A Tissue Factor Bi-Specific T-Cell Engager Provides Effective Targeting and Cytotoxicity Against Cervical Cancer Cell Lines
Abstract
1. Introduction
2. Results
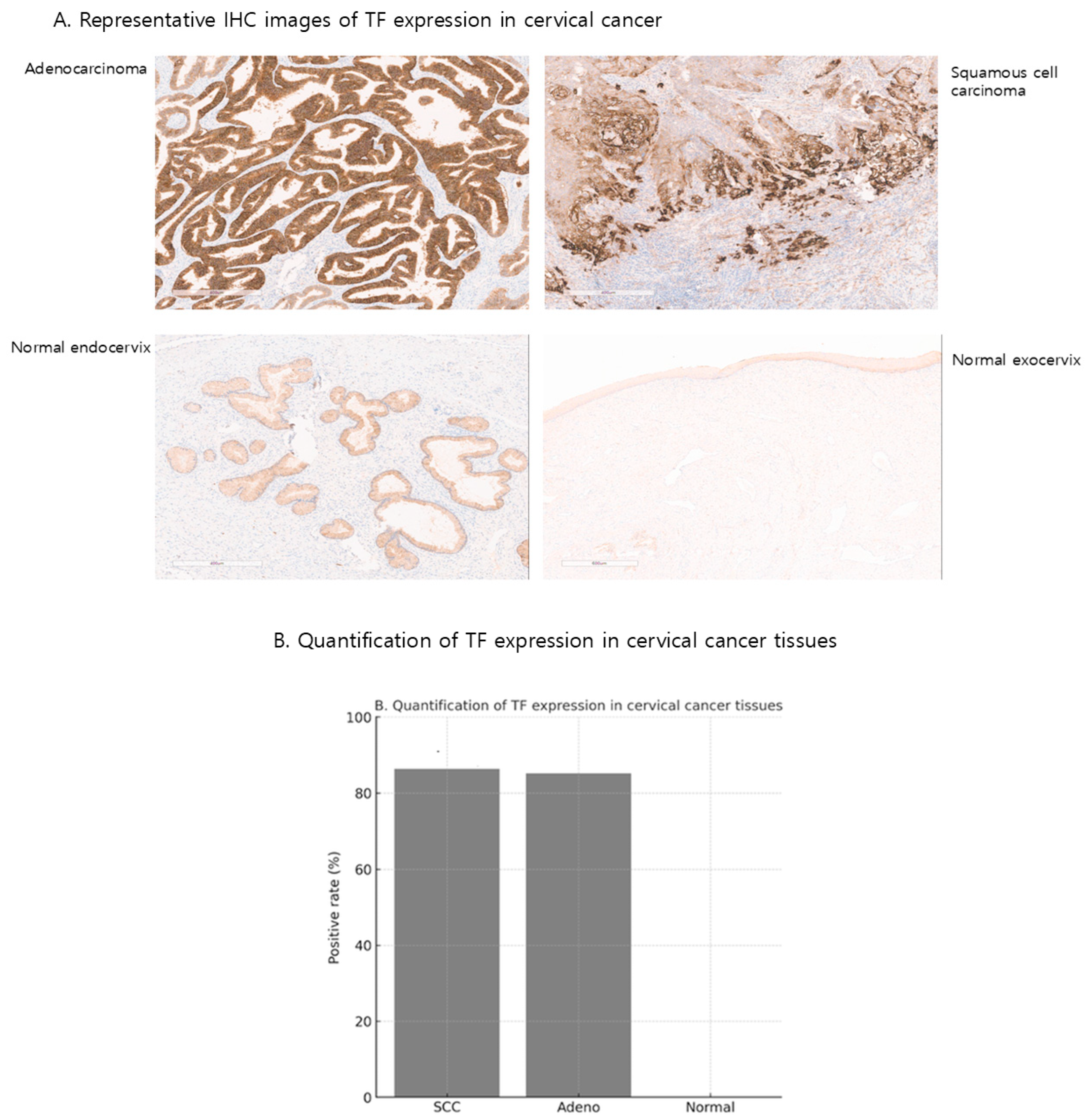
2.1. TF Expression in Cervical Cancer and Normal Human Tissues from Cervix
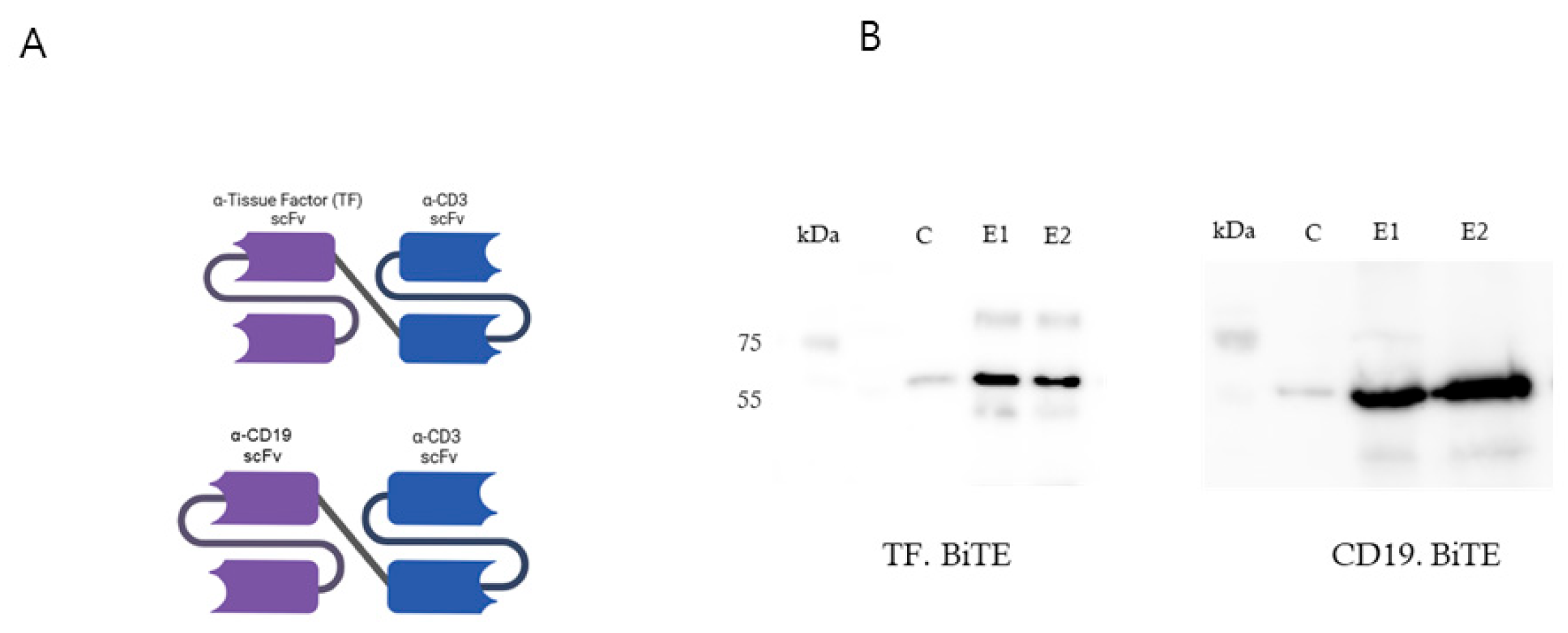
2.2. Design and Production of TF BiTE
2.2.1. Schematic Diagram of TF-BiTE Structure
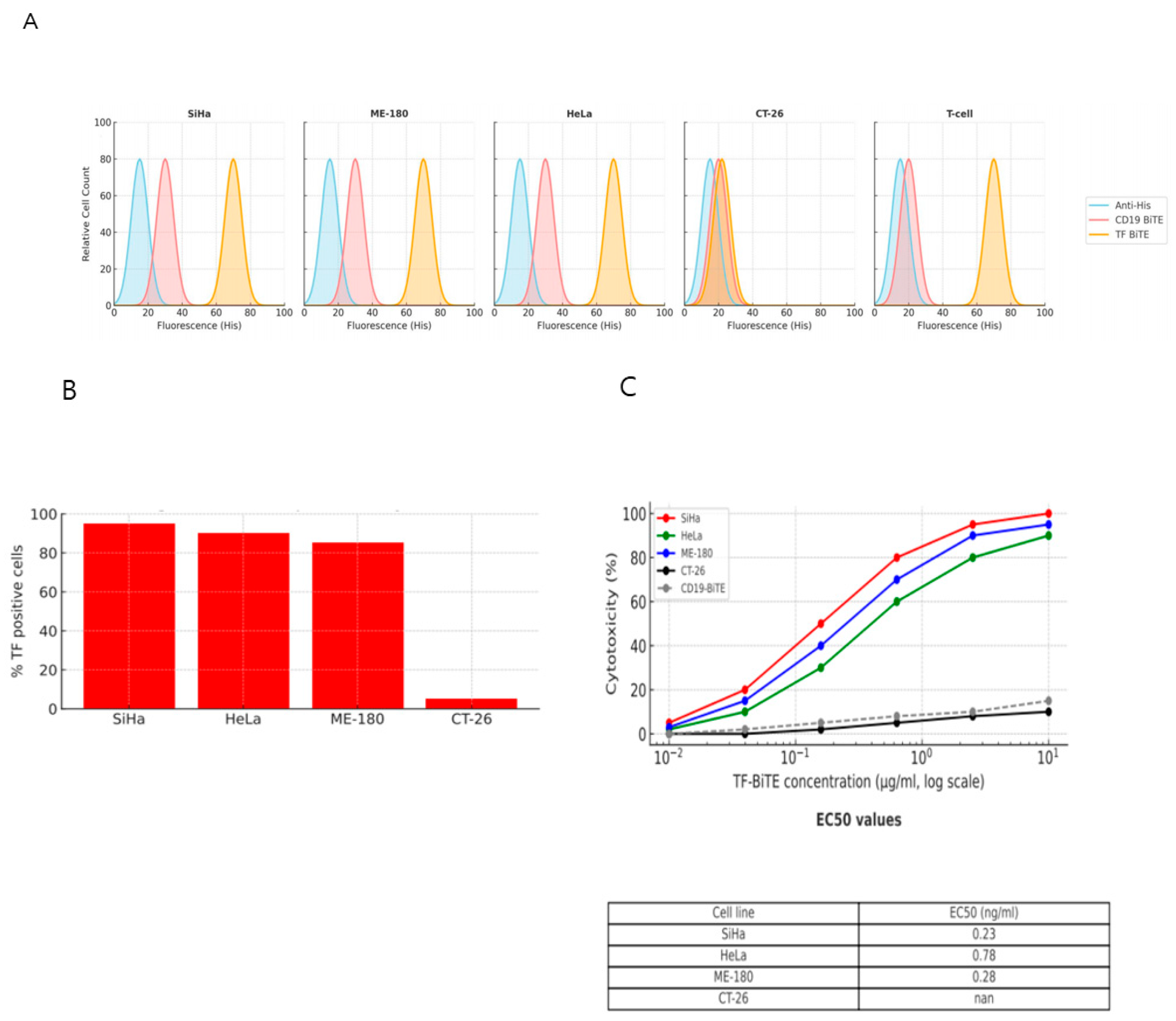
2.2.2. TF-BiTE Directs T Cells to Kill TF-Expressing Cervical Cancer Cells In Vitro
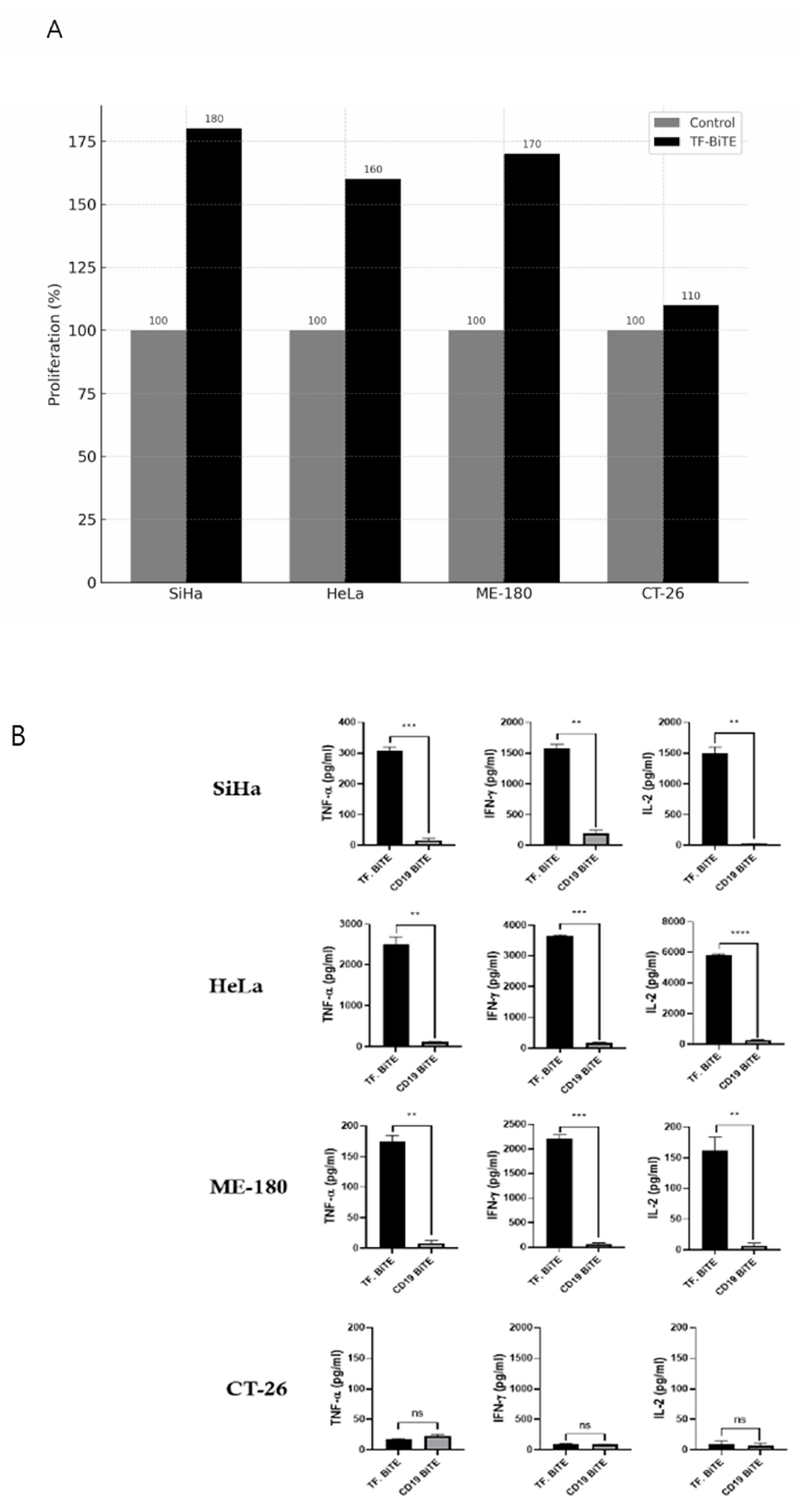
2.3. Preliminary Mechanism Insights
2.3.1. Proliferation Assay and ELISA
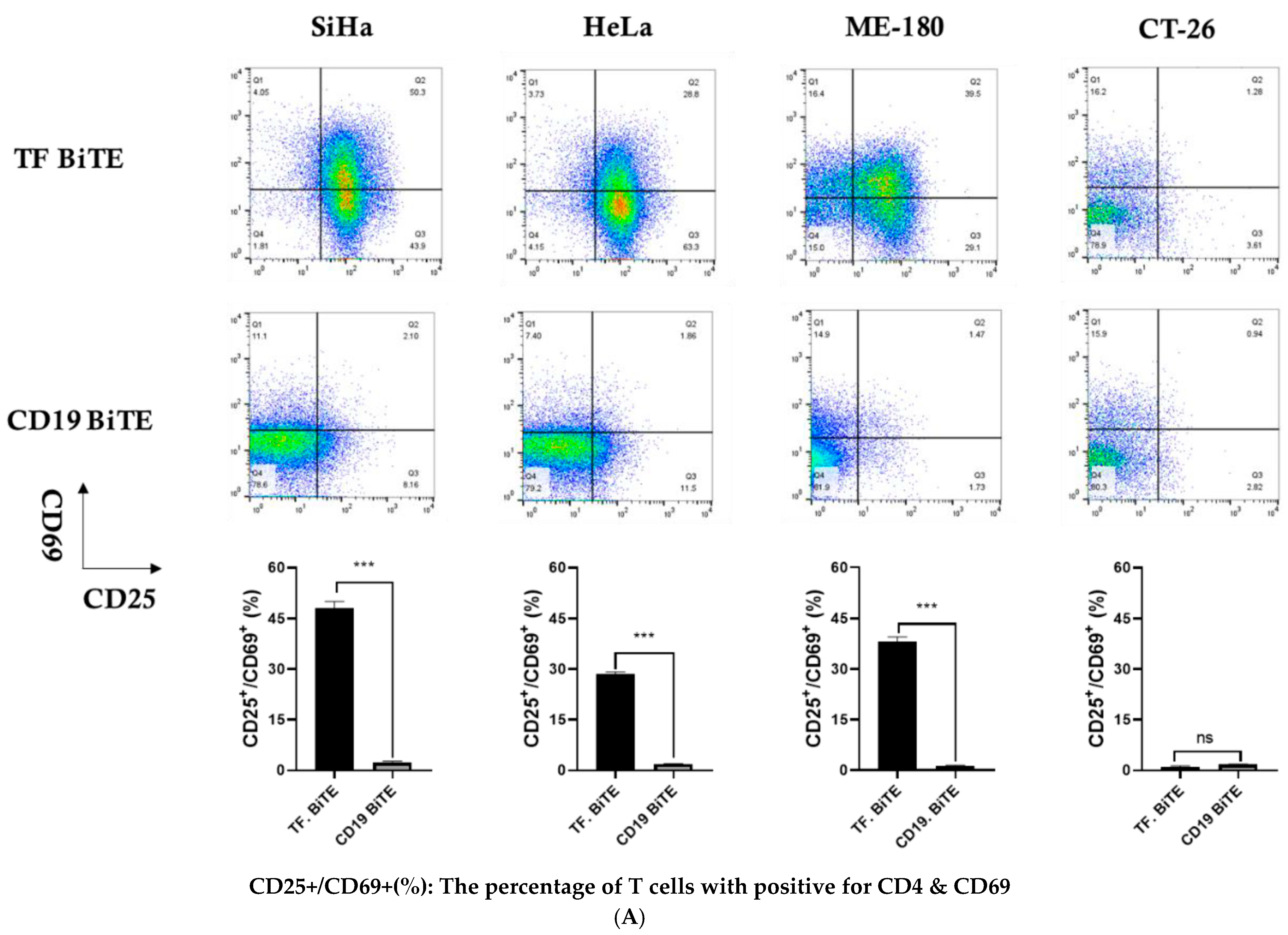
2.3.2. Activation Assay
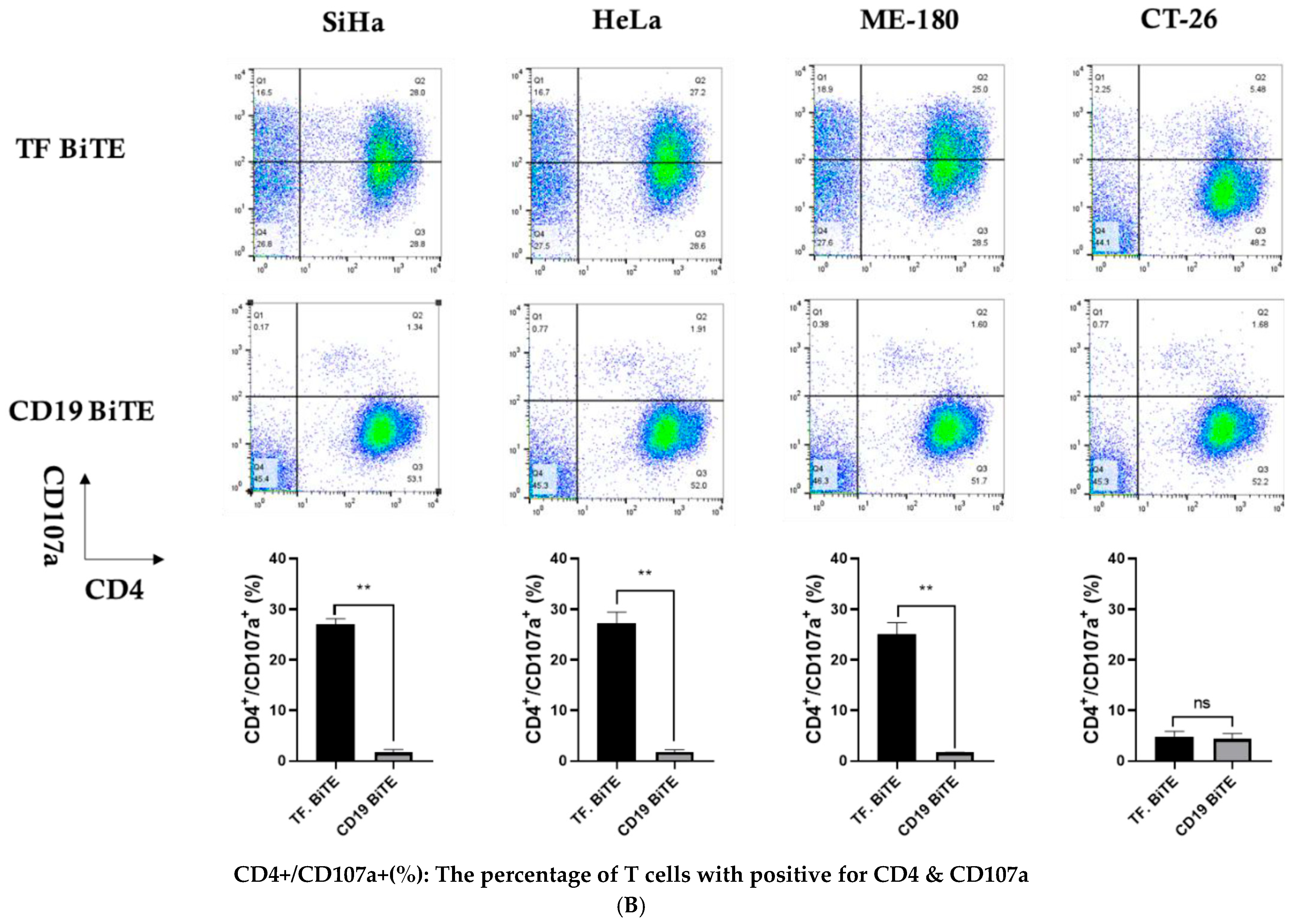
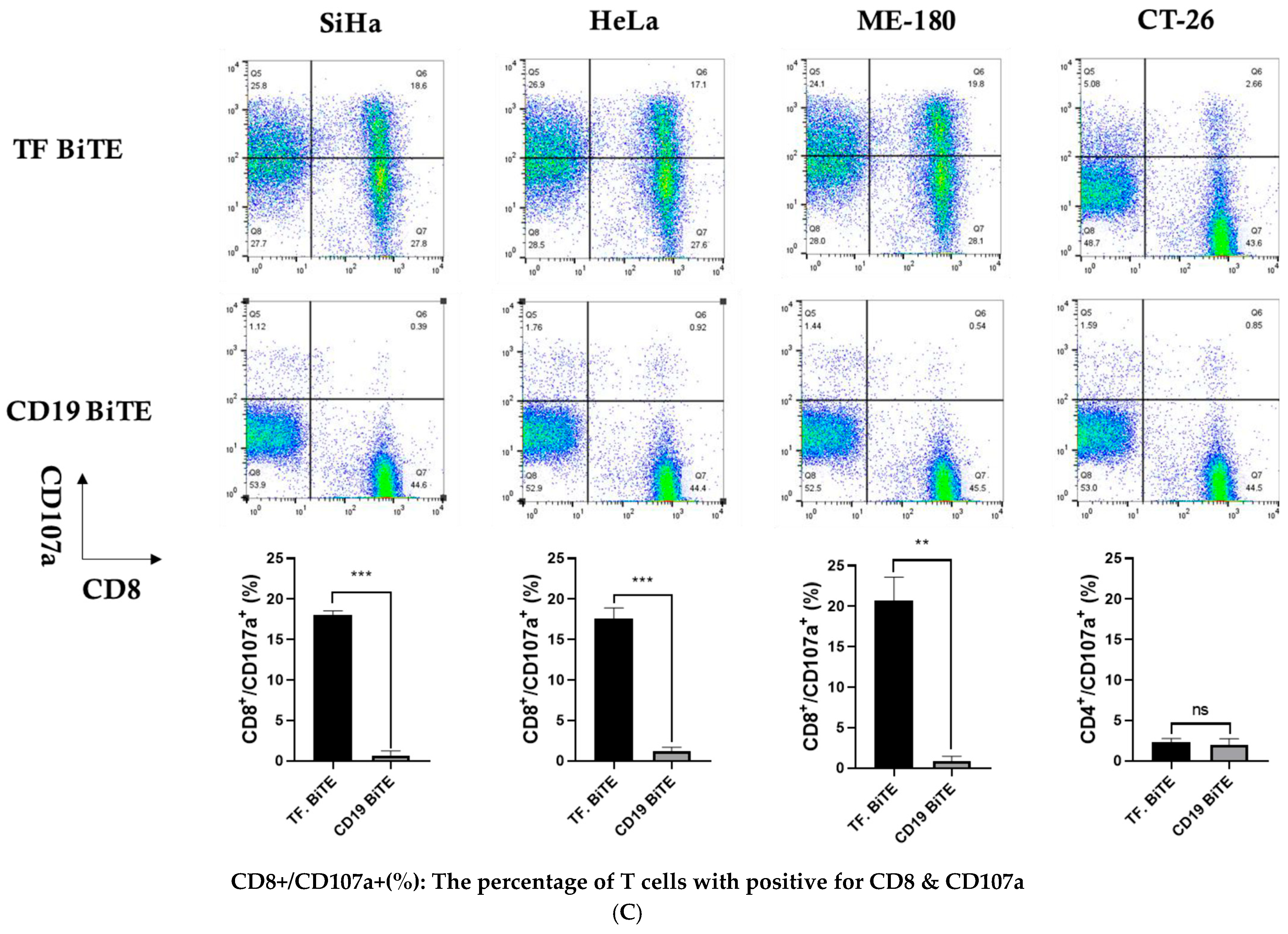
2.3.3. Degranulation Assay
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Patient Samples
4.3. Study Period
4.4. Immunohistochemical Analysis of TF Expression
4.5. Generation and Cloning of TF-BiTE
4.6. Construction and Purification of TF-Targeted BiTE
4.7. In Vitro Cytotoxicity Assay
4.8. Flow Cytometry and Cytokine Quantification
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Usta, E.H.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Manoochehri, H.; Dama, P. Use of CAR T-cell for acute lymphoblastic leukemia (ALL) treatment: A review study. Cancer Gene Ther. 2022, 29, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.; Haber, L.; Kelly, M.P.; Vazzana, K.; Canova, L.; Ram, P.; Pawashe, A.; Finney, J.; Jalal, S.; Chiu, D.; et al. A Mucin 16 bispecific T cell-engaging antibody for the treatment of ovarian cancer. Sci. Transl. Med. 2019, 11, eaau7534. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’BRien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; A Larson, R.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66, Correction in Lancet Oncol. 2015, 16, e158. [Google Scholar] [CrossRef]
- Bacac, M.; Fauti, T.; Sam, J.; Colombetti, S.; Weinzierl, T.; Ouaret, D.; Bodmer, W.; Lehmann, S.; Hofer, T.; Hosse, R.J.; et al. A Novel Carcinoembryonic Antigen T-Cell Bispecific Antibody (CEA TCB) for the Treatment of Solid Tumors. Clin. Cancer Res. 2016, 22, 3286–3297. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Van Dreden, P.; Εlalamy, Ι.; Gerotziafas, G.T. The Role of Tissue Factor in Cancer-Related Hypercoagulability, Tumor Growth, Angiogenesis and Metastasis and Future Therapeutic Strategies. Crit. Rev. Oncog. 2017, 22, 219–248. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin. Thromb. Hemost. 2019, 45, 385–395. [Google Scholar] [CrossRef]
- Breij, E.C.; de Goeij, B.E.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef]
- de Bono, J.S.; Concin, N.; Hong, D.S.; Thistlethwaite, F.C.; Machiels, J.P.; Arkenau, H.T.; Plummer, R.; Jones, R.H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 383–393, Erratum in Lancet Oncol. 2019, 20, e663. [Google Scholar] [CrossRef]
- Gohil, S.H.; Paredes-Moscosso, S.R.; Harrasser, M.; Vezzalini, M.; Scarpa, A.; Morris, E.; Davidoff, A.M.; Sorio, C.; Nathwani, A.C.; Della Peruta, M. An ROR1 bi-specific T-cell engager provides effective targeting and cytotoxicity against a range of solid tumors. Oncoimmunology 2017, 6, e1326437. [Google Scholar] [CrossRef] [PubMed]
- Jitschin, R.; Saul, D.; Braun, M.; Tohumeken, S.; Völkl, S.; Kischel, R.; Lutteropp, M.; Dos Santos, C.; Mackensen, A.; Mougiakakos, D. CD33/CD3-bispecific T-cell engaging (BiTE(R)) antibody construct targets monocytic AML myeloid-derived suppressor cells. J. Immunother. Cancer 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, J.; Yan, Y.; Yao, X.; Fang, L.; Xiong, H.; Liu, Y.; Chu, Q.; Zhou, P.; Wu, K. A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells. J. Exp. Clin. Cancer Res. 2019, 38, 355. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, J.; Xiao, X.; Xie, Y.; Jiang, H.; Zhang, B.; Lu, H.; Yuan, Y.; Han, L.; Zhou, Y.; et al. Characterization of a novel bispecific antibody targeting tissue factor-positive tumors with T cell engagement. Acta Pharm. Sin. B 2022, 12, 1928–1942. [Google Scholar] [CrossRef]
- Kaplon, H.; Crescioli, S.; Chenoweth, A.; Visweswaraiah, J.; Reichert, J.M. Antibodies to watch in 2023. MAbs 2023, 15, 2153410. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518, Correction in N. Engl. J. Med. 2016, 374, 998. [Google Scholar] [CrossRef]
- Yu, L.; Lanqing, G.; Huang, Z.; Xin, X.; Minglin, L.; Fa-Hui, L.; Zou, H.; Min, J. T cell immunotherapy for cervical cancer: Challenges and opportunities. Front. Immunol. 2023, 14, 1105265. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, J.; Zhu, L.; Hu, W.; Liu, B.; Xie, L. Adoptive tumor infiltrating lymphocytes cell therapy for cervical cancer. Hum. Vaccin. Immunother. 2022, 18, 2060019. [Google Scholar] [CrossRef]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Lee, K.-J.; Shin, E.; Park, S.T.; Kim, H.S.; Cho, H.-Y. CT83 Promotes Cancer Progression by Upregulation of PDL1 in Adenocarcinoma of the Cervix. Int. J. Mol. Sci. 2025, 26, 2687. [Google Scholar] [CrossRef] [PubMed]
- Brochet, X.; Lefranc, M.-P.; Giudicelli, V. IMGT/V-QUEST: The highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008, 36, W503–W508. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-j.; Kim, G.; Seo, B.; Jeong, S.Y.; Kim, H.S.; Cho, H.-Y.; Park, S.T. A Tissue Factor Bi-Specific T-Cell Engager Provides Effective Targeting and Cytotoxicity Against Cervical Cancer Cell Lines. Int. J. Mol. Sci. 2025, 26, 8941. https://doi.org/10.3390/ijms26188941
Lee K-j, Kim G, Seo B, Jeong SY, Kim HS, Cho H-Y, Park ST. A Tissue Factor Bi-Specific T-Cell Engager Provides Effective Targeting and Cytotoxicity Against Cervical Cancer Cell Lines. International Journal of Molecular Sciences. 2025; 26(18):8941. https://doi.org/10.3390/ijms26188941
Chicago/Turabian StyleLee, Kyung-jun, Gilhyang Kim, Booseong Seo, Soo Young Jeong, Hyeong Su Kim, Hye-Yon Cho, and Sung Taek Park. 2025. "A Tissue Factor Bi-Specific T-Cell Engager Provides Effective Targeting and Cytotoxicity Against Cervical Cancer Cell Lines" International Journal of Molecular Sciences 26, no. 18: 8941. https://doi.org/10.3390/ijms26188941
APA StyleLee, K.-j., Kim, G., Seo, B., Jeong, S. Y., Kim, H. S., Cho, H.-Y., & Park, S. T. (2025). A Tissue Factor Bi-Specific T-Cell Engager Provides Effective Targeting and Cytotoxicity Against Cervical Cancer Cell Lines. International Journal of Molecular Sciences, 26(18), 8941. https://doi.org/10.3390/ijms26188941




