Methamphetamine Induces Metallothionein 1 Expression and an Inflammatory Phenotype in Primary Human HIV-Infected Macrophages
Abstract
1. Introduction
2. Results
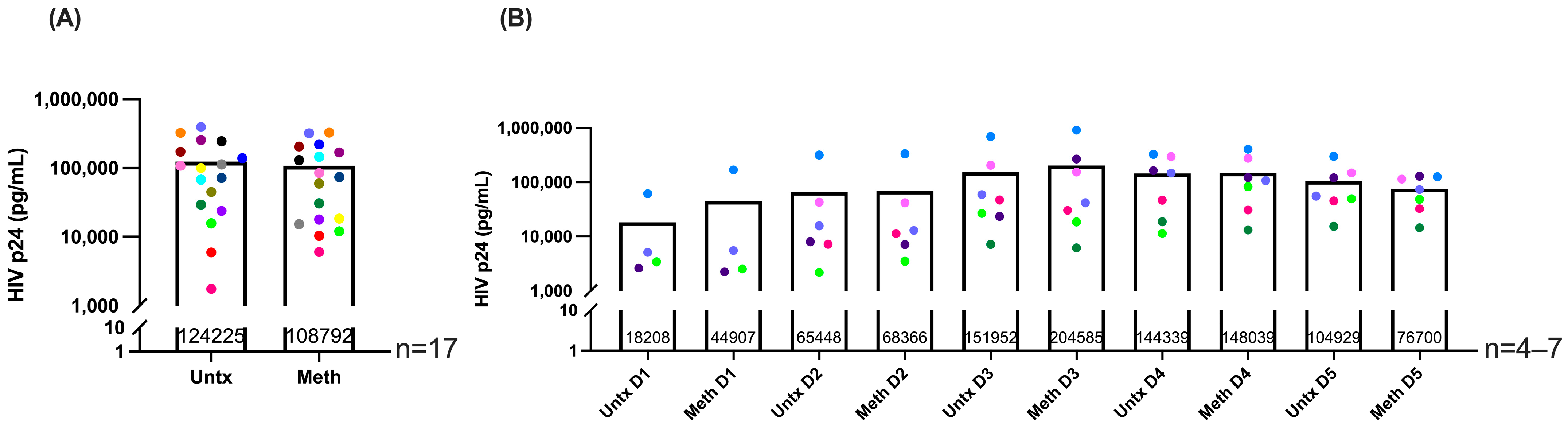
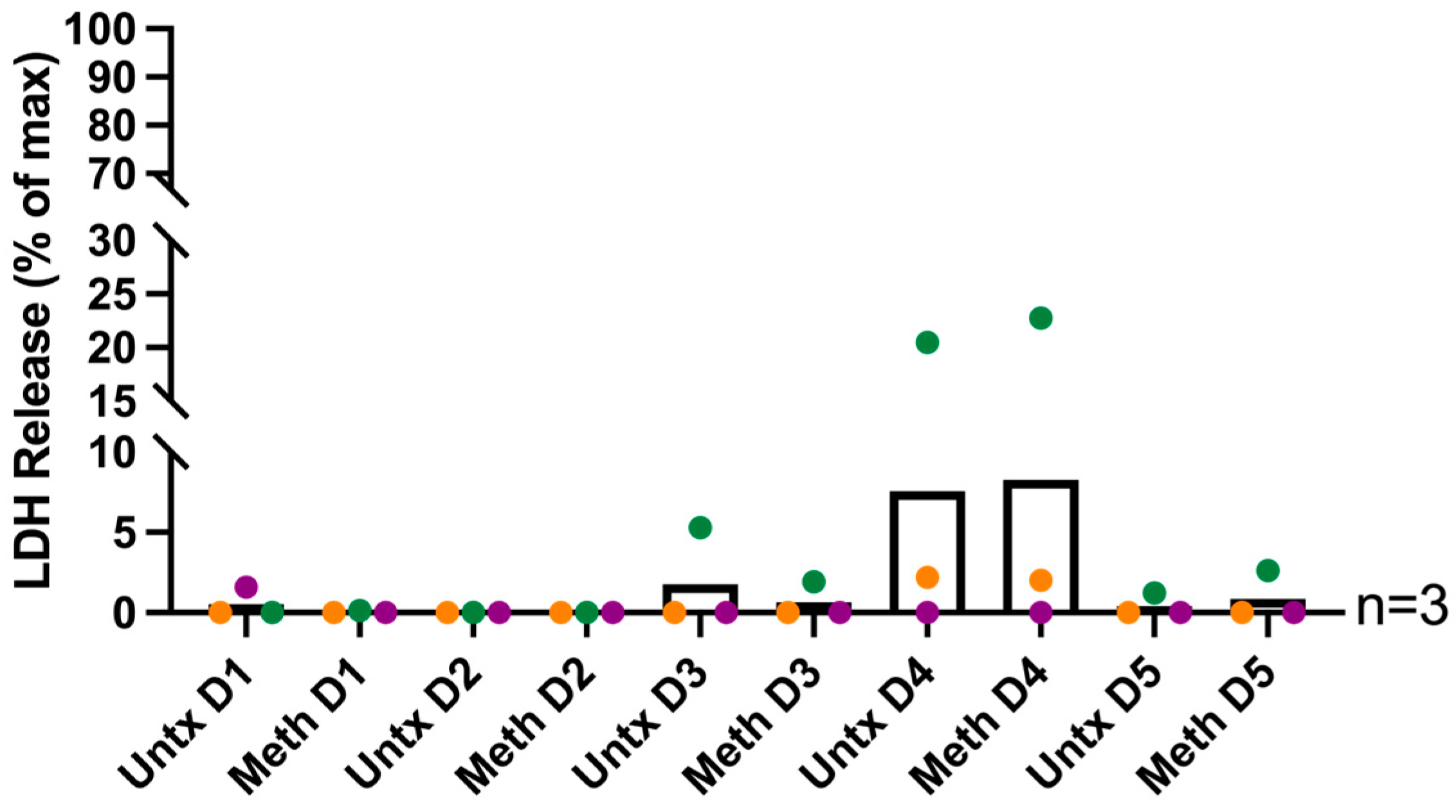
2.1. Methamphetamine Does Not Change HIV Infection Levels and Is Not Toxic to HIV-Infected Macrophages
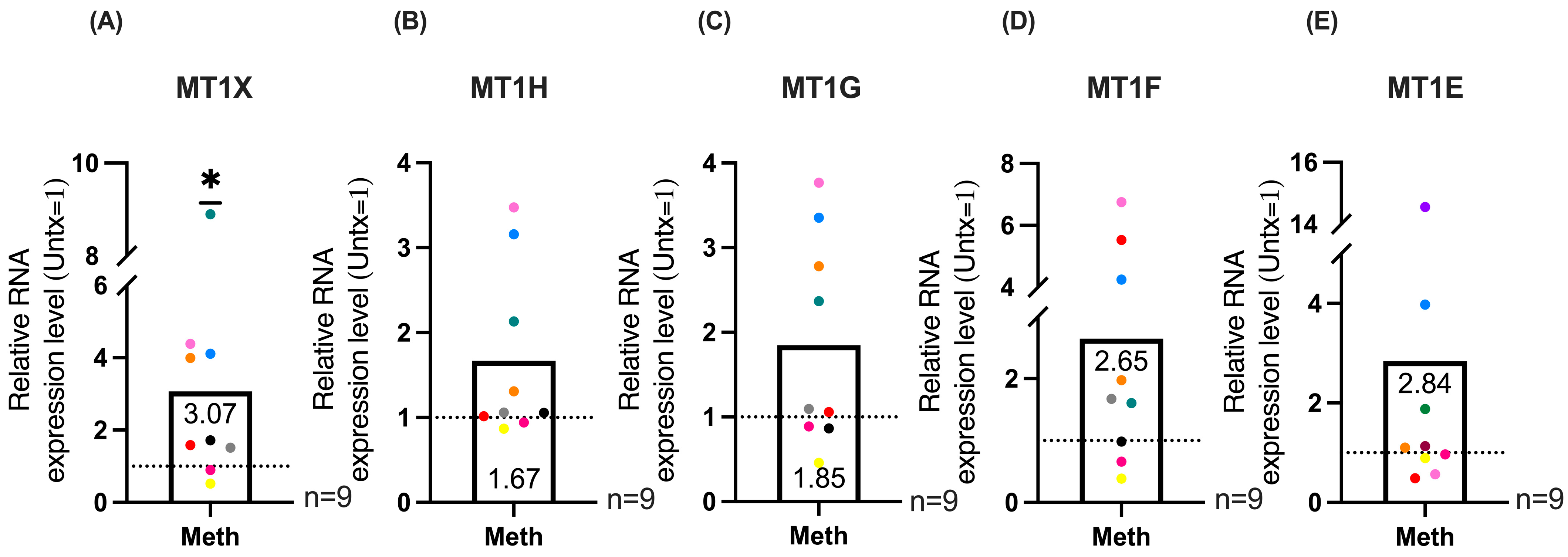
2.2. RNA Sequencing of HIV-Infected Macrophages Identifies Increased Expression of MT1 Genes with Methamphetamine Treatment
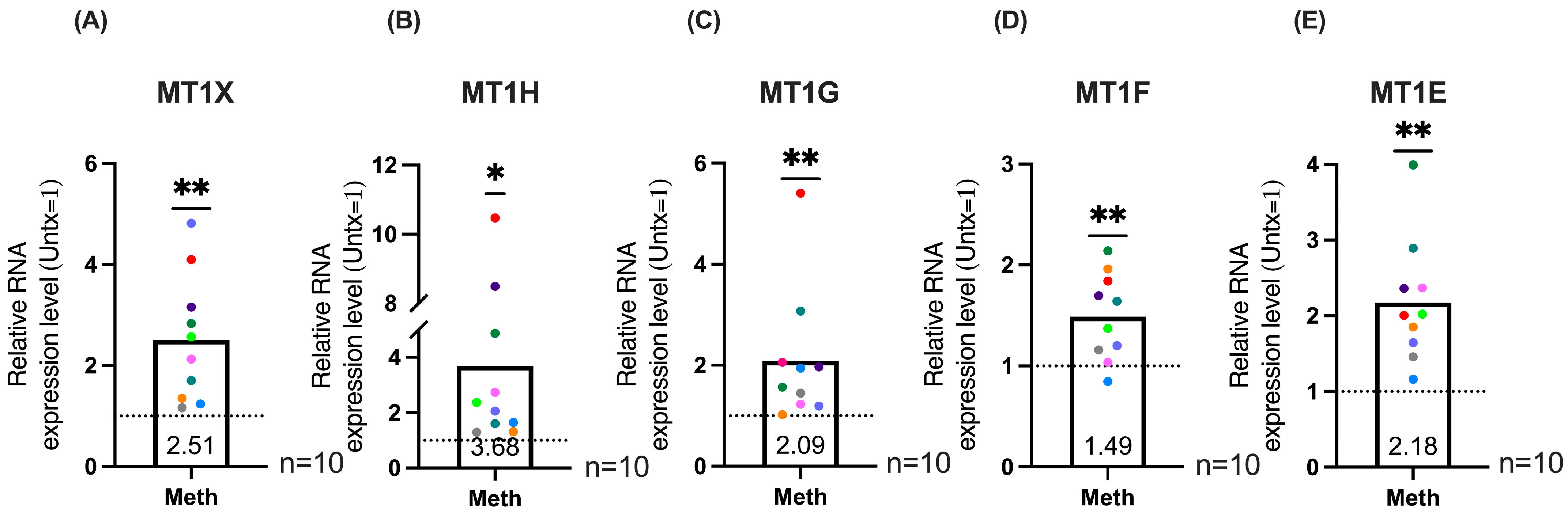
2.3. qRT-PCR Validation of MT1 Genes and Maximal Expression Analysis Demonstrates Significant Increases in MT1 Gene Expression in HIV-Infected Macrophages Treated with Methamphetamine
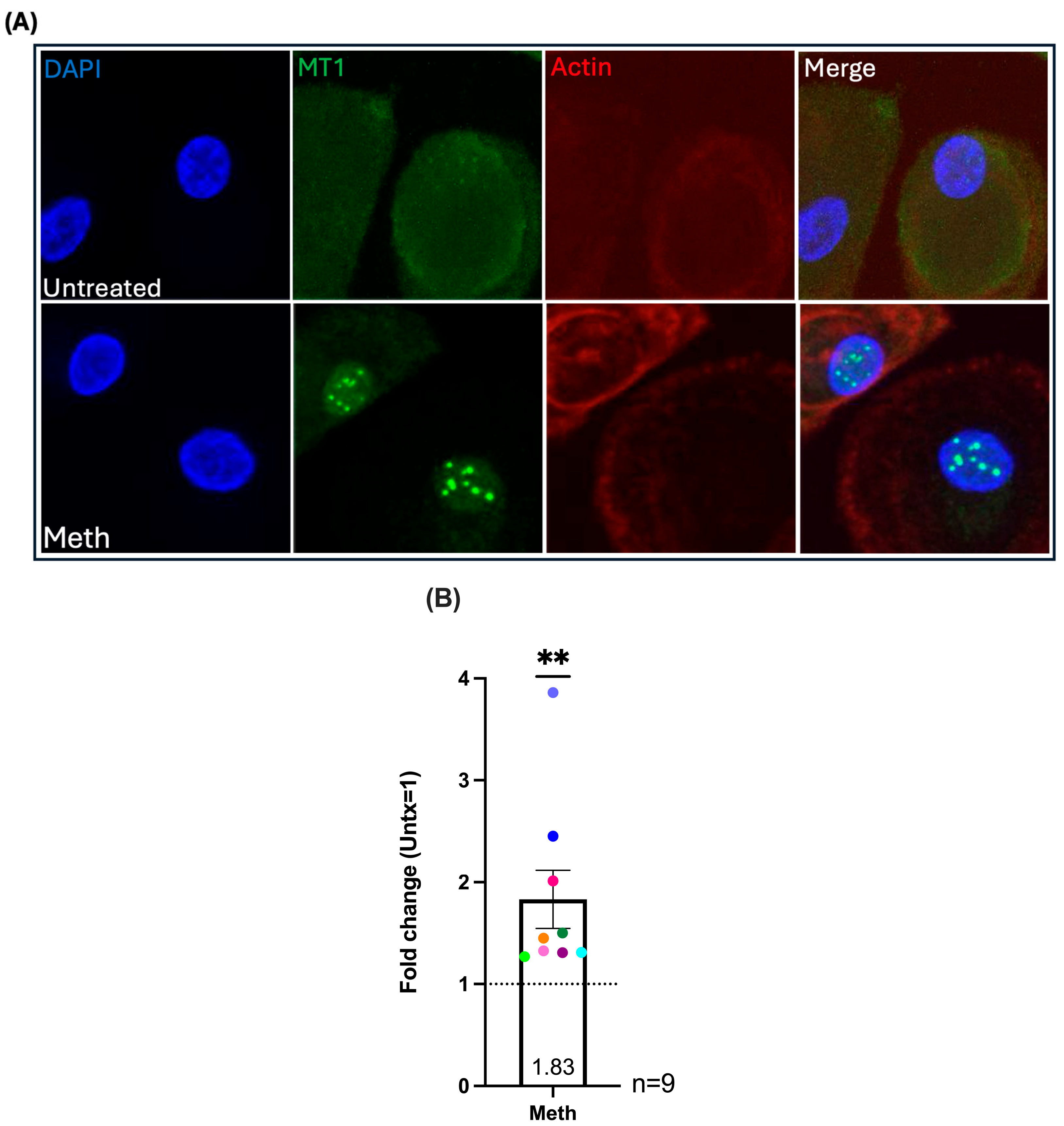
2.4. Methamphetamine Treatment Increases Nuclear Localization of MT1 in HIV-Infected Macrophages
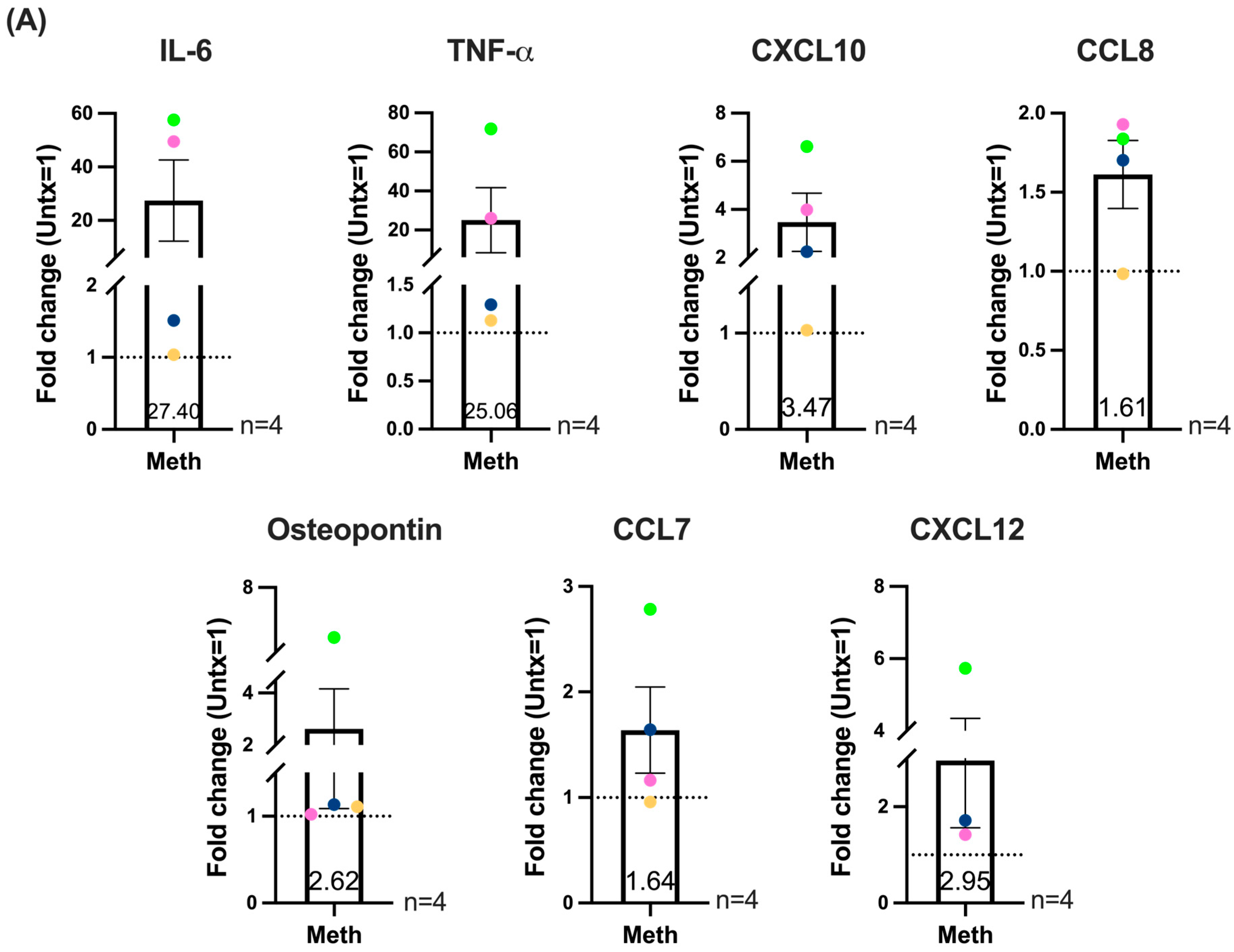
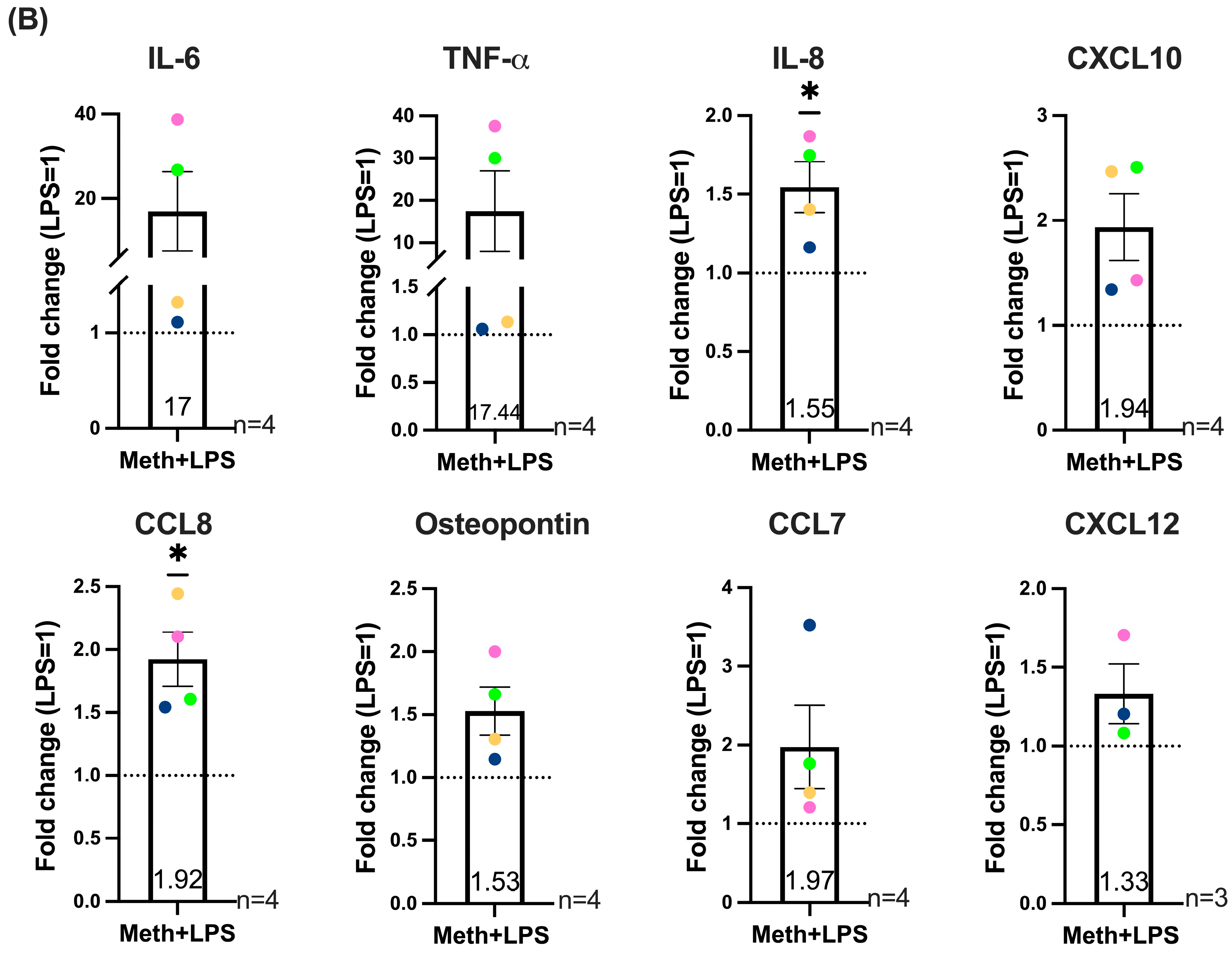
2.5. Methamphetamine in the Absence or Presence of LPS Increases the Production of Inflammatory Mediators Associated with HIV-NCI and CNS Inflammation by HIV-Infected Macrophages
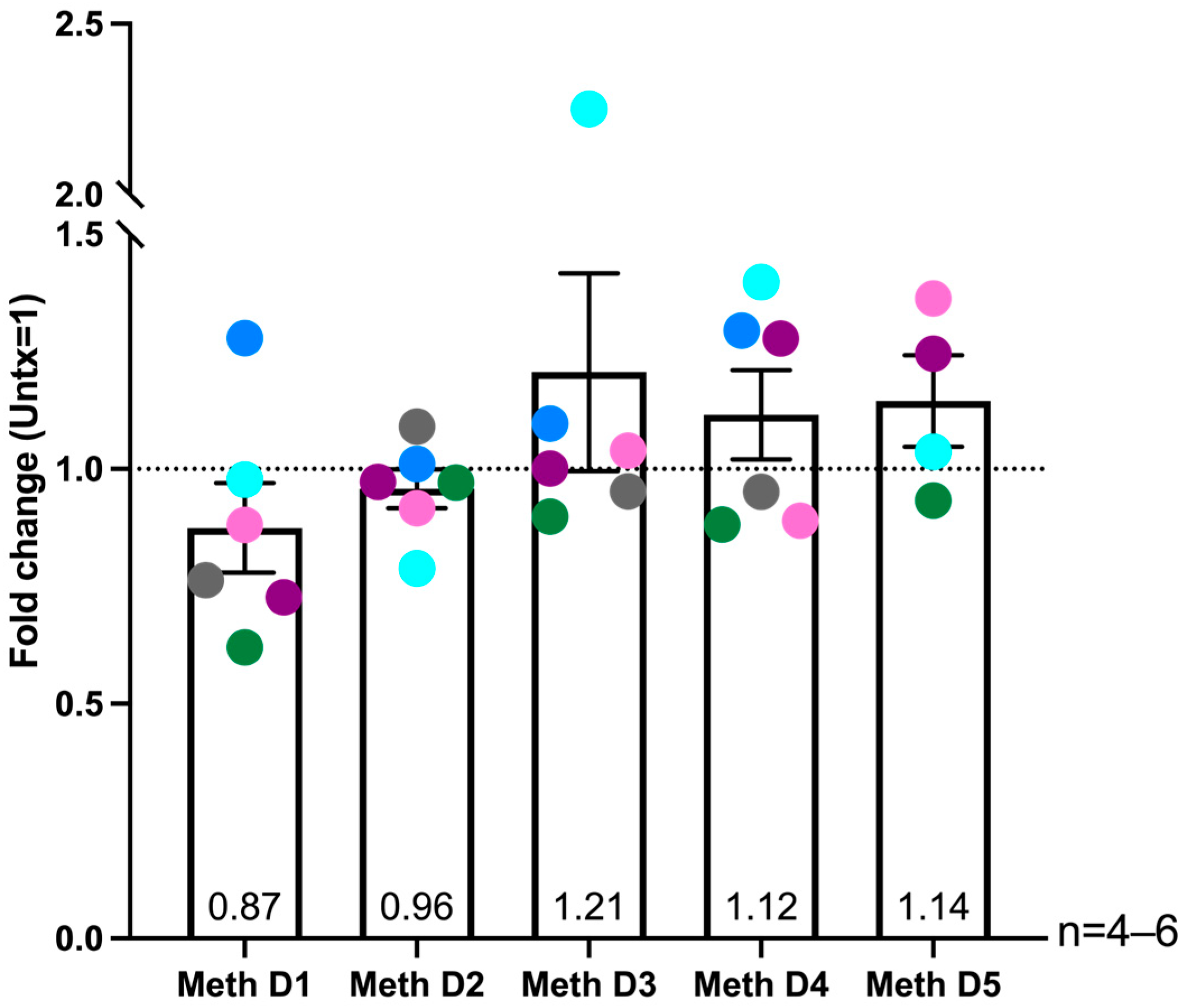
2.6. Donor Specific Increased ROS Levels in HIV-Infected Macrophages After Multiple Days of Methamphetamine Treatment
3. Discussion
4. Materials and Methods
4.1. Monocyte Derived Macrophage Cell Culture
4.2. HIV Infection and Methamphetamine Treatment of Macrophages
4.3. Measurement of HIV Infection Levels
4.4. Measurement of Cell Toxicity
4.5. RNA Sequencing of HIV-Infected Macrophages
4.6. RNA-Seq Data Analysis
4.7. qRT-PCR of MT1 DEG
4.8. MT1 Immunofluorescence and Confocal Microscopy
4.9. Measurement of Inflammatory Mediators
4.10. Measurement of Reactive Oxygen Species
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HIV-NCI | HIV-associated Neurocognitive Impairment |
| HIV | Human immunodeficiency virus |
| PWH | People with HIV |
| PBMC | Peripheral Blood Mononuclear Cells |
| M-CSF | Macrophage colony stimulating factor |
| ROS | Reactive Oxygen Species |
| RNA-seq | RNA Sequencing |
| MT1 | Metallothionein 1 |
| MDM | Monocyte derived macrophages |
| CNS | Central nervous system |
| NF-κB | Nuclear factor κB |
References
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; Leblanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; Woods, S.P.; et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2011, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Lu, Q.; Farrell, M.; Lappin, J.M.; Shi, J.; Lu, L.; Bao, Y. Global prevalence and burden of HIV-associated neurocognitive disorder: A meta-analysis. Neurology 2020, 95, e2610–e2621. [Google Scholar] [CrossRef] [PubMed]
- Zenebe, Y.; Necho, M.; Yimam, W.; Akele, B. Worldwide Occurrence of HIV-Associated Neurocognitive Disorders and Its Associated Factors: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 814362. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.; Mezhir, J.J.; Walsh, K.; Hewitt, R.G. Impact of human immunodeficiency virus type-1-associated cognitive dysfunction on activities of daily living and quality of life. Arch. Clin. Neuropsychol. 2000, 15, 535–544. [Google Scholar] [CrossRef]
- Laverick, R.; Haddow, L.; Daskalopoulou, M.; Lampe, F.; Gilson, R.; Speakman, A.; Antinori, A.; Bruun, T.; Vassilenko, A.; Collins, S.; et al. Self-Reported Decline in Everyday Function, Cognitive Symptoms, and Cognitive Function in People with HIV. J. Acquir. Immune Defic. Syndr. 2017, 76, e74–e83. [Google Scholar] [CrossRef]
- Tozzi, V.; Balestra, P.; Serraino, D.; Bellagamba, R.; Corpolongo, A.; Piselli, P.; Lorenzini, P.; Visco-Comandini, U.; Vlassi, C.; Quartuccio, M.E.; et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res. Hum. Retroviruses 2005, 21, 706–713. [Google Scholar] [CrossRef]
- Patel, S.; Parikh, N.U.; Aalinkeel, R.; Reynolds, J.L.; Dmello, R.; Schwartz, S.A.; Mahajan, S.D. United States National Trends in Mortality, Length of Stay (LOS) and Associated Costs of Cognitive Impairment in HIV Population from 2005 to 2014. AIDS Behav. 2018, 22, 3198–3208. [Google Scholar] [CrossRef]
- Jia, F.F.; Brew, B.J. Neuropathogenesis of acute HIV: Mechanisms, biomarkers, and therapeutic approaches. Curr. Opin. HIV AIDS 2025, 20, 199–208. [Google Scholar] [CrossRef]
- Killingsworth, L.; Spudich, S. Neuropathogenesis of HIV-1: Insights from across the spectrum of acute through long-term treated infection. Semin. Immunopathol. 2022, 44, 709–724. [Google Scholar] [CrossRef]
- Abreu, C.; Shirk, E.N.; Queen, S.E.; Beck, S.E.; Mangus, L.M.; Pate, K.A.M.; Mankowski, J.L.; Gama, L.; Clements, J.E. Brain macrophages harbor latent, infectious simian immunodeficiency virus. AIDS 2019, 33 (Suppl. 2), S181–S188. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Veenhuis, R.T.; Avalos, C.R.; Graham, S.; Parrilla, D.R.; Ferreira, E.A.; Queen, S.E.; Shirk, E.N.; Bullock, B.T.; Li, M.; et al. Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, C.R.; Angelovich, T.A.; Byrnes, S.J.; Waring, E.; Guanizo, A.C.; Trollope, G.S.; Zhou, J.; Vue, J.; Senior, L.; Wanicek, E.; et al. Intact HIV Proviruses Persist in the Brain Despite Viral Suppression with ART. Ann. Neurol. 2022, 92, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Donoso, M.; D’Amico, D.; Valdebenito, S.; Hernandez, C.A.; Prideaux, B.; Eugenin, E.A. Identification, Quantification, and Characterization of HIV-1 Reservoirs in the Human Brain. Cells 2022, 11, 2379. [Google Scholar] [CrossRef]
- Ferreira, E.A.; Clements, J.E.; Veenhuis, R.T. HIV-1 Myeloid Reservoirs—Contributors to Viral Persistence and Pathogenesis. Curr. HIV/AIDS Rep. 2024, 21, 62–74. [Google Scholar] [CrossRef]
- Jamal Eddine, J.; Angelovich, T.A.; Zhou, J.; Byrnes, S.J.; Tumpach, C.; Saraya, N.; Chalmers, E.; Shepherd, R.A.; Tan, A.; Marinis, S.; et al. HIV transcription persists in the brain of virally suppressed people with HIV. PLoS Pathog. 2024, 20, e1012446. [Google Scholar] [CrossRef]
- Ellis, R.J.; Marquine, M.J.; Kaul, M.; Fields, J.A.; Schlachetzki, J.C.M. Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management. Nat. Rev. Neurol. 2023, 19, 668–687, Erratum in Nat. Rev. Neurol. 2023, 19, 787. [Google Scholar] [CrossRef]
- Moschopoulos, C.D.; Alford, K.; Antoniadou, A.; Vera, J.H. Cognitive impairment in people living with HIV: Mechanisms, controversies, and future perspectives. Trends Mol. Med. 2024, 30, 1076–1089. [Google Scholar] [CrossRef]
- Thompson, L.J.; Genovese, J.; Hong, Z.; Singh, M.V.; Singh, V.B. HIV-Associated Neurocognitive Disorder: A Look into Cellular and Molecular Pathology. Int. J. Mol. Sci. 2024, 25, 4697. [Google Scholar] [CrossRef]
- Byrnes, S.J.; Jamal Eddine, J.; Zhou, J.; Chalmers, E.; Wanicek, E.; Osman, N.; Jenkins, T.A.; Roche, M.; Brew, B.J.; Estes, J.D.; et al. Neuroinflammation associated with proviral DNA persists in the brain of virally suppressed people with HIV. Front. Immunol. 2025, 16, 1570692. [Google Scholar] [CrossRef]
- Hileman, C.O.; Funderburg, N.T. Inflammation, Immune Activation, and Antiretroviral Therapy in HIV. Curr. HIV/AIDS Rep. 2017, 14, 93–100. [Google Scholar] [CrossRef]
- Saeb, S.; Wallet, C.; Rohr, O.; Schwartz, C.; Loustau, T. Targeting and eradicating latent CNS reservoirs of HIV-1: Original strategies and new models. Biochem. Pharmacol. 2023, 214, 115679. [Google Scholar] [CrossRef] [PubMed]
- Moszczynska, A.; Callan, S.P. Molecular, Behavioral, and Physiological Consequences of Methamphetamine Neurotoxicity: Implications for Treatment. J. Pharmacol. Exp. Ther. 2017, 362, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Permpoonputtana, K.; Namyen, J.; Buntup, D.; Boontem, P.; Nopparat, C.; Govitrapong, P. Association of Cognitive Impairment and Peripheral Inflammation in Methamphetamine-dependent Patients: A Cross-sectional Study on Neuroinflammatory Markers TNF-alpha and IL-6. Clin. Psychopharmacol. Neurosci. 2025, 23, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.C.; Woods, S.P.; Matt, G.E.; Meyer, R.A.; Heaton, R.K.; Atkinson, J.H.; Grant, I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol. Rev. 2007, 17, 275–297. [Google Scholar] [CrossRef]
- Richesson, D.M.I.; Brown, S.; Linman, S.; Hoenig, J.M. National Survey on Drug Use and Health. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt47095/National%20Report/National%20Report/2023-nsduh-annual-national.pdf (accessed on 5 July 2025).
- Bamford, L.; Rajagopal, A.; Grelotti, D.; Justice-Royster, V.; Karim, A.; Montoya, J. Impact of methamphetamine use on HIV and other health outcomes at an urban HIV medicine clinic. AIDS 2024, 38, 1839–1844. [Google Scholar] [CrossRef]
- Hai, A.H.; Batey, D.S.; Lee, C.S.; Simons, J.N.; Beadleston, A.; Schnall, R. Substance use patterns among U.S. adults with HIV: Identifying priorities for screening and interventions. AIDS Care 2025, 37, 843–854. [Google Scholar] [CrossRef]
- Miao, L.; Wang, H.; Li, Y.; Huang, J.; Wang, C.; Teng, H.; Xu, L.; Yang, X.; Tian, Y.; Yang, G.; et al. Mechanisms and treatments of methamphetamine and HIV-1 co-induced neurotoxicity: A systematic review. Front. Immunol. 2024, 15, 1423263. [Google Scholar] [CrossRef]
- Rippeth, J.D.; Heaton, R.K.; Carey, C.L.; Marcotte, T.D.; Moore, D.J.; Gonzalez, R.; Wolfson, T.; Grant, I.; HNRC Group. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J. Int. Neuropsychol. Soc. 2004, 10, 1–14. [Google Scholar] [CrossRef]
- Soontornniyomkij, V.; Kesby, J.P.; Morgan, E.E.; Bischoff-Grethe, A.; Minassian, A.; Brown, G.G.; Grant, I.; Translational Methamphetamine, A.R.C.G. Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. J. Neuroimmune Pharmacol. 2016, 11, 495–510. [Google Scholar] [CrossRef]
- Carrico, A.W.; Riley, E.D.; Johnson, M.O.; Charlebois, E.D.; Neilands, T.B.; Remien, R.H.; Lightfoot, M.A.; Steward, W.T.; Weinhardt, L.S.; Kelly, J.A.; et al. Psychiatric risk factors for HIV disease progression: The role of inconsistent patterns of antiretroviral therapy utilization. J. Acquir. Immune Defic. Syndr. 2011, 56, 146–150. [Google Scholar] [CrossRef]
- Lai, H.H.; Kuo, Y.C.; Kuo, C.J.; Lai, Y.J.; Chen, M.; Chen, Y.T.; Chen, C.C.; Yen, M.Y.; Hu, B.S.; Wang, T.H.; et al. Methamphetamine Use Associated with Non-adherence to Antiretroviral Treatment in Men Who Have Sex with Men. Sci. Rep. 2020, 10, 7131. [Google Scholar] [CrossRef] [PubMed]
- Feelemyer, J.; Arasteh, K.; Huong, D.T.; Oanh, K.T.H.; Khue, P.M.; Giang, H.T.; Thanh, N.T.T.; Moles, J.P.; Vinh, V.H.; Vallo, R.; et al. Associations between methamphetamine use and lack of viral suppression among a cohort of HIV-positive persons who inject drugs in Hai Phong, Vietnam. AIDS 2020, 34, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, J.A.; Javanbakht, M.; Shover, C.L.; Ragsdale, A.; Brookmeyer, R.; Shoptaw, S.; Gorbach, P.M. Comparative impact of methamphetamine and other drug use on viral suppression among sexual minority men on antiretroviral therapy. Drug Alcohol Depend. 2021, 221, 108622. [Google Scholar] [CrossRef] [PubMed]
- Massanella, M.; Gianella, S.; Schrier, R.; Dan, J.M.; Perez-Santiago, J.; Oliveira, M.F.; Richman, D.D.; Little, S.J.; Benson, C.A.; Daar, E.S.; et al. Methamphetamine Use in HIV-infected Individuals Affects T-cell Function and Viral Outcome during Suppressive Antiretroviral Therapy. Sci. Rep. 2015, 5, 13179. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, S.; Bechet, N.; Gouras, G.; Wen, G. Perivascular macrophages in the central nervous system: Insights into their roles in health and disease. Cell Death Dis. 2025, 16, 350. [Google Scholar] [CrossRef]
- Akiyama, H.; Gummuluru, S. HIV-1 Persistence and Chronic Induction of Innate Immune Responses in Macrophages. Viruses 2020, 12, 711. [Google Scholar] [CrossRef]
- Burdo, T.H.; Lackner, A.; Williams, K.C. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol. Rev. 2013, 254, 102–113. [Google Scholar] [CrossRef]
- Wong, M.E.; Jaworowski, A.; Hearps, A.C. The HIV Reservoir in Monocytes and Macrophages. Front. Immunol. 2019, 10, 1435, Correction in Front. Immunol. 2019, 10, 2517. [Google Scholar] [CrossRef]
- Barbaro, J.M.; Sidoli, S.; Cuervo, A.M.; Berman, J.W. Methamphetamine Dysregulates Macrophage Functions and Autophagy to Mediate HIV Neuropathogenesis. Biomedicines 2022, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Itoh, N.; Kawasaki, A.; Matsuyama, A.; Matsuda, K.; Nakanishi, T.; Tanaka, K. Metallothionein modulates lipopolysaccharide-stimulated tumour necrosis factor expression in mouse peritoneal macrophages. Biochem. J. 2002, 361 Pt 2, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Vasak, M.; Hasler, D.W. Metallothioneins: New functional and structural insights. Curr. Opin. Chem. Biol. 2000, 4, 177–183. [Google Scholar] [CrossRef]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.B.; Agrawal, K.C. Activation of nuclear factor kappaB: Potential role in metallothionein-mediated mitogenic response. Cancer Res. 1998, 58, 2335–2338. [Google Scholar]
- Butcher, H.L.; Kennette, W.A.; Collins, O.; Zalups, R.K.; Koropatnick, J. Metallothionein mediates the level and activity of nuclear factor kappa B in murine fibroblasts. J. Pharmacol. Exp. Ther. 2004, 310, 589–598. [Google Scholar] [CrossRef]
- Cherian, M.G.; Apostolova, M.D. Nuclear localization of metallothionein during cell proliferation and differentiation. Cell Mol. Biol. (Noisy-le-grand) 2000, 46, 347–356. [Google Scholar]
- Cherian, M.G.; Jayasurya, A.; Bay, B.H. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat. Res. 2003, 533, 201–209. [Google Scholar] [CrossRef]
- Dai, H.; Wang, L.; Li, L.; Huang, Z.; Ye, L. Metallothionein 1: A New Spotlight on Inflammatory Diseases. Front. Immunol. 2021, 12, 739918. [Google Scholar] [CrossRef]
- Levadoux, M.; Mahon, C.; Beattie, J.H.; Wallace, H.M.; Hesketh, J.E. Nuclear import of metallothionein requires its mRNA to be associated with the perinuclear cytoskeleton. J. Biol. Chem. 1999, 274, 34961–34966. [Google Scholar] [CrossRef]
- Levadoux-Martin, M.; Hesketh, J.E.; Beattie, J.H.; Wallace, H.M. Influence of metallothionein-1 localization on its function. Biochem. J. 2001, 355 Pt 2, 473–479. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.E.; Jongejan, A.; Monteiro Fortes, J.; Balesar, R.; Rozemuller, A.J.M.; Moerland, P.D.; Huitinga, I.; Swaab, D.F.; Verhaagen, J. Gene-expression profiling of individuals resilient to Alzheimer’s disease reveals higher expression of genes related to metallothionein and mitochondrial processes and no changes in the unfolded protein response. Acta Neuropathol. Commun. 2024, 12, 68, Correction in Acta Neuropathol. Commun. 2025, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Rebollar, D.; Rios, C.; Nava-Ruiz, C.; Mendez-Armenta, M. Metallothionein in Brain Disorders. Oxid. Med. Cell. Longev. 2017, 2017, 5828056. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M. Multifunctional Metallothioneins as a Target for Neuroprotection in Parkinson’s Disease. Antioxidants 2023, 12, 894. [Google Scholar] [CrossRef]
- Talloczy, Z.; Martinez, J.; Joset, D.; Ray, Y.; Gacser, A.; Toussi, S.; Mizushima, N.; Nosanchuk, J.D.; Goldstein, H.; Loike, J.; et al. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008, 4, e28, Erratum in PLoS Pathog. 2008, 4. [Google Scholar] [CrossRef]
- Riviere, G.J.; Gentry, W.B.; Owens, S.M. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J. Pharmacol. Exp. Ther. 2000, 292, 1042–1047. [Google Scholar] [CrossRef]
- Cen, P.; Ye, L.; Su, Q.J.; Wang, X.; Li, J.L.; Lin, X.Q.; Liang, H.; Ho, W.Z. Methamphetamine inhibits Toll-like receptor 9-mediated anti-HIV activity in macrophages. AIDS Res. Hum. Retroviruses 2013, 29, 1129–1137. [Google Scholar] [CrossRef]
- Liang, H.; Wang, X.; Chen, H.; Song, L.; Ye, L.; Wang, S.H.; Wang, Y.J.; Zhou, L.; Ho, W.Z. Methamphetamine enhances HIV infection of macrophages. Am. J. Pathol. 2008, 172, 1617–1624. [Google Scholar] [CrossRef]
- Karin, M.; Haslinger, A.; Heguy, A.; Dietlin, T.; Imbra, R. Transcriptional control mechanisms which regulate the expression of human metallothionein genes. In Metallothionein II; Experientia Supplementum; Springer Nature: Basel, Switzerland, 1987; Volume 52, pp. 401–405. [Google Scholar]
- Moleirinho, A.; Carneiro, J.; Matthiesen, R.; Silva, R.M.; Amorim, A.; Azevedo, L. Gains, losses and changes of function after gene duplication: Study of the metallothionein family. PLoS ONE 2011, 6, e18487. [Google Scholar] [CrossRef]
- West, A.K.; Stallings, R.; Hildebrand, C.E.; Chiu, R.; Karin, M.; Richards, R.I. Human metallothionein genes: Structure of the functional locus at 16q13. Genomics 1990, 8, 513–518. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ogra, Y.; Suzuki, K.T. Nuclear trafficking of metallothionein requires oxidation of a cytosolic partner. J. Cell. Physiol. 2005, 202, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Ramli, F.F.; Rejeki, P.S.; Ibrahim, N.’I.; Abdullayeva, G.; Halim, S. A Mechanistic Review on Toxicity Effects of Methamphetamine. Int. J. Med. Sci. 2025, 22, 482–507. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basilio, J.; Schmid, J.A. NF-κB in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Luo, Z.; Stephenson, S.; Li, H.; Di Germanio, C.; Norris, P.J.; Fuchs, D.; Zetterberg, H.; Gisslen, M.; Price, R.W. Cerebrospinal Fluid and Plasma Lipopolysaccharide Levels in Human Immunodeficiency Virus Type 1 Infection and Associations with Inflammation, Blood-Brain Barrier Permeability, and Neuronal Injury. J. Infect. Dis. 2021, 223, 1612–1620. [Google Scholar] [CrossRef]
- Williams, M.E.; Stein, D.J.; Joska, J.A.; Naude, P.J.W. Cerebrospinal fluid immune markers and HIV-associated neurocognitive impairments: A systematic review. J. Neuroimmunol. 2021, 358, 577649. [Google Scholar] [CrossRef]
- Xin, H.; Yu, N.; Yang, Q.; Zou, X.; An, Z.; Zhou, G. Antioxidative polyphenols attenuate pyocyanin-induced ROS production in neuronal HT22 cell lines. RSC Adv. 2023, 13, 19477–19484. [Google Scholar] [CrossRef]
- Riddle, E.L.; Fleckenstein, A.E.; Hanson, G.R. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006, 8, E413–E418. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2021, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.; Perego, J. CNS Resident Innate Immune Cells: Guardians of CNS Homeostasis. Int. J. Mol. Sci. 2024, 25, 4865. [Google Scholar] [CrossRef] [PubMed]
- Vara-Pérez, M.; Movahedi, K. Border-associated macrophages as gatekeepers of brain homeostasis and immunity. Immunity 2025, 58, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, Y.; Ma, D.; Feng, L.; Yang, F.; An, P.; Su, X.; Feng, J. Neurotoxicity mechanisms and clinical implications of six common recreational drugs. Front. Pharmacol. 2025, 16, 1526270. [Google Scholar] [CrossRef]
- Plankey, M.W.; Ostrow, D.G.; Stall, R.; Cox, C.; Li, X.; Peck, J.A.; Jacobson, L.P. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J. Acquir. Immune Defic. Syndr. 2007, 45, 85–92. [Google Scholar] [CrossRef]
- Liu, X.; Silverstein, P.S.; Singh, V.; Shah, A.; Qureshi, N.; Kumar, A. Methamphetamine increases LPS-mediated expression of IL-8, TNF-α and IL-1β in human macrophages through common signaling pathways. PLoS ONE 2012, 7, e33822. [Google Scholar] [CrossRef]
- Basova, L.V.; Vien, W.; Bortell, N.; Najera, J.A.; Marcondes, M.C.G. Methamphetamine signals transcription of IL1β and TNFα in a reactive oxygen species-dependent manner and interacts with HIV-1 Tat to decrease antioxidant defense mechanisms. Front. Cell. Neurosci. 2022, 16, 911060. [Google Scholar] [CrossRef]
- Sanchez-Alavez, M.; Bortell, N.; Basova, L.; Samad, F.; Marcondes, M.C.G. Macrophages and brown adipocytes cross-communicate to modulate a thermogenic program following methamphetamine exposure. Int. J. Hyperth. 2020, 37, 1368–1382. [Google Scholar] [CrossRef]
- Dutta, D.; Liu, J.; Xu, E.; Xiong, H. Methamphetamine Enhancement of HIV-1 gp120-Mediated NLRP3 Inflammasome Activation and Resultant Proinflammatory Responses in Rat Microglial Cultures. Int. J. Mol. Sci. 2024, 25, 3588. [Google Scholar] [CrossRef]
- Andrews, G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000, 59, 95–104. [Google Scholar] [CrossRef]
- Subramanian Vignesh, K.; Deepe, G.S., Jr. Metallothioneins: Emerging Modulators in Immunity and Infection. Int. J. Mol. Sci. 2017, 18, 2197. [Google Scholar] [CrossRef]
- Ghoshal, K.; Majumder, S.; Zhu, Q.; Hunzeker, J.; Datta, J.; Shah, M.; Sheridan, J.F.; Jacob, S.T. Influenza virus infection induces metallothionein gene expression in the mouse liver and lung by overlapping but distinct molecular mechanisms. Mol. Cell. Biol. 2001, 21, 8301–8317. [Google Scholar] [CrossRef]
- Read, S.A.; Parnell, G.; Booth, D.; Douglas, M.W.; George, J.; Ahlenstiel, G. The antiviral role of zinc and metallothioneins in hepatitis C infection. J. Viral Hepat. 2018, 25, 491–501. [Google Scholar] [CrossRef]
- Schukfeh, N.; Liu, B.; DeLuca, D.S.; Tumpara, S.; Nikolin, C.; Immenschuh, S.; Ure, B.M.; Kuebler, J.F.; Welte, T.; Viemann, D.; et al. Pleural CD14+ monocytes/macrophages of healthy adolescents show a high expression of metallothionein family genes. Eur. J. Immunol. 2023, 53, e2250019. [Google Scholar] [CrossRef] [PubMed]
- Mould, K.J.; Moore, C.M.; McManus, S.A.; McCubbrey, A.L.; McClendon, J.D.; Griesmer, C.L.; Henson, P.M.; Janssen, W.J. Airspace Macrophages and Monocytes Exist in Transcriptionally Distinct Subsets in Healthy Adults. Am. J. Respir. Crit. Care Med. 2021, 203, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Leibbrandt, M.; Koropatnick, J. Activation of human monocytes with lipopolysaccharide induces metallothionein expression and is diminished by zinc. Toxicol. Appl. Pharmacol. 1994, 124, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, M.; van Weyenbergh, J.; Soumillion, A.; Proost, P.; De Ley, M. Induction by zinc of specific metallothionein isoforms in human monocytes. Eur. J. Biochem. 1994, 220, 105–110. [Google Scholar] [CrossRef]
- Raymond, A.D.; Gekonge, B.; Giri, M.S.; Hancock, A.; Papasavvas, E.; Chehimi, J.; Kossenkov, A.V.; Nicols, C.; Yousef, M.; Mounzer, K.; et al. Increased metallothionein gene expression, zinc, and zinc-dependent resistance to apoptosis in circulating monocytes during HIV viremia. J. Leukoc. Biol. 2010, 88, 589–596, Correction in J. Leukoc. Biol. 2010, 88, 1061. [Google Scholar] [CrossRef]
- Trayhurn, P.; Duncan, J.S.; Wood, A.M.; Beattie, J.H. Regulation of metallothionein gene expression and secretion in rat adipocytes differentiated from preadipocytes in primary culture. Horm Metab Res. 2000, 32, 542–547. [Google Scholar] [CrossRef]
- Cisneros, I.E.; Ghorpade, A.; Borgmann, K. Methamphetamine Activates Trace Amine Associated Receptor 1 to Regulate Astrocyte Excitatory Amino Acid Transporter-2 via Differential CREB Phosphorylation During HIV-Associated Neurocognitive Disorders. Front. Neurol. 2020, 11, 593146. [Google Scholar] [CrossRef]
- Nebes, V.L.; Defranco, D.; Morris, S.M., Jr. Cyclic AMP induces metallothionein gene expression in rat hepatocytes but not in rat kidney. Biochem. J. 1988, 255, 741–743. [Google Scholar] [PubMed]
- Saydam, N.; Adams, T.K.; Steiner, F.; Schaffner, W.; Freedman, J.H. Regulation of metallothionein transcription by the metal-responsive transcription factor MTF-1: Identification of signal transduction cascades that control metal-inducible transcription. J. Biol. Chem. 2002, 277, 20438–20445. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, R.E.; Garcia-Zepeda, E.A.; Lim, Y.-C.; Yoshida, M.; Ding, H.A.; Gimbrone, M.A., Jr.; Luster, A.D.; Luscinskas, F.W.; Rosenzweig, A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999, 398, 718–723. [Google Scholar] [CrossRef]
- Chavez, B.; Kiaris, H. Insights on the role of the chemokine CCL8 in pathology. Cell. Signal. 2025, 134, 111951. [Google Scholar] [CrossRef]
- Chomarat, P.; Banchereau, J.; Davoust, J.; Palucka, A.K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 2000, 1, 510–514. [Google Scholar] [CrossRef]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Lu, H.-H.; Sheng, Z.-Q.; Wang, Y.; Zhang, L. Levels of soluble adhesion molecules in patients with various clinical presentations of coronary atherosclerosis. Chin. Med. J. (Engl.) 2010, 123, 3123–3126. [Google Scholar]
- Mikula, T.; Suchacz, M.; Sapula, M.; Wiercinska-Drapalo, A. Significance of Vascular Cell Adhesion Molecule-1 and Tumor Necrosis Factor-Alpha in HIV-Infected Patients. J. Clin. Med. 2022, 11, 514. [Google Scholar] [CrossRef]
- Sharief, M.K.; Ciardi, M.; Noori, M.A.; Thompson, E.J.; Salotti, A.; Sorice, F.; Rossi, F.; Cirelli, A. Free circulating ICAM-1 in serum and cerebrospinal fluid of HIV-1 infected patients correlate with TNF-α and blood-brain barrier damage. Mediat. Inflamm. 1992, 1, 323–328. [Google Scholar] [CrossRef]
- Olmos, G.; Llado, J. Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Fiers, W. Is amyloidogenesis during Alzheimer’s disease due to an IL-1-/IL-6-mediated ‘acute phase response’ in the brain? Immunol. Today 1991, 12, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Misra, V.; Yin, J.; Horiguchi, M.; Uno, H.; Gabuzda, D. Vascular injury markers associated with cognitive impairment in people with HIV on suppressive antiretroviral therapy. AIDS 2023, 37, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Sanchez-Rodriguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of reactive oxygen species in inflammation and cancer. MedComm 2024, 5, e519. [Google Scholar] [CrossRef]
- Thorpe, G.W.; Fong, C.S.; Alic, N.; Higgins, V.J.; Dawes, I.W. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: Oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 2004, 101, 6564–6569. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Leon-Rivera, R.; Veenstra, M.; Donoso, M.; Tell, E.; Eugenin, E.A.; Morgello, S.; Berman, J.W. Central Nervous System (CNS) Viral Seeding by Mature Monocytes and Potential Therapies To Reduce CNS Viral Reservoirs in the cART Era. mBio 2021, 12, e03633-20. [Google Scholar] [CrossRef]
- Veenstra, M.; Leon-Rivera, R.; Li, M.; Gama, L.; Clements, J.E.; Berman, J.W. Mechanisms of CNS Viral Seeding by HIV+CD14+CD16+Monocytes: Establishment and Reseeding of Viral Reservoirs Contributing to HIV-Associated Neurocognitive Disorders. mBio 2017, 8, e01280-17. [Google Scholar] [CrossRef]
| Gene | HIV + Meth vs. HIV Untreated | ||||
|---|---|---|---|---|---|
| Fold Change | |||||
| Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | |
| MT1X | 0.472221266 | 2.333334 | 1.972971 | 1.28114 | 1.437185 |
| MT1H | 0.81538437 | 3.079997 | 2.800008 | 2.060447 | 1.731623 |
| MT1G | 0.330471042 | 8.647055 | 1.759038 | 1.879654 | 1.511675 |
| MT1F | 3.415092533 | 0.961539 | 1.284214 | 1.502437 | 1.587024 |
| MT1E | 0.747127113 | 7.874976 | 1.632653 | 1.072625 | 1.273819 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiselberg, J.; Niu, M.; Hernandez, C.A.; Fox, H.S.; Calderon, T.M.; Berman, J.W. Methamphetamine Induces Metallothionein 1 Expression and an Inflammatory Phenotype in Primary Human HIV-Infected Macrophages. Int. J. Mol. Sci. 2025, 26, 8875. https://doi.org/10.3390/ijms26188875
Weiselberg J, Niu M, Hernandez CA, Fox HS, Calderon TM, Berman JW. Methamphetamine Induces Metallothionein 1 Expression and an Inflammatory Phenotype in Primary Human HIV-Infected Macrophages. International Journal of Molecular Sciences. 2025; 26(18):8875. https://doi.org/10.3390/ijms26188875
Chicago/Turabian StyleWeiselberg, Jessica, Meng Niu, Cristian A. Hernandez, Howard S. Fox, Tina M. Calderon, and Joan W. Berman. 2025. "Methamphetamine Induces Metallothionein 1 Expression and an Inflammatory Phenotype in Primary Human HIV-Infected Macrophages" International Journal of Molecular Sciences 26, no. 18: 8875. https://doi.org/10.3390/ijms26188875
APA StyleWeiselberg, J., Niu, M., Hernandez, C. A., Fox, H. S., Calderon, T. M., & Berman, J. W. (2025). Methamphetamine Induces Metallothionein 1 Expression and an Inflammatory Phenotype in Primary Human HIV-Infected Macrophages. International Journal of Molecular Sciences, 26(18), 8875. https://doi.org/10.3390/ijms26188875







