BA9 Transcriptomics in Huntington’s Disease 80-Gene Signature and MIR219A2-Linked Targets
Abstract
1. Introduction
1.1. Clinical and Genetic Background
1.2. Cortical Involvement and the Brodmann Area 9 (BA9) Context
1.3. Cortical Transcriptomics: Broad Themes from BA9
1.4. Multicellular Remodeling in Human HD Cortex
1.5. MicroRNAs in HD and the Biological Rationale for MIR219A2
1.6. Analytical Strategy: BA9 Re-Analysis and a Compact, Direction-Aware Core Signature
1.7. Positioning Within Current HD Biology
2. Results
2.1. Global Differential Expression in BA9 (GSE64810)
2.2. Top-Ranked Differentially Expressed Genes
2.3. The 80-Gene Core Signature
2.4. MIR219A2 Down-Regulation in BA9 and Target Overlaps with BA9 Up/Down Sets
2.5. Enrichment and PPI Analysis of the Four-Gene Overlap
2.6. Full-Universe MIR219A2 Target Enrichment (Panel-İndependent)
2.7. TF Co-Regulation Signals (MSigDB C3:TFT; BA9 Expressed-Gene Background)
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design and Dataset
4.2. Preprocessing, Filtering, and Differential Expression
4.3. Ranking and Visualization
4.4. Construction of the 80-Gene Core Set
4.5. Validated Target Sets and Enrichment Testing
4.6. miRNA-Centered Integration (MIR219A2)
4.7. Transcription Factor Co-Regulation Analyses
4.8. Cell-Type Marker Context and Sensitivity Analyses
4.9. Computational Validation and Robustness Criteria
4.10. Functional Enrichment, PPI, and Regulator Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Losekoot, M.; van Belzen, M.J.; Seneca, S.; Bauer, P.; Stenhouse, S.A.; Barton, D.E.; European Molecular Genetic Quality, N. EMQN/CMGS best practice guidelines for the molecular genetic testing of Huntington disease. Eur. J. Hum. Genet. 2013, 21, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Schobel, S.; Gantman, E.C.; Mansbach, A.; Borowsky, B.; Konstantinova, P.; Mestre, T.A.; Panagoulias, J.; Ross, C.A.; Zauderer, M.; et al. A biological classification of Huntington’s disease: The Integrated Staging System. Lancet Neurol. 2022, 21, 632–644. [Google Scholar] [CrossRef]
- Donaldson, J.; Powell, S.; Rickards, N.; Holmans, P.; Jones, L. What is the Pathogenic CAG Expansion Length in Huntington’s Disease? J. Huntington’s Dis. 2021, 10, 175–202. [Google Scholar] [CrossRef] [PubMed]
- Scahill, R.I.; Farag, M.; Murphy, M.J.; Hobbs, N.Z.; Leocadi, M.; Langley, C.; Knights, H.; Ciosi, M.; Fayer, K.; Nakajima, M.; et al. Somatic CAG repeat expansion in blood associates with biomarkers of neurodegeneration in Huntington’s disease decades before clinical motor diagnosis. Nat. Med. 2025, 31, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Kalman, L.; Johnson, M.A.; Beck, J.; Berry-Kravis, E.; Buller, A.; Casey, B.; Feldman, G.L.; Handsfield, J.; Jakupciak, J.P.; Maragh, S.; et al. Development of genomic reference materials for Huntington disease genetic testing. Genet. Med. 2007, 9, 719–723. [Google Scholar] [CrossRef]
- Kay, C.; Collins, J.A.; Miedzybrodzka, Z.; Madore, S.J.; Gordon, E.S.; Gerry, N.; Davidson, M.; Slama, R.A.; Hayden, M.R. Huntington disease reduced penetrance alleles occur at high frequency in the general population. Neurology 2016, 87, 282–288. [Google Scholar] [CrossRef]
- Warby, S.C.; Visscher, H.; Collins, J.A.; Doty, C.N.; Carter, C.; Butland, S.L.; Hayden, A.R.; Kanazawa, I.; Ross, C.J.; Hayden, M.R. HTT haplotypes contribute to differences in Huntington disease prevalence between Europe and East Asia. Eur. J. Hum. Genet. 2011, 19, 561–566. [Google Scholar] [CrossRef]
- Wright, G.E.B.; Collins, J.A.; Kay, C.; McDonald, C.; Dolzhenko, E.; Xia, Q.; Becanovic, K.; Drogemoller, B.I.; Semaka, A.; Nguyen, C.M.; et al. Length of Uninterrupted CAG, Independent of Polyglutamine Size, Results in Increased Somatic Instability, Hastening Onset of Huntington Disease. Am. J. Hum. Genet. 2019, 104, 1116–1126. [Google Scholar] [CrossRef]
- Findlay Black, H.; Wright, G.E.B.; Collins, J.A.; Caron, N.; Kay, C.; Xia, Q.; Arning, L.; Bijlsma, E.K.; Squitieri, F.; Nguyen, H.P.; et al. Frequency of the loss of CAA interruption in the HTT CAG tract and implications for Huntington disease in the reduced penetrance range. Genet. Med. 2020, 22, 2108–2113. [Google Scholar] [CrossRef]
- Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium. Identification of Genetic Factors that Modify Clinical Onset of Huntington’s Disease. Cell 2015, 162, 516–526. [Google Scholar] [CrossRef]
- Lee, J.M.; Huang, Y.; Orth, M.; Gillis, T.; Siciliano, J.; Hong, E.; Mysore, J.S.; Lucente, D.; Wheeler, V.C.; Seong, I.S.; et al. Genetic modifiers of Huntington disease differentially influence motor and cognitive domains. Am. J. Hum. Genet. 2022, 109, 885–899. [Google Scholar] [CrossRef]
- McAllister, B.; Donaldson, J.; Binda, C.S.; Powell, S.; Chughtai, U.; Edwards, G.; Stone, J.; Lobanov, S.; Elliston, L.; Schuhmacher, L.N.; et al. Exome sequencing of individuals with Huntington’s disease implicates FAN1 nuclease activity in slowing CAG expansion and disease onset. Nat. Neurosci. 2022, 25, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Genetic Modifiers of Huntington’s Disease, C. Genetic modifiers of somatic expansion and clinical phenotypes in Huntington’s disease highlight shared and tissue-specific effects. Nat. Genet. 2025, 57, 1426–1436. [Google Scholar] [CrossRef]
- Ferguson, R.; Goold, R.; Coupland, L.; Flower, M.; Tabrizi, S.J. Therapeutic validation of MMR-associated genetic modifiers in a human ex vivo model of Huntington disease. Am. J. Hum. Genet. 2024, 111, 1165–1183. [Google Scholar] [CrossRef]
- Migliore, S.; Jankovic, J.; Squitieri, F. Genetic Counseling in Huntington’s Disease: Potential New Challenges on Horizon? Front. Neurol. 2019, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Rosas, H.D.; Salat, D.H.; Lee, S.Y.; Zaleta, A.K.; Pappu, V.; Fischl, B.; Greve, D.; Hevelone, N.; Hersch, S.M. Cerebral cortex and the clinical expression of Huntington’s disease: Complexity and heterogeneity. Brain 2008, 131, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, C.; Wu, N.; Andre, V.M.; Cummings, D.M.; Levine, M.S. The corticostriatal pathway in Huntington’s disease. Prog. Neurobiol. 2007, 81, 253–271. [Google Scholar] [CrossRef]
- Blumenstock, S.; Dudanova, I. Cortical and Striatal Circuits in Huntington’s Disease. Front. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Kloppel, S.; Henley, S.M.; Hobbs, N.Z.; Wolf, R.C.; Kassubek, J.; Tabrizi, S.J.; Frackowiak, R.S. Magnetic resonance imaging of Huntington’s disease: Preparing for clinical trials. Neuroscience 2009, 164, 205–219. [Google Scholar] [CrossRef][Green Version]
- Poudel, G.R.; Egan, G.F.; Churchyard, A.; Chua, P.; Stout, J.C.; Georgiou-Karistianis, N. Abnormal synchrony of resting state networks in premanifest and symptomatic Huntington disease: The IMAGE-HD study. J. Psychiatry Neurosci. 2014, 39, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Pavese, N.; Tai, Y.F.; Kiferle, L.; Mason, S.L.; Brooks, D.J.; Tabrizi, S.J.; Barker, R.A.; Piccini, P. Microglial activation in regions related to cognitive function predicts disease onset in Huntington’s disease: A multimodal imaging study. Hum. Brain Mapp. 2011, 32, 258–270. [Google Scholar] [CrossRef]
- Schippling, S.; Schneider, S.A.; Bhatia, K.P.; Munchau, A.; Rothwell, J.C.; Tabrizi, S.J.; Orth, M. Abnormal motor cortex excitability in preclinical and very early Huntington’s disease. Biol. Psychiatry 2009, 65, 959–965. [Google Scholar] [CrossRef]
- Lorenzano, C.; Dinapoli, L.; Gilio, F.; Suppa, A.; Bagnato, S.; Curra, A.; Inghilleri, M.; Berardelli, A. Motor cortical excitability studied with repetitive transcranial magnetic stimulation in patients with Huntington’s disease. Clin. Neurophysiol. 2006, 117, 1677–1681. [Google Scholar] [CrossRef]
- Pressl, C.; Matlik, K.; Kus, L.; Darnell, P.; Luo, J.D.; Paul, M.R.; Weiss, A.R.; Liguore, W.; Carroll, T.S.; Davis, D.A.; et al. Selective vulnerability of layer 5a corticostriatal neurons in Huntington’s disease. Neuron 2024, 112, 924–941.e910. [Google Scholar] [CrossRef] [PubMed]
- McColgan, P.; Seunarine, K.K.; Gregory, S.; Razi, A.; Papoutsi, M.; Long, J.D.; Mills, J.A.; Johnson, E.; Durr, A.; Roos, R.A.; et al. Topological length of white matter connections predicts their rate of atrophy in premanifest Huntington’s disease. JCI Insight 2017, 2, e92641. [Google Scholar] [CrossRef] [PubMed]
- McColgan, P.; Gregory, S.; Seunarine, K.K.; Razi, A.; Papoutsi, M.; Johnson, E.; Durr, A.; Roos, R.A.C.; Leavitt, B.R.; Holmans, P.; et al. Brain Regions Showing White Matter Loss in Huntington’s Disease Are Enriched for Synaptic and Metabolic Genes. Biol. Psychiatry 2018, 83, 456–465. [Google Scholar] [CrossRef]
- Novak, M.J.; Seunarine, K.K.; Gibbard, C.R.; McColgan, P.; Draganski, B.; Friston, K.; Clark, C.A.; Tabrizi, S.J. Basal ganglia-cortical structural connectivity in Huntington’s disease. Hum. Brain Mapp. 2015, 36, 1728–1740. [Google Scholar] [CrossRef]
- Hensman Moss, D.J.; Flower, M.D.; Lo, K.K.; Miller, J.R.; van Ommen, G.B.; t Hoen, P.A.; Stone, T.C.; Guinee, A.; Langbehn, D.R.; Jones, L.; et al. Huntington’s disease blood and brain show a common gene expression pattern and share an immune signature with Alzheimer’s disease. Sci. Rep. 2017, 7, 44849. [Google Scholar] [CrossRef]
- Dong, X.; Tsuji, J.; Labadorf, A.; Roussos, P.; Chen, J.F.; Myers, R.H.; Akbarian, S.; Weng, Z. The Role of H3K4me3 in Transcriptional Regulation Is Altered in Huntington’s Disease. PLoS ONE 2015, 10, e0144398. [Google Scholar] [CrossRef]
- Seefelder, M.; Kochanek, S. A meta-analysis of transcriptomic profiles of Huntington’s disease patients. PLoS ONE 2021, 16, e0253037. [Google Scholar] [CrossRef]
- Herring, C.A.; Simmons, R.K.; Freytag, S.; Poppe, D.; Moffet, J.J.D.; Pflueger, J.; Buckberry, S.; Vargas-Landin, D.B.; Clement, O.; Echeverria, E.G.; et al. Human prefrontal cortex gene regulatory dynamics from gestation to adulthood at single-cell resolution. Cell 2022, 185, 4428–4447.e4428. [Google Scholar] [CrossRef]
- Hoss, A.G.; Kartha, V.K.; Dong, X.; Latourelle, J.C.; Dumitriu, A.; Hadzi, T.C.; Macdonald, M.E.; Gusella, J.F.; Akbarian, S.; Chen, J.F.; et al. MicroRNAs located in the Hox gene clusters are implicated in Huntington’s disease pathogenesis. PLoS Genet. 2014, 10, e1004188. [Google Scholar] [CrossRef]
- Hyeon, J.W.; Kim, A.H.; Yano, H. Epigenetic regulation in Huntington’s disease. Neurochem. Int. 2021, 148, 105074. [Google Scholar] [CrossRef] [PubMed]
- Al-Dalahmah, O.; Sosunov, A.A.; Shaik, A.; Ofori, K.; Liu, Y.; Vonsattel, J.P.; Adorjan, I.; Menon, V.; Goldman, J.E. Single-nucleus RNA-seq identifies Huntington disease astrocyte states. Acta Neuropathol. Commun. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Paryani, F.; Kwon, J.S.; Ng, C.W.; Jakubiak, K.; Madden, N.; Ofori, K.; Tang, A.; Lu, H.; Xia, S.; Li, J.; et al. Multi-omic analysis of Huntington’s disease reveals a compensatory astrocyte state. Nat. Commun. 2024, 15, 6742. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Park, J.W.; Ramachandran, S.; Zhang, Y.; Tseng, Y.T.; Shen, S.; Waldvogel, H.J.; Curtis, M.A.; Faull, R.L.; Troncoso, J.C.; et al. Transcriptome sequencing reveals aberrant alternative splicing in Huntington’s disease. Hum. Mol. Genet. 2016, 25, 3454–3466. [Google Scholar] [CrossRef]
- Diaz-Castro, B.; Gangwani, M.R.; Yu, X.; Coppola, G.; Khakh, B.S. Astrocyte molecular signatures in Huntington’s disease. Sci. Transl. Med. 2019, 11, eaaw8546. [Google Scholar] [CrossRef]
- Zhou, J.; Wade, S.D.; Graykowski, D.; Xiao, M.F.; Zhao, B.; Giannini, L.A.A.; Hanson, J.E.; van Swieten, J.C.; Sheng, M.; Worley, P.F.; et al. The neuronal pentraxin Nptx2 regulates complement activity and restrains microglia-mediated synapse loss in neurodegeneration. Sci. Transl. Med. 2023, 15, eadf0141. [Google Scholar] [CrossRef]
- Lim, R.G.; Al-Dalahmah, O.; Wu, J.; Gold, M.P.; Reidling, J.C.; Tang, G.; Adam, M.; Dansu, D.K.; Park, H.J.; Casaccia, P.; et al. Huntington disease oligodendrocyte maturation deficits revealed by single-nucleus RNAseq are rescued by thiamine-biotin supplementation. Nat. Commun. 2022, 13, 7791. [Google Scholar] [CrossRef]
- Ferrari Bardile, C.; Garcia-Miralles, M.; Caron, N.S.; Rayan, N.A.; Langley, S.R.; Harmston, N.; Rondelli, A.M.; Teo, R.T.Y.; Waltl, S.; Anderson, L.M.; et al. Intrinsic mutant HTT-mediated defects in oligodendroglia cause myelination deficits and behavioral abnormalities in Huntington disease. Proc. Natl. Acad. Sci. USA 2019, 116, 9622–9627. [Google Scholar] [CrossRef]
- Bostrand, S.M.K.; Seeker, L.A.; Bestard-Cuche, N.; Kazakou, N.L.; Jakel, S.; Kenkhuis, B.; Henderson, N.C.; de Bot, S.T.; van Roon-Mom, W.M.C.; Priller, J.; et al. Mapping the glial transcriptome in Huntington’s disease using snRNAseq: Selective disruption of glial signatures across brain regions. Acta Neuropathol. Commun. 2024, 12, 165. [Google Scholar] [CrossRef]
- Reyes-Ortiz, A.M.; Abud, E.M.; Burns, M.S.; Wu, J.; Hernandez, S.J.; McClure, N.; Wang, K.Q.; Schulz, C.J.; Miramontes, R.; Lau, A.; et al. Single-nuclei transcriptome analysis of Huntington disease iPSC and mouse astrocytes implicates maturation and functional deficits. iScience 2023, 26, 105732. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Chen, Z.; Zhao, X.; Peng, X.; Wu, Y.; Yang, K.; Sun, T. Endothelial Dysfunction in Huntington’s Disease: Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 1432. [Google Scholar] [CrossRef]
- Bjerkan, J.; Kobal, J.; Lancaster, G.; Sesok, S.; Meglic, B.; McClintock, P.V.E.; Budohoski, K.P.; Kirkpatrick, P.J.; Stefanovska, A. The phase coherence of the neurovascular unit is reduced in Huntington’s disease. Brain Commun. 2024, 6, fcae166. [Google Scholar] [CrossRef] [PubMed]
- Dugas, J.C.; Cuellar, T.L.; Scholze, A.; Ason, B.; Ibrahim, A.; Emery, B.; Zamanian, J.L.; Foo, L.C.; McManus, M.T.; Barres, B.A. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 2010, 65, 597–611. [Google Scholar] [CrossRef]
- Zhao, X.; He, X.; Han, X.; Yu, Y.; Ye, F.; Chen, Y.; Hoang, T.; Xu, X.; Mi, Q.S.; Xin, M.; et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 2010, 65, 612–626. [Google Scholar] [CrossRef]
- Galloway, D.A.; Moore, C.S. miRNAs As Emerging Regulators of Oligodendrocyte Development and Differentiation. Front. Cell Dev. Biol. 2016, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Junker, A.; Krumbholz, M.; Eisele, S.; Mohan, H.; Augstein, F.; Bittner, R.; Lassmann, H.; Wekerle, H.; Hohlfeld, R.; Meinl, E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 2009, 132, 3342–3352. [Google Scholar] [CrossRef]
- Teuber-Hanselmann, S.; Meinl, E.; Junker, A. MicroRNAs in gray and white matter multiple sclerosis lesions: Impact on pathophysiology. J. Pathol. 2020, 250, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Moyano, A.L.; Ma, Z.; Deng, Y.; Lin, Y.; Zhao, C.; Zhang, L.; Jiang, M.; He, X.; Ma, Z.; et al. miR-219 Cooperates with miR-338 in Myelination and Promotes Myelin Repair in the CNS. Dev. Cell 2017, 40, 566–582.e565. [Google Scholar] [CrossRef] [PubMed]
- Svaren, J. MicroRNA and transcriptional crosstalk in myelinating glia. Neurochem. Int. 2014, 77, 50–57. [Google Scholar] [CrossRef]
- Dong, X.; Cong, S. MicroRNAs in Huntington’s Disease: Diagnostic Biomarkers or Therapeutic Agents? Front. Cell Neurosci. 2021, 15, 705348. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology, C. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 258. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Labadorf, A.; Hoss, A.G.; Lagomarsino, V.; Latourelle, J.C.; Hadzi, T.C.; Bregu, J.; MacDonald, M.E.; Gusella, J.F.; Chen, J.F.; Akbarian, S.; et al. RNA Sequence Analysis of Human Huntington Disease Brain Reveals an Extensive Increase in Inflammatory and Developmental Gene Expression. PLoS ONE 2015, 10, e0143563. [Google Scholar] [CrossRef]
- Skoufos, G.; Kakoulidis, P.; Tastsoglou, S.; Zacharopoulou, E.; Kotsira, V.; Miliotis, M.; Mavromati, G.; Grigoriadis, D.; Zioga, M.; Velli, A.; et al. TarBase-v9.0 extends experimentally supported miRNA-gene interactions to cell-types and virally encoded miRNAs. Nucleic Acids Res. 2024, 52, D304–D310. [Google Scholar] [CrossRef]
- Cui, S.; Yu, S.; Huang, H.Y.; Lin, Y.C.; Huang, Y.; Zhang, B.; Xiao, J.; Zuo, H.; Wang, J.; Li, Z.; et al. miRTarBase 2025: Updates to the collection of experimentally validated microRNA-target interactions. Nucleic Acids Res. 2025, 53, D147–D156. [Google Scholar] [CrossRef]
- Saba, J.; Couselo, F.L.; Bruno, J.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Neuroinflammation in Huntington’s Disease: A Starring Role for Astrocyte and Microglia. Curr. Neuropharmacol. 2022, 20, 1116–1143. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-kappaB Function in the Nervous System. Front. Immunol. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Siegenthaler, J.A.; Choe, Y.; Patterson, K.P.; Hsieh, I.; Li, D.; Jaminet, S.C.; Daneman, R.; Kume, T.; Huang, E.J.; Pleasure, S.J. Foxc1 is required by pericytes during fetal brain angiogenesis. Biol. Open 2013, 2, 647–659. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, I.M.; Cubelos, B.; Gimenez, C.; Zafra, F. Immunohistochemical localization of the amino acid transporter SNAT2 in the rat brain. Neuroscience 2005, 130, 61–73. [Google Scholar] [CrossRef]
- Menchini, R.J.; Chaudhry, F.A. Multifaceted regulation of the system A transporter Slc38a2 suggests nanoscale regulation of amino acid metabolism and cellular signaling. Neuropharmacology 2019, 161, 107789. [Google Scholar] [CrossRef] [PubMed]
- Kukulowicz, J.; Pietrzak-Lichwa, K.; Klimonczyk, K.; Idlin, N.; Bajda, M. The SLC6A15-SLC6A20 Neutral Amino Acid Transporter Subfamily: Functions, Diseases, and Their Therapeutic Relevance. Pharmacol. Rev. 2023, 76, 142–193. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Tao, L.; Cao, X.; Chen, L. The solute carrier transporters and the brain: Physiological and pharmacological implications. Asian J. Pharm. Sci. 2020, 15, 131–144. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-kappaB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Sutton, G.J.; Poppe, D.; Simmons, R.K.; Walsh, K.; Nawaz, U.; Lister, R.; Gagnon-Bartsch, J.A.; Voineagu, I. Comprehensive evaluation of deconvolution methods for human brain gene expression. Nat. Commun. 2022, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Strasser, B.; Pepe, G.; Burtscher, M.; Kopp, M.; Di Pardo, A.; Maglione, V.; Khamoui, A.V. Brain-Periphery Interactions in Huntington’s Disease: Mediators and Lifestyle Interventions. Int. J. Mol. Sci. 2024, 25, 4696. [Google Scholar] [CrossRef]
- Che, J.; Sun, Y.; Deng, Y.; Zhang, J. Blood-brain barrier disruption: A culprit of cognitive decline? Fluids Barriers CNS 2024, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.S.; Haqqani, A.S.; Sandhu, A.; Aly, A.E.; Findlay Black, H.; Bone, J.N.; McBride, J.L.; Abulrob, A.; Stanimirovic, D.; Leavitt, B.R.; et al. Cerebrospinal fluid biomarkers for assessing Huntington disease onset and severity. Brain Commun. 2022, 4, fcac309. [Google Scholar] [CrossRef]
- Morena, E.; Romano, C.; Marconi, M.; Diamant, S.; Buscarinu, M.C.; Bellucci, G.; Romano, S.; Scarabino, D.; Salvetti, M.; Ristori, G. Peripheral Biomarkers in Manifest and Premanifest Huntington’s Disease. Int. J. Mol. Sci. 2023, 24, 6051. [Google Scholar] [CrossRef]
- Caron, N.S.; Byrne, L.M.; Lemarie, F.L.; Bone, J.N.; Aly, A.E.; Ko, S.; Anderson, C.; Casal, L.L.; Hill, A.M.; Hawellek, D.J.; et al. Elevated plasma and CSF neurofilament light chain concentrations are stabilized in response to mutant huntingtin lowering in the brains of Huntington’s disease mice. Transl. Neurodegener. 2024, 13, 50. [Google Scholar] [CrossRef]
- Machacek, M.; Garcia-Montoya, E.; McColgan, P.; Sanwald-Ducray, P.; Mazer, N.A. NfL concentration in CSF is a quantitative marker of the rate of neurodegeneration in aging and Huntington’s disease: A semi-mechanistic model-based analysis. Front. Neurosci. 2024, 18, 1420198. [Google Scholar] [CrossRef]
- Fodale, V.; Boggio, R.; Daldin, M.; Cariulo, C.; Spiezia, M.C.; Byrne, L.M.; Leavitt, B.R.; Wild, E.J.; Macdonald, D.; Weiss, A.; et al. Validation of Ultrasensitive Mutant Huntingtin Detection in Human Cerebrospinal Fluid by Single Molecule Counting Immunoassay. J. Huntingt. Dis. 2017, 6, 349–361. [Google Scholar] [CrossRef]
- Vauleon, S.; Schutz, K.; Massonnet, B.; Gruben, N.; Manchester, M.; Buehler, A.; Schick, E.; Boak, L.; Hawellek, D.J. Quantifying mutant huntingtin protein in human cerebrospinal fluid to support the development of huntingtin-lowering therapies. Sci. Rep. 2023, 13, 5332. [Google Scholar] [CrossRef] [PubMed]
- Bondulich, M.K.; Phillips, J.; Canibano-Pico, M.; Nita, I.M.; Byrne, L.M.; Wild, E.J.; Bates, G.P. Translatable plasma and CSF biomarkers for use in mouse models of Huntington’s disease. Brain Commun. 2024, 6, fcae030. [Google Scholar] [CrossRef]
- Reed, E.R.; Latourelle, J.C.; Bockholt, J.H.; Bregu, J.; Smock, J.; Paulsen, J.S.; Myers, R.H.; PREDICT-HD CSF ancillary study investigators. MicroRNAs in CSF as prodromal biomarkers for Huntington disease in the PREDICT-HD study. Neurology 2018, 90, e264–e272. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Lorenzo, M.; Perez-Perez, J.; Escaramis, G.; Martinez-Horta, S.; Perez-Gonzalez, R.; Rivas-Asensio, E.; Kulisevsky, J.; Gamez-Valero, A.; Marti, E. Small RNAs in plasma extracellular vesicles define biomarkers of premanifest changes in Huntington’s disease. J. Extracell. Vesicles 2024, 13, e12522. [Google Scholar] [CrossRef] [PubMed]
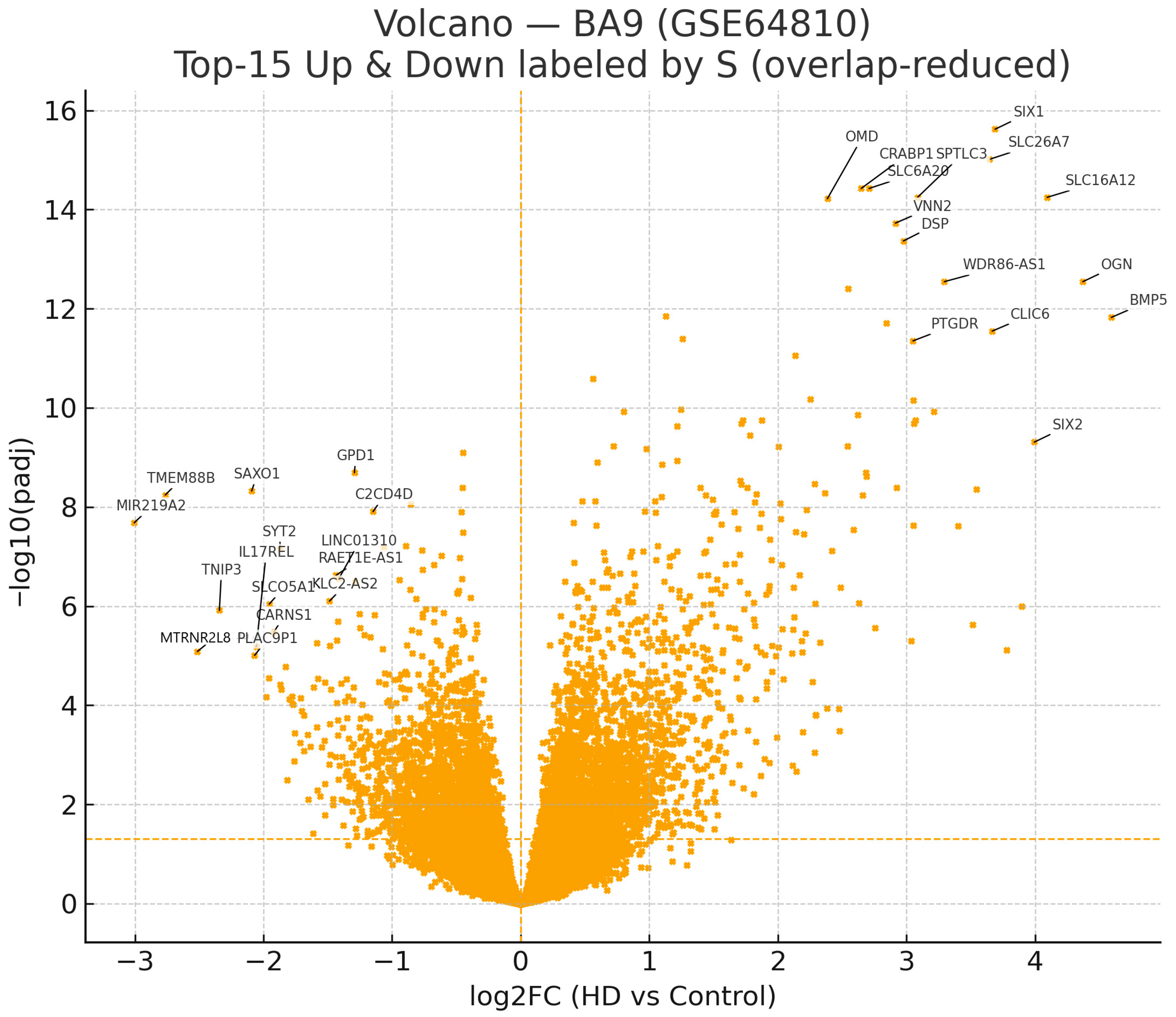
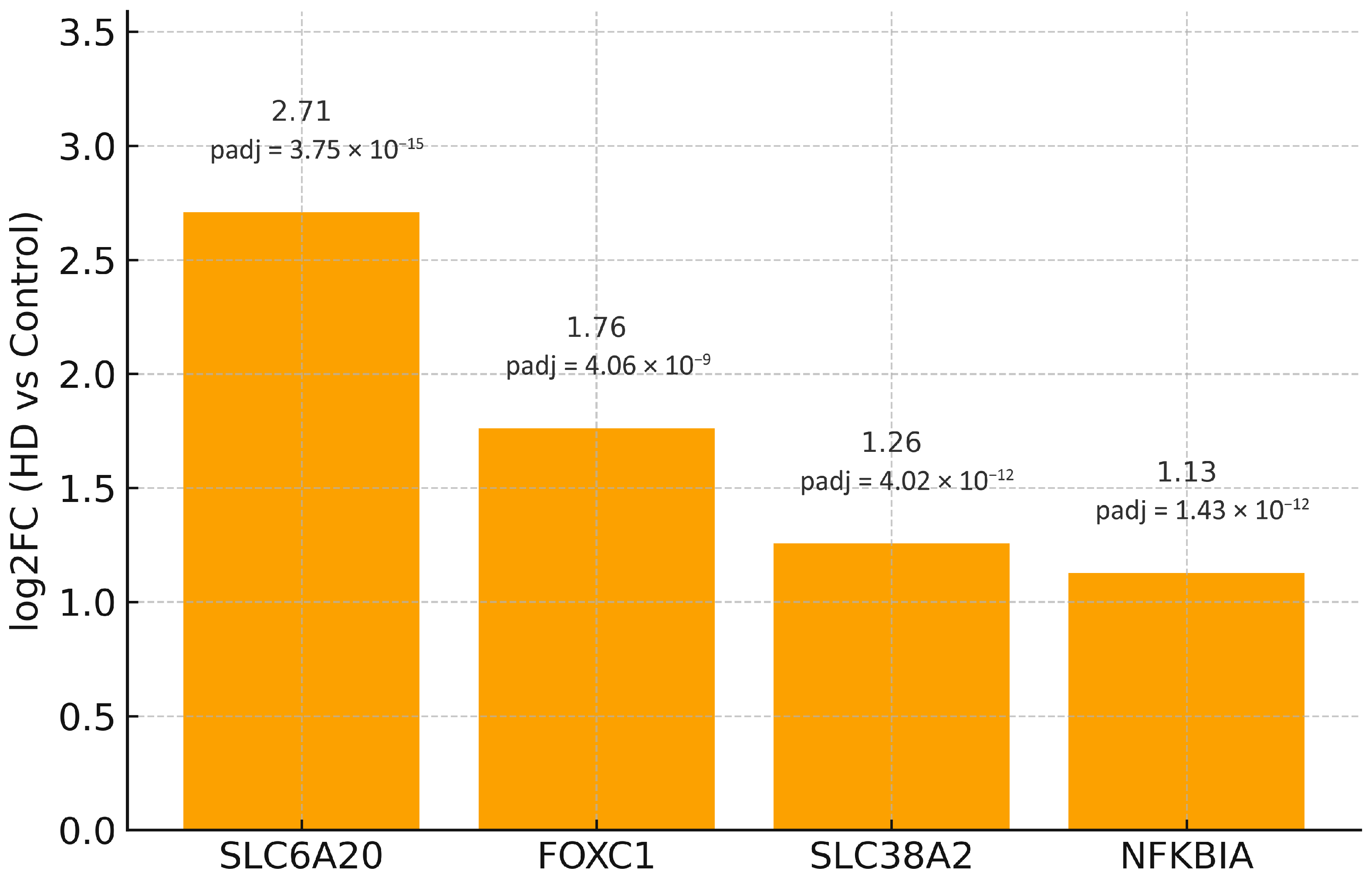

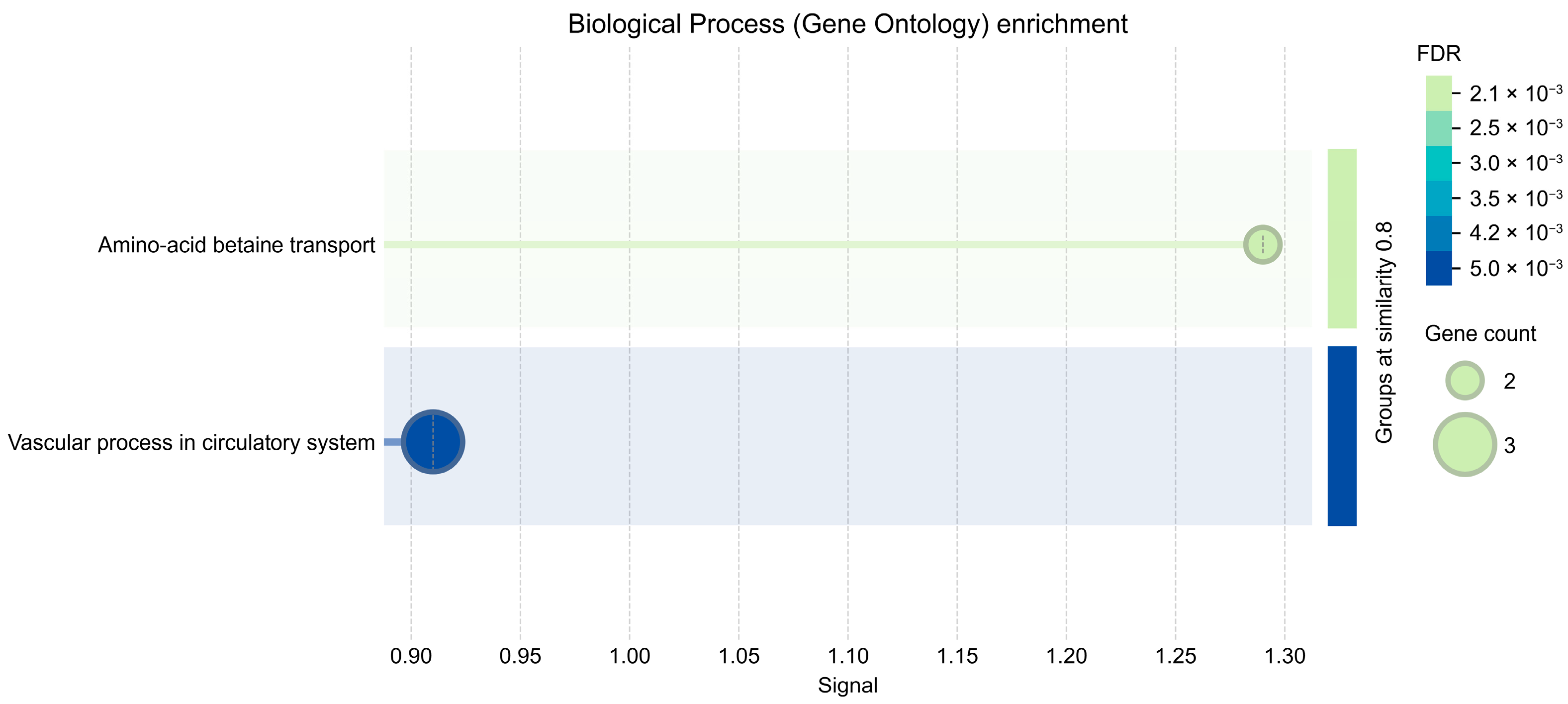
| Symbol | log2FC | padj | S |
|---|---|---|---|
| SLC16A12 | 4.094 | 5.670 × 10−15 | 58.320 |
| SIX1 | 3.689 | 2.400 × 10−16 | 57.615 |
| OGN | 4.372 | 2.850 × 10−13 | 54.842 |
| SLC26A7 | 3.646 | 9.690 × 10−16 | 54.747 |
| BMP5 | 4.593 | 1.500 × 10−12 | 54.305 |
| SPTLC3 | 3.086 | 5.670 × 10−15 | 43.968 |
| CLIC6 | 3.667 | 2.830 × 10−12 | 42.343 |
| WDR86-AS1 | 3.294 | 2.850 × 10−13 | 41.327 |
| VNN2 | 2.913 | 1.880 × 10−14 | 39.985 |
| DSP | 2.976 | 4.300 × 10−14 | 39.779 |
| SLC6A20 | 2.710 | 3.750 × 10−15 | 39.088 |
| CRABP1 | 2.648 | 3.750 × 10−15 | 38.201 |
| SIX2 | 3.994 | 4.910 × 10−10 | 37.182 |
| PTGDR | 3.051 | 4.460 × 10−12 | 34.628 |
| OMD | 2.385 | 6.030 × 10−15 | 33.911 |
| Symbol | log2FC | padj | S |
|---|---|---|---|
| MIR219A2 | −3.005 | 2.080 × 10−8 | 23.088 |
| TMEM88B | −2.764 | 5.730 × 10−9 | 22.781 |
| SAXO1 | −2.092 | 4.760 × 10−9 | 17.414 |
| TNIP3 | −2.343 | 1.220 × 10−6 | 13.856 |
| SYT2 | −1.870 | 6.900 × 10−8 | 13.392 |
| MTRNR2L8 | −2.514 | 8.220 × 10−6 | 12.784 |
| SLCO5A1 | −1.955 | 9.100 × 10−7 | 11.813 |
| GPD1 | −1.293 | 2.040 × 10−9 | 11.236 |
| IL17REL | −2.056 | 6.570 × 10−6 | 10.655 |
| CARNS1 | −1.920 | 3.330 × 10−6 | 10.515 |
| PLAC9P1 | −2.069 | 9.860 × 10−6 | 10.360 |
| RAET1E-AS1 | −1.436 | 2.390 × 10−7 | 9.511 |
| LINC01310 | −1.412 | 2.720 × 10−7 | 9.269 |
| C2CD4D | −1.148 | 1.240 × 10−8 | 9.078 |
| KLC2-AS2 | −1.487 | 7.920 × 10−7 | 9.074 |
| Symbol | log2FC | padj (BH) |
|---|---|---|
| FOXC1 | 1.762 | 4.06 × 10−9 |
| NFKBIA | 1.127 | 1.43 × 10−12 |
| SLC38A2 | 1.257 | 4.02 × 10−12 |
| SLC6A20 | 2.710 | 3.75 × 10−15 |
| Target Set | Size (n) | Overlap (a) | OR | 95% CI | p (One-Sided) | q (BH) |
|---|---|---|---|---|---|---|
| miRTarBase functional-only (luc/qPCR/Western) | 8 | 4 | 6.57 | [1.64, 26.27] | 0.0137 | 0.0137 |
| ENCORI/starBase CLIP-supported | 640 | 138 | 1.84 | [1.52, 2.23] | 2.56 × 10−9 | 7.68 × 10−9 |
| miRTarBase validated any (functional ∪ CLIP) | 42 | 15 | 3.66 | [1.94, 6.88] | 1.87 × 10−4 | 1.87 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öztan, G.; İşsever, H.; Şahin, L. BA9 Transcriptomics in Huntington’s Disease 80-Gene Signature and MIR219A2-Linked Targets. Int. J. Mol. Sci. 2025, 26, 8934. https://doi.org/10.3390/ijms26188934
Öztan G, İşsever H, Şahin L. BA9 Transcriptomics in Huntington’s Disease 80-Gene Signature and MIR219A2-Linked Targets. International Journal of Molecular Sciences. 2025; 26(18):8934. https://doi.org/10.3390/ijms26188934
Chicago/Turabian StyleÖztan, Gözde, Halim İşsever, and Levent Şahin. 2025. "BA9 Transcriptomics in Huntington’s Disease 80-Gene Signature and MIR219A2-Linked Targets" International Journal of Molecular Sciences 26, no. 18: 8934. https://doi.org/10.3390/ijms26188934
APA StyleÖztan, G., İşsever, H., & Şahin, L. (2025). BA9 Transcriptomics in Huntington’s Disease 80-Gene Signature and MIR219A2-Linked Targets. International Journal of Molecular Sciences, 26(18), 8934. https://doi.org/10.3390/ijms26188934






