Blockade of PAR2 Signaling by Punicalagin as a Therapeutic Strategy for Atopic Dermatitis
Abstract
1. Introduction
2. Results
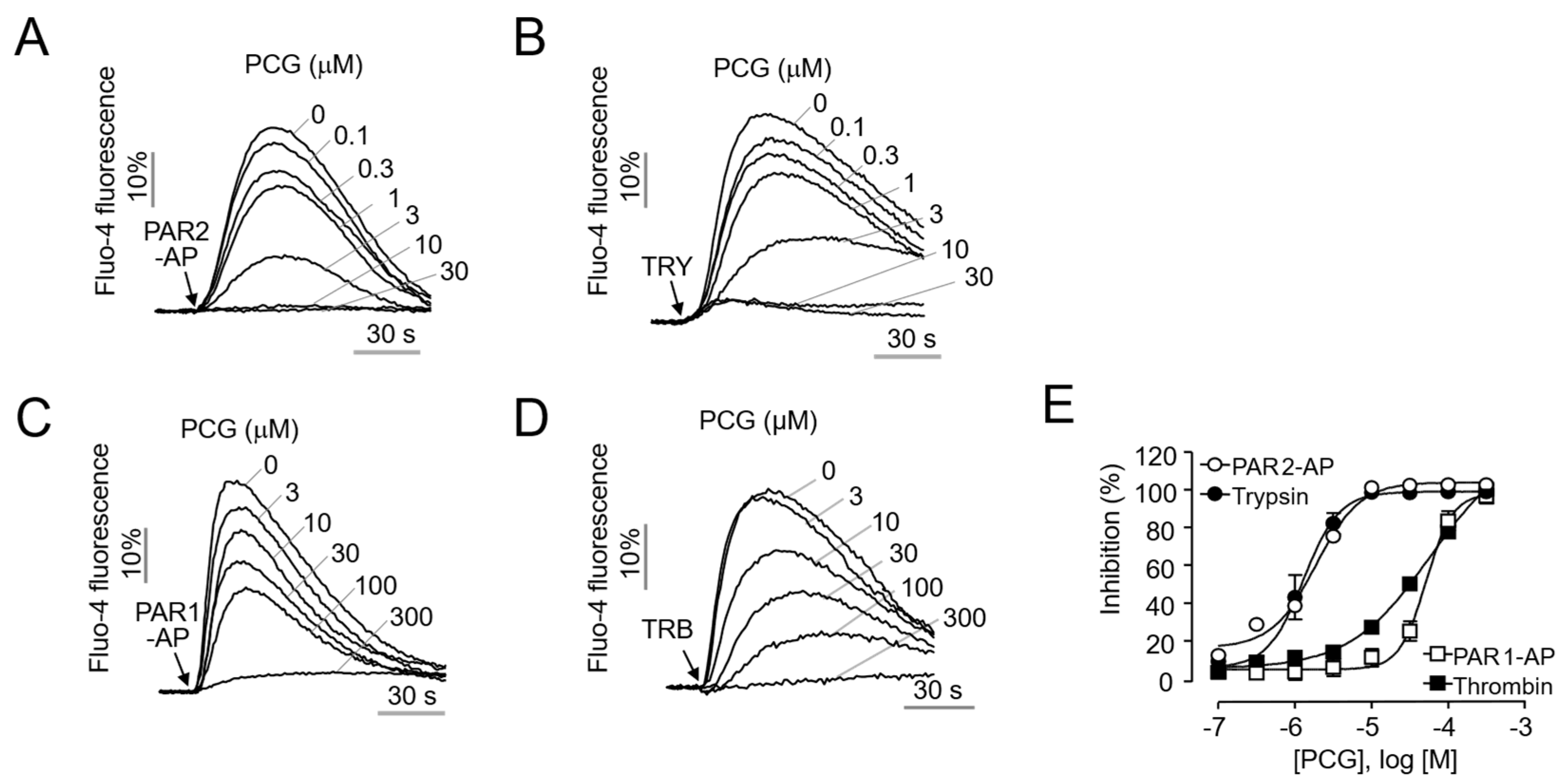
2.1. Selective and Potent Inhibition of PAR2 by PCG in HaCaT Cells
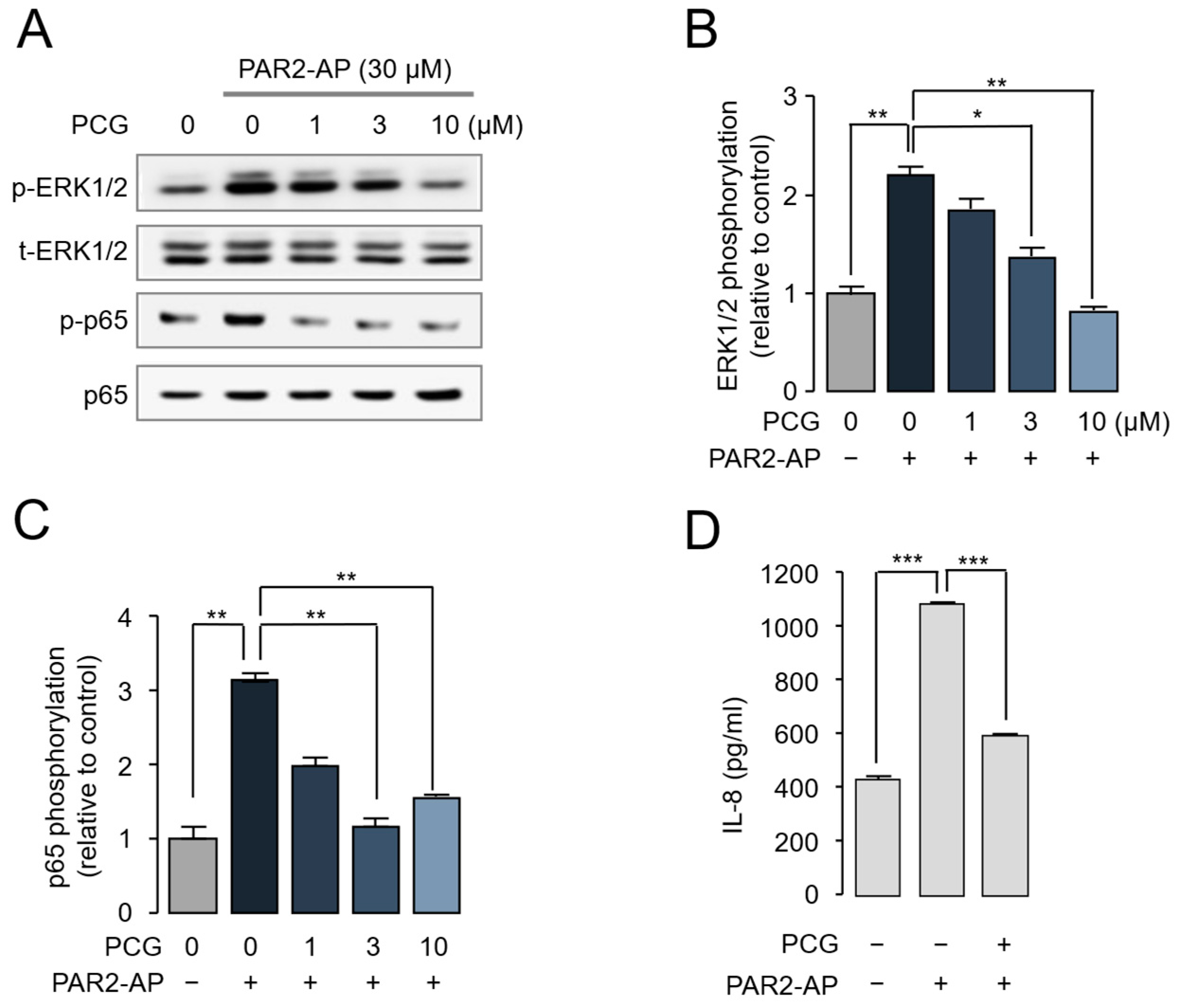
2.2. PCG-Mediated Inhibition of PAR2-Induced ERK1/2 and NF-κB Phosphorylation and IL-8 Release in HaCaT Cells
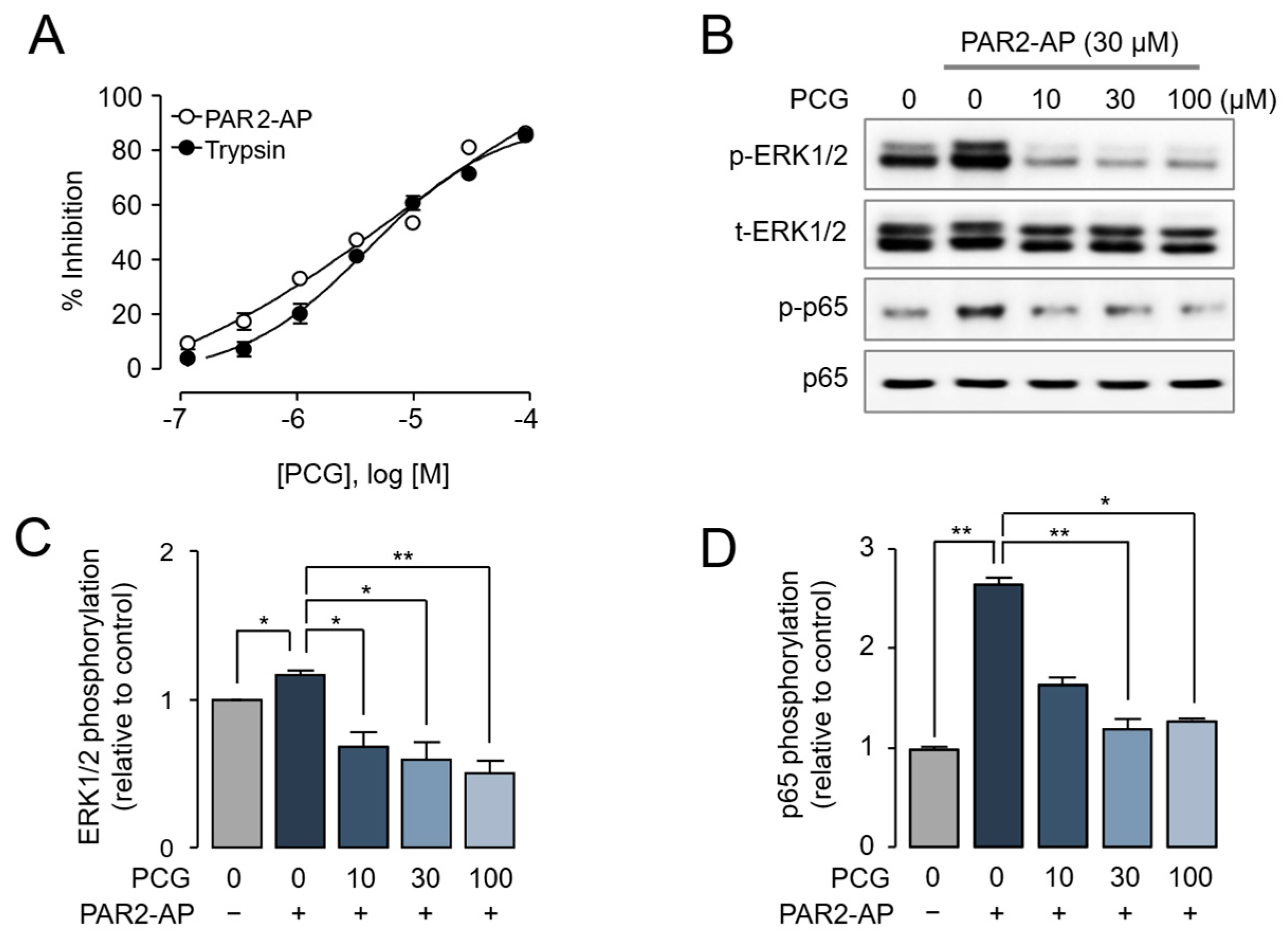
2.3. PCG-Mediated Inhibition of PAR2-Induced ERK1/2 and NF-κB Phosphorylation in Human Dermal Fibroblasts
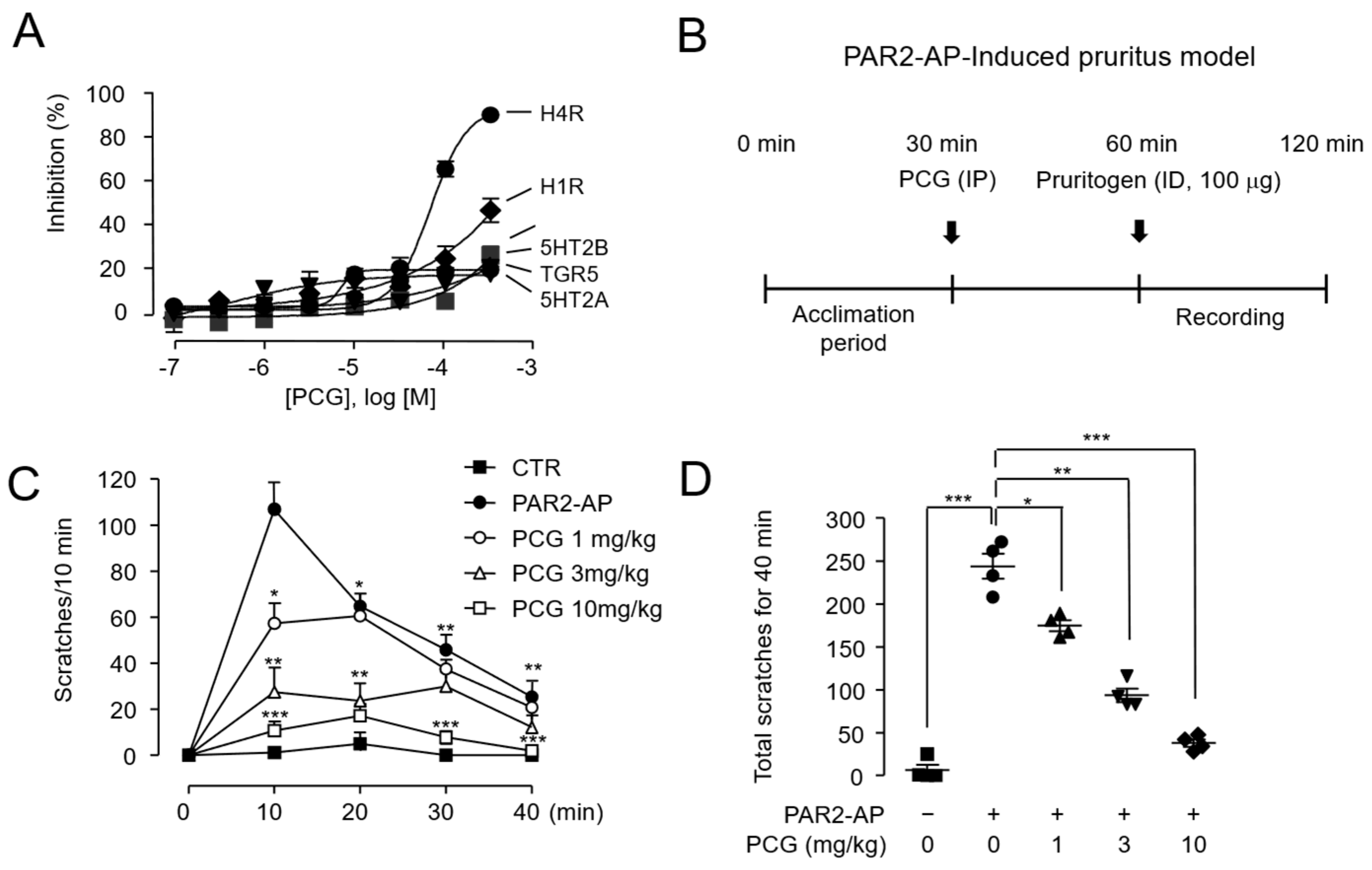
2.4. PCG-Mediated Suppression of PAR2-AP-Induced Scratching in BALB/c Mice
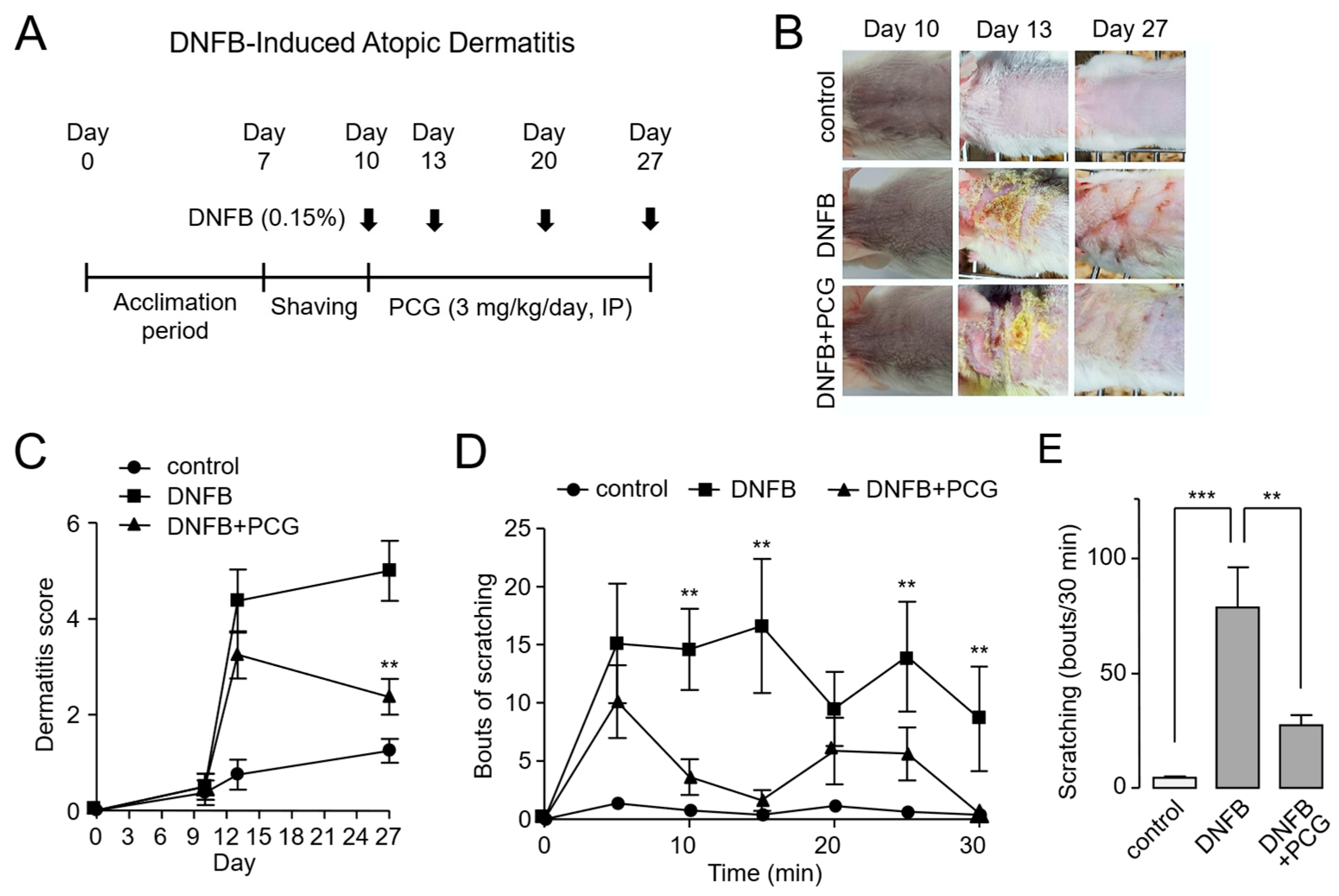
2.5. PCG Attenuates DNFB-Induced Atopic Dermatitis in Mice
2.6. PCG Mediates Therapeutic Mechanisms in DNFB-Induced Atopic Dermatitis
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Cell Lines
4.2. Materials and Reagents
4.3. Intracellular Calcium Measurement
4.4. Immunoblotting
4.5. Cytokine Release (IL-8, ELISA)
4.6. PAR2-AP-Induced Pruritus Model in BALB/c Mice
4.7. DNFB-Induced Atopic Dermatitis Model
4.8. Histological Analysis of Skin Tissue
4.9. Measurement of Serum TSLP Levels by ELISA
4.10. Primary Culture and Calcium Imaging of DRG Neurons
4.11. Data and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSLP | Thymic stromal lymphopoietin |
| PAR2 | Protease-activated receptor 2 |
| GPCR | G protein-coupled receptor |
| KLK | Kallikrein |
| PCG | Punicalagin |
| PAR2-AP | PAR2-activating peptide |
| DNFB | 2,4-dinitrofluorobenzene |
| HaCaT | Human keratinocyte |
| TRY | Trypsin |
| TRB | Thrombin |
| IL-8 | Interleukin-8 |
| PAR2-KO | PAR2 knockout |
| HDF | Human dermal fibroblast |
| DRG | Dorsal root ganglion |
| H&E | Hematoxylin and eosin |
References
- Mortz, C.; Lauritsen, J.; Bindslev-Jensen, C.; Andersen, K. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br. J. Dermatol. 2001, 144, 523–532. [Google Scholar] [CrossRef]
- Kini, S.P.; DeLong, L.K.; Veledar, E.; McKenzie-Brown, A.M.; Schaufele, M.; Chen, S.C. The Impact of Pruritus on Quality of Life: The Skin Equivalent of Pain. Arch. Dermatol. 2011, 147, 1153–1156. [Google Scholar] [CrossRef]
- Paternoster, L.; Standl, M.; Waage, J.; Baurecht, H.; Hotze, M.; Strachan, D.P.; Curtin, J.A.; Bønnelykke, K.; Tian, C.; Takahashi, A.; et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015, 47, 1449–1456. [Google Scholar]
- Hatano, Y.; Terashi, H.; Arakawa, S.; Katagiri, K. Interleukin-4 Suppresses the Enhancement of Ceramide Synthesis and Cutaneous Permeability Barrier Functions Induced by Tumor Necrosis Factor-α and interferon-γ in Human Epidermis. J. Investig. Dermatol. 2005, 124, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Hener, P.; Jiang, H.; Li, M. TSLP Produced by Keratinocytes Promotes Allergen Sensitization through Skin and Thereby Triggers Atopic March in Mice. J. Investig. Dermatol. 2013, 133, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.R.; Kim, B.S. The Itch–Scratch Cycle: A Neuroimmune Perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef]
- Broeders, J.A.; Ali, U.A.; Fischer, G. Systematic review and meta-analysis of randomized clinical trials (RCTs) comparing topical calcineurin inhibitors with topical corticosteroids for atopic dermatitis: A 15-year experience. J. Am. Acad. Dermatol. 2016, 75, 410–419.e3. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2021, 21, 21–40. [Google Scholar] [CrossRef]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect. J. Allergy Clin. Immunol. Pr. 2021, 9, 1053–1065. [Google Scholar] [CrossRef]
- Wahlgren, C.-F.; Hagermark, O.; Bergstrom, R. The antipruritic effect of a sedative and a non-sedative antihistamine in atopic dermatitis. Br. J. Dermatol. 1990, 122, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.K.; Kim, H.J.; Youm, J.-K.; Ahn, S.K.; Choi, E.H.; Sohn, M.H.; Kim, K.-E.; Hong, J.H.; Shin, D.M.; Lee, S.H. Mite and Cockroach Allergens Activate Protease-Activated Receptor 2 and Delay Epidermal Permeability Barrier Recovery. J. Investig. Dermatol. 2008, 128, 1930–1939. [Google Scholar] [CrossRef]
- Fan, M.; Fan, X.; Lai, Y.; Chen, J.; Peng, Y.; Peng, Y.; Xiang, L.; Ma, Y. Protease-Activated Receptor 2 in inflammatory skin disease: Current evidence and future perspectives. Front. Immunol. 2024, 15, 1448952. [Google Scholar] [CrossRef]
- Vu, T.-K.H.; Hung, D.T.; Wheaton, V.I.; Coughlin, S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Oikonomopoulou, K.; Hansen, K.K.; Saifeddine, M.; Tea, I.; Blaber, M.; Blaber, S.I.; Scarisbrick, I.; Andrade-Gordon, P.; Cottrell, G.S.; Bunnett, N.W.; et al. Proteinase-activated Receptors, Targets for Kallikrein Signaling. J. Biol. Chem. 2006, 281, 32095–32112. [Google Scholar] [CrossRef] [PubMed]
- Buhl, T.; Ikoma, A.; Kempkes, C.; Cevikbas, F.; Sulk, M.; Buddenkotte, J.; Akiyama, T.; Crumrine, D.; Camerer, E.; Carstens, E.; et al. Protease-Activated Receptor-2 Regulates Neuro-Epidermal Communication in Atopic Dermatitis. Front. Immunol. 2020, 11, 1740. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-Activated Receptor-2 Mediates Itch: A Novel Pathway for Pruritus in Human Skin. J. Neurosci. 2003, 23, 6176–6180. [Google Scholar] [CrossRef]
- Lee, S.E.; Jeong, S.K.; Lee, S.H. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med. J. 2010, 51, 808–822. [Google Scholar] [CrossRef]
- Tsujii, K.; Andoh, T.; Ui, H.; Lee, J.-B.; Kuraishi, Y.; H, U.; Jb, L. Involvement of Tryptase and Proteinase-Activated Receptor-2 in Spontaneous Itch-Associated Response in Mice With Atopy-like Dermatitis. J. Pharmacol. Sci. 2009, 109, 388–395. [Google Scholar] [CrossRef]
- Nomura, H.; Suganuma, M.; Takeichi, T.; Kono, M.; Isokane, Y.; Sunagawa, K.; Kobashi, M.; Sugihara, S.; Kajita, A.; Miyake, T.; et al. Multifaceted Analyses of Epidermal Serine Protease Activity in Patients with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 913. [Google Scholar] [CrossRef]
- Shimada, S.G.; Shimada, K.A.; Collins, J. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur. J. Pharmacol. 2006, 530, 281–283. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, M.I.; Carstens, E. Excitation of Mouse Superficial Dorsal Horn Neurons by Histamine and/or PAR-2 Agonist: Potential Role in Itch. J. Neurophysiol. 2009, 102, 2176–2183. [Google Scholar] [CrossRef]
- Seo, Y.; Mun, C.H.; Park, S.-H.; Jeon, D.; Kim, S.J.; Yoon, T.; Ko, E.; Jo, S.; Park, Y.-B.; Namkung, W.; et al. Punicalagin Ameliorates Lupus Nephritis via Inhibition of PAR2. Int. J. Mol. Sci. 2020, 21, 4975. [Google Scholar] [CrossRef]
- Hollenberg, M.D.; Compton, S.J. International union of pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol. Rev. 2002, 54, 203–217. [Google Scholar] [CrossRef]
- Nakamura, H.; Aoki, M.; Tamai, K.; Oishi, M.; Ogihara, T.; Kaneda, Y.; Morishita, R. Prevention and regression of atopic dermatitis by ointment containing NF-kB decoy oligodeoxynucleotides in NC/Nga atopic mouse model. Gene Ther. 2002, 9, 1221–1229. [Google Scholar] [CrossRef]
- Zeze, N.; Kido-Nakahara, M.; Tsuji, G.; Maehara, E.; Sato, Y.; Sakai, S.; Fujishima, K.; Hashimoto-Hachiya, A.; Furue, M.; Nakahara, T. Role of ERK Pathway in the Pathogenesis of Atopic Dermatitis and Its Potential as a Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3467. [Google Scholar] [CrossRef]
- Kimata, H.; Lindley, I. Detection of plasma interleukin-8 in atopic dermatitis. Arch. Dis. Child. 1994, 70, 119–122. [Google Scholar] [CrossRef]
- Gruber, B.L.; Marchese, M.J.; Santiago-Schwarz, F.; Martin, C.A.; Zhang, J.; Kew, R.R. Protease-Activated Receptor-2 (PAR-2) Expression in Human Fibroblasts is Regulated by Growth Factors and Extracellular Matrix. J. Investig. Dermatol. 2004, 123, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Wang, N.; He, C.; Jiang, X.; Li, J. Calcium-Based Antimicrobial Peptide Compounds Attenuate DNFB-Induced Atopic Dermatitis-Like Skin Lesions via Th-Cells in BALB/c Mice. Int. J. Mol. Sci. 2022, 23, 11371. [Google Scholar] [CrossRef]
- Li, M.; Messaddeq, N.; Teletin, M.; Pasquali, J.-L.; Metzger, D.; Chambon, P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc. Natl. Acad. Sci. 2005, 102, 14795–14800. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.P.; Garzia, C.; Guha, S.; Fletcher, E.K.; Nguyen, N.; Wieschhaus, A.J.; Ferrer, L.; Covic, L.; Kuliopulos, A. PAR2 Pepducin-Based Suppression of Inflammation and Itch in Atopic Dermatitis Models. J. Investig. Dermatol. 2019, 139, 412–421. [Google Scholar] [CrossRef]
- McIntosh, K.A.; Cunningham, M.R.; Bushell, T.; Plevin, R. The development of proteinase-activated receptor-2 modulators and the challenges involved. Biochem. Soc. Trans. 2020, 48, 2525–2537. [Google Scholar] [CrossRef]
- Trautmann, A.; Akdis, M.; Schmid-Grendelmeier, P.; Disch, R.; Bröcker, E.-B.; Blaser, K.; Akdis, C.A. Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J. Allergy Clin. Immunol. 2001, 108, 839–846. [Google Scholar] [CrossRef]
- Suen, J.; Barry, G.; Lohman, R.; Halili, M.; Cotterell, A.; Le, G.; Fairlie, D. Modulating human proteinase activated receptor 2 with a novel antagonist (GB88) and agonist (GB110). Br. J. Pharmacol. 2011, 165, 1413–1423. [Google Scholar] [CrossRef]
- Steinhoff, M.; Corvera, C.U.; Thoma, M.S.; Kong, W.; McAlpine, B.E.; Caughey, G.H.; Ansel, J.C.; Bunnett, N.W. Proteinase-activated receptor-2 in human skin: Tissue distribution and activation of keratinocytes by mast cell tryptase. Exp. Dermatol. 1999, 8, 282–294. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Tominaga, M.; Yamamoto, S.; Fukuoka, T.; Higashi, T.; Kobayashi, K.; Obata, K.; Yamanaka, H.; Noguchi, K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Investig. 2007, 117, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Lebonvallet, N.; Fluhr, J.W.; Le Gall-Ianotto, C.; Leschiera, R.; Talagas, M.; Reux, A.; Bataille, A.; Brun, C.; Oddos, T.; Pennec, J.-P.; et al. A Re-Innervated in Vitro Skin Model of Non-Histaminergic Itch and Skin Neurogenic Inflammation: PAR2-, TRPV1- and TRPA1-Agonist Induced Functionality. Ski. Heal. Dis. 2021, 1, e66. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, K.; Liu, X.; Zhao, L.; Feng, Z.; Liu, J. Punicalagin Regulates Signaling Pathways in Inflammation-Associated Chronic Diseases. Antioxidants 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Sundström, L.; Geschwindner, S.; Poon, E.K.Y.; Jiang, Y.; Chen, R.; Cooke, R.; Johnstone, S.; Madin, A.; Lim, J.; et al. Protease-activated receptor-2 ligands reveal orthosteric and allosteric mechanisms of receptor inhibition. Commun. Biol. 2020, 3, 1–13. [Google Scholar] [CrossRef]
- Shukla, M.; Gupta, K.; Rasheed, Z.; A Khan, K.; Haqqi, T.M. Bioavailable constituents/metabolites of pomegranate (Punica granatum L) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE2 production in human chondrocytes i n vitro. J. Inflamm. 2008, 5, 9. [Google Scholar] [CrossRef]
- Shabir, I.; Dar, A.H.; Dash, K.K.; Manzoor, S.; Srivastava, S.; Pandey, V.K.; Shams, R.; Bashir, I.; Khan, S.A.; Mukarram, S.A.; et al. Bioactive potential of punicalagin: A comprehensive review. Appl. Food Res. 2024, 4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.; Seo, Y.; Lee, W.-J.; Heo, Y.; Shim, W.-S.; Namkung, W. Blockade of PAR2 Signaling by Punicalagin as a Therapeutic Strategy for Atopic Dermatitis. Int. J. Mol. Sci. 2025, 26, 8920. https://doi.org/10.3390/ijms26188920
Jeon H, Seo Y, Lee W-J, Heo Y, Shim W-S, Namkung W. Blockade of PAR2 Signaling by Punicalagin as a Therapeutic Strategy for Atopic Dermatitis. International Journal of Molecular Sciences. 2025; 26(18):8920. https://doi.org/10.3390/ijms26188920
Chicago/Turabian StyleJeon, Hyejin, Yohan Seo, Wook-Joo Lee, Yunkyung Heo, Won-Sik Shim, and Wan Namkung. 2025. "Blockade of PAR2 Signaling by Punicalagin as a Therapeutic Strategy for Atopic Dermatitis" International Journal of Molecular Sciences 26, no. 18: 8920. https://doi.org/10.3390/ijms26188920
APA StyleJeon, H., Seo, Y., Lee, W.-J., Heo, Y., Shim, W.-S., & Namkung, W. (2025). Blockade of PAR2 Signaling by Punicalagin as a Therapeutic Strategy for Atopic Dermatitis. International Journal of Molecular Sciences, 26(18), 8920. https://doi.org/10.3390/ijms26188920






