An Integrative Bioinformatics Approach to Investigating TIMP3 and Immune Cell Infiltration: Prognostic and Clinicopathological Implications
Abstract
1. Introduction
2. Results
2.1. TIMP3 is Highly Expressed in Normal Cells Compared to CRC
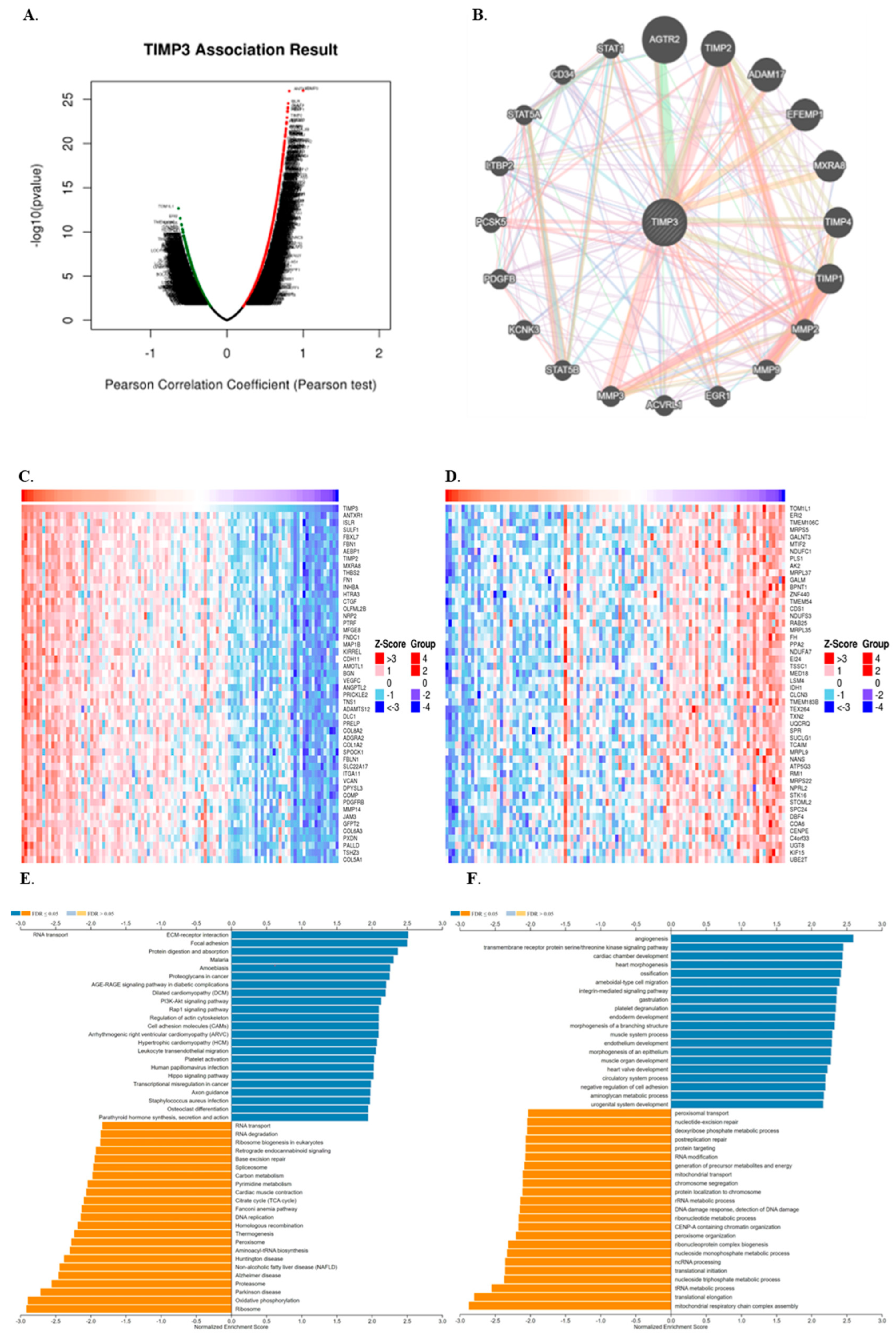
2.2. The Immune Response in CRC Tissues Is Enhanced by TIMP3 and Its Co-Expressed Genes
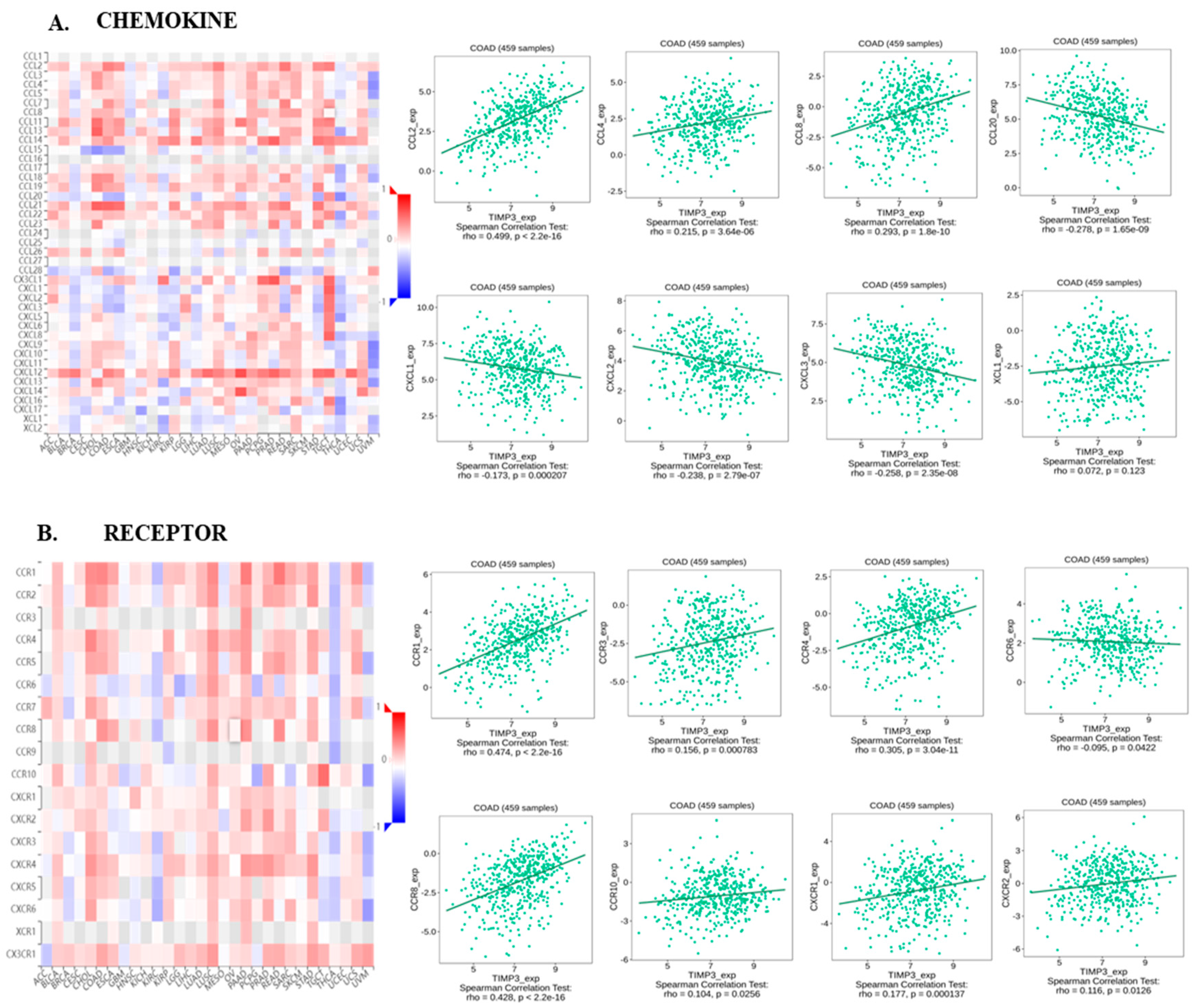
2.3. TIMP3 Displays an Intricate Association with Immune-Related Regulatory Molecules
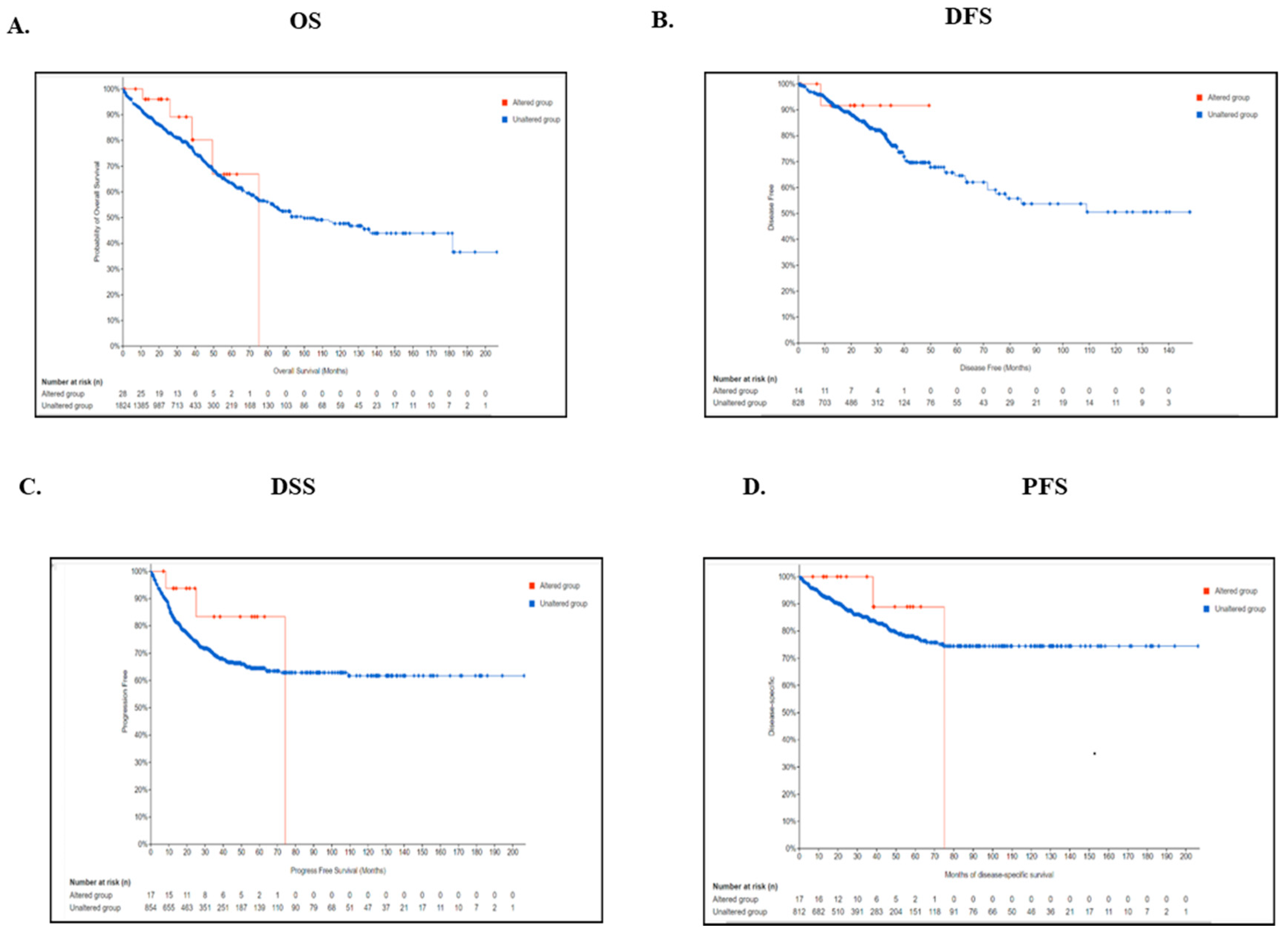
2.4. Genetic Alteration of TIMP3 Is Significantly Associated with Survival of CRC Patients
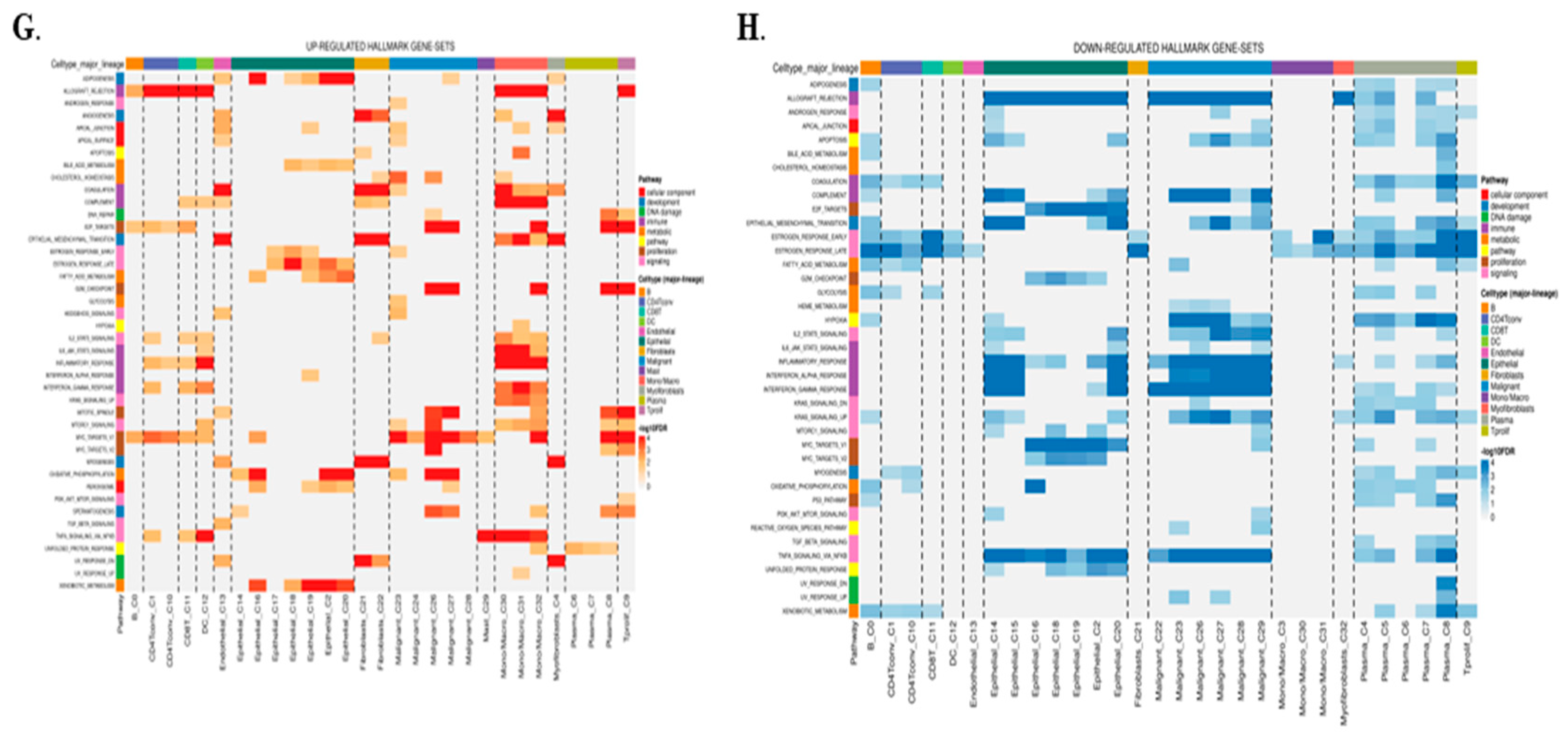
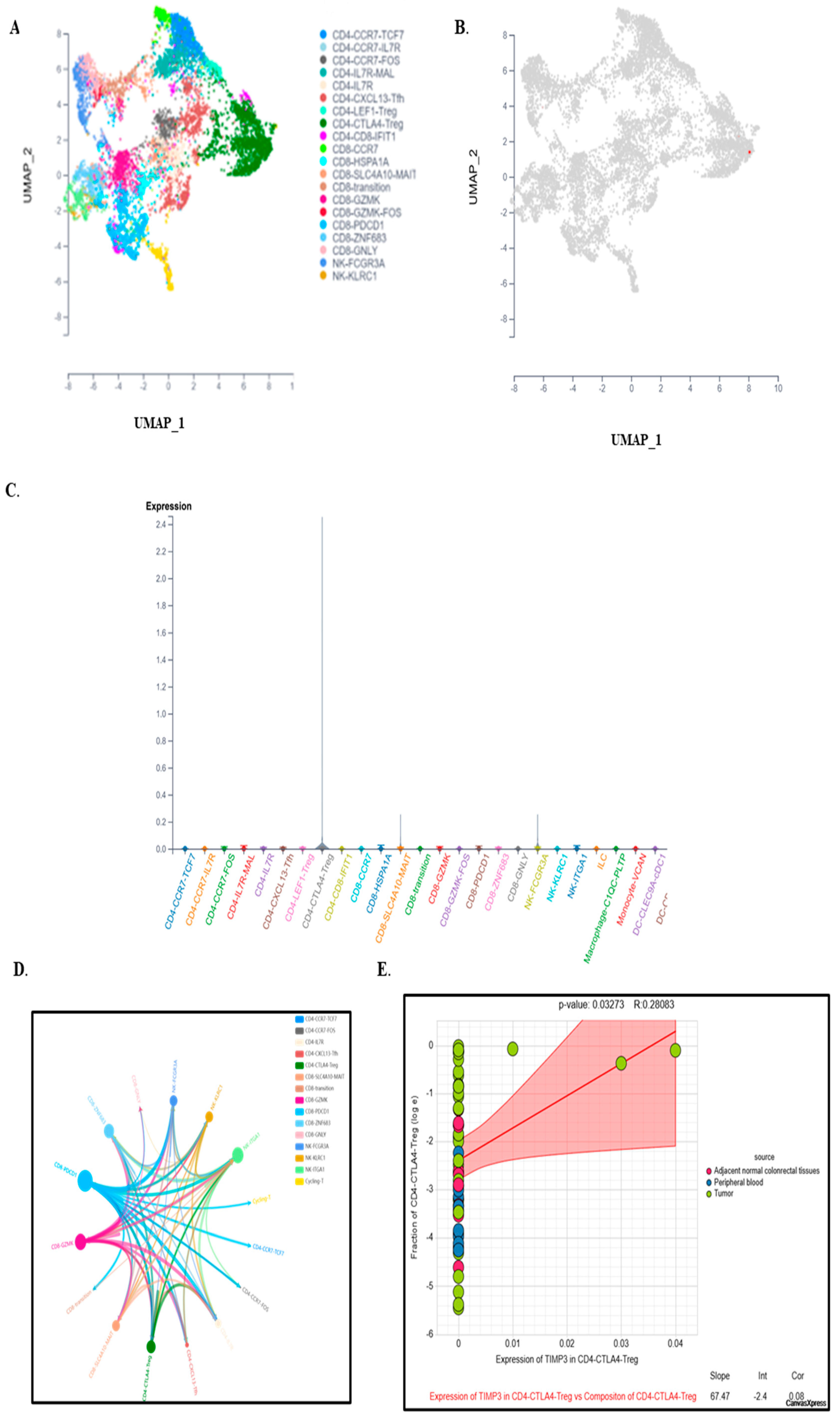
2.5. Single-Cell Expression Analysis Highlights TIMP3’s Role in TME Remodeling and Immune Regulation
2.6. TIMP3 Modulates Immune Outcomes in the Tumor Microenvironment at a Single-Cell Level
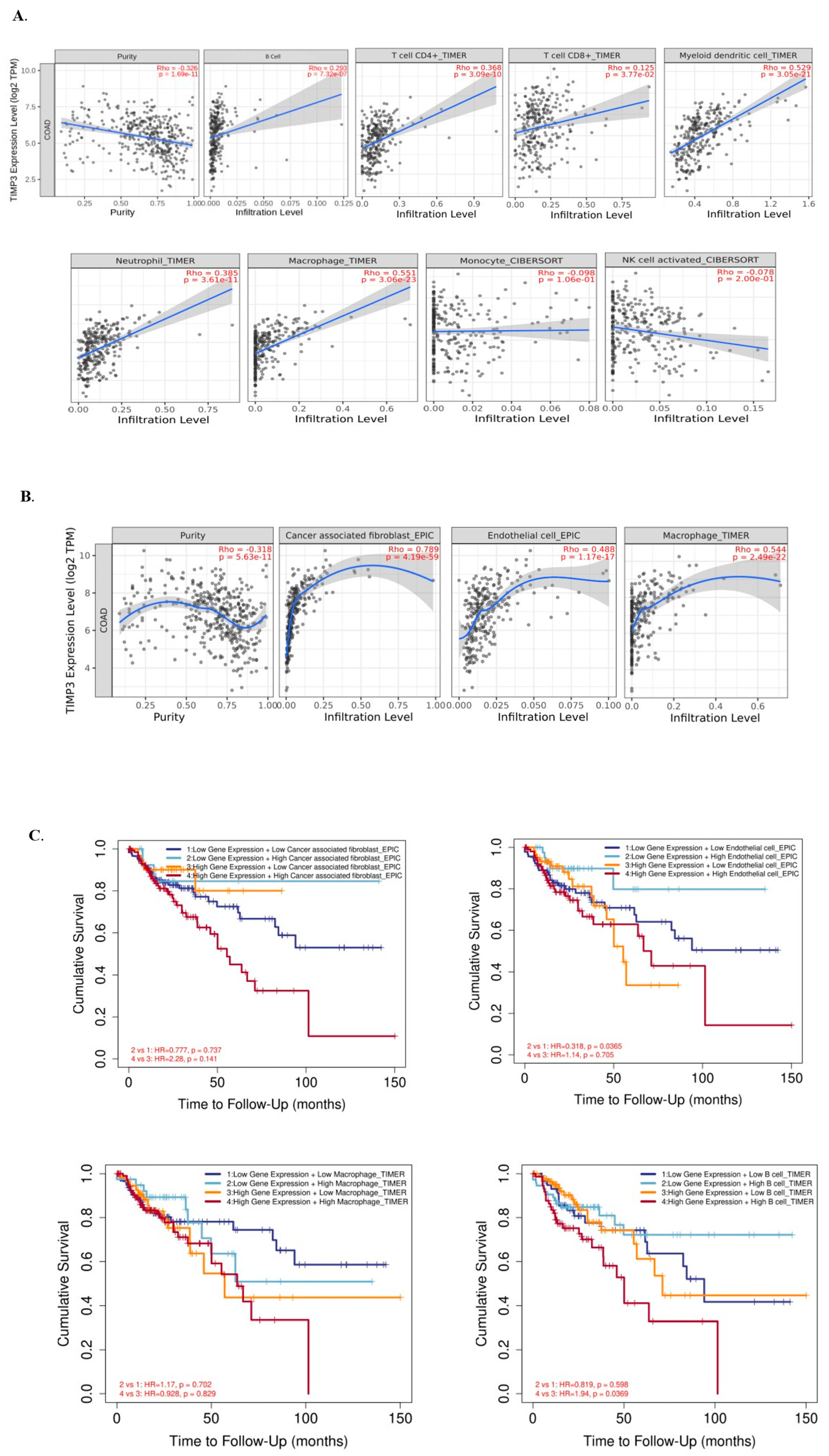
2.7. Immune Cell Infiltration and TIMP3 in Patients with CRC
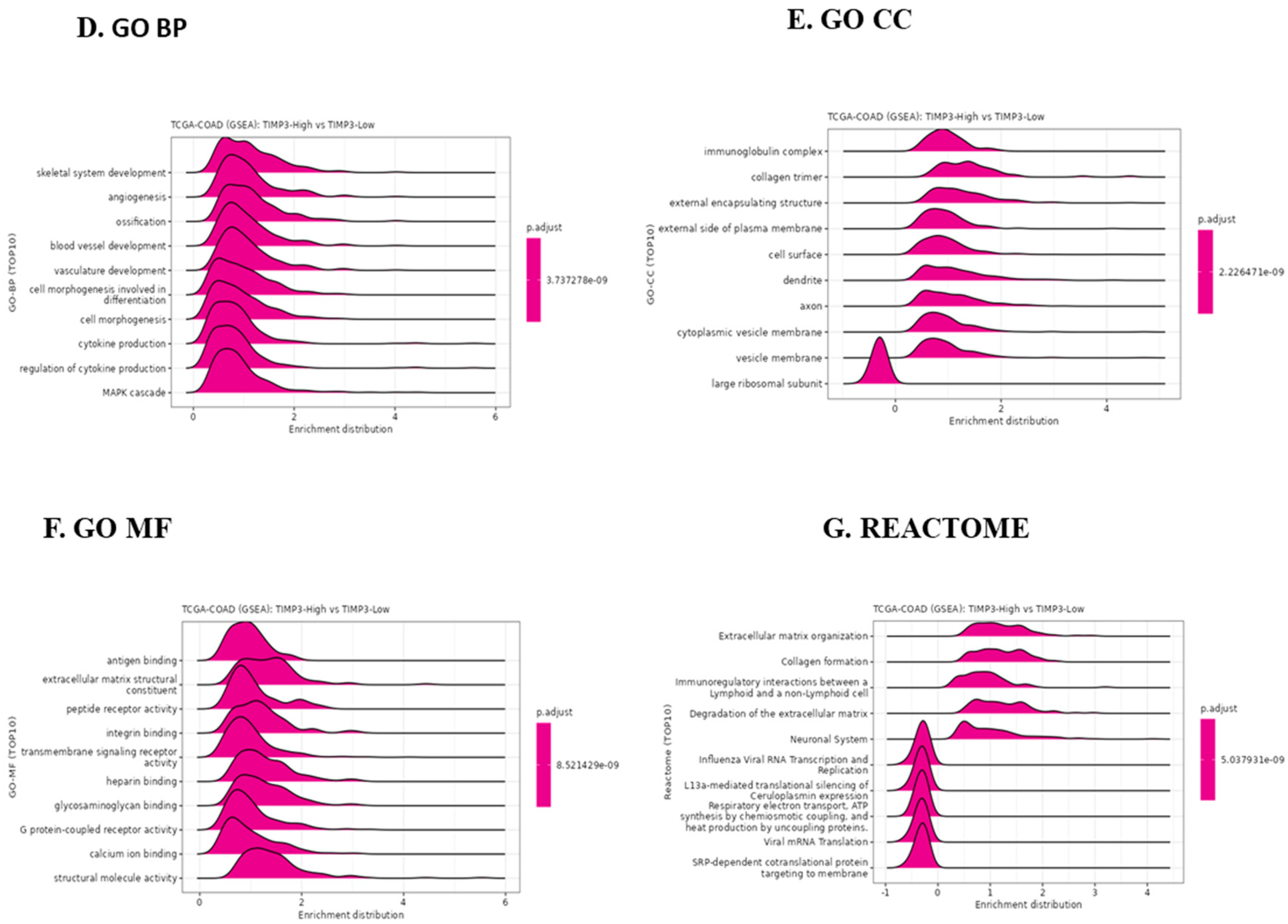
2.8. TIMP3 Affects the Immune Microenvironment of CRC Tissues in TIMP3High and TIMP3 Low Groups, and the Potential Role of TIMP3 in CRC
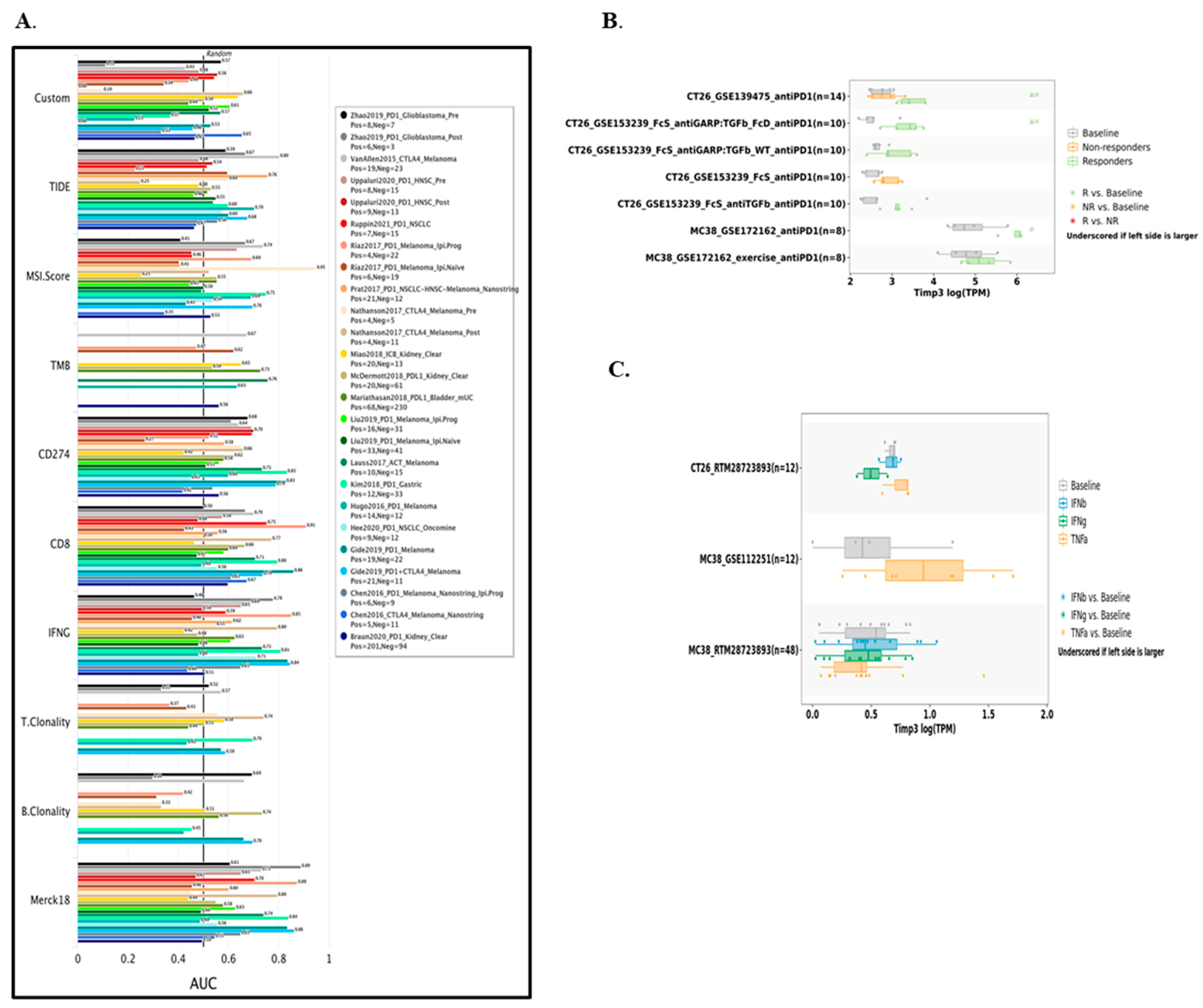
2.9. Predictive Value of TIMP3 in Modulating Immunotherapy
3. Discussion
Limitations
4. Materials and Methods
4.1. Ethics Statement
4.2. Acquisition of Patient Data
4.3. GEPIA
4.4. UALCAN
4.5. LinkedOmics
4.6. GeneMANIA
4.7. TIMER
4.8. TISIDB
4.9. cBioPortal
4.10. TISCH2
4.11. SCTIME
4.12. CAMOIP
4.13. TISMO and TIDE
4.14. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COAD | Colon adenocarcinoma |
| CRC | Colorectal cancer |
| DFS | Disease-free survival |
| ECM | Extracellular matrix |
| FDR | False detection rate |
| GTEx | Genotype–Tissue Expression |
| HR | Hazard ratio |
| KICH | Kidney chromophobe |
| LUAD | Lung adenocarcinoma |
| LUSC | Lung squamous cell carcinoma |
| MMP | Matrix metalloproteinase |
| OS | Overall survival |
| PFS | Progression-free survival |
| READ | Rectal adenocarcinoma |
| RFS | Relapse-free survival |
| TAM | Tumor-associated macrophages |
| TCGA | The Cancer Genome Atlas |
| TIICs | Tumor-infiltrating immune cells |
| TIMP3 | Tissue inhibitor of matrix metalloproteinase |
| TME | Tumor microenvironment |
| TPM | Transcripts per million |
| UCS | Uterine carcinoma |
| VEGF | Vascular endothelial growth factor |
References
- Leslie, A.; Steele, R.J.C. Management of colorectal cancer. Postgrad. Med. J. 2002, 78, 473–478. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Renkonen-Sinisalo, L.; Aarnio, M.; Mecklin, J.P.; Järvinen, H.J. Surveillance improves survival of colorectal cancer in patients with hereditary nonpolyposis colorectal cancer. Cancer Detect. Prev. 2000, 24, 137–142. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Jayasinghe, M.; Prathiraja, O.; Caldera, D.; Jena, R.; Coffie-Pierre, J.A.; Silva, M.S.; Siddiqui, O.S. Colon Cancer Screening Methods: 2023 Update. Cureus 2023, 15, e37509. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, T.I. Screening and surveillance for hereditary colorectal cancer. Intest. Res. 2024, 22, 119–130. [Google Scholar] [CrossRef]
- Vogel, J.D.; Felder, S.I.; Bhama, A.R.; Hawkins, A.T.; Langenfeld, S.J.; Shaffer, V.O.; Thorsen, A.J.; Weiser, M.R.; Chang, G.J.; Lightner, A.L.; et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis. Colon Rectum 2022, 65, 148–177. [Google Scholar] [CrossRef]
- Fornieles, G.; Núñez, M.I.; Expósito, J. Matrix Metalloproteinases and Their Inhibitors as Potential Prognostic Biomarkers in Head and Neck Cancer after Radiotherapy. Int. J. Mol. Sci. 2023, 25, 527. [Google Scholar] [CrossRef]
- Jiang, Y.; Goldberg, I.D.; Shi, Y.E. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 2002, 21, 2245–2252. [Google Scholar] [CrossRef]
- Su, C.-W.; Lin, C.-W.; Yang, W.-E.; Yang, S.-F. TIMP-3 as a therapeutic target for cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919864247. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Y.; Wang, H.; Xu, D.; Meng, X.; Shao, Y.; Lin, C.; Ye, Y.; Qian, H.; Wang, S. Tissue inhibitor of metalloproteinases-3 transfer suppresses malignant behaviors of colorectal cancer cells. Cancer Gene Ther. 2012, 19, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Munoz, W.; Sanchez, O.H.; Di Grappa, M.; English, J.L.; Hill, R.P.; Khokha, R. Enhanced metastatic dissemination to multiple organs by melanoma and lymphoma cells in timp-3−/− mice. Oncogene 2006, 25, 6489–6496. [Google Scholar] [CrossRef] [PubMed]
- English, W.R.; Ireland-Zecchini, H.; Baker, A.H.; Littlewood, T.D.; Bennett, M.R.; Murphy, G.; Pintus, G. Tissue Inhibitor of Metalloproteinase–3 (TIMP-3) induces FAS dependent apoptosis in human vascular smooth muscle cells. PLoS ONE 2018, 13, e0195116. [Google Scholar] [CrossRef]
- Qi, J.H.; Ebrahem, Q.; Ali, M.; Cutler, A.; Bell, B.; Prayson, N.; Sears, J.; Knauper, V.; Murphy, G.; Anand-Apte, B.; et al. Tissue Inhibitor of Metalloproteinases-3 Peptides Inhibit Angiogenesis and Choroidal Neovascularization in Mice. PLoS ONE 2013, 8, e55667. [Google Scholar] [CrossRef]
- Anania, M.C.; Sensi, M.; Radaelli, E.; Miranda, C.; Vizioli, M.G.; Pagliardini, S.; Favini, E.; Cleris, L.; Supino, R.; Formelli, F.; et al. TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene 2011, 30, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Zhang, J.; Song, S.; Dai, D. Promoter methylation and expression of TIMP3 gene in gastric cancer. Diagn. Pathol. 2013, 8, 110. [Google Scholar] [CrossRef]
- Liu, H.-Q.; Song, S.; Wang, J.-H.; Zhang, S.-L. Expression of MMP-3 and TIMP-3 in gastric cancer tissue and its clinical significance. Oncol. Lett. 2011, 2, 1319–1322. [Google Scholar] [CrossRef]
- Dindarloo, M.M.; Fendereski, A.; Kashi, Z.; -Charati, J.Y. Long-Term Effect of TIMP3 Gene Expression on Thyroid Cancer: A Cure Model Analysis. Asian Pac. J. Cancer Prev. 2024, 25, 3627–3634. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, P.; Wang, X.; Lin, Y.; Xu, H.; Mao, R.; Zhu, S.; Lin, T.; Cai, J.; Lin, J.; et al. SORBS2-Mediated inhibition of malignant behaviors in esophageal squamous cell carcinoma through TIMP3. Int. Immunopharmacol. 2024, 142, 113096. [Google Scholar] [CrossRef]
- Han, J.; Jing, Y.; Han, F.; Sun, P. Comprehensive analysis of expression, prognosis and immune infiltration for TIMPs in glioblastoma. BMC Neurol. 2021, 21, 447. [Google Scholar] [CrossRef]
- Mazzoni, M.; Todoerti, K.; Agnelli, L.; Minna, E.; Pagliardini, S.; Di Marco, T.; Borrello, M.G.; Neri, A.; Greco, A. Transcriptomic landscape of TIMP3 oncosuppressor activity in thyroid carcinoma. Cancer Cell Int. 2022, 22, 400. [Google Scholar] [CrossRef]
- Su, C.-W.; Su, B.-F.; Chiang, W.-L.; Yang, S.-F.; Chen, M.-K.; Lin, C.-W. Plasma levels of the tissue inhibitor matrix metalloproteinase-3 as a potential biomarker in oral cancer progression. Int. J. Med. Sci. 2017, 14, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-G.; Mo, H.-M.; Liu, X.-Q.; Li, Y.; Du, L.; Qiao, H.; Fan, Q.-M.; Zhao, J.; Zhang, S.-H.; Tang, T.-T. TIMP3 Overexpression Improves the Sensitivity of Osteosarcoma to Cisplatin by Reducing IL-6 Production. Front. Genet. 2018, 9, 135. [Google Scholar] [CrossRef]
- Huang, H.-L.; Liu, Y.-M.; Sung, T.-Y.; Huang, T.-C.; Cheng, Y.-W.; Liou, J.-P.; Pan, S.-L. TIMP3 expression associates with prognosis in colorectal cancer and its novel arylsulfonamide inducer, MPT0B390, inhibits tumor growth, metastasis and angiogenesis. Theranostics 2019, 9, 6676–6689, Erratum in Theranostics 2021, 11, 2079. [Google Scholar] [CrossRef]
- Lao, V.; Grady, W. The Role of Timp3 in the Pathogenesis of Colorectal Cancer and Timp3 Promoter Methylation as a Potential Predictive Marker for Egfr Inhibitor Therapy. J. Surg. Res. 2012, 172, 306. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Tan, Y.; Beretta, O.; Jiang, D.; Yeap, W.; Tai, J.J.Y.; Wong, W.; Yang, H.; Schwarz, H.; Lim, K.; et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur. J. Immunol. 2012, 42, 89–100. [Google Scholar] [CrossRef]
- Haggar, F.A.; Boushey, R.P. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Verschoor, C.P.; Ma, G.W.; Pawelec, G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front. Immunol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Gu, X.; Fu, M.; Ding, Y.; Ni, H.; Zhang, W.; Zhu, Y.; Tang, X.; Xiong, L.; Li, J.; Qiu, L.; et al. TIMP-3 expression associates with malignant behaviors and predicts favorable survival in HCC. PLoS ONE 2014, 9, e106161. [Google Scholar] [CrossRef]
- Mylona, E.; Magkou, C.; Giannopoulou, I.; Agrogiannis, G.; Markaki, S.; Keramopoulos, A.; Nakopoulou, L. Expression of tissue inhibitor of matrix metalloproteinases (TIMP)-3 protein in invasive breast carcinoma: Relation to tumor phenotype and clinical outcome. Breast Cancer Res. 2006, 8, R57. [Google Scholar] [CrossRef]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2017, 17, 38–53. [Google Scholar] [CrossRef]
- Kao, K.-C.; Vilbois, S.; Tsai, C.-H.; Ho, P.-C. Metabolic communication in the tumour–immune microenvironment. Nat. Cell Biol. 2022, 24, 1574–1583. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xue, R.; Zhu, Z.; Farrukh, H.; Song, W.; Li, T.; Zheng, L.; Pan, C.-X. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp. Hematol. Oncol. 2023, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Genova, C.; Dellepiane, C.; Carrega, P.; Sommariva, S.; Ferlazzo, G.; Pronzato, P.; Gangemi, R.; Filaci, G.; Coco, S.; Croce, M. Therapeutic Implications of Tumor Microenvironment in Lung Cancer: Focus on Immune Checkpoint Blockade. Front. Immunol. 2022, 12, 799455. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Baker, A.H.; George, S.J.; Zaltsman, A.B.; Murphy, G.; Newby, A.C. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br. J. Cancer 1999, 79, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.; Seliger, B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care 2018, 13, 16–21. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef] [PubMed]
- Liaghat, M.; Ferdousmakan, S.; Mortazavi, S.H.; Yahyazadeh, S.; Irani, A.; Banihashemi, S.; Asl, F.S.S.; Akbari, A.; Farzam, F.; Aziziyan, F.; et al. The impact of epithelial-mesenchymal transition (EMT) induced by metabolic processes and intracellular signaling pathways on chemo-resistance, metastasis, and recurrence in solid tumors. Cell Commun. Signal. 2024, 22, 575. [Google Scholar] [CrossRef]
- Speiser, D.E.; Chijioke, O.; Schaeuble, K.; Münz, C. CD4+ T cells in cancer. Nat. Cancer 2023, 4, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Braoudaki, M.; Ahmad, M.S.; Mustafov, D.; Seriah, S.; Siddiqui, M.N.; Siddiqui, S.S. Chemokines and chemokine receptors in colorectal cancer; multifarious roles and clinical impact. Semin. Cancer Biol. 2022, 86, 436–449. [Google Scholar] [CrossRef]
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the immune response to cancer. Immunity 2021, 54, 859–874. [Google Scholar] [CrossRef]
- Bule, P.; Aguiar, S.I.; Aires-Da-Silva, F.; Dias, J.N.R. Chemokine-Directed Tumor Microenvironment Modulation in Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9804. [Google Scholar] [CrossRef]
- Davis, L.N.; Sherbenou, D.W. Emerging Therapeutic Strategies to Overcome Drug Resistance in Multiple Myeloma. Cancers 2021, 13, 1686. [Google Scholar] [CrossRef]
- Maes, K.; Mondino, A.; Lasarte, J.J.; Agirre, X.; Vanderkerken, K.; Prosper, F.; Breckpot, K. Epigenetic Modifiers: Anti-Neoplastic Drugs with Immunomodulating Potential. Front. Immunol. 2021, 12, 652160. [Google Scholar] [CrossRef]
- Yuan, L.-Q.; Liu, Y.-S.; Luo, X.-H.; Guo, L.-J.; Xie, H.; Lu, Y.; Wu, X.-P.; Liao, E.-Y. Recombinant tissue metalloproteinase inhibitor-3 protein induces apoptosis of murine osteoblast MC3T3-E1. Amino Acids 2008, 35, 123–127. [Google Scholar] [CrossRef]
- Span, P.N.; Lindberg, R.L.; Manders, P.; Tjan-Heijnen, V.C.; Heuvel, J.J.; Beex, L.V.; Sweep, C. Tissue inhibitors of metalloproteinase expression in human breast cancer: TIMP-3 is associated with adjuvant endocrine therapy success. J. Pathol. 2004, 202, 395–402. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef] [PubMed]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38 (Suppl. S2), W214–W220. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, G.; Lu, Y.; Zuo, S.; Duan, D.; Luo, X.; Zhao, H.; Li, J.; Zeng, Z.; Chen, Q.; et al. TIMER3: An enhanced resource for tumor immune analysis. Nucleic Acids Res. 2025, 53, W534–W541. [Google Scholar] [CrossRef]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor–immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404, Erratum in Cancer Discov. 2012, 2, 960. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Dong, X.; Sun, D.; Liu, Z.; Yue, J.; Wang, H.; Li, T.; Wang, C. TISCH2: Expanded datasets and new tools for single-cell transcriptome analyses of the tumor microenvironment. Nucleic Acids Res. 2023, 51, D1425–D1431. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Meng, Q.; Zhang, W.; Zheng, R.; Li, X.; Cheng, T.; Hu, D.; Gao, X. Single-Cell Analysis of the Pan-Cancer Immune Microenvironment and scTIME Portal. Cancer Immunol. Res. 2021, 9, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Qi, C.; Wei, T.; Li, M.; Cheng, Q.; Liu, Z.; Luo, P.; Zhang, J. CAMOIP: A web server for comprehensive analysis on multi-omics of immunotherapy in pan-cancer. Brief. Bioinform. 2022, 23, bbac129. [Google Scholar] [CrossRef]
- Zeng, Z.; Wong, C.J.; Yang, L.; Ouardaoui, N.; Li, D.; Zhang, W.; Gu, S.; Zhang, Y.; Liu, Y.; Wang, X.; et al. TISMO: Syngeneic mouse tumor database to model tumor immunity and immunotherapy response. Nucleic Acids Res. 2022, 50, D1391–D1397. [Google Scholar] [CrossRef]
- Jiang, P.; Gu, S.; Pan, D.; Fu, J.; Sahu, A.; Hu, X.; Li, Z.; Traugh, N.; Bu, X.; Li, B.; et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 2018, 24, 1550–1558. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhola, N.; Jaiswal, A.K.; Saluja, D. An Integrative Bioinformatics Approach to Investigating TIMP3 and Immune Cell Infiltration: Prognostic and Clinicopathological Implications. Int. J. Mol. Sci. 2025, 26, 8867. https://doi.org/10.3390/ijms26188867
Bhola N, Jaiswal AK, Saluja D. An Integrative Bioinformatics Approach to Investigating TIMP3 and Immune Cell Infiltration: Prognostic and Clinicopathological Implications. International Journal of Molecular Sciences. 2025; 26(18):8867. https://doi.org/10.3390/ijms26188867
Chicago/Turabian StyleBhola, Neelam, Amit K. Jaiswal, and Daman Saluja. 2025. "An Integrative Bioinformatics Approach to Investigating TIMP3 and Immune Cell Infiltration: Prognostic and Clinicopathological Implications" International Journal of Molecular Sciences 26, no. 18: 8867. https://doi.org/10.3390/ijms26188867
APA StyleBhola, N., Jaiswal, A. K., & Saluja, D. (2025). An Integrative Bioinformatics Approach to Investigating TIMP3 and Immune Cell Infiltration: Prognostic and Clinicopathological Implications. International Journal of Molecular Sciences, 26(18), 8867. https://doi.org/10.3390/ijms26188867







