1.330 | 3.497 | |
| TSC | 1.734 | 1.360 | 1.439 | 2.370 | 1.423 | 2.071 | −0.40 | |
| 6 | 1.775 | 1.333 | 1.505 | 1.563 | 1.526 | 1.825 | ||
| D | MCD | 1.689 | 1.404 | 1.396 | 3.473 | 1.333 | 3.469 | |
| TSD | 1.724 | 1.361 | 1.438 | 2.237 | 1.409 | 2.172 | −0.31 | |
| 7 | 1.777 | 1.333 | 1.502 | 1.563 | 1.546 | 1.784 | ||
| Path | Transition | ∆H | ∆S | ∆G |
|---|---|---|---|---|
| E | 5→TS1E | 41.7 | −3.3 | 42.7 |
| 5→IE | 15.9 | −4.8 | 17.4 | |
| 5→TS2E | 21.9 | −2.4 | 22.6 | |
| 5→3 + N(H)Me2 | 21.1 | 44.7 | 7.8 | |
| F | THF·BH3 + 5→TSF | 5.0 | −43.4 | 18.0 |
| THF·BH3 + 5→IF + THF | −4.7 | −13.8 | −0.6 | |
| G | THF·BH3 + 5→TS1G | 8.7 | −39.9 | 20.6 |
| THF·BH3 + 5→I1G + THF | 11.2 | −5.6 | 12.8 | |
| I1G→TS2G | 36.0 | −5.1 | 37.5 | |
| I1G→I2G | 2.0 | −6.0 | 3.8 | |
| I1G→TS3G | 13.2 | −1.0 | 13.4 | |
| I1G→I3G + N(H)Me2 | 19.7 | 43.4 | 6.7 | |
| THF + I3G→TS4G | 0.9 | −31.6 | 10.3 | |
| THF + I3G→3 + THF·BH3 | −9.7 | 6.8 | −11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowski, M.; Dresler, E.; Jasiński, R. On the Question of the Regio-Orientation, Stereo-Orientation and Molecular Mechanism in the Cascade Cycloaddition/Rearrangement/Elimination Processes Leading to Nitro-Substituted Thiopyran Analogs: DFT Computational Study. Int. J. Mol. Sci. 2025, 26, 8948. https://doi.org/10.3390/ijms26188948
Sadowski M, Dresler E, Jasiński R. On the Question of the Regio-Orientation, Stereo-Orientation and Molecular Mechanism in the Cascade Cycloaddition/Rearrangement/Elimination Processes Leading to Nitro-Substituted Thiopyran Analogs: DFT Computational Study. International Journal of Molecular Sciences. 2025; 26(18):8948. https://doi.org/10.3390/ijms26188948
Chicago/Turabian StyleSadowski, Mikołaj, Ewa Dresler, and Radomir Jasiński. 2025. "On the Question of the Regio-Orientation, Stereo-Orientation and Molecular Mechanism in the Cascade Cycloaddition/Rearrangement/Elimination Processes Leading to Nitro-Substituted Thiopyran Analogs: DFT Computational Study" International Journal of Molecular Sciences 26, no. 18: 8948. https://doi.org/10.3390/ijms26188948
APA StyleSadowski, M., Dresler, E., & Jasiński, R. (2025). On the Question of the Regio-Orientation, Stereo-Orientation and Molecular Mechanism in the Cascade Cycloaddition/Rearrangement/Elimination Processes Leading to Nitro-Substituted Thiopyran Analogs: DFT Computational Study. International Journal of Molecular Sciences, 26(18), 8948. https://doi.org/10.3390/ijms26188948







