Immunopeptidome Landscape During Brucella melitensis Infection in Mice
Abstract
1. Introduction
2. Results and Discussion
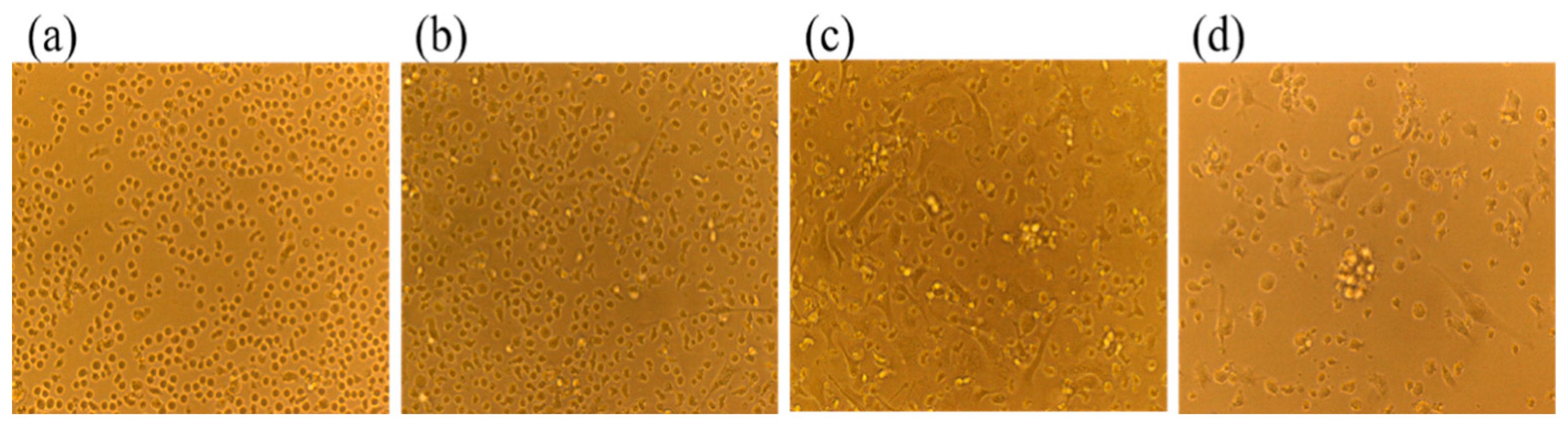
2.1. Morphological Observation of BMDCs in Mice
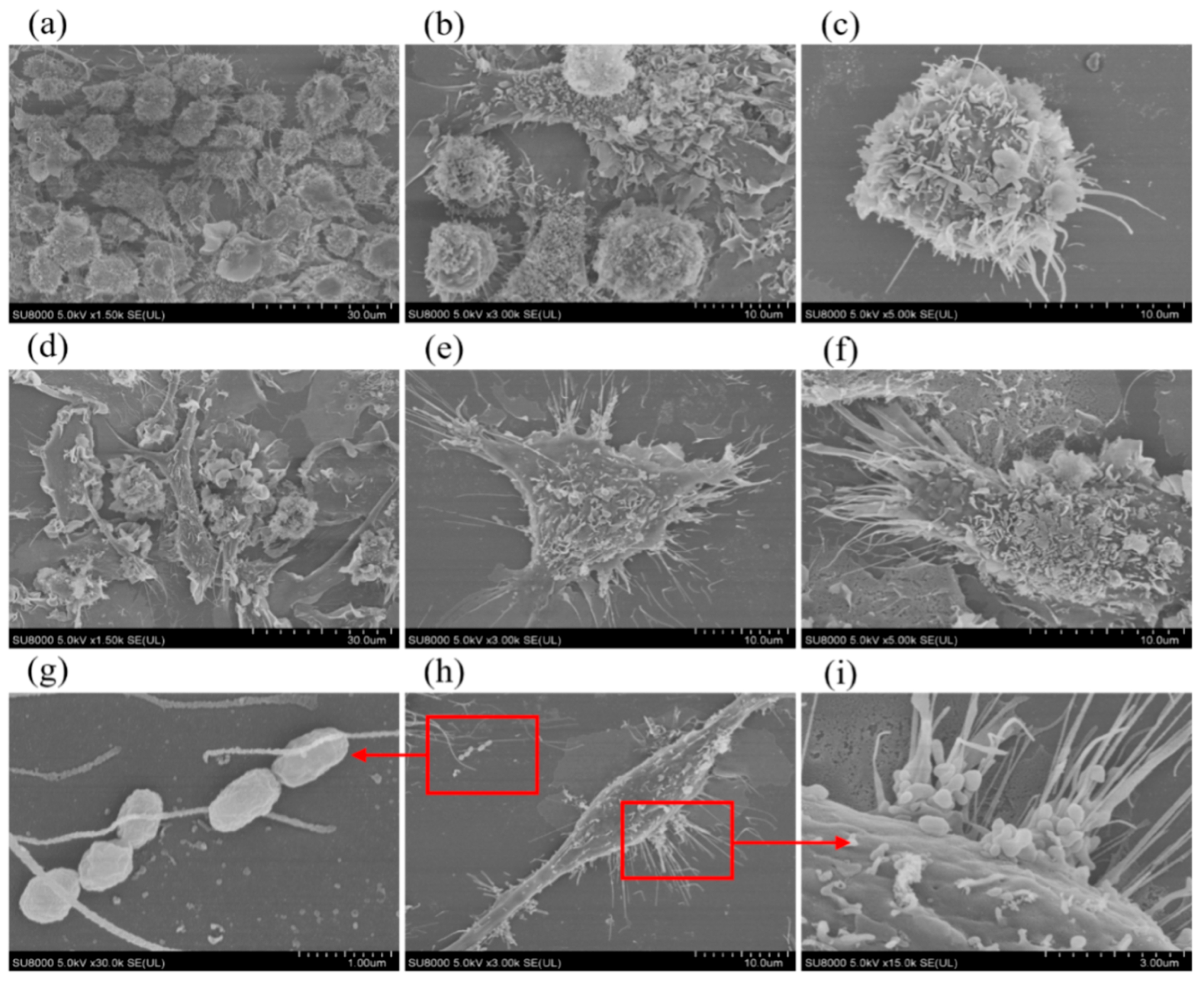
2.2. Scanning Electron Microscope Observation of Cell Surface Morphology Before and After Brucella melitensis M5+gfp Infestation
2.3. Laser Confocal Observation of Intracellular Fluorescence After Infestation
2.4. Identification of Peptide Sequences of the Brucella melitensis M5+gfp Immunopeptidome
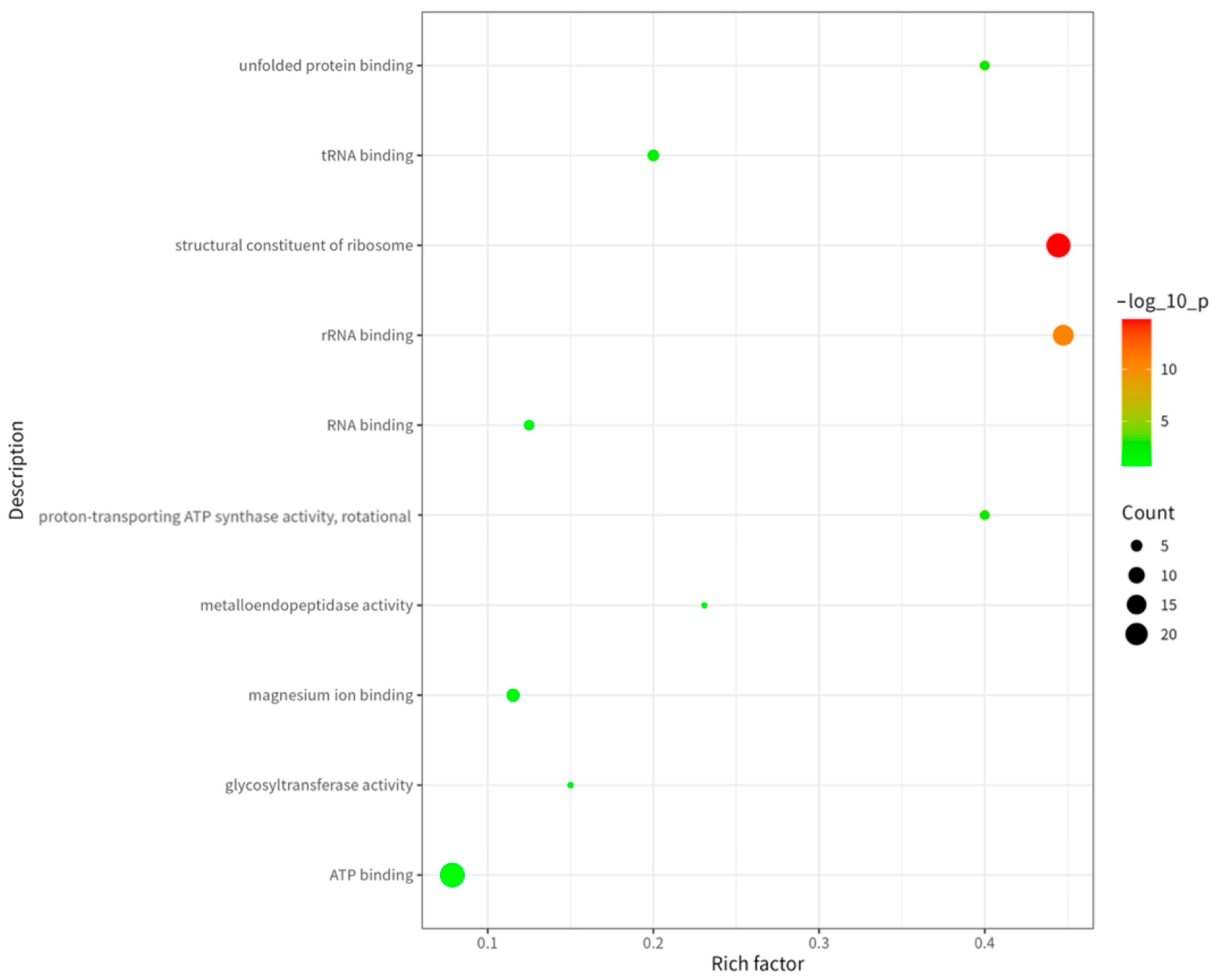
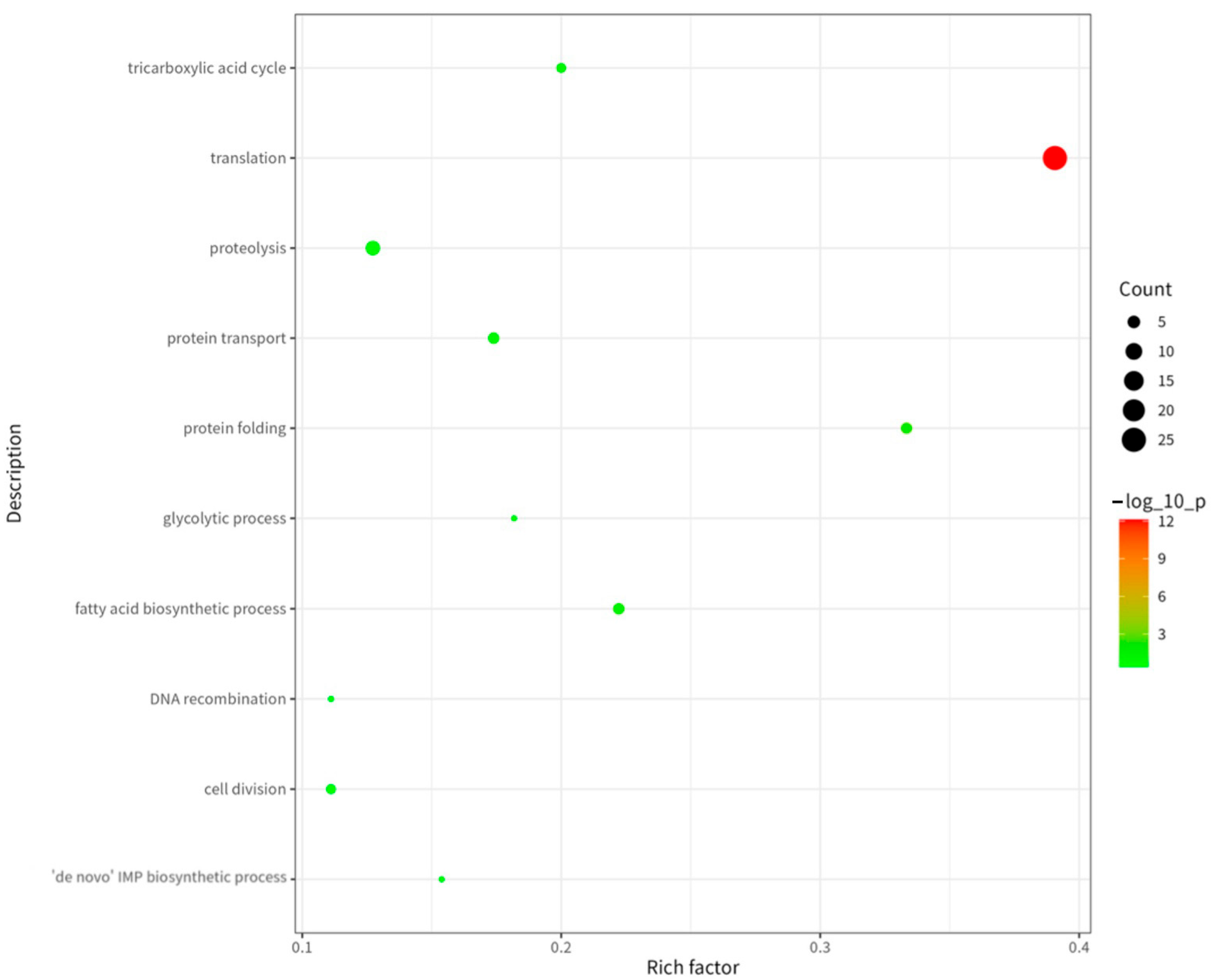
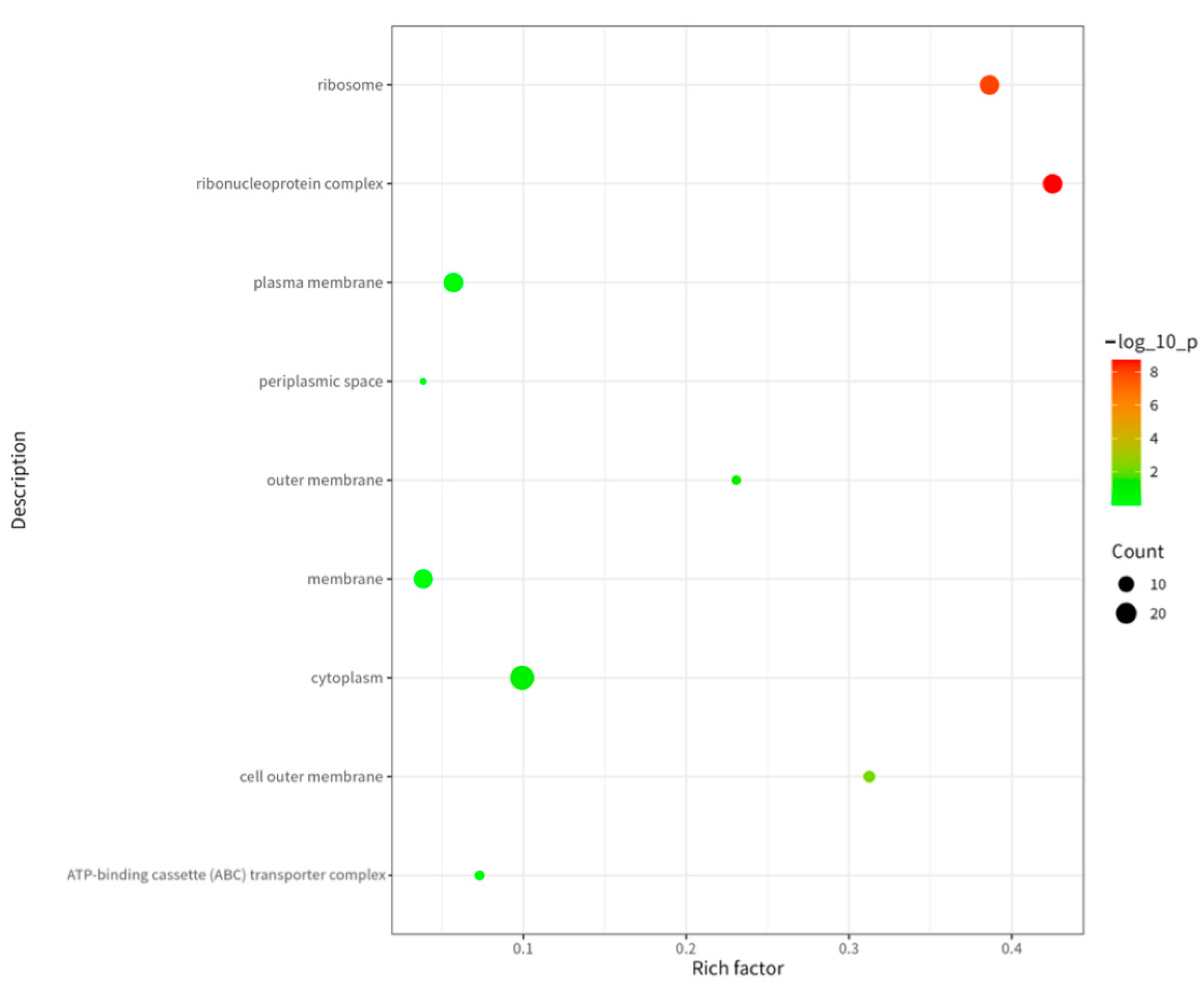
2.5. GO and KEGG Analysis of Proteins Corresponding to Peptide Sequences of Brucella melitensis M5+gfp Immunopeptide Group
Functional, Structural, and Metabolic Classification of MHC-II-Bound Brucella melitensis Antigens
- 1.
- Functional classification revealed four major categories among the 183 source proteins.
- 2.
- Metabolic pathway classification identified three core functional groups.
- 3.
- Structural classification revealed three principal categories.
3. Materials and Methods
3.1. Experimental Materials and Instruments
3.2. Main Reagent Preparation
3.2.1. Preparation of Complete RPMI 1640 Culture Medium
3.2.2. Preparation of 10% RPMI 1640 Culture Medium
3.3. Experimental Replication Design
3.4. Induction Culture of Mouse BMDC
3.5. Morphological Observation of BMDC in Mice
3.6. Brucella melitensis M5+gfp Infested Mouse BMDCs
3.7. Scanning Electron Microscope Observation of Cell Surface Morphology Before and After Infestation
3.8. Laser Confocal Observation of Intracellular Fluorescence After Infestation
3.9. Acquisition of MHC II-Brucella melitensis M5+gfp Immunopeptidome Complexes
3.10. Isolation, Purification and Characterization of the Brucella melitensis M5+gfp Immunopeptidome
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMDCs | Bone marrow-derived dendritic cell |
| MHC II | Major Histocompatibility Complex Class II |
| Co-IP | Co-immunoprecipitation |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| APC | Antigen-Presenting Cell |
| HLA | Human Leukocyte Antigen |
| MOI | Multiplicity of Infection |
| LSCM | Laser Scanning Confocal Microscopy |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| IL-4 | Interleukin-4 |
| TEM | Transmission Electron Microscope |
| SEM | Scanning Electron Microscope |
References
- Chavda, V.P.; Redwan, E.M. SARS-CoV-2: Immunopeptidomics and Other Immunological Studies. Vaccines 2022, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Smith, M.R.; Zhao, H. Construction and Screening of an Antigen-Derived Peptide Library Displayed on Yeast Cell Surface for CD4+ T Cell Epitope Identification. Methods Mol. Biol. 2019, 2024, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Mommen, G.P.; Marino, F.; Meiring, H.D.; Poelen, M.C.; van Gaans-van den Brink, J.A.; Mohammed, S.; Heck, A.J.; van Els, C.A. Sampling From the Proteome to the Human Leukocyte Antigen-DR (HLA-DR) Ligandome Proceeds Via High Specificity. Mol. Cell Proteomics 2016, 15, 1412–1423. [Google Scholar] [CrossRef]
- Millot, P. The major histocompatibility complex of sheep (OLA) and two minor loci. Anim. Blood Groups Biochem. Genet. 1978, 9, 115–121. [Google Scholar] [CrossRef]
- Socorro Ruiz-Palma, M.D.; Avila-Calderon, E.D.; Aguilera-Arreola, M.G.; Lopez-Merino, A.; Ruiz, E.A.; Morales-Garcia, M.D.R.; Lopez-Villegas, E.O.; Gomez-Lunar, Z.; Arellano-Reynoso, B.; Contreras-Rodriguez, A. Comparative proteomic analysis of outer membrane vesicles from Brucella suis, Brucella ovis, Brucella canis and Brucella neotomae. Arch. Microbiol. 2021, 203, 1611–1626. [Google Scholar] [CrossRef]
- Shakir, R. Brucellosis. J. Neurol. Sci. 2021, 420, 117280. [Google Scholar] [CrossRef]
- Yeh, H.I.; Yu, Y.C.; Kuo, P.L.; Tsai, C.K.; Huang, H.T.; Hwang, T.C. Functional stability of CFTR depends on tight binding of ATP at its degenerate ATP-binding site. J. Physiol. 2021, 599, 4625–4642. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, G.M.; Korepanov, A.P.; Korobeinikova, A.V.; Garber, M.B. Bacterial 5S rRNA-binding proteins of the CTC family. Biochemistry 2008, 73, 1405–1417. [Google Scholar] [CrossRef]
- Hektor, H.J.; Kloosterman, H.; Dijkhuizen, L. Identification of a magnesium-dependent NAD(P)(H)-binding domain in the nicotinoprotein methanol dehydrogenase from Bacillus methanolicus. J. Biol. Chem. 2002, 277, 46966–46973. [Google Scholar] [CrossRef]
- Sorokina, I.; Mushegian, A. The role of the backbone torsion in protein folding. Biol. Direct 2016, 11, 64. [Google Scholar] [CrossRef]
- Jentoft, I.M.A.; Bauerlein, F.J.B.; Welp, L.M.; Cooper, B.H.; Petrovic, A.; So, C.; Penir, S.M.; Politi, A.Z.; Horokhovskyi, Y.; Takala, I.; et al. Mammalian oocytes store proteins for the early embryo on cytoplasmic lattices. Cell 2023, 186, 5308–5327. [Google Scholar] [CrossRef]
- Luciano-Mateo, F.; Cabre, N.; Fernandez-Arroyo, S.; Baiges-Gaya, G.; Hernandez-Aguilera, A.; Rodriguez-Tomas, E.; Munoz-Pinedo, C.; Menendez, J.A.; Camps, J.; Joven, J. Chemokine C-C motif ligand 2 overexpression drives tissue-specific metabolic responses in the liver and muscle of mice. Sci. Rep. 2020, 10, 11954. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Singh, A.K.; Wang, J.; Applegate, T. Functional role of branched chain amino acids in poultry: A review. Poult. Sci. 2022, 101, 101715. [Google Scholar] [CrossRef] [PubMed]
- Berod, L.; Sparwasser, T. Influencing immune cell differentiation by microbe-associated immunomodulatory metabolites (meta-MAMPs). J. Reprod. Immunol. 2016, 115, 48. [Google Scholar] [CrossRef]
- Frietze, K.K.; Pappy, A.L., 2nd; Melson, J.W.; O’Driscoll, E.E.; Tyler, C.M.; Perlman, D.H.; Boulanger, L.M. Cryptic protein-protein interaction motifs in the cytoplasmic domain of MHCI proteins. BMC Immunol. 2016, 17, 24. [Google Scholar] [CrossRef]
- Ratner, D.; Orning, M.P.; Lien, E. Bacterial secretion systems and regulation of inflammasome activation. J. Leukoc. Biol. 2017, 101, 165–181. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Y.; Sun, Y.; Liu, S.; Lin, Z.; Xue, Q. Copper only SOD repeat proteins likely act as an extracellular superoxide dismutase in oyster antioxidant defense. Sci. Rep. 2025, 15, 20465. [Google Scholar] [CrossRef]
- Singh, E.; Gupta, A.; Singh, P.; Jain, M.; Muthukumaran, J.; Singh, R.P.; Singh, A.K. Exploring mammalian heme peroxidases: A comprehensive review on the structure and function of myeloperoxidase, lactoperoxidase, eosinophil peroxidase, thyroid peroxidase and peroxidasin. Arch. Biochem. Biophys. 2024, 761, 110155. [Google Scholar] [CrossRef]
- Kaim, G.; Prummer, M.; Sick, B.; Zumofen, G.; Renn, A.; Wild, U.P.; Dimroth, P. Coupled rotation within single F0F1 enzyme complexes during ATP synthesis or hydrolysis. FEBS Lett. 2002, 525, 156–163. [Google Scholar] [CrossRef]
- Lee, V.M.; Szepesi, B.; Hansen, R.J. Gender-linked differences in dietary induction of hepatic glucose-6 phosphate dehydrogenase, 6-phosphogluconate dehydrogenase and malic enzyme in the rat. J. Nutr. 1986, 116, 1547–1554. [Google Scholar] [CrossRef]
- Rubio Gomez, M.A.; Ibba, M. Aminoacyl-tRNA synthetases. RNA 2020, 26, 910–936. [Google Scholar] [CrossRef]
- Welty, R.; Rau, M.; Pabit, S.; Dunstan, M.S.; Conn, G.L.; Pollack, L.; Hall, K.B. Ribosomal Protein L11 Selectively Stabilizes a Tertiary Structure of the GTPase Center rRNA Domain. J. Mol. Biol. 2020, 432, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.; Ron, E.Z. Regulation and organization of the groE and dnaK operons in Eubacteria. FEMS Microbiol. Lett. 1996, 138, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, H.; Du, L.; Chou, S.H.; Liu, H.; Liu, Y.; Liu, F.; Qian, G. A TonB-dependent receptor regulates antifungal HSAF biosynthesis in Lysobacter. Sci. Rep. 2016, 6, 26881. [Google Scholar] [CrossRef]
- Bilsing, F.L.; Anlauf, M.T.; Hachani, E.; Khosa, S.; Schmitt, L. ABC Transporters in Bacterial Nanomachineries. Int. J. Mol. Sci. 2023, 24, 6227. [Google Scholar] [CrossRef]
- Smith, T.G.; Pereira, L.; Hoover, T.R. Helicobacter pylori FlhB processing-deficient variants affect flagellar assembly but not flagellar gene expression. Microbiology 2009, 155, 1170–1180. [Google Scholar] [CrossRef]
- Grant, M.M. Pyruvate Kinase, Inflammation and Periodontal Disease. Pathogens 2021, 10, 784. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-CoA Carboxylases and Diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef]
- Boros, K.; Gal, L.; Gal, C.A.; Wäscher, M.; Tomoiagă, R.B.; Toşa, M.I.; Pietruszka, J.; Bencze, L.C. Immobilization of d-amino acid dehydrogenase from Ureibacillus thermosphaericus. Process Biochem. 2024, 140, 45–55. [Google Scholar] [CrossRef]
- Ohnishi, T.; Ohnishi, S.T.; Salerno, J.C. Five decades of research on mitochondrial NADH-quinone oxidoreductase (complex I). Biol. Chem. 2018, 399, 1249–1264. [Google Scholar] [CrossRef]
- Nunn, A.V.; Norris, P.; Hawk, J.L.; Cox, T.M. Zinc chelatase in human lymphocytes: Detection of the enzymatic defect in erythropoietic protoporphyria. Anal. Biochem. 1988, 174, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Cloeckaert, A.; Verger, J.M.; Grayon, M.; Vizcaino, N. Molecular and immunological characterization of the major outer membrane proteins of Brucella. FEMS Microbiol. Lett. 1996, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sinnige, T.; Weingarth, M.; Daniels, M.; Boelens, R.; Bonvin, A.M.; Houben, K.; Baldus, M. Conformational Plasticity of the POTRA 5 Domain in the Outer Membrane Protein Assembly Factor BamA. Structure 2015, 23, 1317–1324. [Google Scholar] [CrossRef][Green Version]
- Peng, H.; Zhao, Y.; Chen, J.; Huo, J.; Zhang, Y.; Xiao, T. Knockdown of ribosomal protein S3 causes preimplantation developmental arrest in mice. Theriogenology 2019, 129, 77–81. [Google Scholar] [CrossRef]
- Stacpoole, P.W. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J. Natl. Cancer Inst. 2017, 109, djx071. [Google Scholar] [CrossRef]
- Allen-Worthington, K.; Xie, J.; Brown, J.L.; Edmunson, A.M.; Dowling, A.; Navratil, A.M.; Scavelli, K.; Yoon, H.; Kim, D.G.; Bynoe, M.S.; et al. The F0F1 ATP Synthase Complex Localizes to Membrane Rafts in Gonadotrope Cells. Mol. Endocrinol. 2016, 30, 996–1011. [Google Scholar] [CrossRef][Green Version]
- Papich, M.G. An American Veterinary Medical Association perspective. J. Am. Vet. Med. Assoc. 1995, 207, 871–874. [Google Scholar] [CrossRef]
- Hu, M.W.; Liu, P.T.; Yan, G.; Gao, J. The fluorescent characterization and analysis of two Brucella attenuated vaccine infecting mouse macrophagocyte. China Anim. Husb. Vet. Med. 2016, 43, 1944–1950. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Sheng, Y.; Li, T.; Wang, K.; Geng, F.; Li, Y.; Gao, J. Immunopeptidome Landscape During Brucella melitensis Infection in Mice. Int. J. Mol. Sci. 2025, 26, 8874. https://doi.org/10.3390/ijms26188874
Jin J, Sheng Y, Li T, Wang K, Geng F, Li Y, Gao J. Immunopeptidome Landscape During Brucella melitensis Infection in Mice. International Journal of Molecular Sciences. 2025; 26(18):8874. https://doi.org/10.3390/ijms26188874
Chicago/Turabian StyleJin, Jing, Yaming Sheng, Tingting Li, Kang Wang, Fanghao Geng, Yi Li, and Jianfeng Gao. 2025. "Immunopeptidome Landscape During Brucella melitensis Infection in Mice" International Journal of Molecular Sciences 26, no. 18: 8874. https://doi.org/10.3390/ijms26188874
APA StyleJin, J., Sheng, Y., Li, T., Wang, K., Geng, F., Li, Y., & Gao, J. (2025). Immunopeptidome Landscape During Brucella melitensis Infection in Mice. International Journal of Molecular Sciences, 26(18), 8874. https://doi.org/10.3390/ijms26188874




