Functional Recovery by Transplantation of Human iPSC-Derived A2B5 Positive Neural Progenitor Cell After Spinal Cord Injury in Mice
Abstract
1. Introduction
2. Results
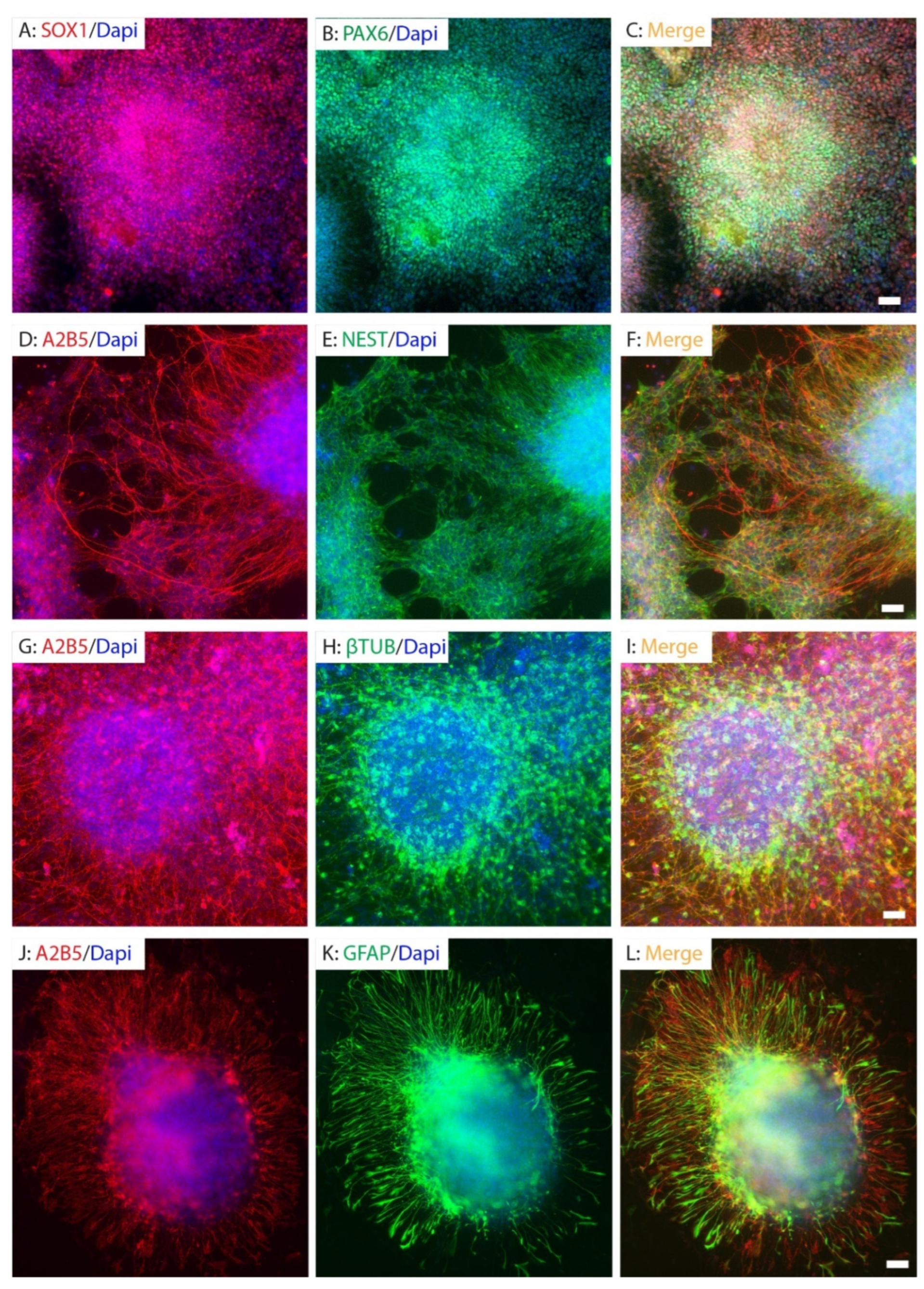
2.1. Human iPSC Reprogramming and Neural Differentiation In Vitro
2.2. Purification and Differentiation of hiPSC-Derived Neural Progenitor Cells In Vitro
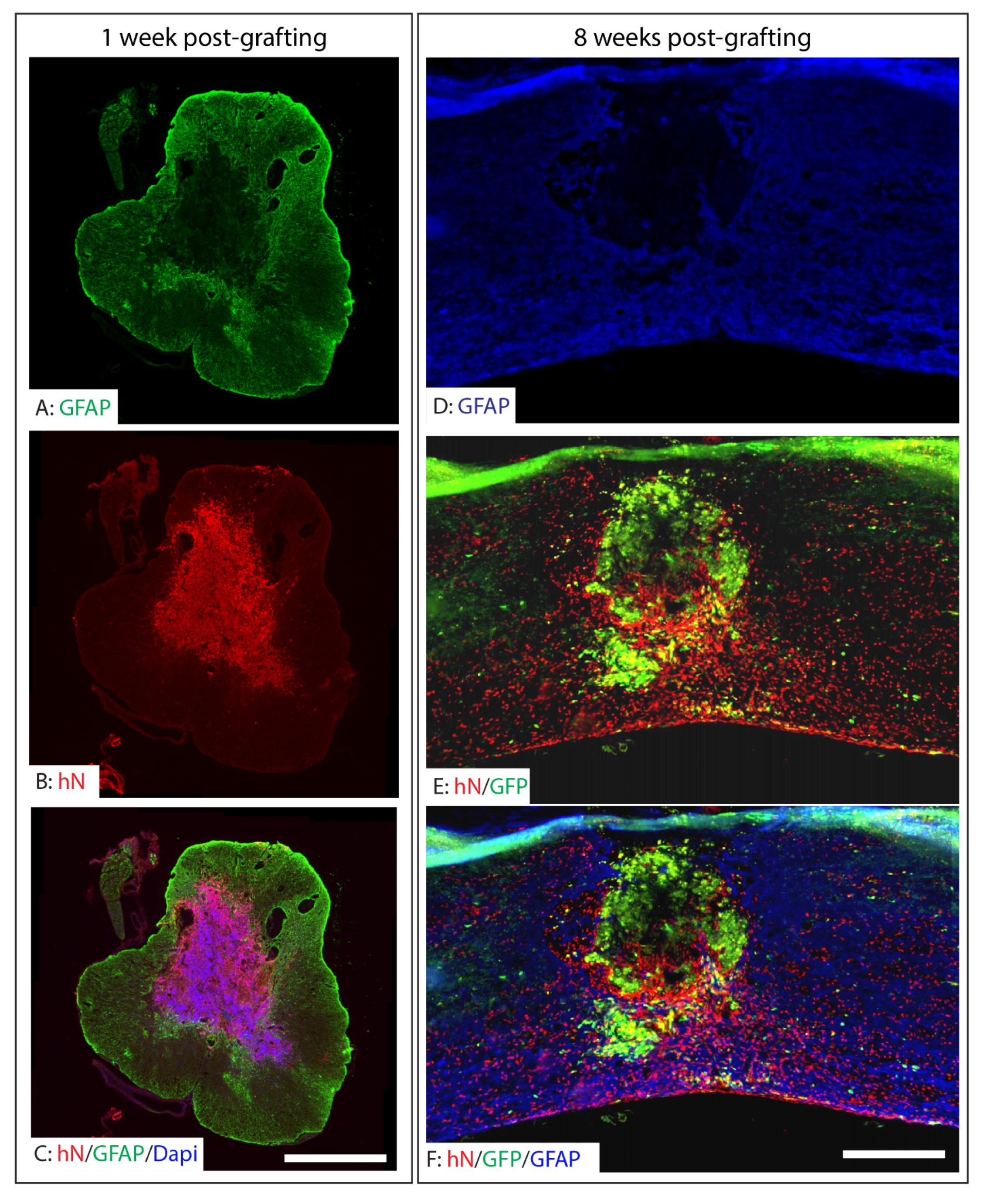
2.3. Survival, Integration, and Migration of Grafted iPSC-Derived Neural Progenitor Cells
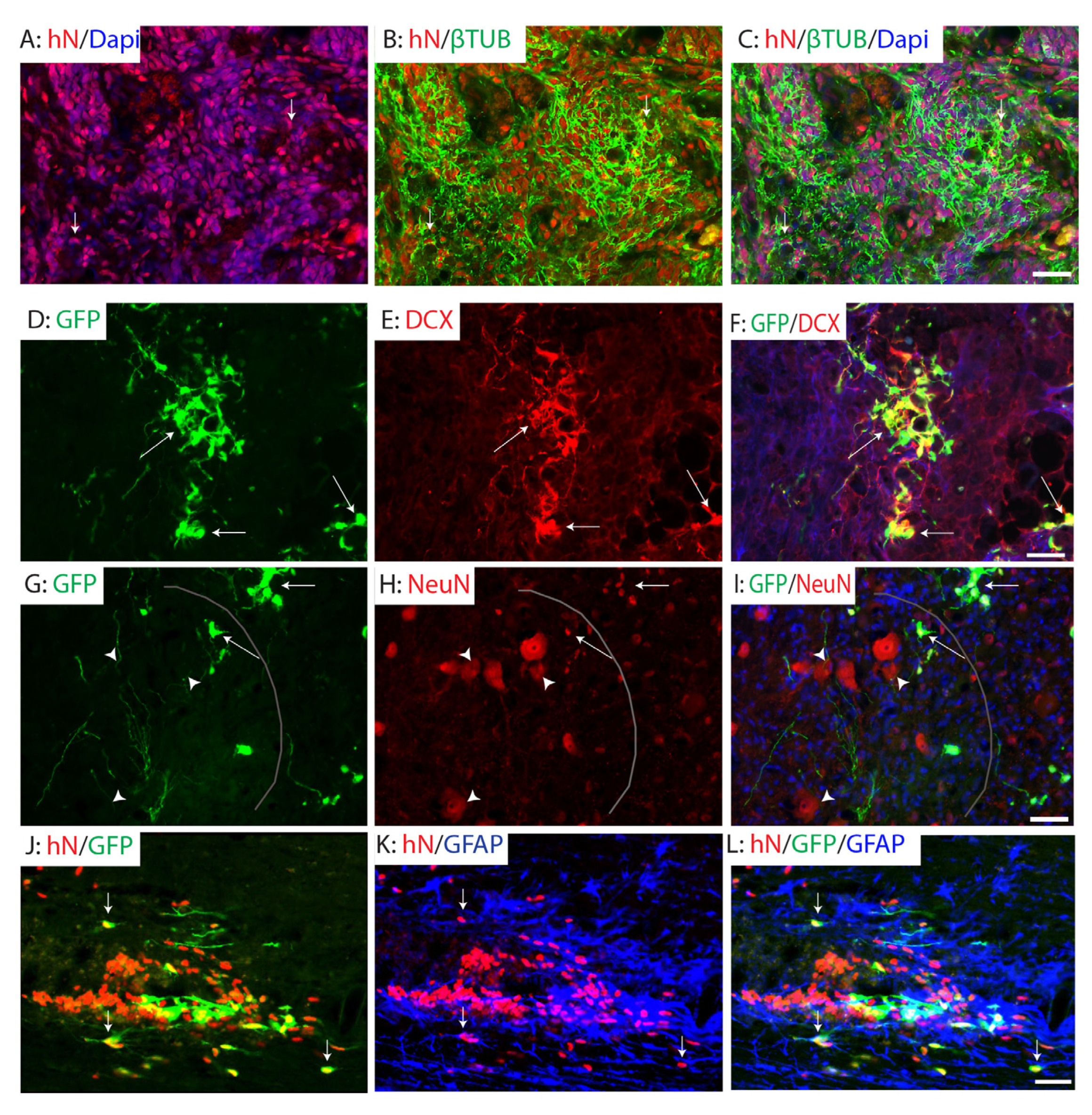
2.4. Differentiation of Grafted NPCs Following SCI
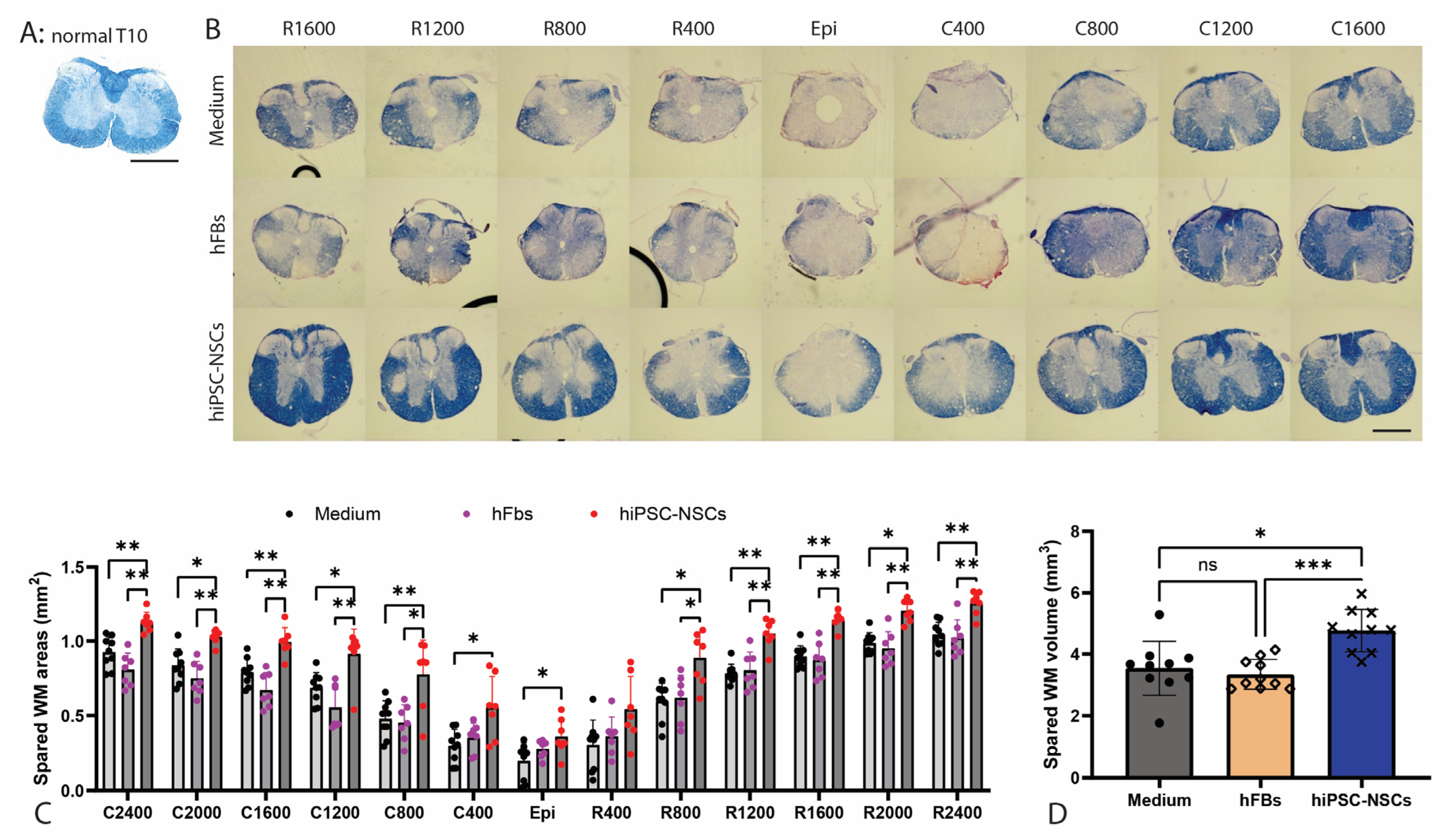
2.5. Preservation of White Matter Following Transplantation of A2B5+ iPSC-NPCs in SCI
2.6. Functional Recovery After Transplantation of Human iPSC-Derived NPCs After SCI
3. Discussion
4. Materials and Methods
4.1. Human iPSC Reprogramming
4.2. Cell Culture
4.3. Differentiation of iPSCs into Neural Progenitor Cells (NPCs)
4.4. Purification of A2B5+ NPCs by FACS
4.5. In Vitro Differentiation of NPCs
4.6. Cell Preparation for Transplantation
4.7. Animals
4.8. Thoracic Contusion SCI and Cell Transplantation
4.9. Behavioral Assessments
4.9.1. Basso Mouse Scale (BMS)
4.9.2. Elevated Gradient Beam-Walking
4.10. Immunofluorescence and Immunohistochemistry
4.11. Assessment of Spared White Matter by EC Staining
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemos, N.; Fernandes, G.L.; Ribeiro, A.M.; Maia-Lemos, P.S.; Contiero, W.; Croos-Bezerra, V.; Tomlison, G.; Faber, J.; Oliveira, A.S.B.; Girao, M. Rehabilitation of People With Chronic Spinal Cord Injury Using a Laparoscopically Implanted Neurostimulator: Impact on Mobility and Urinary, Anorectal, and Sexual Functions. Neuromodulation 2023, 26, 233–245. [Google Scholar] [CrossRef]
- Baroudi, M.; Rezk, A.; Daher, M.; Balmaceno-Criss, M.; Gregoryczyk, J.G.; Sharma, Y.; McDonald, C.L.; Diebo, B.G.; Daniels, A.H. Management of traumatic spinal cord injury: A current concepts review of contemporary and future treatment. Injury 2024, 55, 111472. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.A.; Sharma, K.D.; Xie, J.Y. Delving into the recent advancements of spinal cord injury treatment: A review of recent progress. Neural Regen. Res. 2022, 17, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Zipser, C.M.; Cragg, J.J.; Guest, J.D.; Fehlings, M.G.; Jutzeler, C.R.; Anderson, A.J.; Curt, A. Cell-based and stem-cell-based treatments for spinal cord injury: Evidence from clinical trials. Lancet Neurol. 2022, 21, 659–670. [Google Scholar] [CrossRef]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef]
- Sugai, K.; Nakamura, M.; Okano, H.; Nagoshi, N. Stem cell therapies for spinal cord injury in humans: A review of recent clinical research. Brain Spine 2025, 5, 104207. [Google Scholar] [CrossRef]
- Inoue, M.; Yamaguchi, R.; He, C.C.J.; Ikeda, A.; Okano, H.; Kohyama, J. Current status and prospects of regenerative medicine for spinal cord injury using human induced pluripotent stem cells: A review. Stem Cell Investig. 2023, 10, 6. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Borys, B.; Karimi-Abdolrezaee, S. Neural stem cell therapies for spinal cord injury repair: An update on recent preclinical and clinical advances. Brain 2024, 147, 766–793. [Google Scholar] [CrossRef]
- Pieczonka, K.; Nakashima, H.; Nagoshi, N.; Yokota, K.; Hong, J.; Badner, A.; Chio, J.C.T.; Shibata, S.; Khazaei, M.; Fehlings, M.G. Human Spinal Oligodendrogenic Neural Progenitor Cells Enhance Pathophysiological Outcomes and Functional Recovery in a Clinically Relevant Cervical Spinal Cord Injury Rat Model. Stem Cells Transl. Med. 2023, 12, 603–616. [Google Scholar] [CrossRef]
- Salazar, D.L.; Uchida, N.; Hamers, F.P.; Cummings, B.J.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS ONE 2010, 5, e12272. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Zheng, Y.; Gallegos, C.M.; Xue, H.; Li, S.; Kim, D.H.; Zhou, H.; Xia, X.; Liu, Y.; Cao, Q. Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Promotes Forelimb Functional Recovery after Cervical Spinal Cord Injury. Cells 2022, 11, 2765. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Okada, Y.; Itakura, G.; Iwai, H.; Nishimura, S.; Yasuda, A.; Nori, S.; Hikishima, K.; Konomi, T.; Fujiyoshi, K.; et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE 2012, 7, e52787. [Google Scholar] [CrossRef] [PubMed]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef]
- van Gorp, S.; Leerink, M.; Kakinohana, O.; Platoshyn, O.; Santucci, C.; Galik, J.; Joosten, E.A.; Hruska-Plochan, M.; Goldberg, D.; Marsala, S.; et al. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res. Ther. 2013, 4, 57. [Google Scholar] [CrossRef]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950.E6. [Google Scholar] [CrossRef]
- Lu, P.; Ceto, S.; Wang, Y.; Graham, L.; Wu, D.; Kumamaru, H.; Staufenberg, E.; Tuszynski, M.H. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J. Clin. Investig. 2017, 127, 3287–3299. [Google Scholar] [CrossRef]
- Han, X.; Chen, M.; Wang, F.; Windrem, M.; Wang, S.; Shanz, S.; Xu, Q.; Oberheim, N.A.; Bekar, L.; Betstadt, S.; et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 2013, 12, 342–353. [Google Scholar] [CrossRef]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef]
- Tsuji, O.; Miura, K.; Okada, Y.; Fujiyoshi, K.; Mukaino, M.; Nagoshi, N.; Kitamura, K.; Kumagai, G.; Nishino, M.; Tomisato, S.; et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl. Acad. Sci. USA 2010, 107, 12704–12709. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Abematsu, M.; Falk, A.; Tsujimura, K.; Sanosaka, T.; Juliandi, B.; Semi, K.; Namihira, M.; Komiya, S.; Smith, A.; et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells 2012, 30, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Sugai, K.; Okano, H.; Nakamura, M. Regenerative Medicine for Spinal Cord Injury Using Induced Pluripotent Stem Cells. Spine Surg. Relat. Res. 2024, 8, 22–28. [Google Scholar] [CrossRef]
- Zeng, C.W. Stem Cell-Based Approaches for Spinal Cord Injury: The Promise of iPSCs. Biology 2025, 14, 314. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 348–362. [Google Scholar] [CrossRef]
- Ban, H.; Nishishita, N.; Fusaki, N.; Tabata, T.; Saeki, K.; Shikamura, M.; Takada, N.; Inoue, M.; Hasegawa, M.; Kawamata, S.; et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc. Natl. Acad. Sci. USA 2011, 108, 14234–14239. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Li, S.; Xue, H.; Schmitt, K.; Hergenroeder, G.W.; Wu, J.; Zhang, Y.; Kim, D.H.; Cao, Q. Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Res. 2017, 19, 55–64. [Google Scholar] [CrossRef]
- Kunitomi, A.; Hirohata, R.; Arreola, V.; Osawa, M.; Kato, T.M.; Nomura, M.; Kawaguchi, J.; Hara, H.; Kusano, K.; Takashima, Y.; et al. Improved Sendai viral system for reprogramming to naive pluripotency. Cell Rep. Methods 2022, 2, 100317. [Google Scholar] [CrossRef]
- Nori, S.; Okada, Y.; Nishimura, S.; Sasaki, T.; Itakura, G.; Kobayashi, Y.; Renault-Mihara, F.; Shimizu, A.; Koya, I.; Yoshida, R.; et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015, 4, 360–373. [Google Scholar] [CrossRef]
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M.; et al. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 2009, 27, 743–745. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, G.S.; Walsh, F.S.; Nirenberg, M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc. Natl. Acad. Sci. USA 1979, 76, 4913–4917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Lee, J.C.; Xue, H.; Pevny, L.H.; Kaprielian, Z.; Rao, M.S. Oligodendrocyte and astrocyte development in rodents: An in situ and immunohistological analysis during embryonic development. Glia 2002, 40, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Noble, M.; Mayer-Proschel, M. A tripotential glial precursor cell is present in the developing spinal cord. Proc. Natl. Acad. Sci. USA 1998, 95, 3996–4001. [Google Scholar] [CrossRef]
- Lepore, A.C.; O’Donnell, J.; Kim, A.S.; Williams, T.; Tuteja, A.; Rao, M.S.; Kelley, L.L.; Campanelli, J.T.; Maragakis, N.J. Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PLoS ONE 2011, 6, e25968. [Google Scholar] [CrossRef]
- Kawabata, S.; Takano, M.; Numasawa-Kuroiwa, Y.; Itakura, G.; Kobayashi, Y.; Nishiyama, Y.; Sugai, K.; Nishimura, S.; Iwai, H.; Isoda, M.; et al. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Okubo, T.; Iwanami, A.; Kohyama, J.; Itakura, G.; Kawabata, S.; Nishiyama, Y.; Sugai, K.; Ozaki, M.; Iida, T.; Matsubayashi, K.; et al. Pretreatment with a gamma-Secretase Inhibitor Prevents Tumor-like Overgrowth in Human iPSC-Derived Transplants for Spinal Cord Injury. Stem Cell Rep. 2016, 7, 649–663. [Google Scholar] [CrossRef]
- Kajikawa, K.; Imaizumi, K.; Shinozaki, M.; Shibata, S.; Shindo, T.; Kitagawa, T.; Shibata, R.; Kamata, Y.; Kojima, K.; Nagoshi, N.; et al. Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol. Brain 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Nagoshi, N.; Kohyama, J.; Tsuji, O.; Shinozaki, M.; Shibata, S.; Kase, Y.; Matsumoto, M.; Nakamura, M.; Okano, H. Treatment with a Gamma-Secretase Inhibitor Promotes Functional Recovery in Human iPSC- Derived Transplants for Chronic Spinal Cord Injury. Stem Cell Rep. 2018, 11, 1416–1432. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Feng, B.; Amponsah, A.E.; He, J.; Guo, R.; Liu, B.; Du, X.; Liu, X.; Zhang, S.; Lv, F.; et al. hiPSC-derived NSCs effectively promote the functional recovery of acute spinal cord injury in mice. Stem Cell Res. Ther. 2021, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, P.; Hernandez, J.; Giraldo, E.; Gonzalez-Perez, M.A.; Alastrue-Agudo, A.; Elkhenany, H.; Vicent, M.J.; Navarro, X.; Edel, M.; Moreno-Manzano, V. Human-Induced Neural and Mesenchymal Stem Cell Therapy Combined with a Curcumin Nanoconjugate as a Spinal Cord Injury Treatment. Int. J. Mol. Sci. 2021, 22, 5966. [Google Scholar] [CrossRef]
- Shiga, Y.; Shiga, A.; Mesci, P.; Kwon, H.; Brifault, C.; Kim, J.H.; Jeziorski, J.J.; Nasamran, C.; Ohtori, S.; Muotri, A.R.; et al. Tissue-type plasminogen activator-primed human iPSC-derived neural progenitor cells promote motor recovery after severe spinal cord injury. Sci. Rep. 2019, 9, 19291. [Google Scholar] [CrossRef]
- Fuhrmann, T.; Tam, R.Y.; Ballarin, B.; Coles, B.; Elliott, D.I.; van der Kooy, D.; Nagy, A.; Tator, C.H.; Morshead, C.M. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 2016, 83, 23–36. [Google Scholar] [CrossRef]
- Amemori, T.; Ruzicka, J.; Romanyuk, N.; Jhanwar-Uniyal, M.; Sykova, E.; Jendelova, P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res. Ther. 2015, 6, 257. [Google Scholar] [CrossRef]
- Romanyuk, N.; Amemori, T.; Turnovcova, K.; Prochazka, P.; Onteniente, B.; Sykova, E.; Jendelova, P. Beneficial Effect of Human Induced Pluripotent Stem Cell-Derived Neural Precursors in Spinal Cord Injury Repair. Cell Transpl. 2015, 24, 1781–1797. [Google Scholar] [CrossRef]
- Itakura, G.; Kawabata, S.; Ando, M.; Nishiyama, Y.; Sugai, K.; Ozaki, M.; Iida, T.; Ookubo, T.; Kojima, K.; Kashiwagi, R.; et al. Fail-Safe System against Potential Tumorigenicity after Transplantation of iPSC Derivatives. Stem Cell Rep. 2017, 8, 673–684. [Google Scholar] [CrossRef]
- Itakura, G.; Kobayashi, Y.; Nishimura, S.; Iwai, H.; Takano, M.; Iwanami, A.; Toyama, Y.; Okano, H.; Nakamura, M. Controlling immune rejection is a fail-safe system against potential tumorigenicity after human iPSC-derived neural stem cell transplantation. PLoS ONE 2015, 10, e0116413. [Google Scholar] [CrossRef]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef]
- Tohyama, S.; Fujita, J.; Hishiki, T.; Matsuura, T.; Hattori, F.; Ohno, R.; Kanazawa, H.; Seki, T.; Nakajima, K.; Kishino, Y.; et al. Glutamine Oxidation Is Indispensable for Survival of Human Pluripotent Stem Cells. Cell Metab. 2016, 23, 663–674. [Google Scholar] [CrossRef]
- Kojima, K.; Miyoshi, H.; Nagoshi, N.; Kohyama, J.; Itakura, G.; Kawabata, S.; Ozaki, M.; Iida, T.; Sugai, K.; Ito, S.; et al. Selective Ablation of Tumorigenic Cells Following Human Induced Pluripotent Stem Cell-Derived Neural Stem/Progenitor Cell Transplantation in Spinal Cord Injury. Stem Cells Transl. Med. 2019, 8, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Ceto, S.; Sekiguchi, K.J.; Takashima, Y.; Nimmerjahn, A.; Tuszynski, M.H. Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury. Cell Stem Cell 2020, 27, 430–440.E5. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.F.; Lee-Kubli, C.; Kumamaru, H.; Kadoya, K.; Tuszynski, M.H. Comprehensive Monosynaptic Rabies Virus Mapping of Host Connectivity with Neural Progenitor Grafts after Spinal Cord Injury. Stem Cell Rep. 2017, 8, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, G.H.; Tuszynski, M.H. Regeneration of Corticospinal Axons into Neural Progenitor Cell Grafts After Spinal Cord Injury. Neurosci. Insights 2020, 15, 2633105520974000. [Google Scholar] [CrossRef]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.N.; Strobl, H.; et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016, 22, 479–487. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; van der Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef]
- Lu, P.; Jones, L.L.; Snyder, E.Y.; Tuszynski, M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 2003, 181, 115–129. [Google Scholar] [CrossRef]
- Hawryluk, G.W.; Mothe, A.; Wang, J.; Wang, S.; Tator, C.; Fehlings, M.G. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012, 21, 2222–2238. [Google Scholar] [CrossRef]
- Kokaia, Z.; Martino, G.; Schwartz, M.; Lindvall, O. Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat. Neurosci. 2012, 15, 1078–1087. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumamaru, H.; Vokes, T.J.; Tran, A.N.; Shevinsky, C.A.; Graham, L.; Archuleta, K.; Limon, K.R.; Lu, P.; Blesch, A.; et al. An improved method for generating human spinal cord neural stem cells. Exp. Neurol. 2024, 376, 114779. [Google Scholar] [CrossRef] [PubMed]
- Kumamaru, H.; Kadoya, K.; Adler, A.F.; Takashima, Y.; Graham, L.; Coppola, G.; Tuszynski, M.H. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods 2018, 15, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef]
- Kirkeby, A.; Main, H.; Carpenter, M. Pluripotent stem-cell-derived therapies in clinical trial: A 2025 update. Cell Stem Cell 2025, 32, 10–37, Erratum in Cell Stem Cell 2025, 32, 329–331. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, Y.; Bu, P.; Qi, X.; Fan, C.; Li, F.; Kim, D.H.; Cao, Q. Apolipoprotein E as a novel therapeutic neuroprotection target after traumatic spinal cord injury. Exp. Neurol. 2018, 299, 97–108. [Google Scholar] [CrossRef]
- Cao, Q.; He, Q.; Wang, Y.; Cheng, X.; Howard, R.M.; Zhang, Y.; DeVries, W.H.; Shields, C.B.; Magnuson, D.S.; Xu, X.M.; et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J. Neurosci. 2010, 30, 2989–3001. [Google Scholar] [CrossRef]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef]
- Gu, Z.; Li, F.; Zhang, Y.P.; Shields, L.B.; Hu, X.; Zheng, Y.; Yu, P.; Zhang, Y.; Cai, J.; Vitek, M.P.; et al. Apolipoprotein E Mimetic Promotes Functional and Histological Recovery in Lysolecithin-Induced Spinal Cord Demyelination in Mice. J. Neurol. Neurophysiol. 2013, 2014, 10. [Google Scholar] [CrossRef]
- Hill, R.L.; Zhang, Y.P.; Burke, D.A.; DeVries, W.H.; Zhang, Y.; Magnuson, D.S.; Whittemore, S.R.; Shields, C.B. Anatomical and functional outcomes following a precise, graded, dorsal laceration spinal cord injury in C57BL/6 mice. J. Neurotrauma 2009, 26, 1–15. [Google Scholar] [CrossRef]
- Gallegos, C.; Carey, M.; Zheng, Y.; He, X.; Cao, Q.L. Reaching and Grasping Training Improves Functional Recovery After Chronic Cervical Spinal Cord Injury. Front. Cell Neurosci. 2020, 14, 110. [Google Scholar] [CrossRef]


| Medium Ctrl | hFB Ctrl | hNPC Graft | |
|---|---|---|---|
| Sample size by power analysis | N = 16 | N = 16 | N = 16 |
| Exp. 1 | N = 10 | N = 10 | N = 10 |
| attrition | N = 2 a | N = 1 b | N = 1 c |
| BMS | N = 8 | N = 9 | N = 9 |
| Beam-walking | N = 8 | N = 9 | N = 9 |
| IHC | N = 6 | N = 9 | |
| EC staining | N = 5 | N = 5 | N = 5 |
| Exp. 2 | N = 10 | N = 10 | N = 10 |
| attrition | N = 1 d | N = 1 e | N = 1 f |
| BMS | N = 9 | N = 9 | N = 9 |
| Beam-walking | N = 9 | N = 9 | N = 9 |
| IHC | N = 3 | N = 9 | |
| EC staining | N = 5 | N = 5 | N = 5 |
| Final sample size for behavioral tests | N = 17 | N = 18 | N = 18 |
| Final sample size for EC staining | N = 10 | N = 10 | N = 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Chen, X.; Bu, P.; Xue, H.; Kim, D.H.; Zhou, H.; Xia, X.; Liu, Y.; Cao, Q. Functional Recovery by Transplantation of Human iPSC-Derived A2B5 Positive Neural Progenitor Cell After Spinal Cord Injury in Mice. Int. J. Mol. Sci. 2025, 26, 8940. https://doi.org/10.3390/ijms26188940
Zheng Y, Chen X, Bu P, Xue H, Kim DH, Zhou H, Xia X, Liu Y, Cao Q. Functional Recovery by Transplantation of Human iPSC-Derived A2B5 Positive Neural Progenitor Cell After Spinal Cord Injury in Mice. International Journal of Molecular Sciences. 2025; 26(18):8940. https://doi.org/10.3390/ijms26188940
Chicago/Turabian StyleZheng, Yiyan, Xiaohui Chen, Ping Bu, Haipeng Xue, Dong H. Kim, Hongxia Zhou, Xugang Xia, Ying Liu, and Qilin Cao. 2025. "Functional Recovery by Transplantation of Human iPSC-Derived A2B5 Positive Neural Progenitor Cell After Spinal Cord Injury in Mice" International Journal of Molecular Sciences 26, no. 18: 8940. https://doi.org/10.3390/ijms26188940
APA StyleZheng, Y., Chen, X., Bu, P., Xue, H., Kim, D. H., Zhou, H., Xia, X., Liu, Y., & Cao, Q. (2025). Functional Recovery by Transplantation of Human iPSC-Derived A2B5 Positive Neural Progenitor Cell After Spinal Cord Injury in Mice. International Journal of Molecular Sciences, 26(18), 8940. https://doi.org/10.3390/ijms26188940







