Preclinical Efficacy of the Estrogen Receptor Degrader Fulvestrant in Combination with RAF/MEK Clamp Avutometinib and FAK Inhibitor in a Low-Grade Serous Ovarian Cancer Animal Model with Acquired Resistance to Chemotherapy and Aromatase Inhibitor
Abstract
1. Introduction
2. Results
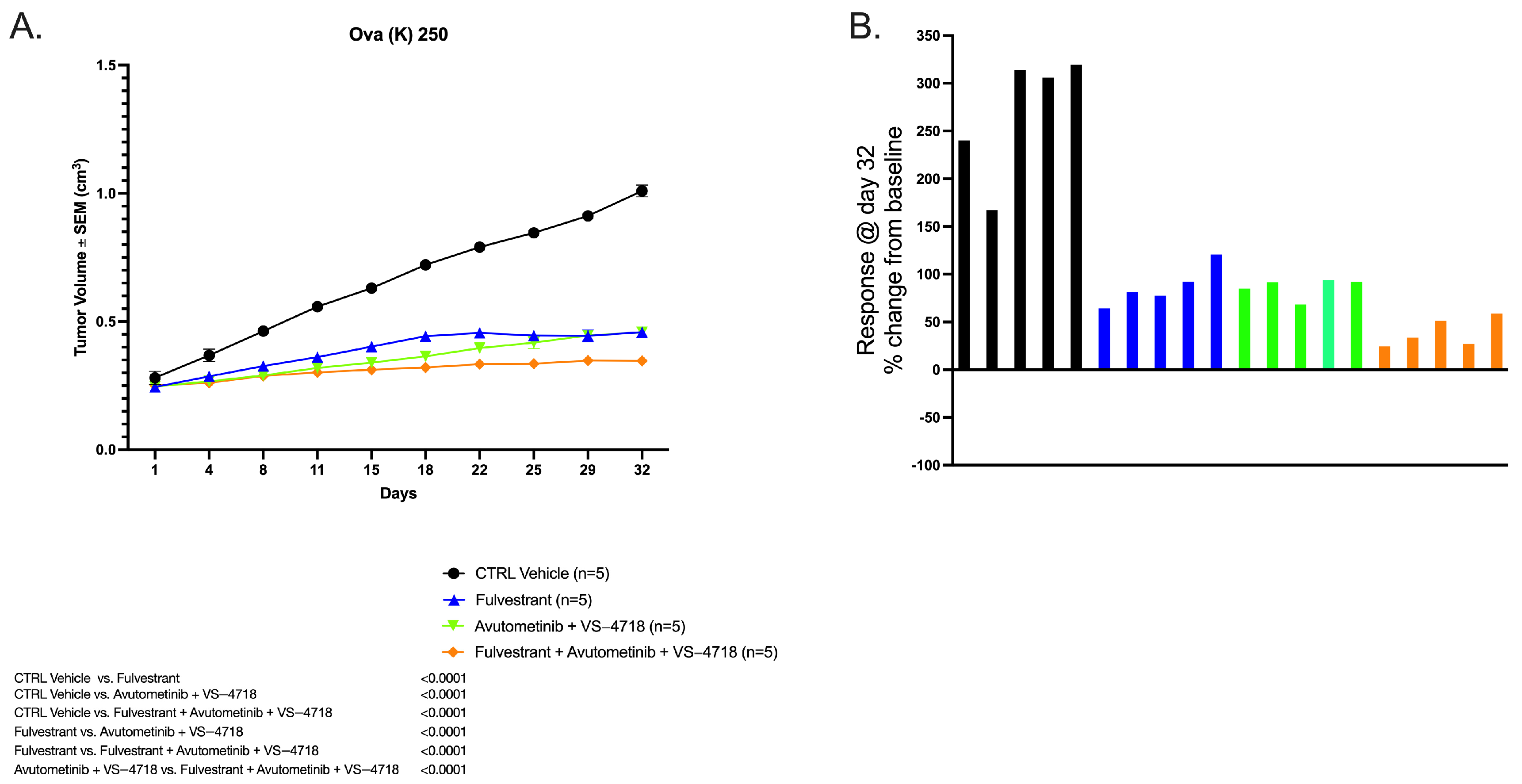
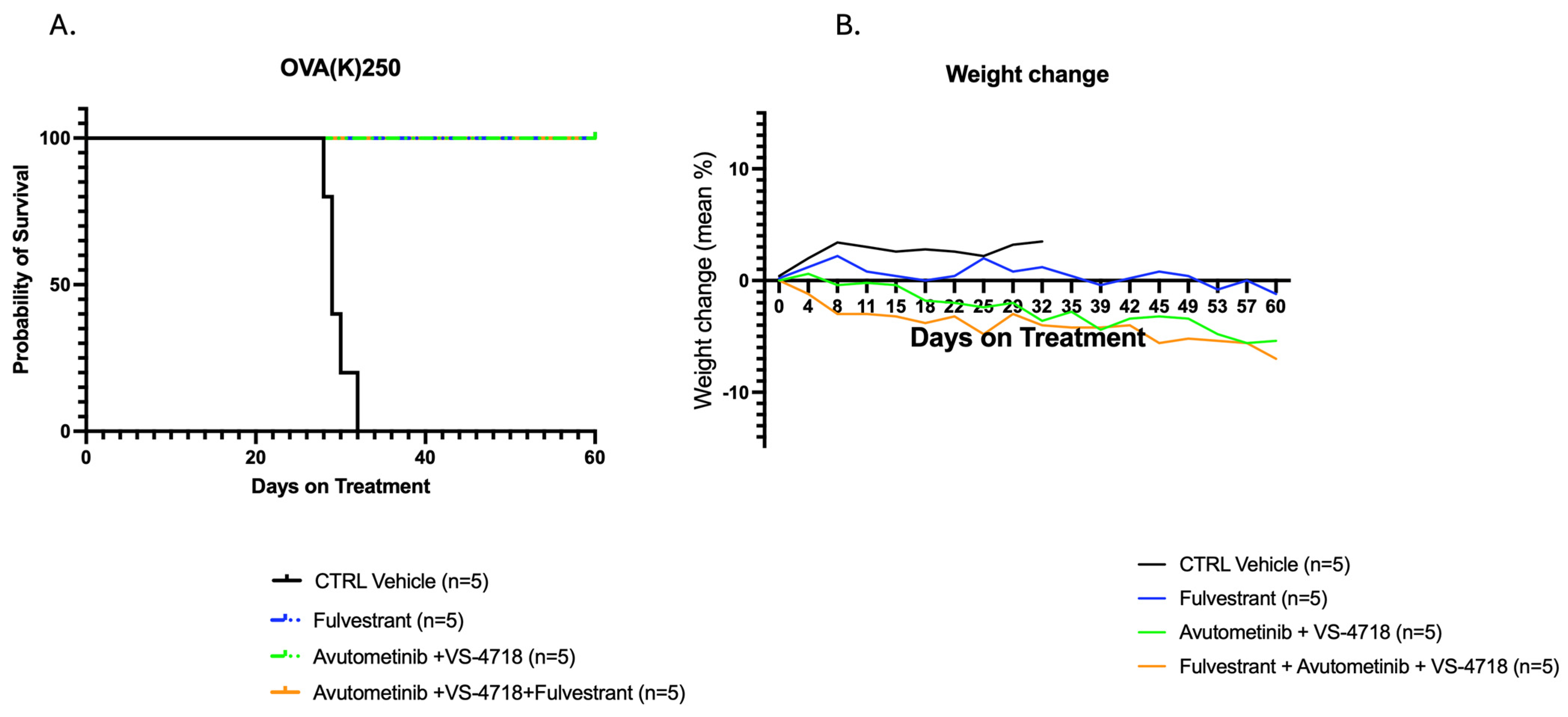
2.1. In Vivo Antitumor Activity of Avutometinib, VS-4718, and Fulvestrant Versus Control
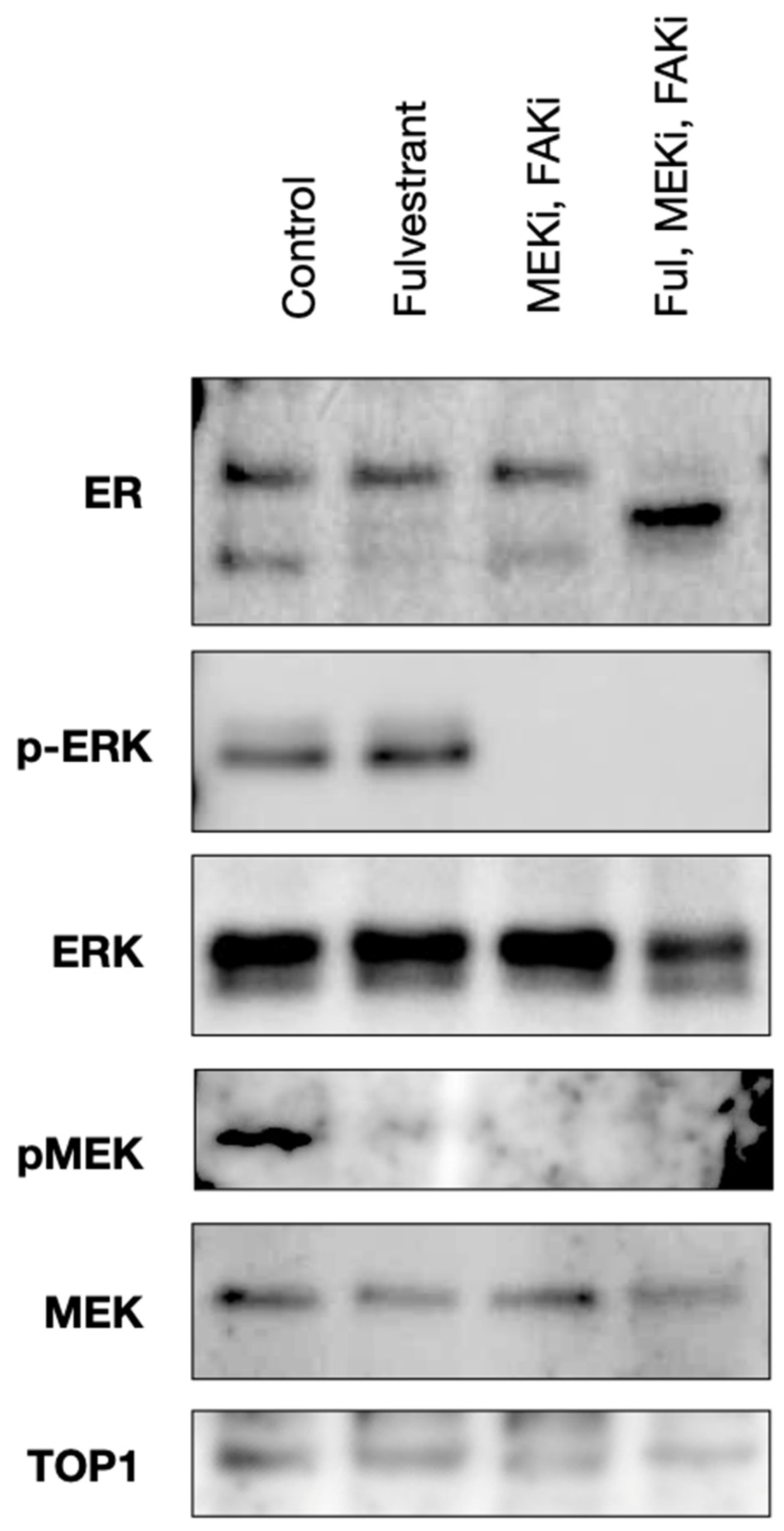
2.2. Avutometinib, VS-4718, and Fulvestrant Treatment Inhibits ERK and FAK Activation in an Ex Vivo Model of LGSOC
3. Discussion
4. Materials and Methods
4.1. Specimen Collection and Formation of PDX Models
4.2. Avutometinib, Defactinib, VS-4718, and Fulvestrant
4.3. In Vivo Treatment
4.4. Ex Vivo Tumor Tissue Culture and Treatment
4.5. Western Blot
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LGSOC | Low-Grade Serous Ovarian Cancer |
| HGSOC | High-Grade Serous Ovarian Cancer |
| ORR | Overall response rate |
| FAK | Focal Adhesion Kinase |
| FAKi | Focal Adhesion Kinase Inhibitor |
| PDX | Patient-Derived Xenograft |
| OS | Overall Survival |
| IC | Confidence Interval |
References
- Matsuo, K.; Machida, H.; Matsuzaki, S.; Grubbs, B.H.; Klar, M.; Roman, L.D.; Sood, A.K.; Gershenson, D.M.; Wright, J.D. Evolving population-based statistics for rare epithelial ovarian cancers. Gynecol. Oncol. 2020, 157, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.D.; Horkayne-Szakaly, I.; Haiba, M.; Boice, C.R.; Kurman, R.J.; Ronnett, B.M. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int. J. Gynecol. Pathol. 2004, 23, 41–44. [Google Scholar] [CrossRef] [PubMed]
- De Decker, K.; Wenzel, H.H.B.; Bart, J.; van der Aa, M.A.; Kruitwagen, R.; Nijman, H.W.; Kruse, A.J. Stage, treatment and survival of low-grade serous ovarian carcinoma in the Netherlands: A nationwide study. Acta Obstet. Gynecol. Scand. 2023, 102, 246–256. [Google Scholar] [CrossRef]
- Bogani, G.; Leone Roberti Maggiore, U.; Paolini, B.; Diito, A.; Martinelli, F.; Lorusso, D.; Raspagliesi, F. The detrimental effect of adopting interval debulking surgery in advanced stage low-grade serous ovarian cancer. J. Gynecol. Oncol. 2019, 30, e4. [Google Scholar] [CrossRef]
- Grabowski, J.P.; Harter, P.; Heitz, F.; Pujade-Lauraine, E.; Reuss, A.; Kristensen, G.; Ray-Coquard, I.; Heitz, J.; Traut, A.; Pfisterer, J.; et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol. Oncol. 2016, 140, 457–462. [Google Scholar] [CrossRef]
- Monk, B.J.; Grisham, R.N.; Banerjee, S.; Kalbacher, E.; Mirza, M.R.; Romero, I.; Vuylsteke, P.; Coleman, R.L.; Hilpert, F.; Oza, A.M.; et al. MILO/ENGOT-ov11: Binimetinib Versus Physician’s Choice Chemotherapy in Recurrent or Persistent Low-Grade Serous Carcinomas of the Ovary, Fallopian Tube, or Primary Peritoneum. J. Clin. Oncol. 2020, 38, 3753–3762. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Miller, A.; Brady, W.E.; Paul, J.; Carty, K.; Rodgers, W.; Millan, D.; Coleman, R.L.; Moore, K.N.; Banerjee, S.; et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): An international, randomised, open-label, multicentre, phase 2/3 trial. Lancet 2022, 399, 541–553. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Sun, C.C.; Bodurka, D.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Deavers, M.; Malpica, A.L.; Kavanagh, J.J. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol. Oncol. 2009, 114, 48–52. [Google Scholar] [CrossRef]
- Jones, S.; Wang, T.L.; Kurman, R.J.; Nakayama, K.; Velculescu, V.E.; Vogelstein, B.; Kinzler, K.W.; Papadopoulos, N.; Shih Ie, M. Low-grade serous carcinomas of the ovary contain very few point mutations. J. Pathol. 2012, 226, 413–420. [Google Scholar] [CrossRef]
- Hunter, S.M.; Anglesio, M.S.; Ryland, G.L.; Sharma, R.; Chiew, Y.E.; Rowley, S.M.; Doyle, M.A.; Li, J.; Gilks, C.B.; Moss, P.; et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget 2015, 6, 37663–37677. [Google Scholar] [CrossRef]
- Singer, G.; Oldt, R., 3rd; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih Ie, M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef]
- Llaurado Fernandez, M.; Dawson, A.; Kim, H.; Lam, N.; Russell, H.; Bruce, M.; Bittner, M.; Hoenisch, J.; Scott, S.A.; Talhouk, A.; et al. Hormone receptor expression and outcomes in low-grade serous ovarian carcinoma. Gynecol. Oncol. 2020, 157, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Sun, C.C.; Westin, S.N.; Eyada, M.; Cobb, L.P.; Nathan, L.C.; Sood, A.K.; Malpica, A.; Hillman, R.T.; Wong, K.K. The genomic landscape of low-grade serous ovarian/peritoneal carcinoma and its impact on clinical outcomes. Gynecol. Oncol. 2022, 165, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Manning-Geist, B.; Gordhandas, S.; Liu, Y.L.; Zhou, Q.; Iasonos, A.; Paula, A.D.; Mandelker, D.; Roche, K.L.; Zivanovic, O.; Maio, A.; et al. MAPK Pathway Genetic Alterations Are Associated with Prolonged Overall Survival in Low-Grade Serous Ovarian Carcinoma. Clin. Cancer Res. 2022, 28, 4456–4465. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chenard-Poirier, M.; Roda, D.; de Miguel, M.; Harris, S.J.; Candilejo, I.M.; Sriskandarajah, P.; Xu, W.; Scaranti, M.; Constantinidou, A.; et al. Intermittent schedules of the oral RAF-MEK inhibitor CH5126766/VS-6766 in patients with RAS/RAF-mutant solid tumours and multiple myeloma: A single-centre, open-label, phase 1 dose-escalation and basket dose-expansion study. Lancet Oncol. 2020, 21, 1478–1488, Erratum in Lancet Oncol. 2021, 22, e42. [Google Scholar] [CrossRef]
- Schwock, J.; Dhani, N.; Hedley, D.W. Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin. Ther. Targets 2010, 14, 77–94. [Google Scholar] [CrossRef]
- Dawson, J.C.; Serrels, A.; Stupack, D.G.; Schlaepfer, D.D.; Frame, M.C. Targeting FAK in anticancer combination therapies. Nat. Rev. Cancer 2021, 21, 313–324. [Google Scholar] [CrossRef]
- Kang, Y.; Hu, W.; Ivan, C.; Dalton, H.J.; Miyake, T.; Pecot, C.V.; Zand, B.; Liu, T.; Huang, J.; Jennings, N.B.; et al. Role of Focal Adhesion Kinase in Regulating YB-1-Mediated Paclitaxel Resistance in Ovarian Cancer. JNCI-J. Natl. Cancer Inst. 2013, 105, 1485–1495. [Google Scholar] [CrossRef]
- McNamara, B.; Demirkiran, C.; Hartwich, T.M.P.; Bellone, S.; Manavella, D.; Mutlu, L.; Greenman, M.; Zipponi, M.; Yang-Hartwich, Y.; Yang, K.; et al. Preclinical efficacy of RAF/MEK clamp avutometinib in combination with FAK inhibition in low grade serous ovarian cancer. Gynecol. Oncol. 2024, 183, 133–140. [Google Scholar] [CrossRef]
- Demirkiran, C.; Greenman, M.; Bellone, S.; McNamara, B.; Hartwich, T.M.P.; Manavella, D.; Mutlu, L.; Zipponi, M.; Yang-Hartwich, Y.; Yang, K.; et al. Preclinical in vitro and in vivo activity of the RAF/MEK clamp avutometinib in combination with FAK inhibition in uterine carcinosarcomas. Gynecol. Oncol. 2024, 187, 12–20. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Sun, C.C.; Iyer, R.B.; Malpica, A.L.; Kavanagh, J.J.; Bodurka, D.C.; Schmeler, K.; Deavers, M. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 2012, 125, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Bodurka, D.C.; Coleman, R.L.; Lu, K.H.; Malpica, A.; Sun, C.C. Hormonal Maintenance Therapy for Women with Low-Grade Serous Cancer of the Ovary or Peritoneum. J. Clin. Oncol. 2017, 35, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Babaier, A.; Mal, H.; Alselwi, W.; Ghatage, P. Low-Grade Serous Carcinoma of the Ovary: The Current Status. Diagnostics 2022, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M. The life and times of low-grade serous carcinoma of the ovary. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Shotton, R.; Ren, X.; Randhawa, M.; Tilby, M.; Vazquez, I.; Williams, S.; Glasspool, R.M.; Gourley, C.; Clamp, A.R.; Mitchell, C.L.; et al. Real-world outcomes in patients treated with trametinib for low grade serous ovarian carcinoma. Ann. Oncol. 2021, 32, S740. [Google Scholar] [CrossRef]
- Grisham, R.N.; Vergote, I.; Banerjee, S.; Drill, E.; Kalbacher, E.; Mirza, M.R.; Romero, I.; Vuylsteke, P.; Coleman, R.L.; Hilpert, F.; et al. Molecular Results and Potential Biomarkers Identified from the Phase 3 MILO/ENGOT-ov11 Study of Binimetinib versus Physician Choice of Chemotherapy in Recurrent Low-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2023, 29, 4068–4075. [Google Scholar] [CrossRef]
- Banerjee, S.N.; Ring, K.L.; Van Niewenhuysen, E.; Fabbro, M.; Aghajanian, C.; Oaknin, A.; Colombo, N.; Santin, A.D.; Clamp, A.R.; Moore, K.N.; et al. Initial Efficacy and Safety Results from Engot-Ov60/Gog-3052/Ramp 201: A Phase 2 Study of Avutometinib (Vs-6766) ± Defactinib in Recurrent Low-Grade Serous Ovarian Cancer (Lgsoc). Int. J. Gynecol. Cancer 2023, 33, A20–A21. [Google Scholar] [CrossRef]
- Fader, A.N.; Bergstrom, J.; Jernigan, A.; Tanner, E.J., 3rd; Roche, K.L.; Stone, R.L.; Levinson, K.L.; Ricci, S.; Wethingon, S.; Wang, T.L.; et al. Primary cytoreductive surgery and adjuvant hormonal monotherapy in women with advanced low-grade serous ovarian carcinoma: Reducing overtreatment without compromising survival? Gynecol. Oncol. 2017, 147, 85–91. [Google Scholar] [CrossRef]
- McLaughlin, P.M.J.; Klar, M.; Zwimpfer, T.A.; Dutilh, G.; Vetter, M.; Marth, C.; du Bois, A.; Schade-Brittinger, C.; Reuss, A.; Bommer, C.; et al. Maintenance Therapy with Aromatase Inhibitor in epithelial Ovarian Cancer (MATAO): Study protocol of a randomized double-blinded placebo-controlled multi-center phase III Trial. BMC Cancer 2022, 22, 508. [Google Scholar] [CrossRef]
- Hou, J.Y.; Rodriguez-Gabin, A.; Samaweera, L.; Hazan, R.; Goldberg, G.L.; Horwitz, S.B.; McDaid, H.M. Exploiting MEK Inhibitor-Mediated Activation of ERα for Therapeutic Intervention in ER-Positive Ovarian Carcinoma. PLoS ONE 2013, 8, e54103. [Google Scholar] [CrossRef]
- Nawaz, Z.; Lonard, D.M.; Dennis, A.P.; Smith, C.L.; O’Malley, B.W. Proteasome-dependent degradation of the human estrogen receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 1858–1862. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirkiran, C.; Bellone, S.; Ettorre, V.M.; Mansolf, M.; Hartwich, T.M.P.; McNamara, B.; Greenman, M.; Yang-Hartwich, Y.; Ratner, E.; Santin, N.G.; et al. Preclinical Efficacy of the Estrogen Receptor Degrader Fulvestrant in Combination with RAF/MEK Clamp Avutometinib and FAK Inhibitor in a Low-Grade Serous Ovarian Cancer Animal Model with Acquired Resistance to Chemotherapy and Aromatase Inhibitor. Int. J. Mol. Sci. 2025, 26, 8924. https://doi.org/10.3390/ijms26188924
Demirkiran C, Bellone S, Ettorre VM, Mansolf M, Hartwich TMP, McNamara B, Greenman M, Yang-Hartwich Y, Ratner E, Santin NG, et al. Preclinical Efficacy of the Estrogen Receptor Degrader Fulvestrant in Combination with RAF/MEK Clamp Avutometinib and FAK Inhibitor in a Low-Grade Serous Ovarian Cancer Animal Model with Acquired Resistance to Chemotherapy and Aromatase Inhibitor. International Journal of Molecular Sciences. 2025; 26(18):8924. https://doi.org/10.3390/ijms26188924
Chicago/Turabian StyleDemirkiran, Cem, Stefania Bellone, Victoria M. Ettorre, Miranda Mansolf, Tobias Max Philipp Hartwich, Blair McNamara, Michelle Greenman, Yang Yang-Hartwich, Elena Ratner, Niccoló G. Santin, and et al. 2025. "Preclinical Efficacy of the Estrogen Receptor Degrader Fulvestrant in Combination with RAF/MEK Clamp Avutometinib and FAK Inhibitor in a Low-Grade Serous Ovarian Cancer Animal Model with Acquired Resistance to Chemotherapy and Aromatase Inhibitor" International Journal of Molecular Sciences 26, no. 18: 8924. https://doi.org/10.3390/ijms26188924
APA StyleDemirkiran, C., Bellone, S., Ettorre, V. M., Mansolf, M., Hartwich, T. M. P., McNamara, B., Greenman, M., Yang-Hartwich, Y., Ratner, E., Santin, N. G., Sethi, N., Palmieri, L., Coma, S., Pachter, J. A., Ottum, S., & Santin, A. D. (2025). Preclinical Efficacy of the Estrogen Receptor Degrader Fulvestrant in Combination with RAF/MEK Clamp Avutometinib and FAK Inhibitor in a Low-Grade Serous Ovarian Cancer Animal Model with Acquired Resistance to Chemotherapy and Aromatase Inhibitor. International Journal of Molecular Sciences, 26(18), 8924. https://doi.org/10.3390/ijms26188924





