Immunonutrition: Another Player on the MASLD Field
Abstract
1. Introduction
2. Background
3. Immunonutrients in MASLD
3.1. Amino Acids
3.1.1. Cysteine
3.1.2. Arginine
3.1.3. Glutamine
3.1.4. Branched-Chain Amino Acids
3.2. Polyphenols
3.3. Vitamins
3.3.1. Vitamin C
3.3.2. Vitamin E
3.4. Trace Elements
Zinc
3.5. Fatty Acids
3.6. Nucleotides
4. Impact of Immunonutrients on MASLD Diet Pattern
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| NF-κB | Nuclear Factor kappa B |
| IR | Insulin Resistance |
| ω-3 | Omega-3 Fatty Acid |
| ROS | Reactive Oxygen Species |
| HSC | Hepatic Stellate Cells |
| AMPK | AMP activated protein kinase |
| mTOR | Mechanistic Target of Rapamycin |
| SIRT1 | Sirtuin 1 |
| SREBP1c | Sterol Regulatory Element-Binding Protein |
| Zn | Zinc |
| SPARC | Secretory Acidic Protein Rich in Cysteine |
| SLD | Steatotic Liver Disease |
| NAC | N-acetylcysteine |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| TNF-α | Tumor Necrosis Factor-alpha |
| HFD | High-Fat Diet |
| BCAA | Branched Chain Amino acids |
| MedDiet | Mediterranean Diet |
| NAD | Nicotinamide Adenine Dinucleotide |
| EGCG | Epigallocatechin-3-gallate |
| PUFA | Polyunsaturated Fatty Acids |
| NR | Nicotinamide Riboside |
| NMN | Nicotinamide Mononucleotide |
References
- Pérez, C.T.; Amarelle, C.G.; Novo, N.R.; Rodríguez, G.L.; Gil, B.M.; Carballeira, R.P.; Gutiérrez, F.P.; Armesto, R.A.; Blanco, A.C.; López, M.A.B.; et al. Immunonutrition, Evidence and Experiences. Nutr. Hosp. 2022, 40, 186–199. [Google Scholar] [CrossRef]
- Singh, R.; Gopalan, S.; Sibal, A. Immunonutrition. Indian J. Pediatr. 2002, 69, 417–419. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The Global Burden of Metabolic Disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef]
- Haas, J.T.; Francque, S.; Staels, B. Pathophysiology and Mechanisms of Nonalcoholic Fatty Liver Disease. Annu. Rev. Physiol. 2016, 78, 181–205. [Google Scholar] [CrossRef]
- Hoogerland, J.A.; Staels, B.; Dombrowicz, D. Immune-Metabolic Interactions in Homeostasis and the Progression to NASH. Trends Endocrinol. Metab. 2022, 33, 690–709. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A Systemic Metabolic Disorder with Cardiovascular and Malignant Complications. Gut 2024, 7, 691–702. [Google Scholar] [CrossRef]
- Chona, M.C.; Basto, L.M.L.; Ospina, C.P.; Coronado, A.C.P.; Silva, M.P.G.; Marín, M.; Vallejos, A.; Osmán, G.E.C.; Saavedra, C.; Rojas, J.D.; et al. Preoperative Immunonutrition and Postoperative Outcomes in Patients with Cancer Undergoing Major Abdominal Surgery: Retrospective Cohort Study. Clin. Nutr. ESPEN 2025, 65, 324–330. [Google Scholar] [CrossRef]
- McCarthy, M.S.; Martindale, R.G. Immunonutrition in Critical Illness: What Is the Role? Nutr. Clin. Pract. 2018, 33, 348–358. [Google Scholar] [CrossRef]
- Ricci, C.; Serbassi, F.; Alberici, L.; Ingaldi, C.; Gaetani, L.; De Raffele, E.; Pironi, L.; Sasdelli, A.S.; Mosconi, C.; Di Marco, M.C.; et al. Immunonutrition in Patients Who Underwent Major Abdominal Surgery: A Comprehensive Systematic Review and Component Network Metanalysis Using GRADE and CINeMA Approaches. Surgery 2023, 174, 1401–1409. [Google Scholar] [CrossRef]
- Beevi, S.S.; Pottakkat, B. Effect of Immunonutrition on the Liver Function Status of End-Stage Liver Disease Patients Waiting/Referred for Liver Transplant: A Randomized Controlled Trial. Cureus 2023, 15, e36923. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Vitamin C: A Review on Its Role in the Management of Metabolic Syndrome. Int. J. Med. Sci. 2020, 17, 1625–1638. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Yu, J.-H.; Sun, J.-H.; Ma, W.-Q.; Wang, J.-J.; Sun, G.-J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 725. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated Mechanisms of MASLD Pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef]
- Feng, J.; MengHuan, L.; TingTing, Y.; XueJie, Y.; HaiNing, G. Research Progress on AMPK in the Pathogenesis and Treatment of MASLD. Front. Immunol. 2025, 16, 1558041. [Google Scholar] [CrossRef]
- Marcondes-de-Castro, I.A.; Reis-Barbosa, P.H.; Marinho, T.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. AMPK/MTOR Pathway Significance in Healthy Liver and Non-alcoholic Fatty Liver Disease and Its Progression. J. Gastroenterol. Hepatol. 2023, 38, 1868–1876. [Google Scholar] [CrossRef]
- Idilman, I.S.; Ozdeniz, I.; Karcaaltincaba, M. Hepatic Steatosis: Etiology, Patterns, and Quantification. Semin. Ultrasound CT MRI 2016, 37, 501–510. [Google Scholar] [CrossRef]
- Pollock, G.R.Y.; Van Way, C.W. Immune-Enhancing Nutrition in Surgical Critical Care. Mo. Med. 2012, 109, 388–392. [Google Scholar]
- Beevi Safeena, S. Are Immunonutrition a Novel Therapy for Liver Diseases? Medicon Med. Sci. 2022, 4, 55–57. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Bai, X.; Li, Z.; Huang, S.; Lu, D.; Li, J.; Wang, Y.; Han, L.; Xia, K.; et al. Metabolic and Hepatic Biomarkers Associated with MASLD in the Chinese Population. Sci. Rep. 2025, 15, 31593. [Google Scholar] [CrossRef]
- Raczyńska, A.; Leszczyńska, T.; Skotnicki, P.; Koronowicz, A. The Impact of Immunomodulatory Components Used in Clinical Nutrition—A Narrative Review. Nutrients 2025, 17, 752. [Google Scholar] [CrossRef]
- de Jong, J.C.W.; van Rooijen, K.S.; Stigter, E.C.A.; Gülersönmez, M.C.; de Zoete, M.R.; Top, J.; Baars, M.J.D.; Vercoulen, Y.; Kuipers, F.; van Mil, S.W.C.; et al. Dietary Cystine Restriction Increases the Proliferative Capacity of the Small Intestine of Mice. PLoS ONE 2024, 19, e0290493. [Google Scholar] [CrossRef]
- Wong, S.L.I.; Sukkar, M.B. The SPARC Protein: An Overview of Its Role in Lung Cancer and Pulmonary Fibrosis and Its Potential Role in Chronic Airways Disease. Br. J. Pharmacol. 2017, 174, 3–14. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. Change of Some Oxidative Stress Parameters after Supplementation with Whey Protein Isolate in Patients with Type 2 Diabetes. Nutrition 2020, 73, 110700. [Google Scholar] [CrossRef]
- Mazzolini, G.; Atorrasagasti, C.; Onorato, A.; Peixoto, E.; Schlattjan, M.; Sowa, J.-P.; Sydor, S.; Gerken, G.; Canbay, A. SPARC Expression Is Associated with Hepatic Injury in Rodents and Humans with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2018, 8, 725. [Google Scholar] [CrossRef]
- Sarem, M.; Znaidak, R.; Macías, M.; Rey, R. Las Células Estrelladas Del Hígado: Su Importancia En Condiciones Normales y Patológicas. Gastroenterol. Hepatol. 2006, 29, 93–101. [Google Scholar] [CrossRef]
- Atorrasagasti, C.; Onorato, A.M.; Mazzolini, G. The Role of SPARC (Secreted Protein Acidic and Rich in Cysteine) in the Pathogenesis of Obesity, Type 2 Diabetes, and Non-Alcoholic Fatty Liver Disease. J. Physiol. Biochem. 2023, 79, 815–831. [Google Scholar] [CrossRef]
- Tran, M.; Mostofa, G.; Picard, M.; Wu, J.; Wang, L.; Shin, D.-J. SerpinA3N Deficiency Attenuates Steatosis and Enhances Insulin Signaling in Male Mice. J. Endocrinol. 2023, 256, e220073. [Google Scholar] [CrossRef]
- Rague, J. N-Acetylcysteine. In History of Modern Clinical Toxicology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–212. [Google Scholar]
- Hang, W.; Shu, H.; Wen, Z.; Liu, J.; Jin, Z.; Shi, Z.; Chen, C.; Wang, D.W. N-Acetyl Cysteine Ameliorates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease and Intracellular Triglyceride Accumulation by Preserving Mitochondrial Function. Front. Pharmacol. 2021, 12, 636204. [Google Scholar] [CrossRef]
- Nikbaf-Shandiz, M.; Adeli, S.; Faghfouri, A.H.; Khademi, F.; Jamilian, P.; Zarezadeh, M.; Ebrahimi-Mamaghani, M. The Efficacy of N-Acetylcysteine in Improving Liver Function: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. PharmaNutrition 2023, 24, 100343. [Google Scholar] [CrossRef]
- Grimble, R.F. Basics in Clinical Nutrition: Immunonutrition—Nutrients Which Influence Immunity: Effect and Mechanism of Action. E-Spen Eur. E J. Clin. Nutr. Metab. 2009, 4, e10–e13. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Campas-Baypoli, O.N.; Cantú-Soto, E.U.; de la Mora-López, G.S. Influence of Other Nutrients (e.g., l-Arginine, Taurine, and Choline) on Liver Diseases. In Influence of Nutrients, Bioactive Compounds, and Plant Extracts in Liver Diseases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–208. [Google Scholar]
- d’Unienville, N.M.A.; Blake, H.T.; Coates, A.M.; Hill, A.M.; Nelson, M.J.; Buckley, J.D. Effect of Food Sources of Nitrate, Polyphenols, L-Arginine and L-Citrulline on Endurance Exercise Performance: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Int. Soc. Sports Nutr. 2021, 18, 76. [Google Scholar] [CrossRef]
- Navarro, L.A.; Wree, A.; Povero, D.; Berk, M.P.; Eguchi, A.; Ghosh, S.; Papouchado, B.G.; Erzurum, S.C.; Feldstein, A.E. Arginase 2 Deficiency Results in Spontaneous Steatohepatitis: A Novel Link between Innate Immune Activation and Hepatic de Novo Lipogenesis. J. Hepatol. 2015, 62, 412–420. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.Y.; Gee, H.Y. Lactobacillus Plantarum Ameliorates NASH-Related Inflammation by Upregulating l-Arginine Production. Exp. Mol. Med. 2023, 55, 2332–2345. [Google Scholar] [CrossRef] [PubMed]
- Otani, L.; Nishi, H.; Koyama, A.; Akasaka, Y.; Taguchi, Y.; Toyoshima, Y.; Yamanaka, D.; Hakuno, F.; Jia, H.; Takahashi, S.-I.; et al. Low-Arginine and Low-Protein Diets Induce Hepatic Lipid Accumulation through Different Mechanisms in Growing Rats. Nutr. Metab. 2020, 17, 60. [Google Scholar] [CrossRef]
- Abu-Serie, M.M.; El-Gamal, B.A.; El-Kersh, M.A.; El-Saadani, M.A. Investigation into the Antioxidant Role of Arginine in the Treatment and the Protection for Intralipid-Induced Non-Alcoholic Steatohepatitis. Lipids Health Dis. 2015, 14, 128. [Google Scholar] [CrossRef]
- Ma, W.; Heianza, Y.; Huang, T.; Wang, T.; Sun, D.; Zheng, Y.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Dietary Glutamine, Glutamate and Mortality: Two Large Prospective Studies in US Men and Women. Int. J. Epidemiol. 2018, 47, 311–320. [Google Scholar] [CrossRef]
- Kapalka, G.M. Substances Involved in Neurotransmission. In Nutritional and Herbal Therapies for Children and Adolescents; Elsevier: Amsterdam, The Netherlands, 2010; pp. 71–99. [Google Scholar]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Chashmniam, S.; Ghafourpour, M.; Rezaei Farimani, A.; Gholami, A.; Ghoochani, B.F.N.M. Metabolomic Biomarkers in the Diagnosis of Non-Alcoholic Fatty Liver Disease. Hepat. Mon. 2019, 19, e92244. [Google Scholar] [CrossRef]
- Li, J.; Ghazwani, M.; Liu, K.; Huang, Y.; Chang, N.; Fan, J.; He, F.; Li, L.; Bu, S.; Xie, W.; et al. Regulation of Hepatic Stellate Cell Proliferation and Activation by Glutamine Metabolism. PLoS ONE 2017, 12, e0182679. [Google Scholar] [CrossRef]
- Yin, X.; Peng, J.; Gu, L.; Liu, Y.; Li, X.; Wu, J.; Xu, B.; Zhuge, Y.; Zhang, F. Targeting Glutamine Metabolism in Hepatic Stellate Cells Alleviates Liver Fibrosis. Cell Death Dis. 2022, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Hyun, J.; Premont, R.T.; Choi, S.S.; Michelotti, G.A.; Swiderska-Syn, M.; Dalton, G.D.; Thelen, E.; Rizi, B.S.; Jung, Y.; et al. Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells. Gastroenterology 2018, 154, 1465–1479.e13. [Google Scholar] [CrossRef] [PubMed]
- Schemitt, E.G.; Colares, J.R.; Hartmann, R.M.; Morgan-Martins, M.I.; Marroni, C.A.; Tuñón, M.J.; Marroni, N.P. Efecto de La Glutamina En El Estrés Oxidativo y La Inflamación En Un Modelo de Rata Con Insuficiencia Hepática Fulminante. Nutr. Hosp. 2016, 33, 92. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, F.; Lin, N.; Ye, J.; Zheng, Q.; Ding, G. Effects of Glutamine on Oxidative Stress and Nuclear Factor-ΚB Expression in the Livers of Rats with Nonalcoholic Fatty Liver Disease. Exp. Ther. Med. 2014, 7, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H. The Role of Cytokines in Non-Alcoholic Fatty Liver Disease. Dig. Dis. 2010, 28, 179–185. [Google Scholar] [CrossRef]
- Xu, B.; Wang, M.; Pu, L.; Shu, C.; Li, L.; Han, L. Association of Dietary Intake of Branched-Chain Amino Acids with Long-Term Risks of CVD, Cancer and All-Cause Mortality. Public Health Nutr. 2022, 25, 3390–3400. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Lee, H.; Provido, S.M.P.; Chung, G.H.; Hong, S.; Yu, S.H.; Lee, C.B.; Lee, J.E. Dietary Intakes of Branched-Chain Amino Acids and Plasma Lipid Profiles among Filipino Women in Korea: The Filipino Women’s Diet and Health Study (FiLWHEL). Nutr. J. 2023, 22, 34. [Google Scholar] [CrossRef]
- Cuomo, P.; Capparelli, R.; Iannelli, A.; Iannelli, D. Role of Branched-Chain Amino Acid Metabolism in Type 2 Diabetes, Obesity, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 4325. [Google Scholar] [CrossRef]
- Honda, T.; Ishigami, M.; Luo, F.; Lingyun, M.; Ishizu, Y.; Kuzuya, T.; Hayashi, K.; Nakano, I.; Ishikawa, T.; Feng, G.-G.; et al. Branched-Chain Amino Acids Alleviate Hepatic Steatosis and Liver Injury in Choline-Deficient High-Fat Diet Induced NASH Mice. Metabolism 2017, 69, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-McKeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched Chain Amino Acid Metabolism Profiles in Progressive Human Nonalcoholic Fatty Liver Disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Surugihalli, C.; Muralidaran, V.; Ryan, C.E.; Patel, K.; Zhao, D.; Sunny, N.E. Branched-Chain Amino Acids Alter Cellular Redox to Induce Lipid Oxidation and Reduce de Novo Lipogenesis in the Liver. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E299–E313. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. The Polyphenols, Naturally Occurring Compounds with Beneficial Effects on Cardiovascular Disease. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar]
- Safer, A.-M.; Afzal, M.; Nomani, M.; Mousa, S.A. Green Tea Extract in the Management of Hepatic Fibrosis. In Tea in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2013; pp. 903–909. [Google Scholar]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Rafiei, H.; Omidian, K.; Bandy, B. Dietary Polyphenols Protect Against Oleic Acid-Induced Steatosis in an In Vitro Model of NAFLD by Modulating Lipid Metabolism and Improving Mitochondrial Function. Nutrients 2019, 11, 541. [Google Scholar] [CrossRef]
- Osborn, L.J.; Schultz, K.; Massey, W.; DeLucia, B.; Choucair, I.; Varadharajan, V.; Banerjee, R.; Fung, K.; Horak, A.J.; Orabi, D.; et al. A Gut Microbial Metabolite of Dietary Polyphenols Reverses Obesity-Driven Hepatic Steatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2202934119. [Google Scholar] [CrossRef]
- Fogacci, F.; Avagimyan, A.; Cesaro, A.; Bernardi, M.; Perone, F.; Giovannini, M.; Cicero, A.F.G. The Effect of Highly Bioavailable Forms of Curcumin on Lipoprotein(a) Plasma Levels: A Systematic Review and Meta-Analysis of Randomized Clinical Studies. Prostaglandins Other Lipid Mediat. 2025, 178, 106994. [Google Scholar] [CrossRef]
- Rajabinasab, F.; Tabatabaei, F.S.A.; Kheirandish, A.; Hajimirzaei, P.; Abolfazli, S.; Aromolaran, A.S.; Jamialahmadi, T.; Sahebkar, A. Effect of Curcumin on Cardiometabolic Diseases in the Elderly: A Systematic Review of Randomized Controlled Trials. Ageing Res. Rev. 2025, 110, 102801. [Google Scholar] [CrossRef]
- Goleij, P.; Rezaee, A.; Lam, H.Y.; Tabari, M.A.K.; Ouf, N.; Alijanzadeh, D.; Sanaye, P.M.; Larsen, D.S.; Daglia, M.; Khan, H.; et al. From Bench to Bedside: Exploring Curcumin-Driven Signaling Pathways in Immune Cells for Cancer Management. Inflammopharmacology 2025, 33, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef] [PubMed]
- Lukkunaprasit, T.; Tansawet, A.; Boonmanunt, S.; Sobhonslidsuk, A.; McKay, G.J.; Attia, J.; Thakkinstian, A. An Updated Meta-Analysis of Effects of Curcumin on Metabolic Dysfunction-Associated Fatty Liver Disease Based on Available Evidence from Iran and Thailand. Sci. Rep. 2023, 13, 5824. [Google Scholar] [CrossRef]
- Bayram, H.M.; Majoo, F.M.; Ozturkcan, A. Polyphenols in the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease: An Update of Preclinical and Clinical Studies. Clin. Nutr. ESPEN 2021, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wang, J.; Xie, X.; Xu, L.; Tang, S. Green Tea Polyphenols Attenuate Hepatic Steatosis, and Reduce Insulin Resistance and Inflammation in High-Fat Diet-Induced Rats. Int. J. Mol. Med. 2019, 44, 1523–1530. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Wang, Y.; Zhang, Q.; Li, J.; Zhong, P.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-Gallate Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via Inhibition of Apoptosis and Promotion of Autophagy through the ROS/MAPK Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5599997. [Google Scholar] [CrossRef]
- Du, Y.; Paglicawan, L.; Soomro, S.; Abunofal, O.; Baig, S.; Vanarsa, K.; Hicks, J.; Mohan, C. Epigallocatechin-3-Gallate Dampens Non-Alcoholic Fatty Liver by Modulating Liver Function, Lipid Profile and Macrophage Polarization. Nutrients 2021, 13, 599. [Google Scholar] [CrossRef]
- Ngu, M.H.; Norhayati, M.N.; Rosnani, Z.; Zulkifli, M.M. Curcumin as Adjuvant Treatment in Patients with Non-Alcoholic Fatty Liver (NAFLD) Disease: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2022, 68, 102843. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Huang, N.; Guan, F.; Luo, H.; Chen, L.; Wei, G.; Li, M.; Lin, Z.; Su, Z.; et al. Curcumin Alleviates High-Fat Diet-Induced Nonalcoholic Steatohepatitis via Improving Hepatic Endothelial Function with Microbial Biotransformation in Rats. J. Agric. Food Chem. 2023, 71, 10338–10348. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, X.; Li, Y.; Liang, Y.; Hong, T.; Yang, J.; Cao, Z.; Mai, H.; Yao, J.; Zhang, T.; et al. Curcumin Supplementation Alleviates Hepatic Fat Content Associated with Modulation of Gut Microbiota-Dependent Bile Acid Metabolism in Patients with Nonalcoholic Simple Fatty Liver Disease: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2024, 120, 66–79. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, L.; Mei, L.; Zhao, X.; Han, P.; Liu, J.; Meng, C.; Li, R.; Zhong, R.; Wang, K.; et al. Vitamin C and Vitamin D3 Alleviate Metabolic-Associated Fatty Liver Disease by Regulating the Gut Microbiota and Bile Acid Metabolism via the Gut-Liver Axis. Front. Pharmacol. 2023, 14, 1163694. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Hon, S.L. Vitamin C (Ascorbic Acid). In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 805–807. [Google Scholar]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary Habits and Their Relations to Insulin Resistance and Postprandial Lipemia in Nonalcoholic Steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, Y.-J.; Baek, S.-M.; Kang, K.-K.; Kim, T.-U.; Yim, J.-H.; Kim, H.-Y.; Han, S.-H.; Choi, S.-K.; Park, S.-J.; et al. Mega-Dose Vitamin C Ameliorates Nonalcoholic Fatty Liver Disease in a Mouse Fast-Food Diet Model. Nutrients 2022, 14, 2195. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, X.; Yang, H.; Wu, P.; Wang, S.; Cao, D.; Guo, X.; Xu, Z.; Gao, J.; Zhang, W.; et al. Effects of Oral Vitamin C Supplementation on Liver Health and Associated Parameters in Patients With Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Nutr. 2021, 8, 745609. [Google Scholar] [CrossRef]
- Fatima, G.; Dzupina, A.; Mahdi, A.A.; Fedacko, J.; Magomedova, A.; Yousif, N.G. Role of Vitamin-E as a Vital Nutrient in Health and Diseases. Indian J. Clin. Biochem. 2025, 1–14. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin e in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [CrossRef]
- Nagashimada, M.; Ota, T. Role of Vitamin E in Nonalcoholic Fatty Liver Disease. IUBMB Life 2019, 71, 516–522. [Google Scholar] [CrossRef]
- Pervez, M.A.; Khan, D.A.; Mirza, S.A.; Slehria, A.U.R.; Nisar, U.; Aamir, M. Comparison of Delta-Tocotrienol and Alpha-Tocopherol Effects on Hepatic Steatosis and Inflammatory Biomarkers in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blind Active-Controlled Trial. Complement. Ther. Med. 2022, 70, 102866. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Lahmi, A.; Oryan, S.; Eidi, A.; Rohani, A.H. Comparative Effects of Thymol and Vitamin E on Nonalcoholic Fatty Liver Disease in Male Wistar Rats. Braz. J. Biol. 2024, 84, e268781. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and Its Importance for Human Health: An Integrative Review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Sangeetha, V.J.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Zinc Nutrition and Human Health: Overview and Implications. eFood 2022, 3, e17. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc Status Is Associated with Inflammation, Oxidative Stress, Lipid, and Glucose Metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Cooper-Capetini, V.; De Vasconcelos, D.; Martins, A.; Hirabara, S.; Donato, J., Jr.; Carpinelli, A.; Abdulkader, F. Zinc Supplementation Improves Glucose Homeostasis in High Fat-Fed Mice by Enhancing Pancreatic β-Cell Function. Nutrients 2017, 9, 1150. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-D.; Zhang, H.; Rios, R.S.; Li, Y.-Y.; Zhu, P.-W.; Jin, Y.; Ma, H.-L.; Tang, L.-J.; Li, G.; Huang, O.-Y.; et al. J-Shaped Relationship between Serum Zinc Levels and the Severity of Hepatic Necro-Inflammation in Patients with MAFLD. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1259–1265. [Google Scholar] [CrossRef]
- Barbara, M.; Mindikoglu, A.L. The Role of Zinc in the Prevention and Treatment of Nonalcoholic Fatty Liver Disease. Metab. Open 2021, 11, 100105. [Google Scholar] [CrossRef]
- Rezaei, S.M.A.; Mohammadi, F.; Eftekhari, M.H.; Ejtehadi, F.; Ghaem, H.; Mohammadipoor, N. The Effects of Zinc Supplementation on the Metabolic Factors in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. BMC Nutr. 2023, 9, 138. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Human Requirement for N-3 Polyunsaturated Fatty Acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R.C. Dietary Intakes and Food Sources of Omega-6 and Omega-3 Polyunsaturated Fatty Acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Spooner, M.H.; Jump, D.B. Nonalcoholic Fatty Liver Disease and Omega-3 Fatty Acids: Mechanisms and Clinical Use. Annu. Rev. Nutr. 2023, 43, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 Fatty Acids and Non-Alcoholic Fatty Liver Disease: Evidence of Efficacy and Mechanism of Action. Mol. Asp. Med. 2018, 64, 135–146. [Google Scholar] [CrossRef]
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; Van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary Bioactive Fatty Acids as Modulators of Immune Function: Implications on Human Health. Nutrients 2019, 11, 2974. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, E673–E691. [Google Scholar] [CrossRef]
- Musazadeh, V.; Karimi, A.; Malekahmadi, M.; Ahrabi, S.S.; Dehghan, P. Omega-3 Polyunsaturated Fatty Acids in the Treatment of Non-alcoholic Fatty Liver Disease: An Umbrella Systematic Review and Meta-analysis. Clin. Exp. Pharmacol. Physiol. 2023, 50, 327–334. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Abdelmalek, M.F.; Suzuki, A.; Cummings, O.W.; Chojkier, M. No Significant Effects of Ethyl-Eicosapentanoic Acid on Histologic Features of Nonalcoholic Steatohepatitis in a Phase 2 Trial. Gastroenterology 2014, 147, 377–384.e1. [Google Scholar] [CrossRef]
- Argo, C.K.; Patrie, J.T.; Lackner, C.; Henry, T.D.; de Lange, E.E.; Weltman, A.L.; Shah, N.L.; Al-Osaimi, A.M.; Pramoonjago, P.; Jayakumar, S.; et al. Effects of N-3 Fish Oil on Metabolic and Histological Parameters in NASH: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Hepatol. 2015, 62, 190–197. [Google Scholar] [CrossRef]
- Gil, A. Modulation of the Immune Response Mediated by Dietary Nucleotides. Eur. J. Clin. Nutr. 2002, 56, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Dall, M.; Hassing, A.S.; Treebak, J.T. NAD+ and NAFLD—Caution, Causality and Careful Optimism. J. Physiol. 2022, 600, 1135–1154. [Google Scholar] [CrossRef]
- Jiang, R.; Zhou, Y.; Wang, S.; Pang, N.; Huang, Y.; Ye, M.; Wan, T.; Qiu, Y.; Pei, L.; Jiang, X.; et al. Nicotinamide Riboside Protects against Liver Fibrosis Induced by CCl4 via Regulating the Acetylation of Smads Signaling Pathway. Life Sci. 2019, 225, 20–28. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Beaulant, A.; Dia, M.; Pillot, B.; Chauvin, M.-A.; Ji-Cao, J.; Durand, C.; Bendridi, N.; Chanon, S.; Vieille-Marchiset, A.; Da Silva, C.C.; et al. Endoplasmic Reticulum-Mitochondria Miscommunication Is an Early and Causal Trigger of Hepatic Insulin Resistance and Steatosis. J. Hepatol. 2022, 77, 710–722. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Yu, Q.; Bao, T.; Dai, C.; Jiang, L.; Niu, K.; Yang, J.; Wang, S.; Wu, X. Alleviation of Hepatic Insulin Resistance and Steatosis with NMN via Improving Endoplasmic Reticulum–Mitochondria Miscommunication in the Liver of HFD Mice. Biomed. Pharmacother. 2024, 175, 116682. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.W.; Holmes, H.E.; Hu-Seliger, T.; Butt, R.W.; Harrison, S.A.; Mozaffarian, D.; Chen, O.; Guarente, L. Nicotinamide Riboside and Pterostilbene Reduces Markers of Hepatic Inflammation in NAFLD: A Double-blind, Placebo-controlled Clinical Trial. Hepatology 2023, 78, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-J.; Sun, S.-J.; Fu, J.-T.; Ouyang, S.-X.; Zhao, Q.-J.; Su, L.; Ji, Q.-X.; Sun, D.-Y.; Zhu, J.-H.; Zhang, G.-Y.; et al. NAD+-Boosting Therapy Alleviates Nonalcoholic Fatty Liver Disease via Stimulating a Novel Exerkine Fndc5/Irisin. Theranostics 2021, 11, 4381–4402. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mo, J.; Paolella, L.M.; Perry, C.E.; Toth, J.; Hugo, M.M.; Chu, Q.; Tong, Q.; Chellappa, K.; Baur, J.A. SIRT3 Is Required for Liver Regeneration but Not for the Beneficial Effect of Nicotinamide Riboside. JCI Insight 2021, 6, e147193. [Google Scholar] [CrossRef]
- Lu, X.; Yang, R.; Chen, Y.; Chen, D. Evaluating the Therapeutic Efficacy of NAD Supplementation in Management of Metabolic Dysfunction-Associated Steatotic Liver Disease: Key Considerations. Clin. Mol. Hepatol. 2024, 30, 582–584. [Google Scholar] [CrossRef]
- Hadefi, A.; Arvanitakis, M.; Trépo, E.; Zelber-Sagi, S. Dietary Strategies in Non-alcoholic Fatty Liver Disease Patients: From Evidence to Daily Clinical Practice, a Systematic Review. United Eur. Gastroenterol. J. 2023, 11, 663–689. [Google Scholar] [CrossRef]
- Saavedra, Y.; Mena, V.; Priken, K. Efecto de La Dieta Mediterránea Sobre Indicadores Histológicos y Pruebas de Imagen En Enfermedad de Hígado Graso No Alcohólico. Gastroenterol. Hepatol. 2022, 45, 350–360. [Google Scholar] [CrossRef]
- Aller, R.; Izaola, O.; de la Fuente, B.; De Luis Román, D.A. Mediterranean diet is associated with liver histology in patients with non-alcoholic fatty liver disease. Nutr. Hosp. 2015, 32, 2518–2524. [Google Scholar] [CrossRef]
- Miryan, M.; Darbandi, M.; Moradi, M.; Najafi, F.; Soleimani, D.; Pasdar, Y. Relationship between the Mediterranean Diet and Risk of Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Analysis of the RaNCD Cohort. Front. Nutr. 2023, 10, 1062008. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver Clinical Practice Guidelines for the Diagnosis and Management of Metabolic Associated Fatty Liver Disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wai-Sun Wong, V.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Arab, J.P.; Idalsoaga, F.; Perelli, J.; Vega, J.; Dirchwolf, M.; Carreño, J.; Samith, B.; Valério, C.; Moreira, R.O.; et al. Updated Recommendations for the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) by the Latin American Working Group. Ann. Hepatol. 2025, 30, 101903. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Retraction and Republication: Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N Engl J Med 2013;368:1279-90. N. Engl. J. Med. 2018, 378, 2441–2442, Correction in N. Engl. J. Med. 2018, 378, e34. https://doi.org/10.1056/NEJMoa1800389. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean Diet and Health: A Comprehensive Overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Dayi, T.; Ozgoren, M. Effects of the Mediterranean Diet on the Components of Metabolic Syndrome. J. Prev. Med. Hyg. 2022, 63, E56–E64. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Costanzo, S.; Di Castelnuovo, A.; Gialluisi, A.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; et al. Increased Adherence to a Mediterranean Diet Is Associated With Reduced Low-Grade Inflammation after a 12.7-Year Period: Results From the Moli-Sani Study. J. Acad. Nutr. Diet. 2023, 123, 783–795.e7. [Google Scholar] [CrossRef]
- Bettermann, E.L.; Hartman, T.J.; Easley, K.A.; Ferranti, E.P.; Jones, D.P.; Quyyumi, A.A.; Vaccarino, V.; Ziegler, T.R.; Alvarez, J.A. Higher Mediterranean Diet Quality Scores and Lower Body Mass Index Are Associated with a Less-Oxidized Plasma Glutathione and Cysteine Redox Status in Adults. J. Nutr. 2018, 148, 245–253. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Zelicha, H.; Kloting, N.; Kaplan, A.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Chassidim, Y.; Bluher, M.; Ceglarek, U.; Isermann, B.; et al. The Effect of High-Polyphenol Mediterranean Diet on Visceral Adiposity: The DIRECT PLUS Randomized Controlled Trial. BMC Med. 2022, 20, 327. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of Green-Mediterranean Diet on Intrahepatic Fat: The DIRECT PLUS Randomised Controlled Trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The Effectiveness and Acceptability of Mediterranean Diet and Calorie Restriction in Non-Alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef]
- Vazeux-Blumental, N.; Manicacci, D.; Tenaillon, M. The Milpa, from Mesoamerica to Present Days, a Multicropping Traditional Agricultural System Serving Agroecology. C. R. Biol. 2024, 347, 159–173. [Google Scholar] [CrossRef]
- Huerta-Álvarez, A.; Arellano, M.; Chávez-Méndez, C.A.; Carpinteyro-Espin, P.; Palacios-Reyes, C.; Pérez-Escobar, J. Milpa Diet for MASLD in Mesoamerican Populations: Feasibility, Advantages, and Future Perspectives. Life 2025, 15, 812. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, O.A.; Luna-Vital, D.A.; Morales-Hernandez, N.; Contreras, J.; Villaseñor-Tapia, E.C.; Fragoso-Medina, J.A.; Mojica, L. Nutritional, Bioactive Components and Health Properties of the Milpa Triad System Seeds (Corn, Common Bean and Pumpkin). Front. Nutr. 2023, 10, 1169675. [Google Scholar] [CrossRef]
- Luo, J.; Wei, Y.; Fan, Z.; Li, B.; Li, L.; Chai, Y.; Xing, Z.; Qiu, Y.; Lu, W.; Fang, Z. Gut Microbiota as a Metabolic Mediator to Prevent or Attenuate MASLD. Crit. Rev. Food Sci. Nutr. 2025, 1–17. [Google Scholar] [CrossRef]
- Lau, H.C.-H.; Zhang, X.; Yu, J. Gut Microbiome in Metabolic Dysfunction-Associated Steatotic Liver Disease and Associated Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 619–638. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef]
- Meadows, V.; Antonio, J.M.; Ferraris, R.P.; Gao, N. Ruminococcus Gnavus in the Gut: Driver, Contributor, or Innocent Bystander in Steatotic Liver Disease? FEBS J. 2025, 292, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The Effects of the Green-Mediterranean Diet on Cardiometabolic Health Are Linked to Gut Microbiome Modifications: A Randomized Controlled Trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
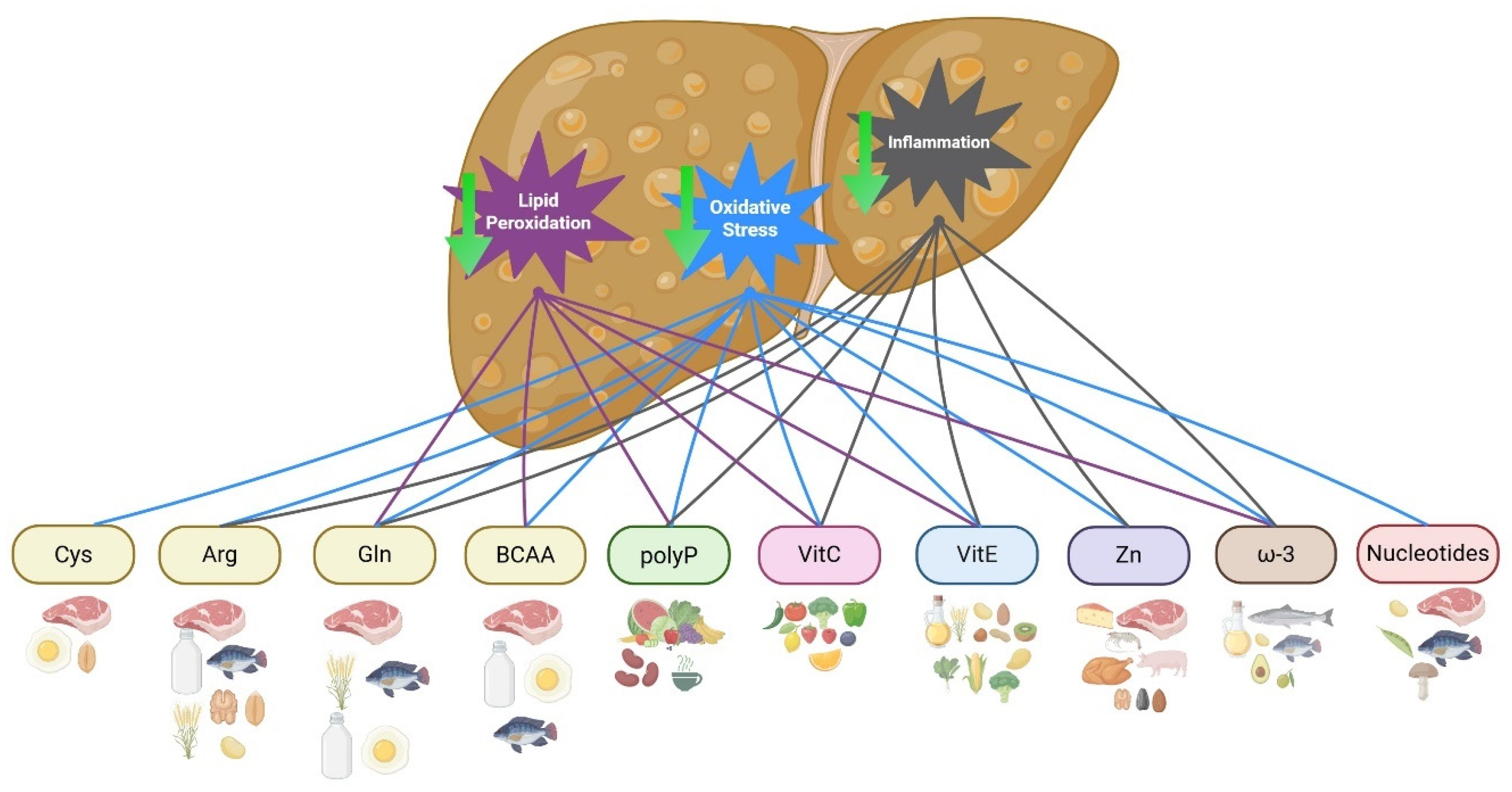
| Immunonutrient | Foods |
|---|---|
| Cysteine | Meat, egg, and whole grains |
| Arginine | Meat, fish, nuts, wheat germ, whole grains, soy, and dairy products |
| Glutamine | Meat, eggs, fish, dairy products, wheat, tofu, and fermented products |
| BCAA | Meat, fish, dairy, eggs |
| Polyphenols | Vegetables, fruits, cocoa, legumes, curcuma, green tea, corn, beans, amaranth, chia |
| Vitamin C | Citrus fruits, berries, tomatoes, peppers, corn, leafy greens, broccoli, kale, and parsley |
| Vitamin E | Vegetable oils (wheat germ, sunflower, corn, soybean), nuts, almonds, peanuts, hazelnuts, green leafy vegetables, broccoli and spinach, kiwi, mango, and fortified cereals |
| Zinc | Seafood, beef, pork, chicken, cheese, almonds, sunflowers, pumpkin seeds, and cashews |
| ω-3 fatty acids | Flaxseed, canola, and soybean oils, pumpkin seeds, peanuts, chia, avocado, salmon, tuna, sardines, cod, and hake |
| Cysteine | Meat, egg, and whole grains |
| Nucleotides | Meat, fish, beans, peas, lentil, mushroom |
| Type | Immunonutrient | Year Author Type of Study | Population | Intervention | Principal Findings |
|---|---|---|---|---|---|
| Amino acids | Cysteine | 2021 Hang W et al. [32] Basic | HFD induced MASLD mice | NAC vs. PBS | Decrease hepatic TG accumulation Preserved mitochondrial function Preventing ROS production |
| 2023 Tran M et al. [30] Basic | SerpinAN3 knock-out MASLD induced mice | Induced SerpinAN3 | Decrease in leptin and insulin levels Improved glucose tolerance | ||
| Arginine | 2015 Abu-Serie MM et al. [40] Basic | Induced steatohepatitis murine model | 500 mg/kg Arginine | Decrease levels of lipid peroxidation and TNF-α Decrease the activity of CYP2E1 Increase the activity of nitric oxide and endothelial nitric oxide | |
| Glutamine | 2014 Lin Z et al. [49] Basic | HFD-induced MASLD rats | 1 g/kg/day Glutamine | Decrease in oxidative stress, TNF-α, and MDA | |
| BCAA | 2016 Honda T et al. [54] Basic | CDHFD MASLD murine model | 0.23 g isoleucine/g + 0.46 g leucine/g + 0.28 g valine/g | Decrease in serum ALT and glucose Decrease in hepatic TG Decrease fatty acid synthetase activity | |
| Polyphenols | EGCG | 2021 Wu D et al. [69] Basic | Liver cell line and HFD-induced MASLD murine model | 50 mg/kg/day EGCG | Decrease mitochondrial-dependent apoptosis Increase autophagy |
| 2021 Du Y et al. [70] Basic | Fructose + HFD-induced MASLD murine model | EGCG low dose (25 mg/kg/day) vs. high dose (50 mg/kg/day) | Decrease in serum cholesterol Decrease hepatic TG Decrease in steatosis and steatohepatitis scores | ||
| Curcumin | 2023 Wu J et al. [76] Basic | HFD-induced MASLD murine model | Curcumin supplement | Improvement of hepatic endothelial function Decrease hepatic lipid accumulation and inflammation | |
| 2023 Lukkunaprasit T et al. [66] Meta-analysis | 16 RCTs in MASLD patients | Curcumin supplementation | Decrease in BMI Improvement of AST, ALT Decrease fasting glucose and total cholesterol Steatosis resolution Decrease steatosis severity | ||
| 2024 He Y et al. [73] RCT | 80 MASLD patients | 500 mg/day Curcumin vs. placebo | Decrease hepatic steatosis (dB/m) Decrease in BMI, free fatty acids, TG, fasting glucose, and HbA1c | ||
| Vitamins | Vitamin C | 2021 He Z et al. [79] RCT | 84 MASLD patients | 1000 mg/day of Vitamin C | Decrease AST and ALT levels Improvement of HOMA-IR |
| 2022 Lee S et al. [78] Basic | HFD-induced MASLD mice | Megadose of Vitamin C | Less lobular inflammation and fat accumulation Decrease TG and free fatty acids accumulation | ||
| Vitamin E | 2010 Sanyal AJ et al. [84] RCT—PIVENS study | 247 NASH patients | 800 IU/day of Vitamin E | Decrease ALT and AST levels Decrease in lobular inflammation and hepatocellular ballooning Decrease in NAS Score | |
| 2024 Lahmi A et al. [86] Basic | HFD-induced MASLD murine model | 200 mg/kg/day of Vitamin E and 200 mg/kg/day of Vitamin E + 50 mg/kg/day of thymol | Decrease in AST and ALT levels Cytoarchitectural changes in hepatocytes | ||
| Trace element | Zinc | 2023 Razei SMA et al. [93] RCT | 50 MASLD patients | 30 mg/day Zinc Gluconate vs. placebo | Decrease in AST, LDL, and total cholesterol levels |
| Fatty acids | ω-3 | 2023 Musazadeh V et al. [101] Meta-analysis | 6561 MASLD patients | ω-3 supplementation | Decrease in AST, ALT, and GGT levels |
| Nucleotides | NAD+ | 2021 Li D et al. [111] Basic | NAFLD murine model | NAD+-Boosting supplementation | Reversion of liver steatosis |
| NR | 2021 Mukherjee et al. [112] Basic | Hepatocyte-specific SIRT1-ko mice | 500 mg/day of NR | Hepatic regeneration Improve mitochondrial function Decrease hepatic TG | |
| NMN | 2023 Dellinger, RW et al. [110] RCT | 111 MASLD patients | NR+Pterostilbene supplementation | Decrease in AST and GGT serum levels | |
| 2024 Li Y et al. [109] Basic | HFD SLD induced murine model | 500 mg/L od NMN on pure water | Improve IR and mitochondrial dysfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Méndez, I.; Uribe, M.; Juárez-Hernández, E. Immunonutrition: Another Player on the MASLD Field. Int. J. Mol. Sci. 2025, 26, 8928. https://doi.org/10.3390/ijms26188928
López-Méndez I, Uribe M, Juárez-Hernández E. Immunonutrition: Another Player on the MASLD Field. International Journal of Molecular Sciences. 2025; 26(18):8928. https://doi.org/10.3390/ijms26188928
Chicago/Turabian StyleLópez-Méndez, Iván, Misael Uribe, and Eva Juárez-Hernández. 2025. "Immunonutrition: Another Player on the MASLD Field" International Journal of Molecular Sciences 26, no. 18: 8928. https://doi.org/10.3390/ijms26188928
APA StyleLópez-Méndez, I., Uribe, M., & Juárez-Hernández, E. (2025). Immunonutrition: Another Player on the MASLD Field. International Journal of Molecular Sciences, 26(18), 8928. https://doi.org/10.3390/ijms26188928






