The Female Reproductive Tract Microbiota and Endometrial Cancer: A Systematic Review
Abstract
1. Introduction
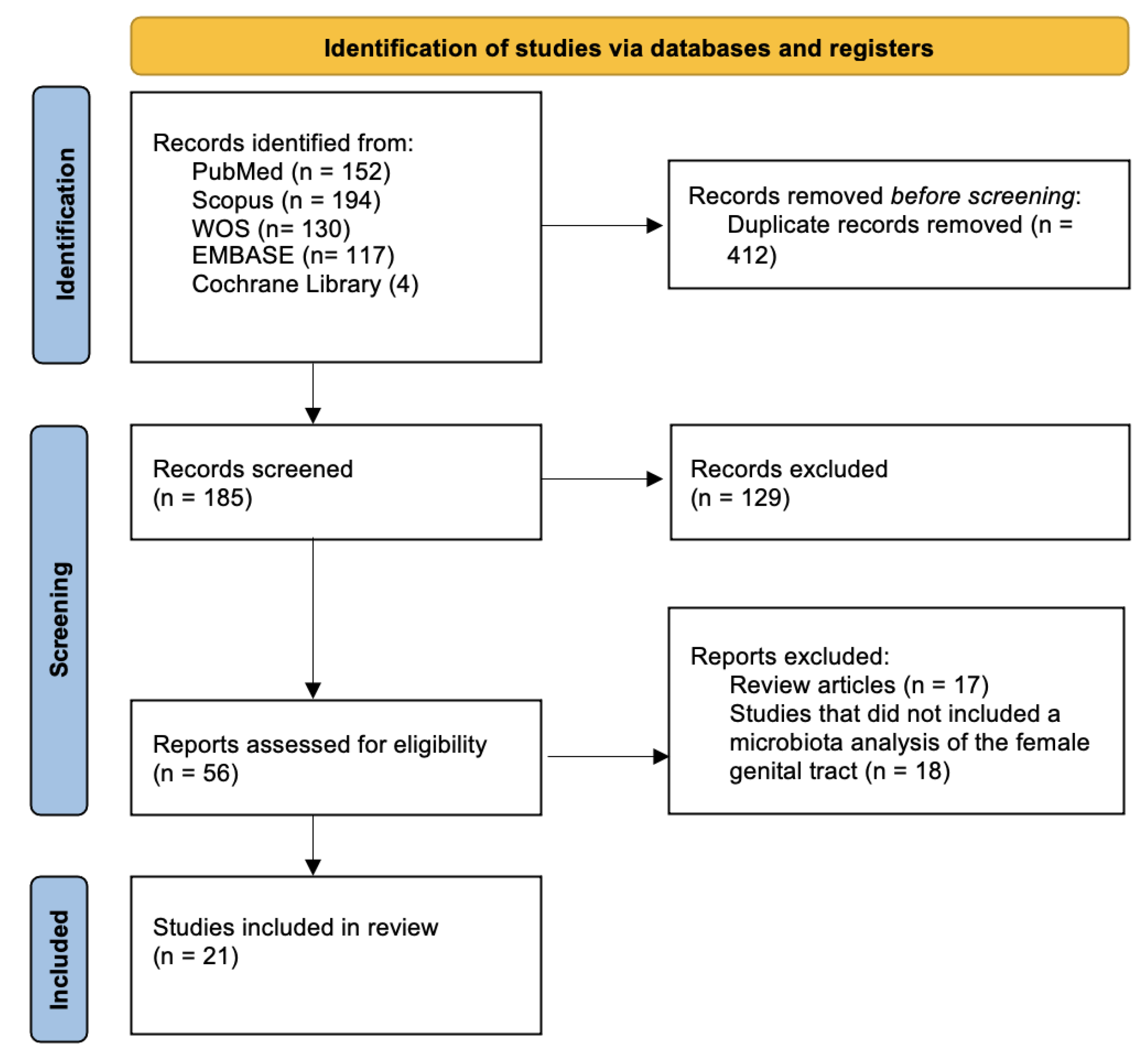
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy and Eligibility Criteria
2.3. Study Selection and Data Extraction
3. Results
3.1. Endometrial Microbiota in Endometrial Cancer
3.1.1. Bacteria
3.1.2. Fungi and Viruses
| First Author and Year | Sampling Method | Sample Size | Study Results |
|---|---|---|---|
| Deligdisch et al., 2013 [26] | Formalin-fixed paraffin-embedded (FFPE) tissues from hysterectomy and endometrial scraping; DNA sequencing performed. | EC n = 56, controls n = 33 | HMTV env gene sequences and protein detected in 23.2% of EC cases, 0% in controls. Expression confirmed by nested PCR and immunohistochemistry, suggesting viral involvement in carcinogenesis. |
| Li et al., 2021 [18] | Endometrial tissue samples analyzed using 16S rRNA sequencing. | EC n = 30, controls n = 10 | Prevotella and Pelomonas enriched in EC tissues. Prevotella abundance correlated with increased D-dimer and FDPs, and with genes involved in fibrin degradation (e.g., PRSS33, CPB2, XBP1). Combined markers (Prevotella + DD + FDPs) had high diagnostic potential (AUC = 0.86). |
| Chen et al., 2021 [15] | Endometrial biopsies analyzed using meta-transcriptomic sequencing. | EC n = 9 EC, normal group n = 8 | Identified 5576 active bacterial species and 381 archaeal species in EC patients. Key species and pathways (e.g., Apelin, Wnt, IL-17) linked to tumor migration and host-microbiota metabolic crosstalk. Microbes potentially influence EMT and unfolded protein response. Among the most abundant species in the EC group: Clostridium botulinum, Mycoplasma hyopneumoniae, Bacillus cereus, Pasteurella multocida. 17 species showed significant differences between EC group and control group (e.g., Borrelia coriaceae ↑ in EC; Streptococcus mitis ↓ in EC. |
| Chao et al., 2022 [16] | Endometrial lavage fluid collected via transcervical catheter. | EH, n = 18; EC, n = 7; metastatic EC, n = 2; benign endometrial lesions, n = 8 | Found over-representation of Bacillus pseudofirmus and Stenotrophomonas rhizophila in EC/EH patients. Suggested link between plastic-degrading bacteria and endometrial carcinogenesis. Microbiota function associated with fatty acid and amino acid metabolism |
| Kaakoush et al., 2022 [17] | Endometrial brushings or tissue biopsies analyzed via 16S rRNA sequencing. | EC n = 30, benign n = 30 | Endometrial microbiota clustered into three community types. Cancer samples showed reduced Lactobacillus, with Lactobacillus iners enriched in controls. Obesity influenced community type prevalence but not Lactobacillus abundance. Similar microbiota between tumor and adjacent normal tissue. |
| Hawkins et al., 2022 [21] | Endometrial biopsies from patients undergoing hysterectomy, analyzed by 16S sequencing. | EC n = 30, benign n = 30 | Higher microbial diversity in ECs from Black vs. White women. Tumors from Black women had more Firmicutes, Cyanobacteria, and OD1. Lactobacillus acidophilus enriched in Black women. Differences may contribute to racial disparities in EC outcomes. |
| Wang et al., 2022 [23] | Endometrial samples from hysterectomy procedures, 16S rRNA sequencing. | EC n = 28, pericancer n = 28 | EC tissues showed higher alpha diversity and were enriched with Prevotella, Atopobium, Anaerococcus, Dialister, Porphyromonas, and Peptoniphilus. Lactobacillus dominated in adjacent non-EC tissues. Microbiota differences correlated with clinical stage, pH, and Lactobacillus abundance. |
| Leoni et al., 2024 [20] | Endometrial biopsies from patients undergoing hysterectomy, analyzed by 16S sequencing. | EC n = 8, controls n = 6 | Confirmed low bacterial abundance in endometrium. Metabarcoding revealed higher prevalence of pathogenic genera in EC tissues. Cutibacterium more abundant in EC; Ralstonia more abundant in controls. No significant differences in diversity between groups. |
| Han et al., 2024 [19] | Endometrial tissue samples analyzed using 16S rRNA sequencing, and the ITS1 for the study of the uterine fungal microbiome. | EC n = 33, EH n = 15, benign n = 15. | EC and EH showed increased alpha diversity and shift in microbiome structure, especially fungal composition. Penicillium sp. enriched in EC/EH, Sarocladium in controls. Dysbiosis correlated with pro-inflammatory cytokines (IL-6, IL-11, TGF-β) and β-glucuronidase activity, implicating estrogen-like metabolic disruption. |
| Ying et al., 2024 [22] | Endometrial biopsies analyzed using 16S rRNA sequencing. | benign n = 53, EH n = 15 (including 2 AEH). | Patients with endometrial hyperplasia had significantly lower alpha diversity and increased abundance of Delftia, Serratia, and Stenotrophomonas. These bacteria showed diagnostic potential for EH with AUCs around 71–75%. Suggests potential for microbiota-based biomarkers. |
| Gonzalez-Bosquet et al., 2023 [14] | Tumor tissue samples analyzed via 16S rRNA. | EC n = 62, Controls n = 36, HSOC n = 112 | Microbial diversity correlated with somatic variation. Specific bacterial taxa (e.g., Leclercia, Desulfobulbaceae) associated with high-grade serous ovarian cancer (HGSC) and endometrioid endometrial cancer (EEC). Pathway analyses suggested potential for early cancer detection. |
| Kuźmycz et al., 2025 [24] | Endometrial canal swabs collected pre-hysterectomy; 16S rRNA sequencing. | EC n = 16, endometrial myoma n = 13. | Higher microbial alpha- and beta-diversity in EC samples. Anaerococcus significantly enriched in EC and capable of adhering to uterine fibroblasts and inducing ROS production. Suggests a potential role in inflammation-mediated carcinogenesis. |
3.2. Cervicovaginal Microbiota in Endometrial Cancer
3.3. Functional and In Vitro Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CST | Community State Type (vaginal microbial community type) |
| EC | Endometrial Cancer |
| EH | Endometrial Hyperplasia |
| AEH | Atypical Endometrial Hyperplasia |
| EMT | Epithelial–Mesenchymal Transition |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| HGSC/HSOC | High-Grade Serous Ovarian Cancer |
| ITS1 | Internal Transcribed Spacer 1 |
| ROS | Reactive Oxygen Species |
| USC | Uterine Serous Carcinoma |
References
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human Microbiome: An Academic Update on Human Body Site Specific Surveillance and Its Possible Role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The Gut Microbiota-Immune-Brain Axis: Therapeutic Implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Microbiota Implications in Endocrine-Related Diseases: From Development to Novel Therapeutic Approaches. Biomedicines 2024, 12, 221. [Google Scholar] [CrossRef]
- Cullin, N.; Azevedo Antunes, C.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and Cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef]
- Chase, D.; Goulder, A.; Zenhausern, F.; Monk, B.; Herbst-Kralovetz, M. The Vaginal and Gastrointestinal Microbiomes in Gynecologic Cancers: A Review of Applications in Etiology, Symptoms and Treatment. Gynecol. Oncol. 2015, 138, 190–200. [Google Scholar] [CrossRef]
- Zhang, B.; Mohd Sahardi, N.F.N.; Di, W.; Long, X.; Shafiee, M.N. The Gut-Endometrium Axis: Exploring the Role of Microbiome in the Pathogenesis and Treatment of Endometrial Cancer-A Narrative Review. Cancers 2025, 17, 1044. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk Factors for Endometrial Cancer: An Umbrella Review of the Literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef]
- Pollet, R.M.; D’Agostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Patterson, L.M.; Creekmore, B.C.; Isenberg, H.N.; et al. An Atlas of β-Glucuronidases in the Human Intestinal Microbiome. Struct. Lond. Engl. 1993 2017, 25, 967–977.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The Microbiota Continuum along the Female Reproductive Tract and Its Relation to Uterine-Related Diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Stroup, D.F. Meta-Analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bosquet, J.; McDonald, M.E.; Bender, D.P.; Smith, B.J.; Leslie, K.K.; Goodheart, M.J.; Devor, E.J. Microbial Communities in Gynecological Cancers and Their Association with Tumor Somatic Variation. Cancers 2023, 15, 3316. [Google Scholar] [CrossRef]
- Chen, P.; Guo, Y.; Jia, L.; Wan, J.; He, T.; Fang, C.; Li, T. Interaction Between Functionally Activate Endometrial Microbiota and Host Gene Regulation in Endometrial Cancer. Front. Cell Dev. Biol. 2021, 9, 727286. [Google Scholar] [CrossRef]
- Chao, A.; Chao, A.-S.; Lin, C.-Y.; Weng, C.H.; Wu, R.-C.; Yeh, Y.-M.; Huang, S.-S.; Lee, Y.-S.; Lai, C.-H.; Huang, H.-J.; et al. Analysis of Endometrial Lavage Microbiota Reveals an Increased Relative Abundance of the Plastic-Degrading Bacteria Bacillus Pseudofirmus and Stenotrophomonas Rhizophila in Women with Endometrial Cancer/Endometrial Hyperplasia. Front. Cell. Infect. Microbiol. 2022, 12, 1031967. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Olzomer, E.M.; Kosasih, M.; Martin, A.R.; Fargah, F.; Lambie, N.; Susic, D.; Hoehn, K.L.; Farrell, R.; Byrne, F.L. Differences in the Active Endometrial Microbiota across Body Weight and Cancer in Humans and Mice. Cancers 2022, 14, 2141. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gu, Y.; He, Q.; Huang, J.; Song, Y.; Wan, X.; Li, Y. Integrated Analysis of Microbiome and Transcriptome Data Reveals the Interplay Between Commensal Bacteria and Fibrin Degradation in Endometrial Cancer. Front. Cell. Infect. Microbiol. 2021, 11, 748558. [Google Scholar] [CrossRef]
- Han, X.; Zheng, J.; Zhang, L.; Zhao, Z.; Cheng, G.; Zhang, W.; Qu, P. Endometrial Microbial Dysbiosis and Metabolic Alteration Promote the Development of Endometrial Cancer. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2024, 167, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Leoni, C.; Vinci, L.; Marzano, M.; D’Erchia, A.M.; Dellino, M.; Cox, S.N.; Vitagliano, A.; Visci, G.; Notario, E.; Filomena, E.; et al. Endometrial Cancer: A Pilot Study of the Tissue Microbiota. Microorganisms 2024, 12, 1090. [Google Scholar] [CrossRef]
- Hawkins, G.M.; Burkett, W.C.; McCoy, A.N.; Nichols, H.B.; Olshan, A.F.; Broaddus, R.; Merker, J.D.; Weissman, B.; Brewster, W.R.; Roach, J.; et al. Differences in the Microbial Profiles of Early Stage Endometrial Cancers between Black and White Women. Gynecol. Oncol. 2022, 165, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Xu, G.; Wang, H.; Wang, Y. An Altered Uterine Microbiota with Endometrial Hyperplasia. BMC Microbiol. 2024, 24, 258. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Su, H.; Shi, L.; Chen, B.; Zhang, S. Endometrial Microbiota from Endometrial Cancer and Paired Pericancer Tissues in Postmenopausal Women: Differences and Clinical Relevance. Menopause 2022, 29, 1168–1175. [Google Scholar] [CrossRef]
- Kuźmycz, O.; Kowalczyk, A.; Bolanowska, A.; Drozdzowska, A.; Lach, J.; Wierzbińska, W.; Kluz, T.; Stączek, P. A Comprehensive Analysis of the Uterine Microbiome in Endometrial Cancer Patients—Identification of Anaerococcus as a Potential Biomarker and Carcinogenic Cofactor. Front. Cell. Infect. Microbiol. 2025, 15, 1511625. [Google Scholar] [CrossRef]
- Liu, J.; Qu, Y.; Li, Y.-Y.; Xu, Y.-L.; Yan, Y.-F.; Qin, H. Exploring Prognostic Microbiota Markers in Patients with Endometrial Carcinoma: Intratumoral Insights. Heliyon 2024, 10, e27879. [Google Scholar] [CrossRef] [PubMed]
- Deligdisch, L.; Marin, T.; Lee, A.T.; Etkind, P.; Holland, J.F.; Melana, S.; Pogo, B.G.T. Human Mammary Tumor Virus (HMTV) in Endometrial Carcinoma. Int. J. Gynecol. Cancer 2013, 23, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Semertzidou, A.; Whelan, E.; Smith, A.; Ng, S.; Roberts, L.; Brosens, J.J.; Marchesi, J.R.; Bennett, P.R.; MacIntyre, D.A.; Kyrgiou, M. Microbial Signatures and Continuum in Endometrial Cancer and Benign Patients. Microbiome 2024, 12, 118. [Google Scholar] [CrossRef]
- Hakimjavadi, H.; George, S.H.; Taub, M.; Dodds, L.V.; Sanchez-Covarrubias, A.P.; Huang, M.; Pearson, J.M.; Slomovitz, B.M.; Kobetz, E.N.; Gharaibeh, R.; et al. The Vaginal Microbiome Is Associated with Endometrial Cancer Grade and Histology. Cancer Res. Commun. 2022, 2, 447–455. [Google Scholar] [CrossRef]
- Gressel, G.M.; Usyk, M.; Frimer, M.; Kuo, D.Y.S.; Burk, R.D. Characterization of the Endometrial, Cervicovaginal and Anorectal Microbiota in Post-Menopausal Women with Endometrioid and Serous Endometrial Cancers. PLoS ONE 2021, 16, e0259188. [Google Scholar] [CrossRef]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential Contribution of the Uterine Microbiome in the Development of Endometrial Cancer. Genome Med. 2016, 8, 122. [Google Scholar] [CrossRef]
- Walsh, D.M.; Hokenstad, A.N.; Chen, J.; Sung, J.; Jenkins, G.D.; Chia, N.; Nelson, H.; Mariani, A.; Walther-Antonio, M.R.S. Postmenopause as a Key Factor in the Composition of the Endometrial Cancer Microbiome (ECbiome). Sci. Rep. 2019, 9, 19213. [Google Scholar] [CrossRef]
- Barczyński, B.; Frąszczak, K.; Grywalska, E.; Kotarski, J.; Korona-Głowniak, I. Vaginal and Cervical Microbiota Composition in Patients with Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 8266. [Google Scholar] [CrossRef]
- Caselli, E.; Soffritti, I.; D’Accolti, M.; Piva, I.; Greco, P.; Bonaccorsi, G. Atopobium Vaginae And Porphyromonas Somerae Induce Proinflammatory Cytokines Expression In Endometrial Cells: A Possible Implication For Endometrial Cancer? Cancer Manag. Res. 2019, 11, 8571–8575. [Google Scholar] [CrossRef] [PubMed]
- Crooks, T.A.; Madison, J.D.; Walsh, D.M.; Herbert, W.G.; Jeraldo, P.R.; Chia, N.; Cliby, W.A.; Kaufmann, S.H.; Walther-Antonio, M.R.S. Porphyromonas Somerae Invasion of Endometrial Cancer Cells. Front. Microbiol. 2021, 12, 674835. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qin, L.-H.; Li, L.; Wei, Q.-Y.; Long, L.; Liao, J.-Y. The Causal Relationship between the Gut Microbiota and Endometrial Cancer: A Mendelian Randomization Study. BMC Cancer 2025, 25, 248. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, D.V.; Wang, D.; Liu, L.; Andreu-Sánchez, S.; Zhang, Y.; Ruiz-Moreno, A.J.; Peng, H.; Plomp, N.; Del Castillo-Izquierdo, Á.; Gacesa, R.; et al. Host genetic regulation of human gut microbial structural variation. Nature 2024, 625, 813–821. [Google Scholar] [CrossRef]
- Qin, Y.; Havulinna, A.S.; Liu, Y.; Jousilahti, P.; Ritchie, S.C.; Tokolyi, A.; Sanders, J.G.; Valsta, L.; Brożyńska, M.; Zhu, Q.; et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 2022, 54, 134–142. [Google Scholar] [CrossRef]
- Abdill, R.J.; Graham, S.P.; Rubinetti, V.; Ahmadian, M.; Hicks, P.; Chetty, A.; McDonald, D.; Ferretti, P.; Gibbons, E.; Rossi, M.; et al. Integration of 168,000 samples reveals global patterns of the human gut microbiome. Cell 2025, 188, 1100–1118.e17. [Google Scholar] [CrossRef]
| First Author and Year | Sampling Method | Sample Size | Study Results |
|---|---|---|---|
| Gressel et al., 2021 [29] | Sterile swabs from vaginal fornices, ectocervix, rectum, and endometrial cavity during hysterectomy; 16S rRNA. | Controls n = 10, endometrioid EC n = 14, serous EC n = 11. | Significant differences in microbial beta-diversity among niches; USC group showed reduced alpha-diversity in uterine samples and distinct microbial signatures across sites. Cervicovaginal Lactobacillus depletion and uterine Pseudomonas elevation were biomarkers for USC. Microbiota composition could distinguish USC from controls (p = 0.042). |
| Semertzidou et al., 2024 [27] | Samples collected from the vagina, cervix and endometrium. 16S rRNA sequencing. | EC n = 37 EC, control n = 24 | Endometrial cancer patients showed in cervix and rectum reduced Lactobacillus (especially L. crispatus), increased bacterial diversity, and enrichment of Porphyromonas, Prevotella, Peptoniphilus, Anaerococcus. L. crispatus-conditioned media had anti-proliferative effect on endometrial organoids. |
| Walther-António et al., 2016 [30] | Vaginal, cervical, Fallopian, ovarian, and urine samples; 16S rDNA sequencing. | benign n = 10, EH n = 4 (including 1 AEH), EC n = 17. | Cancer and hyperplasia samples showed significantly different microbiome profiles vs. benign. A. vaginae and Porphyromonas sp. correlated with EC, especially with high vaginal pH. Increased alpha diversity in EC and EH compared to benign. Suggests microbial contribution to tumorigenesis. |
| Walsh et al., 2019 [31] | Vaginal and cervical swabs obtained preoperatively, 16S rRNA sequencing. | EC n = 66, benign n = 75, HP n = 7. | Postmenopausal status, obesity, and high vaginal pH significantly increased vaginal microbiome diversity. Porphyromonas somerae was most enriched in EC patients and proposed as a potential biomarker (AUC = 76.7%). P. somerae detected in 100% of Type II EC cases. No differences regarding α-diversity between cancer and control group. |
| Hakimjavadi et al., 2022 [28] | Vaginal swabs taken pre-surgery using sterile swabs; DNA extracted and shotgun metagenomic sequencing performed. | Benign n = 1, low-grade EC n = 30, high-grade EC n = 20. | Microbial α- and β-diversity correlated with tumor grade. Fusobacterium ulcerans and Prevotella bivia were enriched in high-grade EC. Vaginal microbiome profiles predicted cancer presence and grade with high accuracy (AUC up to 0.88). No differences regarding α-diversity between cancer and control group. |
| Barczyński et al., 2023 [32] | Cervical and vaginal swabs collected intraoperatively post-anesthesia, pre-douching; 16S rRNA sequencing. | EC n = 48, hyperplasia n = 21, benign n = 27. | Significant cervicovaginal dysbiosis observed in EC patients. Mobiluncus curtisii and Dialister pneumosintes were more frequent in cancer patients, while Lactobacillus iners was more common in benign cases. Suggests potential role of microbiota in carcinogenesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizza, R.; Belli, F.; Fabene, P.; Salari, V.; Casprini, C.; Corrado, G.; Laganà, A.S.; Zorzato, P.C.; Bosco, M.; Porcari, I.; et al. The Female Reproductive Tract Microbiota and Endometrial Cancer: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 8877. https://doi.org/10.3390/ijms26188877
Vizza R, Belli F, Fabene P, Salari V, Casprini C, Corrado G, Laganà AS, Zorzato PC, Bosco M, Porcari I, et al. The Female Reproductive Tract Microbiota and Endometrial Cancer: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(18):8877. https://doi.org/10.3390/ijms26188877
Chicago/Turabian StyleVizza, Riccardo, Francesco Belli, Paolo Fabene, Valentina Salari, Chiara Casprini, Giacomo Corrado, Antonio Simone Laganà, Pier Carlo Zorzato, Mariachiara Bosco, Irene Porcari, and et al. 2025. "The Female Reproductive Tract Microbiota and Endometrial Cancer: A Systematic Review" International Journal of Molecular Sciences 26, no. 18: 8877. https://doi.org/10.3390/ijms26188877
APA StyleVizza, R., Belli, F., Fabene, P., Salari, V., Casprini, C., Corrado, G., Laganà, A. S., Zorzato, P. C., Bosco, M., Porcari, I., Uccella, S., & Garzon, S. (2025). The Female Reproductive Tract Microbiota and Endometrial Cancer: A Systematic Review. International Journal of Molecular Sciences, 26(18), 8877. https://doi.org/10.3390/ijms26188877










