Dual Therapeutic Impact of AXL Inhibitor AB-329: Chemotherapy Sensitization and Immune Microenvironment Reprogramming in TNBC
Abstract
1. Introduction
2. Results
2.1. AXL Expression Is Elevated in TNBC and Targetable by an AXL-Specific Inhibitor, AB-329
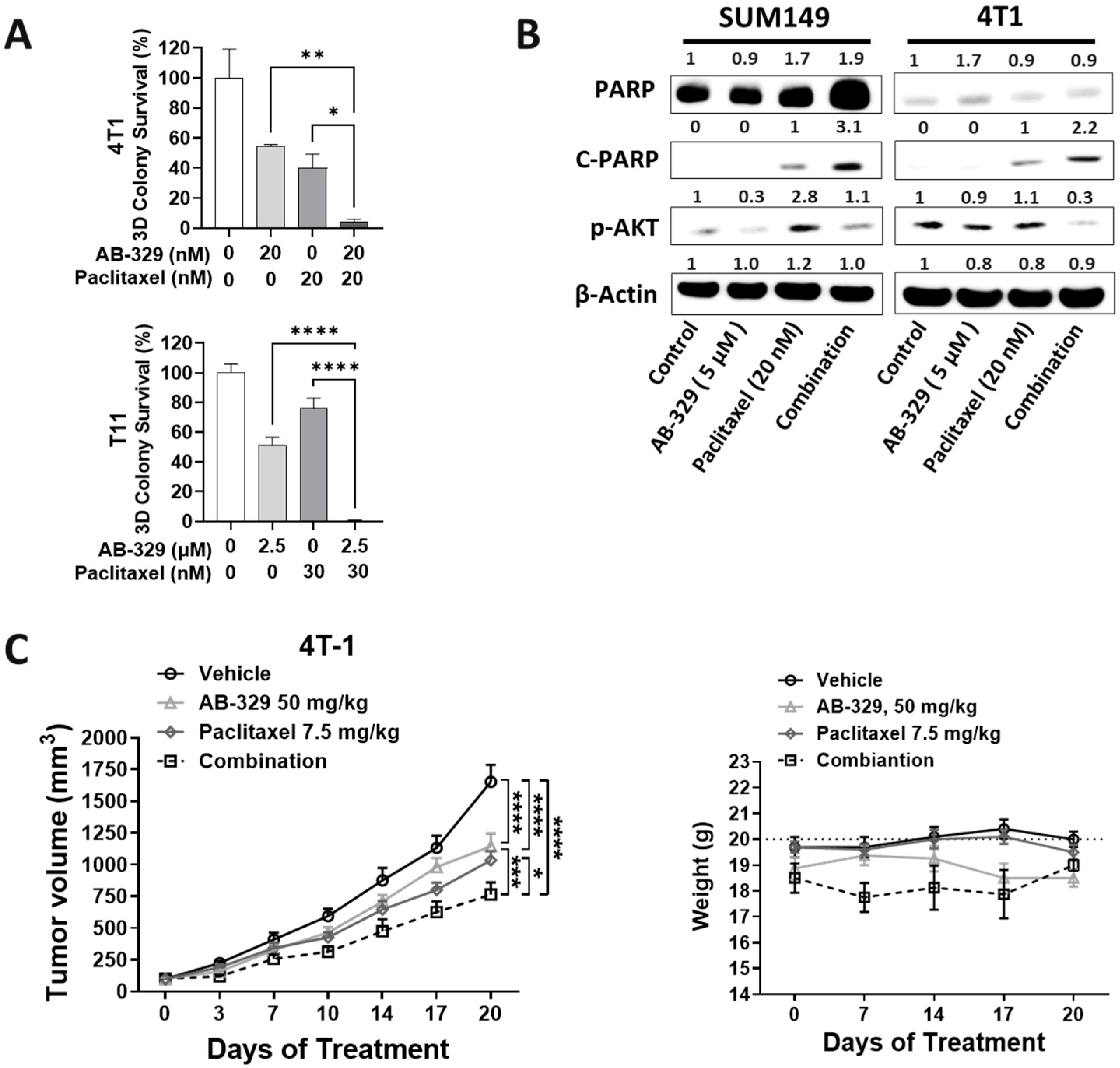
2.2. AB-329 Combined with Paclitaxel Synergistically Inhibited the Proliferation, Migration, and Invasion of TNBC Cells
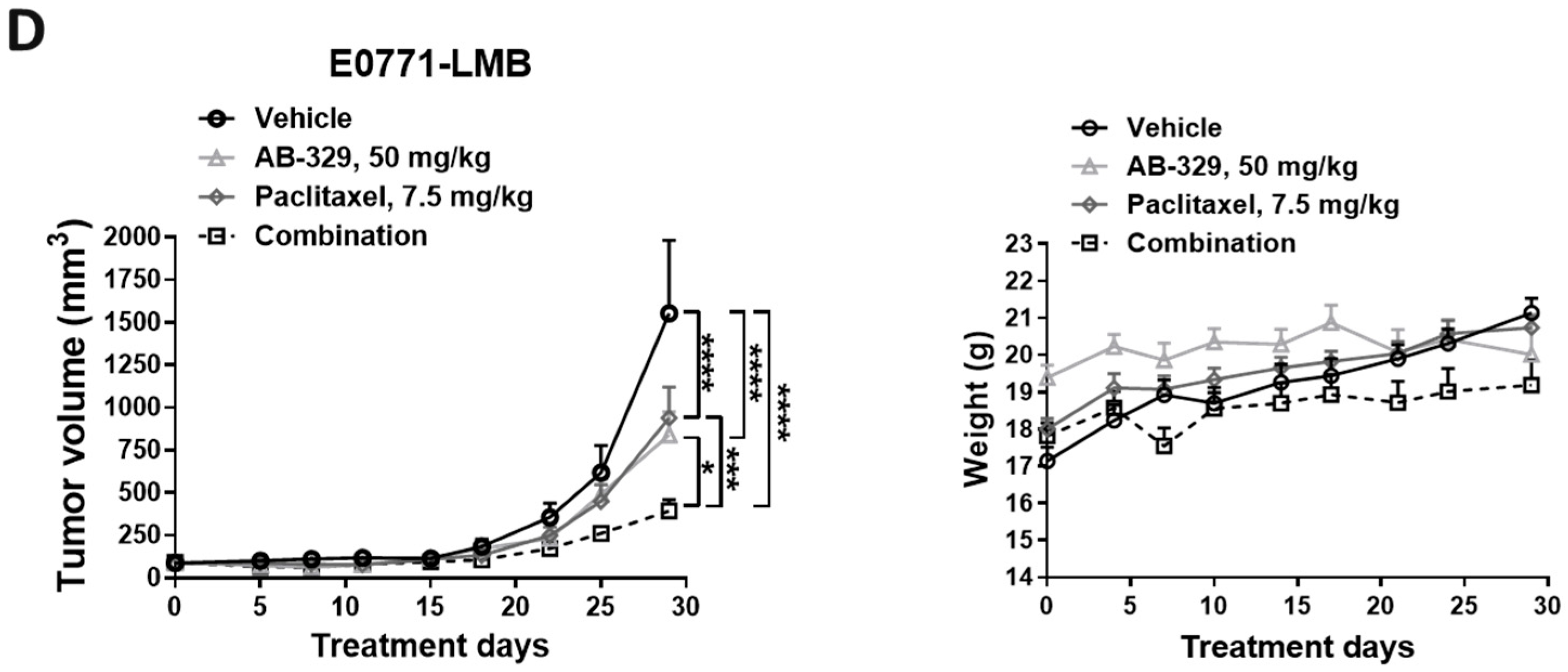
2.3. AB-329 Combined with Paclitaxel Synergistically Reduced Tumor Growth in Murine TNBC Models
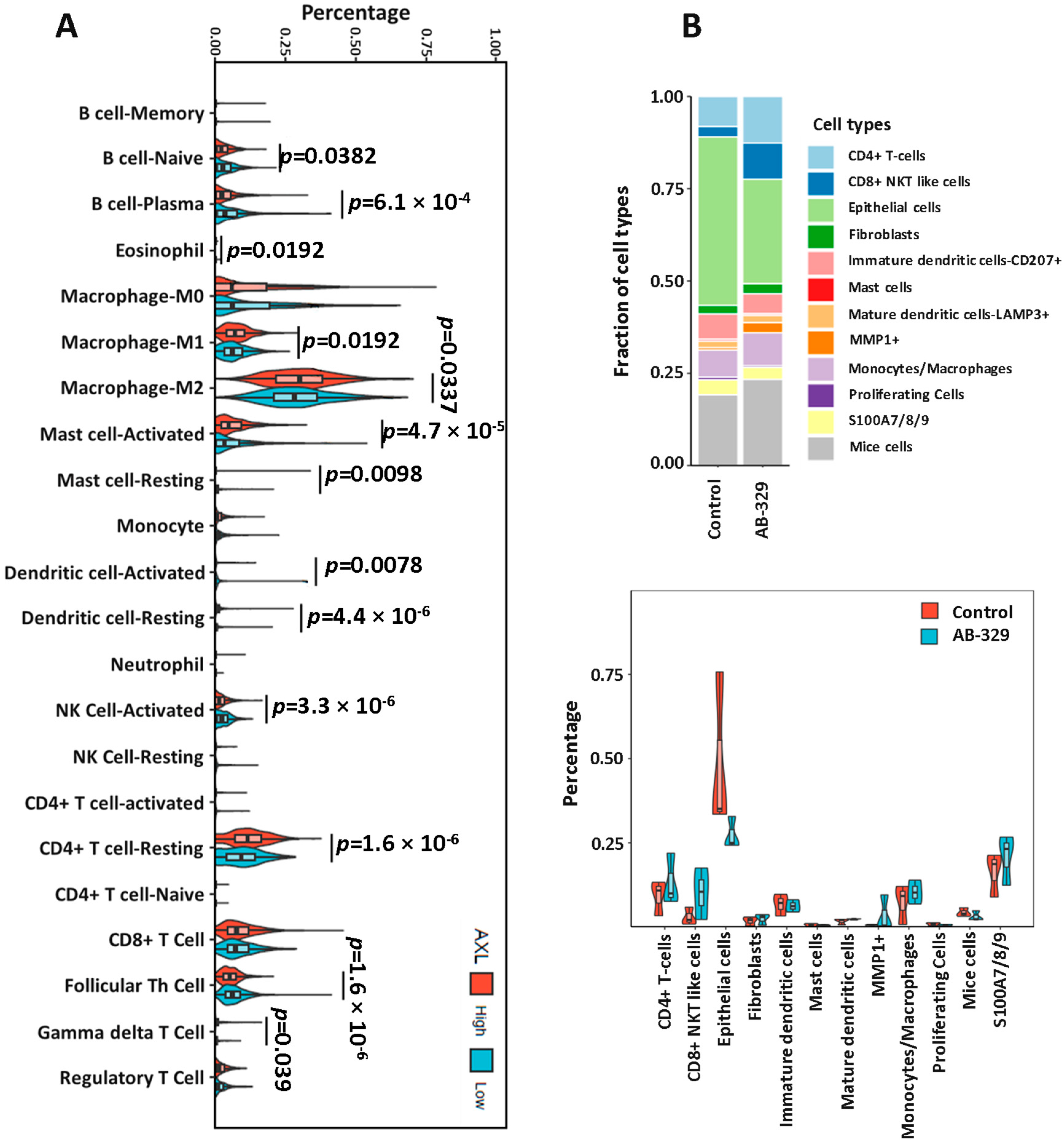
2.4. AXL Inhibition Enhances Activated NK Cell Infiltration in Breast Cancer Tissues
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Clonogenic Assay
4.3. Soft Agar Assay
4.4. Gene Expression
4.5. Western Blot
4.6. Cell Migration Assay and Cell Invasion Assay
4.7. TNBC Mouse Model
4.8. Immune Cell Fraction in SUM149 Humanized Mice Model
4.9. Human Breast Cancer TCGA Data
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TNBC | Triple-negative breast cancer |
| EMT | Epithelial–mesenchymal transition |
| NK | Natural killer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER-2 | Human epidermal growth factor receptor 2 |
| BL1 | Basal-like 1 |
| BL2 | Basal-like 2 |
| M | Mesenchymal |
| MSL | Mesenchymal stem-like |
| IM | Immunomodulatory |
| LAR | Luminal androgen receptor |
| TAM | TYRO3-AXL-MERTK |
| GAS6 | Growth arrest-specific protein 6 |
| DMFS | Distant-metastasis-free survival |
| TCGA | The Cancer Genome Atlas |
| CSC | Cancer stem cell |
References
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K.; et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: A single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer 2007, 110, 876–884. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer 2008, 113, 2638–2645. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, C.; Xu, X.; Li, A.; Cai, Q.; Long, X. Androgen receptor, EGFR, and BRCA1 as biomarkers in triple-negative breast cancer: A meta-analysis. Biomed. Res. Int. 2015, 2015, 357485. [Google Scholar] [CrossRef]
- Gluz, O.; Liedtke, C.; Gottschalk, N.; Pusztai, L.; Nitz, U.; Harbeck, N. Triple-negative breast cancer--current status and future directions. Ann. Oncol. 2009, 20, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.D.; Zhu, R.; Zhan, M.; Rodriguez, A.A.; Yang, W.; Wong, S.; Makris, A.; Lehmann, B.D.; Chen, X.; Mayer, I.; et al. Identification of prognosis-relevant subgroups in patients with chemoresistant triple-negative breast cancer. Clin. Cancer Res. 2013, 19, 2723–2733. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Ding, Y.C.; Steele, L.; Warden, C.; Wilczynski, S.; Mortimer, J.; Yuan, Y.; Neuhausen, S.L. Molecular subtypes of triple-negative breast cancer in women of different race and ethnicity. Oncotarget 2019, 10, 198–208. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Terragno, M.; Vetrova, A.; Semenov, O.; Sayan, A.E.; Kriajevska, M.; Tulchinsky, E. Mesenchymal-epithelial transition and AXL inhibitor TP-0903 sensitise triple-negative breast cancer cells to the antimalarial compound, artesunate. Sci. Rep. 2024, 14, 425. [Google Scholar] [CrossRef]
- Gjerdrum, C.; Tiron, C.; Hoiby, T.; Stefansson, I.; Haugen, H.; Sandal, T.; Collett, K.; Li, S.; McCormack, E.; Gjertsen, B.T.; et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. USA 2010, 107, 1124–1129. [Google Scholar] [CrossRef]
- Bottai, G.; Raschioni, C.; Szekely, B.; Di Tommaso, L.; Szasz, A.M.; Losurdo, A.; Gyorffy, B.; Acs, B.; Torrisi, R.; Karachaliou, N.; et al. AXL-associated tumor inflammation as a poor prognostic signature in chemotherapy-treated triple-negative breast cancer patients. NPJ Breast Cancer 2016, 2, 16033. [Google Scholar] [CrossRef]
- Tang, Y.; Zang, H.; Wen, Q.; Fan, S. AXL in cancer: A modulator of drug resistance and therapeutic target. J. Exp. Clin. Cancer Res. 2023, 42, 148. [Google Scholar] [CrossRef]
- Jimbo, T.; Hatanaka, M.; Komatsu, T.; Taira, T.; Kumazawa, K.; Maeda, N.; Suzuki, T.; Ota, M.; Haginoya, N.; Isoyama, T.; et al. DS-1205b, a novel selective inhibitor of AXL kinase, blocks resistance to EGFR-tyrosine kinase inhibitors in a non-small cell lung cancer xenograft model. Oncotarget 2019, 10, 5152–5167. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Shiraishi, Y.; Murakami, H.; Horinouchi, H.; Toyozawa, R.; Takeda, M.; Uno, M.; Crawford, N.; McGill, J.; Jimbo, T.; et al. Phase 1 study of DS-1205c combined with gefitinib for EGFR mutation-positive non-small cell lung cancer. Cancer Med. 2023, 12, 7090–7104. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, M.K.; Beauchamp-Perez, F.D.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene 2014, 33, 1316–1324. [Google Scholar] [CrossRef]
- Wilson, C.; Ye, X.; Pham, T.; Lin, E.; Chan, S.; McNamara, E.; Neve, R.M.; Belmont, L.; Koeppen, H.; Yauch, R.L.; et al. AXL inhibition sensitizes mesenchymal cancer cells to antimitotic drugs. Cancer Res. 2014, 74, 5878–5890. [Google Scholar] [CrossRef]
- Vuoriluoto, K.; Haugen, H.; Kiviluoto, S.; Mpindi, J.P.; Nevo, J.; Gjerdrum, C.; Tiron, C.; Lorens, J.B.; Ivaska, J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 2011, 30, 1436–1448. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, S.; Gerber, D.E. AXL Inhibitors: Status of Clinical Development. Curr. Oncol. Rep. 2023, 25, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Boysen, J.; Nelson, M.; Secreto, C.; Warner, S.L.; Bearss, D.J.; Lesnick, C.; Shanafelt, T.D.; Kay, N.E.; Ghosh, A.K. Targeted Axl Inhibition Primes Chronic Lymphocytic Leukemia B Cells to Apoptosis and Shows Synergistic/Additive Effects in Combination with BTK Inhibitors. Clin. Cancer Res. 2015, 21, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Goswami, S.; Thall, P.F.; Wang, X.; Yuan, Y.; Jonasch, E.; Gao, J.; Campbell, M.T.; Shah, A.Y.; Corn, P.G.; et al. A phase 1-2 trial of sitravatinib and nivolumab in clear cell renal cell carcinoma following progression on antiangiogenic therapy. Sci. Transl. Med. 2022, 14, eabm6420. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampa, D.R.; Fuson, J.A.; Liu, H.; Pan, M.; Qin, Y.; Deng, Y.; Ueno, N.T.; Lee, J. Dual Therapeutic Impact of AXL Inhibitor AB-329: Chemotherapy Sensitization and Immune Microenvironment Reprogramming in TNBC. Int. J. Mol. Sci. 2025, 26, 8896. https://doi.org/10.3390/ijms26188896
Rampa DR, Fuson JA, Liu H, Pan M, Qin Y, Deng Y, Ueno NT, Lee J. Dual Therapeutic Impact of AXL Inhibitor AB-329: Chemotherapy Sensitization and Immune Microenvironment Reprogramming in TNBC. International Journal of Molecular Sciences. 2025; 26(18):8896. https://doi.org/10.3390/ijms26188896
Chicago/Turabian StyleRampa, Dileep Reddy, Jon A. Fuson, Huey Liu, Max Pan, Yujia Qin, Youping Deng, Naoto T. Ueno, and Jangsoon Lee. 2025. "Dual Therapeutic Impact of AXL Inhibitor AB-329: Chemotherapy Sensitization and Immune Microenvironment Reprogramming in TNBC" International Journal of Molecular Sciences 26, no. 18: 8896. https://doi.org/10.3390/ijms26188896
APA StyleRampa, D. R., Fuson, J. A., Liu, H., Pan, M., Qin, Y., Deng, Y., Ueno, N. T., & Lee, J. (2025). Dual Therapeutic Impact of AXL Inhibitor AB-329: Chemotherapy Sensitization and Immune Microenvironment Reprogramming in TNBC. International Journal of Molecular Sciences, 26(18), 8896. https://doi.org/10.3390/ijms26188896







