Thyroid Hormones Regulate Postprandial Glucose Metabolism by Regulating SGLT1 Expression in the Small Intestine in Rats and Mice
Abstract
1. Introduction
2. Results
2.1. Thyroid Receptors in the Small Intestine: Distribution and Impact on SGLT1 Expression
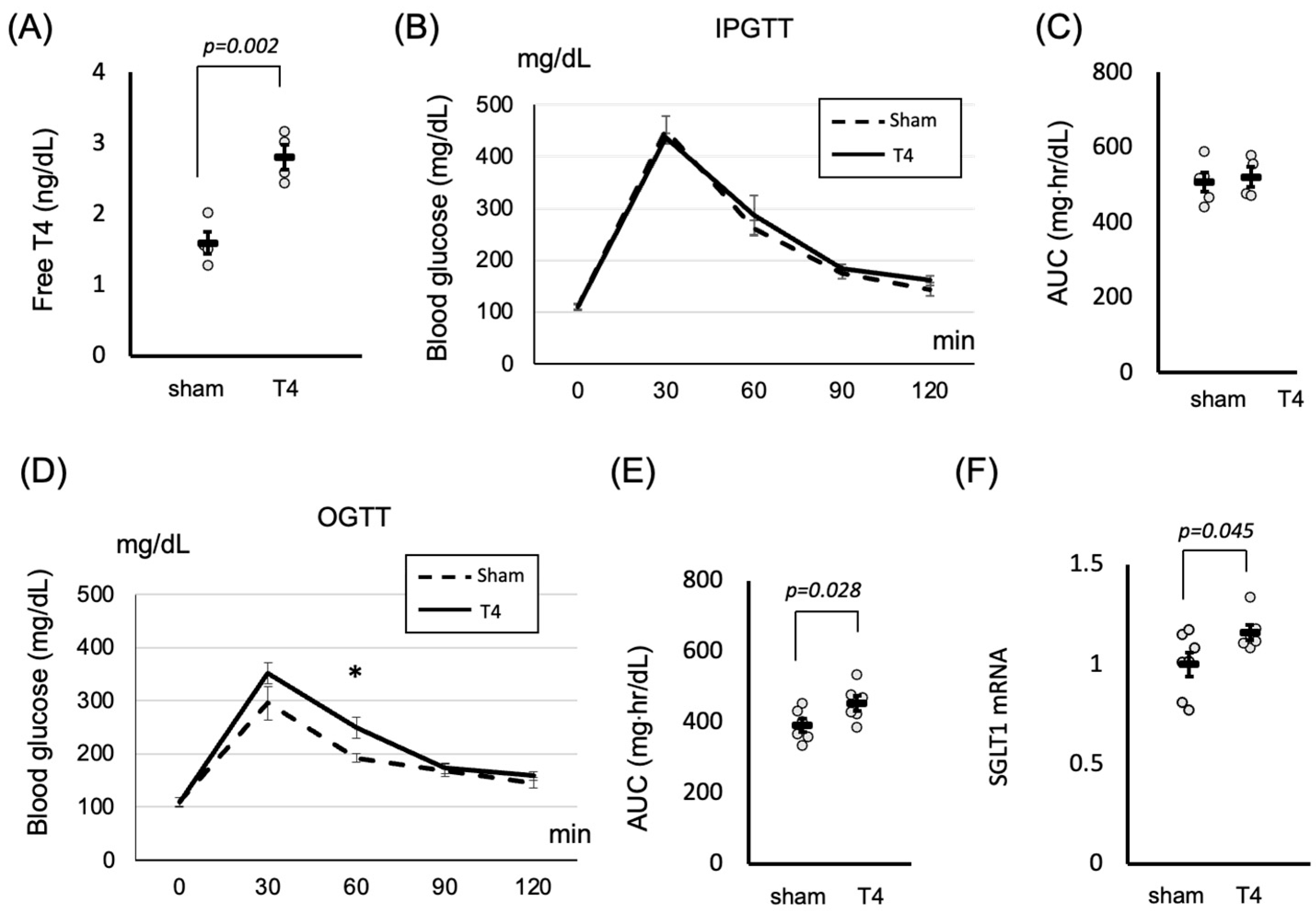
2.2. Thyroid Hormones Influence SGLT1 Expression and Postprandial Glucose Metabolism in the Small Intestine
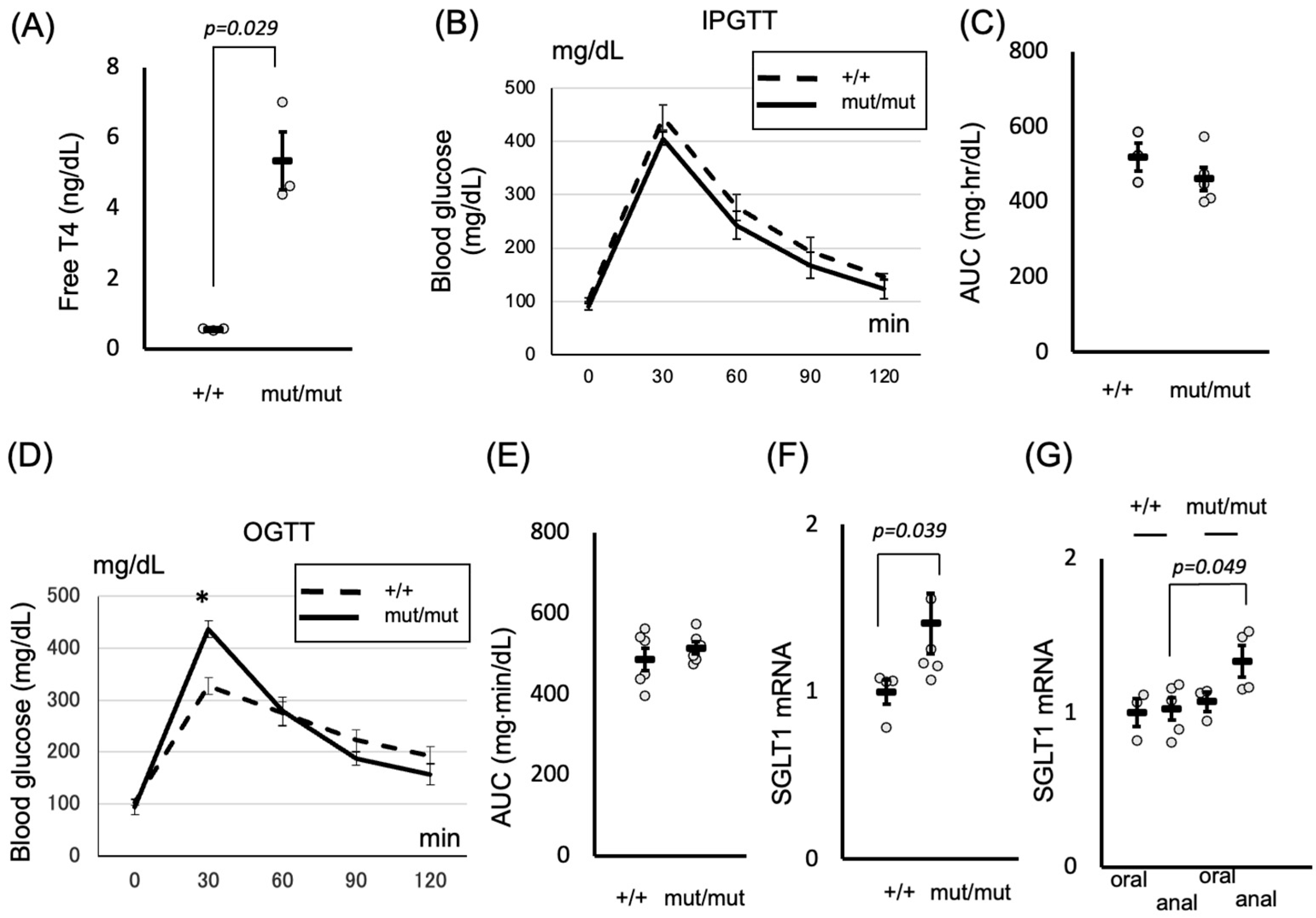
2.3. Role of Thyroid Hormones in Postprandial Hyperglycemia: Insights from the TRβΔ337T Knock-In Mouse Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Thyroid Hormone Measurement
4.3. RNA Extraction and Real-Time Quantitative Reverse Transcription PCR
4.4. Glucose Tolerance Tests
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The DECODE study group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 1999, 354, 617–621. [Google Scholar] [CrossRef]
- DECODA Study Group; International Diabetes Epidemiology Group. Cardiovascular risk profile assessment in glucose-intolerant Asian individuals—An evaluation of the World Health Organization two-step strategy: The DECODA Study (Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia). Diabet. Med. 2002, 19, 549–557. [Google Scholar] [CrossRef]
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata diabetes study. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef]
- Miller, M.E.; Bonds, D.E.; Gerstein, H.C.; Seaquist, E.R.; Bergenstal, R.M.; Calles-Escandon, J.; Childress, R.D.; Craven, T.E.; Cuddihy, R.M.; Dailey, G.; et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: Post hoc epidemiological analysis of the Accord study. BMJ 2010, 340, b5444. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Griffen, S.C.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M.; DEPICT-1 Investigators. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 864–876. [Google Scholar] [CrossRef]
- Rahmoune, H.; Thompson, P.W.; Ward, J.M.; Smith, C.D.; Hong, G.; Brown, J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005, 54, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. SGLT2 inhibitors: Physiology and pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed]
- Tsimihodimos, V.; Filippas-Ntekouan, S.; Elisaf, M. SGLT1 inhibition: Pros and cons. Eur. J. Pharmacol. 2018, 838, 153–156. [Google Scholar] [CrossRef]
- Zorzano, A.; Palacín, M.; Gumà, A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol. Scand. 2005, 183, 43–58. [Google Scholar] [CrossRef]
- Crunkhorn, S.; Patti, M.E. Links between thyroid hormone action, oxidative metabolism, and diabetes risk? Thyroid 2008, 18, 227–237. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Nishi, M. Diabetes mellitus and thyroid diseases. Diabetol. Int. 2018, 9, 108–112. [Google Scholar] [CrossRef]
- O’Meara, N.M.; Blackman, J.D.; Sturis, J.; Polonsky, K.S. Alterations in the kinetics of C-peptide and insulin secretion in hyperthyroidism. J. Clin. Endocrinol. Metab. 1993, 76, 79–84. [Google Scholar] [CrossRef]
- Kurozumi, A.; Okada, Y.; Tanaka, Y. Changes in glucose intolerance after treatment with antithyroid drugs in patients with Graves’ disease using continuous glucose monitoring: A pilot study. Intern. Med. 2023, 62, 1259–1263. [Google Scholar] [CrossRef]
- Shen, D.C.; Davidson, M.B.; Kuo, S.W.; Sheu, W.H. Peripheral and hepatic insulin antagonism in hyperthyroidism. J. Clin. Endocrinol. Metab. 1988, 66, 565–569. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Baker, B.; Marsh, H.; Mandarino, L.; Rizza, R.; Bergman, R.; Haymond, M.; Gerich, J. Effect of thyroid hormone excess on action, secretion, and metabolism of insulin in humans. Am. J. Physiol. 1985, 248, E593–E601. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef] [PubMed]
- Flamant, F.; Samarut, J. Thyroid hormone receptors: Lessons from knockout and knock-in mutant mice. Trends Endocrinol. Metab. 2003, 14, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Fraichard, A.; Chassande, O.; Plateroti, M.; Roux, J.P.; Trouillas, J.; Dehay, C.; Legrand, C.; Gauthier, K.; Kedinger, M.; Malaval, L.; et al. The T3R alpha gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 1997, 16, 4412–4420. [Google Scholar] [CrossRef]
- Gauthier, K.; Plateroti, M.; Harvey, C.B.; Williams, G.R.; Weiss, R.E.; Refetoff, S.; Willott, J.F.; Sundin, V.; Roux, J.P.; Malaval, L.; et al. Genetic analysis reveals different functions for the products of the thyroid hormone receptor alpha locus. Mol. Cell. Biol. 2001, 21, 4748–4760. [Google Scholar] [CrossRef]
- Bochukova, E.; Schoenmakers, N.; Agostini, M.; Schoenmakers, E.; Rajanayagam, O.; Keogh, J.M.; Henning, E.; Reinemund, J.; Gevers, E.; Sarri, M.; et al. A mutation in the thyroid hormone receptor alpha gene. N. Engl. J. Med. 2012, 366, 243–249. [Google Scholar] [CrossRef]
- Moran, C.; Chatterjee, K. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Suraci, E.; Arcidiacono, G.P.; Cimellaro, A.; Mignogna, C.; Presta, I.; Andreozzi, F.; Hribal, M.L.; Perticone, F.; Donato, G.; et al. Duodenal sodium/glucose cotransporter 1 expression under fasting conditions is associated with postload hyperglycemia. J. Clin. Endocrinol. Metab. 2017, 102, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.; Wood, I.S.; Palejwala, A.; Ellis, A.; Shirazi-Beechey, S.P. Expression of monosaccharide transporters in intestine of diabetic humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G241–G248. [Google Scholar] [CrossRef]
- Lambadiari, V.; Mitrou, P.; Maratou, E.; Raptis, A.E.; Tountas, N.; Raptis, S.A.; Dimitriadis, G. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine 2011, 39, 28–32. [Google Scholar] [CrossRef]
- Matosin-Matekalo, M.; Mesonero, J.E.; Delezay, O.; Poiree, J.C.; Ilundain, A.A.; Brot-Laroche, E. Thyroid hormone regulation of the Na+/glucose cotransporter SGLT1 in Caco-2 cells. Biochem. J. 1998, 334, 633–640. [Google Scholar] [CrossRef]
- Brent, G.A. Tissue-specific actions of thyroid hormone: Insights from animal models. Rev. Endocr. Metab. Disord. 2000, 1, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Lehmann, A.; Hornby, P.J. Intestinal SGLT1 in metabolic health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G887–G898. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Inoue, R.; Matsumoto, M.; Yajima, T.; Ushida, K.; Iwanaga, T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem. Cell Biol. 2011, 135, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Curty, F.H.; Borges, P.P.; Lee, C.E.; Abel, E.D.; Elmquist, J.K.; Cohen, R.N.; Wondisford, F.E. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc. Natl Acad. Sci. USA 2001, 98, 3998–4003. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Ghezzi, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Barrio, J.R.; Wright, E.M. Intestinal absorption of glucose in mice as determined by positron emission tomography. J. Physiol. 2018, 596, 2473–2489. [Google Scholar] [CrossRef]
- Nakamura, C.; Ishizuka, N.; Yokoyama, K.; Yazaki, Y.; Tatsumi, F.; Ikumi, N.; Hempstock, W.; Ikari, A.; Yoshino, Y.; Hayashi, H. Regulatory mechanisms of glucose absorption in the mouse proximal small intestine during fasting and feeding. Sci. Rep. 2023, 13, 10838. [Google Scholar] [CrossRef]
- Lei, J.; Nowbar, S.; Mariash, C.N.; Ingbar, D.H. Thyroid hormone stimulates Na-K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L762–L772. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Yan, Y.; Niu, Z.; Sun, C.; Li, P.; Shen, S.; Liu, S.; Wu, Y.; Yun, C.; Jiao, T.; Jia, S.; et al. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nat. Commun. 2022, 13, 6408. [Google Scholar] [CrossRef]
- Doong, M.L.; Wang, J.W.; Chung, S.C.; Liu, J.Y.; Hwang, C.; Hwang, C.Y.; Day, C.H.; Liu, Y.F.; Young, T.K.; Ho, L.L.; et al. Regulation of thyroid hormones in the secretion of insulin and gastric inhibitory polypeptide in male rats. Metabolism 1997, 46, 154–158. [Google Scholar] [CrossRef]
- Osei, K.; Falko, J.M.; O’Dorisio, T.M.; Adam, D.R. Gastric inhibitory polypeptide (GIP) responses after oral glucose ingestion in hyperthyroidism. Diabetes Care 1985, 8, 436–439. [Google Scholar] [CrossRef]
- Alemdar, S.; Yilmaz, N.; Ozdem, S.; Sari, R. Incretin levels in patients with hypothyroidism and the evaluation of incretin levels alterations with treatment. Asian Biomed. 2019, 13, 3–9. [Google Scholar] [CrossRef]
- Chiamolera, M.I.; Sidhaye, A.R.; Matsumoto, S.; He, Q.; Hashimoto, K.; Ortiga-Carvalho, T.M.; Wondisford, F.E. Fundamentally distinct roles of thyroid hormone receptor isoforms in a thyrotroph cell line are due to differential DNA binding. Mol. Endocrinol. 2012, 26, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Nakajima, Y.; Shibusawa, N.; Horiguchi, K.; Matsumoto, S.; Yamada, E.; Tomaru, T.; Ishii, S.; Ozawa, A.; Ishizuka, T.; et al. Pituitary NR4A1 is negatively regulated by thyroid hormone without direct binding of thyroid hormone receptors on the gene. Mol. Cell. Endocrinol. 2018, 461, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, S.; Doherty, T.M.; Kenneth, J.; TB Trials Study Group. Comparison of different standards for real-time PCR-based absolute quantification. J. Immunol. Methods 2010, 354, 34–39. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, S.; Yoshino, S.; Okada, S.; Horiguchi, K.; Hashimoto, K.; Yamada, E. Thyroid Hormones Regulate Postprandial Glucose Metabolism by Regulating SGLT1 Expression in the Small Intestine in Rats and Mice. Int. J. Mol. Sci. 2025, 26, 8854. https://doi.org/10.3390/ijms26188854
Matsumoto S, Yoshino S, Okada S, Horiguchi K, Hashimoto K, Yamada E. Thyroid Hormones Regulate Postprandial Glucose Metabolism by Regulating SGLT1 Expression in the Small Intestine in Rats and Mice. International Journal of Molecular Sciences. 2025; 26(18):8854. https://doi.org/10.3390/ijms26188854
Chicago/Turabian StyleMatsumoto, Shunichi, Satoshi Yoshino, Shuichi Okada, Kazuhiko Horiguchi, Koshi Hashimoto, and Eijiro Yamada. 2025. "Thyroid Hormones Regulate Postprandial Glucose Metabolism by Regulating SGLT1 Expression in the Small Intestine in Rats and Mice" International Journal of Molecular Sciences 26, no. 18: 8854. https://doi.org/10.3390/ijms26188854
APA StyleMatsumoto, S., Yoshino, S., Okada, S., Horiguchi, K., Hashimoto, K., & Yamada, E. (2025). Thyroid Hormones Regulate Postprandial Glucose Metabolism by Regulating SGLT1 Expression in the Small Intestine in Rats and Mice. International Journal of Molecular Sciences, 26(18), 8854. https://doi.org/10.3390/ijms26188854





