CUD003, a Novel Curcumin Derivative, Ameliorates LPS-Induced Impairment of Endothelium-Dependent Relaxation and Vascular Inflammation in Mice
Abstract
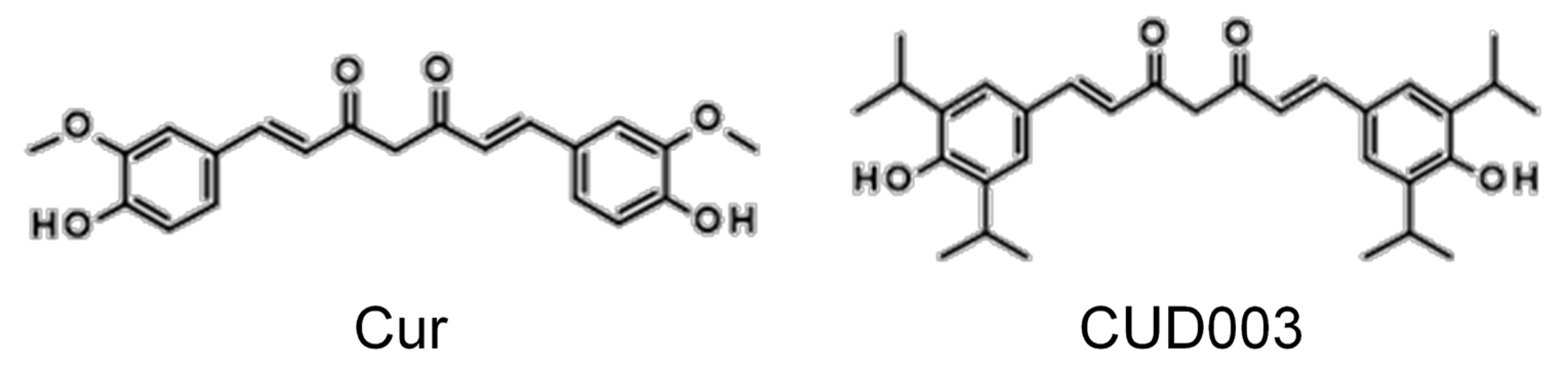
1. Introduction
2. Results
2.1. Survival Rates and Body Weight Changes
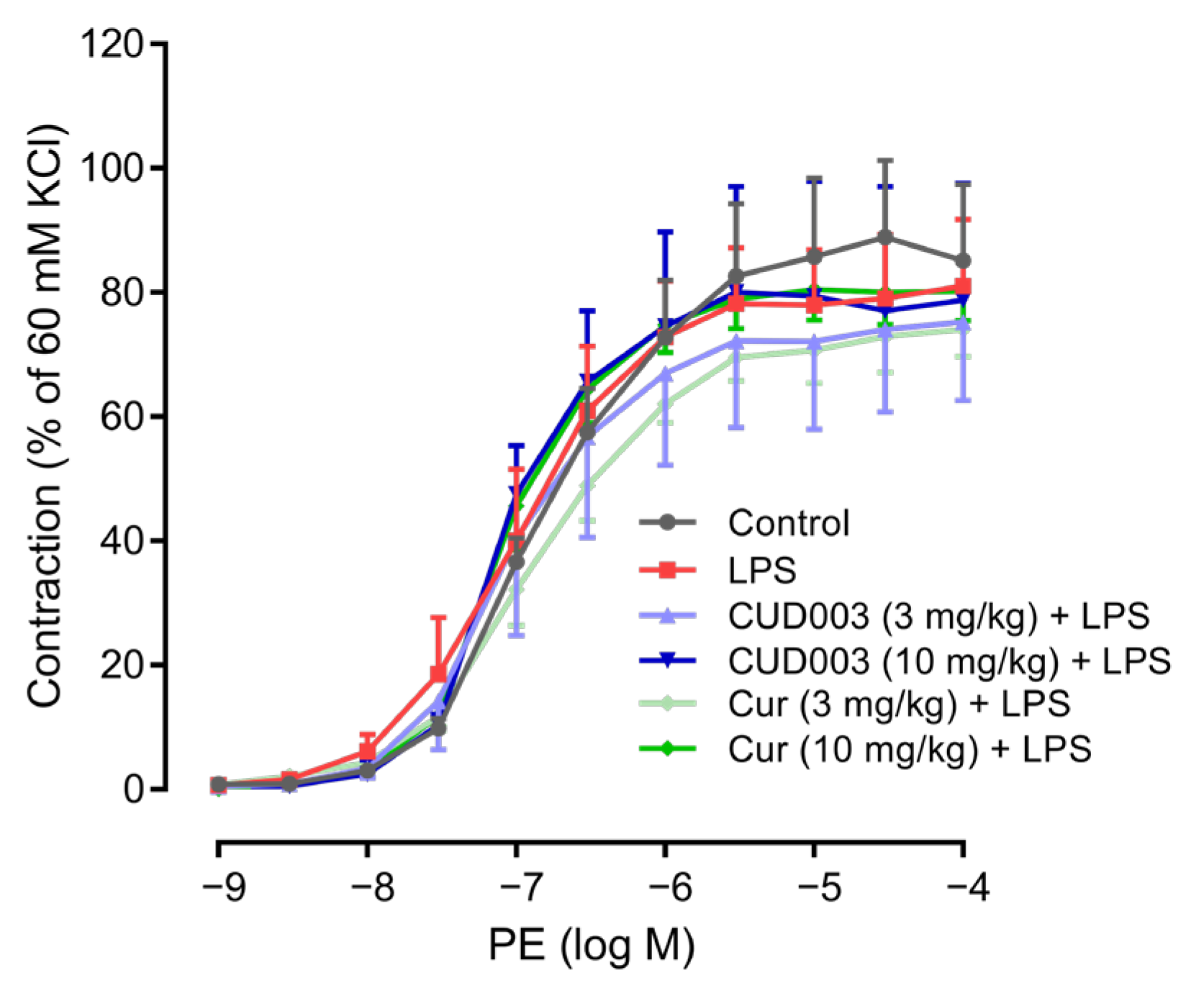
2.2. Restoration of LPS-Impaired Endothelium-Dependent Vasorelaxation by CUD003
2.3. Restoration of LPS-Induced Loss of eNOS Expression in the Thoracic Aorta by CUD003
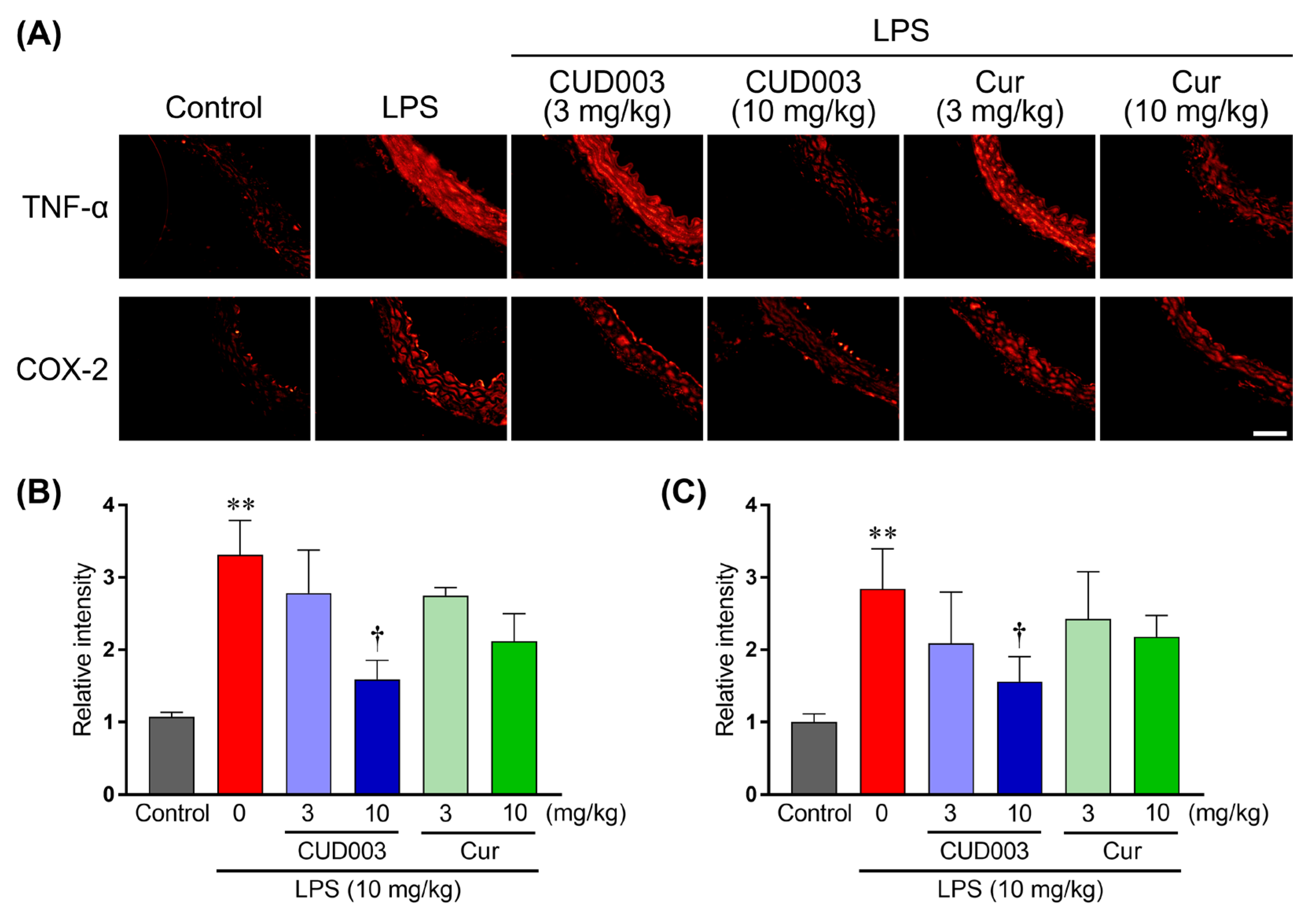
2.4. Inhibition of LPS-Induced Inflammatory Responses in the Thoracic Aorta by CUD003
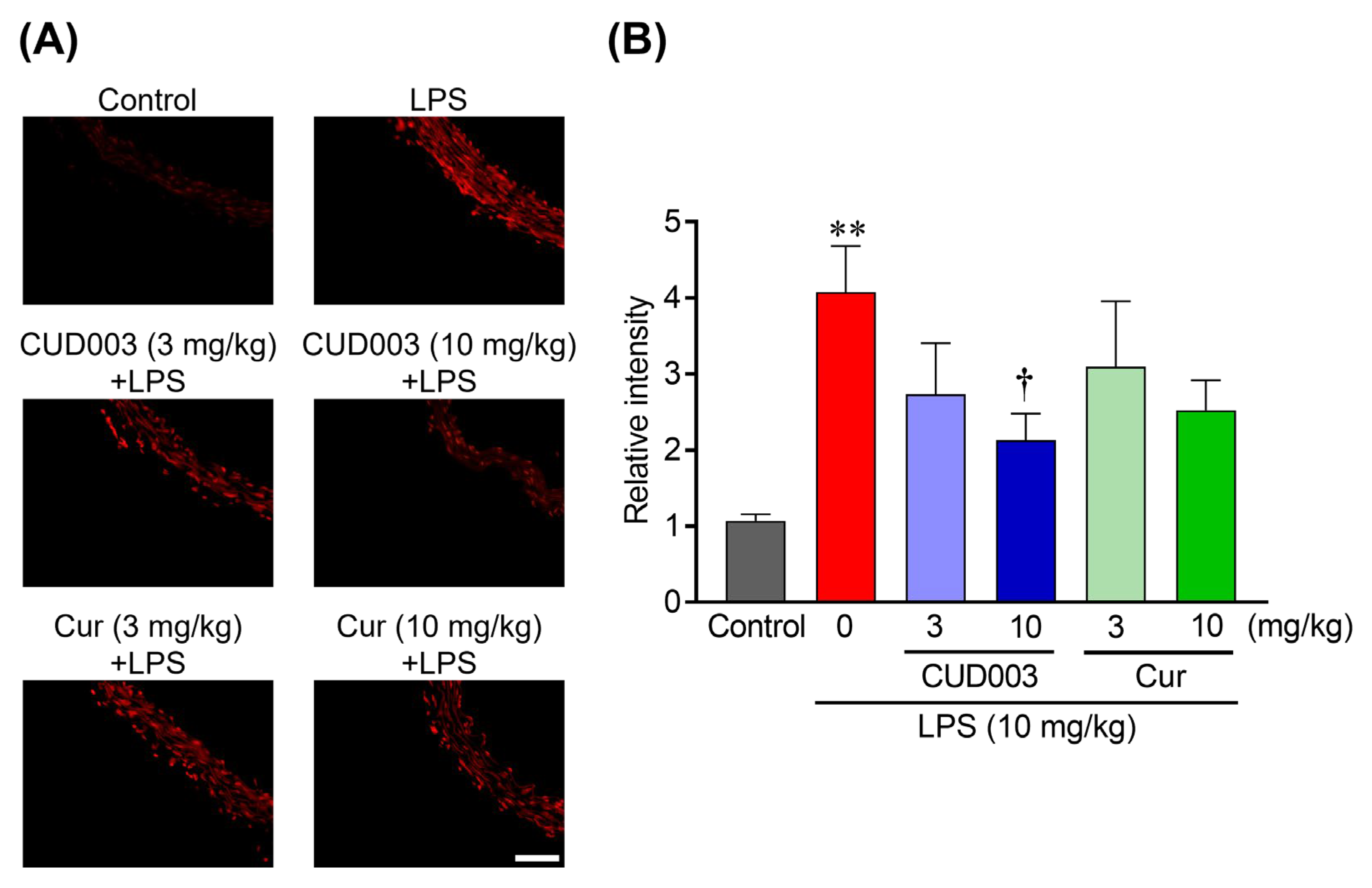
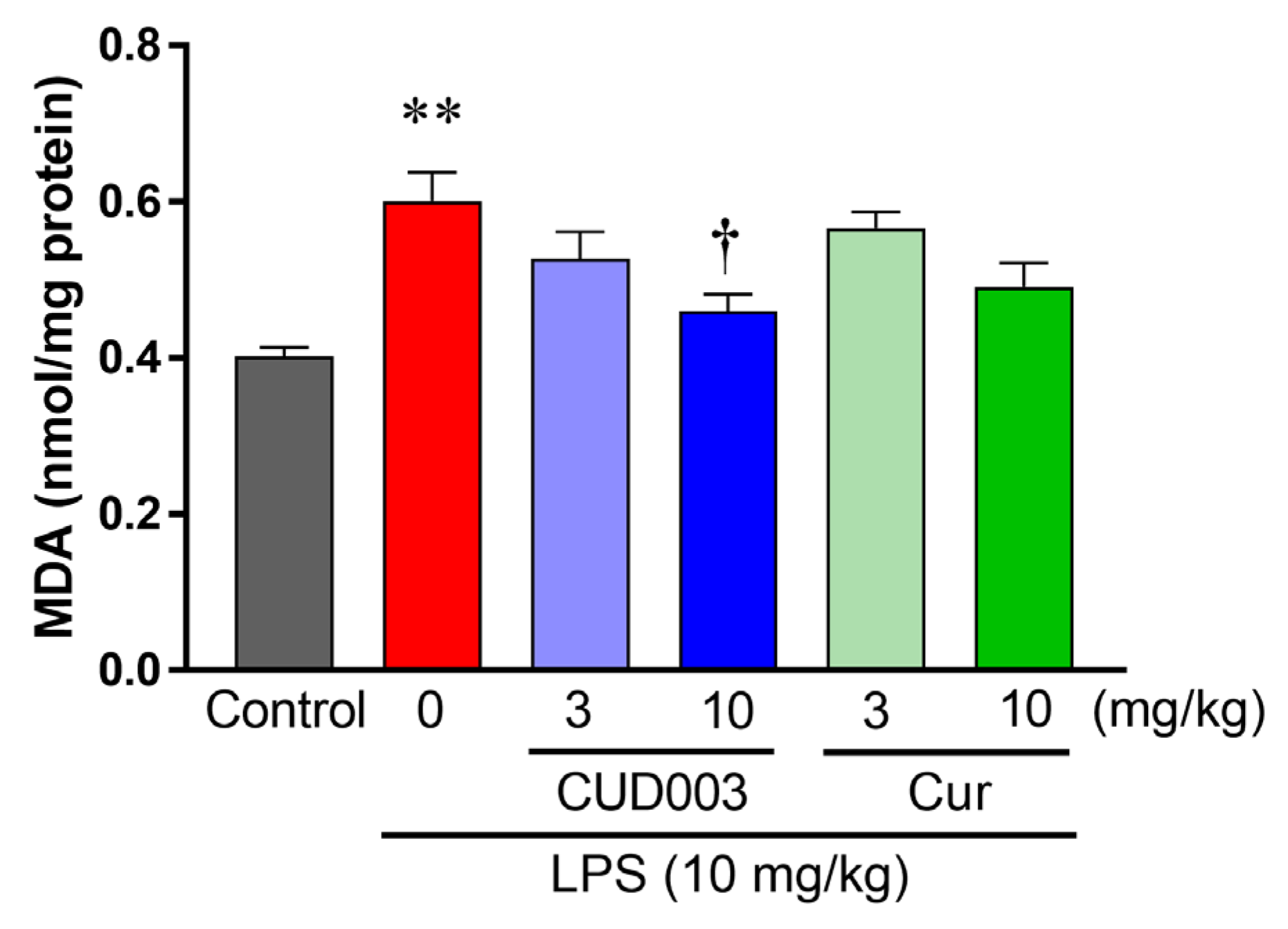
2.5. Inhibition of LPS-Induced ROS Generation and Lipid Peroxidation in the Aorta by CUD003
2.6. Antioxidant Properties of CUD003
2.7. In Silico ADMET Predictions
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Treatment
4.4. Measurement of Vascular Reactivity
4.5. Immunohistochemistry
4.6. Analysis of O2− Production by Dihydroethidium Staining
4.7. Malondialdehyde Assay
4.8. Free Radical Scavenging and Lipid Peroxidation Inhibitory Activities of CUD003
4.9. In Silico Absorption, Distribution, Metabolism, Excretion, and Toxicity Prediction
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| ADMET | Absorption, distribution, metabolism, excretion, and toxicity |
| ANOVA | Analysis of variance |
| ARE | Antioxidant response element |
| CUD003 | Curcumin derivative 003 |
| Cur | Curcumin |
| COX-2 | Cyclooxygenase-2 |
| DHE | Dihydroethidium |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EC50 | Half maximal effective concentration |
| Emax | Maximum effect |
| eNOS | Endothelial nitric oxide synthase |
| HO-1 | Heme oxygenase 1 |
| IC50 | Half maximal inhibitory concentration |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| MAPKs | Mitogen-activated protein kinases |
| MDA | Malondialdehyde |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NF-κB | Nuclear factor kappa B |
| NQO-1 | NAD(P)H quinone dehydrogenase 1 |
| PE | Phenylephrine |
| O2− | Superoxide |
| pD2 | Negative logarithm of the EC50 |
| ROS | Reactive oxygen species |
| SNP | Sodium nitroprusside |
| TBARS | Thiobarbituric acid-reactive substances |
| TNF | Tumor necrosis factor |
| TLR | Toll-like receptor |
| TPSA | Topological polar surface area |
| VCAM-1 | Vascular cell adhesion molecule 1 |
References
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Roşian, Ş.H.; Boarescu, I.; Boarescu, P.-M. Antioxidant and Anti-Inflammatory Effects of Bioactive Compounds in Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 1379. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Józkowiak, M.; Niebora, J.; Domagała, D.; Data, K.; Partyńska, A.; Kulus, M.; Kotrych, K.; Podralska, M.; Górska, A.; Chwiłkowska, A.; et al. New Insights into Endothelial Cell Physiology and Pathophysiology. Biomed. Pharmacother. 2025, 190, 118415. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Grylls, A.; Seidler, K.; Neil, J. Link between Microbiota and Hypertension: Focus on LPS/TLR4 Pathway in Endothelial Dysfunction and Vascular Inflammation, and Therapeutic Implication of Probiotics. Biomed. Pharmacother. 2021, 137, 111334. [Google Scholar] [CrossRef]
- Fock, E.M.; Parnova, R.G. Protective Effect of Mitochondria-Targeted Antioxidants against Inflammatory Response to Lipopolysaccharide Challenge: A Review. Pharmaceutics 2021, 13, 144. [Google Scholar] [CrossRef]
- Kalyan, M.; Tousif, A.H.; Sonali, S.; Vichitra, C.; Sunanda, T.; Praveenraj, S.S.; Ray, B.; Gorantla, V.R.; Rungratanawanich, W.; Mahalakshmi, A.M.; et al. Role of Endogenous Lipopolysaccharides in Neurological Disorders. Cells 2022, 11, 4038. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Salari, A.; Kheirkhah, J.; Ghorbani, Z. The Effects of Probiotics on Inflammation, Endothelial Dysfunction, and Atherosclerosis Progression: A Mechanistic Overview. Heart Lung Circ. 2022, 31, e45–e71. [Google Scholar] [CrossRef]
- Ayub, H.; Islam, M.; Saeed, M.; Ahmad, H.; Al-Asmari, F.; Ramadan, M.F.; Alissa, M.; Arif, M.A.; Rana, M.U.J.; Subtain, M.; et al. On the Health Effects of Curcumin and Its Derivatives. Food Sci. Nutr. 2024, 12, 8623–8650. [Google Scholar] [CrossRef]
- Kaur, K.; Al-Khazaleh, A.K.; Bhuyan, D.J.; Li, F.; Li, C.G. A Review of Recent Curcumin Analogues and Their Antioxidant, Anti-Inflammatory, and Anticancer Activities. Antioxidants 2024, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, R.; Jin, S.; Feng, X. Curcumin, a Plant Polyphenol with Multiple Physiological Functions of Improving Antioxidation, Anti-Inflammation, Immunomodulation and Its Application in Poultry Production. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1890–1905. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Ghanaatian, N.; Lashgari, N.-A.; Abdolghaffari, A.H.; Rajaee, S.M.; Panahi, Y.; Barreto, G.E.; Butler, A.E.; Sahebkar, A. Curcumin as a Therapeutic Candidate for Multiple Sclerosis: Molecular Mechanisms and Targets. J. Cell. Physiol. 2019, 234, 12237–12248. [Google Scholar] [CrossRef] [PubMed]
- Ahmadabady, S.; Beheshti, F.; Shahidpour, F.; Khordad, E.; Hosseini, M. A Protective Effect of Curcumin on Cardiovascular Oxidative Stress Indicators in Systemic Inflammation Induced by Lipopolysaccharide in Rats. Biochem. Biophys. Rep. 2021, 25, 100908. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.; Niu, X. Curcumin Inhibits LPS-Induced Inflammation in Rat Vascular Smooth Muscle Cells in Vitro via ROS-Relative TLR4-MAPK/NF-κB Pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef]
- Lu, W.; Jiang, J.-P.; Hu, J.; Wang, J.; Zheng, M.-Z. Curcumin Protects against Lipopolysaccharide-Induced Vasoconstriction Dysfunction via Inhibition of Thrombospondin-1 and Transforming Growth Factor-Β1. Exp. Ther. Med. 2014, 9, 377–383. [Google Scholar] [CrossRef][Green Version]
- Vanucci-Bacqué, C.; Bedos-Belval, F. Anti-Inflammatory Activity of Naturally Occuring Diarylheptanoids—A Review. Bioorg. Med. Chem. 2021, 31, 115971. [Google Scholar] [CrossRef]
- Sun, D.-J.; Zhu, L.-J.; Zhao, Y.-Q.; Zhen, Y.-Q.; Zhang, L.; Lin, C.-C.; Chen, L.-X. Diarylheptanoid: A Privileged Structure in Drug Discovery. Fitoterapia 2020, 142, 104490. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem.—Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef] [PubMed]
- Lund, D.D.; Brooks, R.M.; Faraci, F.M.; Heistad, D.D. Role of Angiotensin II in Endothelial Dysfunction Induced by Lipopolysaccharide in Mice. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H3726–H3731. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Lau, Y.S.; Murugan, D.; Vanhoutte, P.M.; Mustafa, M.R. Paeonol Attenuates LPS-Induced Endothelial Dysfunction and Apoptosis by Inhibiting BMP4 and TLR4 Signaling Simultaneously but Independently. J. Pharmacol. Exp. Ther. 2018, 364, 420–432. [Google Scholar] [CrossRef]
- Bányai, B.; Répás, C.; Miklós, Z.; Johnsen, J.; Horváth, E.M.; Benkő, R. Delta 9-Tetrahydrocannabinol Conserves Cardiovascular Functions in a Rat Model of Endotoxemia: Involvement of Endothelial Molecular Mechanisms and Oxidative-Nitrative Stress. PLoS ONE 2023, 18, e0287168. [Google Scholar] [CrossRef]
- Uğurel, S.S.; Kuşçu, N.; Özenci, Ç.Ç.; Dalaklıoğlu, S.; Taşatargil, A. Resveratrol Prevented Lipopolysaccharide-Induced Endothelial Dysfunction in Rat Thoracic Aorta Through Increased eNOS Expression. Balk. Med. J. 2016, 33, 138–143. [Google Scholar] [CrossRef]
- Izadparast, F.; Riahi-Zajani, B.; Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Protective Effect of Berberine against LPS-Induced Injury in the Intestine: A Review. Cell Cycle 2022, 21, 2365–2378. [Google Scholar] [CrossRef]
- Kim, J.-D.; Jain, A.; Fang, L. Mitigating Vascular Inflammation by Mimicking AIBP Mechanisms: A New Therapeutic End for Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 10314. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Kashtalap, V.V.; Rozhkova, U.V.; Maksaeva, A.O.; Sukhorukov, V.N.; Orekhov, A.N. The Role of Cell Junctions in Atherosclerosis: Implications for Inflammation, Endothelial Dysfunction, and Plaque Stability. J. Physiol. Biochem. 2025, 81, 573–587. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin Attenuates Inflammatory Responses by Suppressing TLR4-Mediated NF-κB Signaling Pathway in Lipopolysaccharide-Induced Mastitis in Mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Xie, Q.-F.; Cheng, J.-J.; Chen, J.-F.; Feng, Y.-C.; Lin, G.-S.; Xu, Y. Comparation of Anti-Inflammatory and Antioxidantactivities of Curcumin, Tetrahydrocurcuminand Octahydrocurcuminin LPS-Stimulated RAW264.7 Macrophages. Evid.-Based Complement. Altern. Med. ECAM 2020, 2020, 8856135. [Google Scholar] [CrossRef]
- Liu, X.; Guan, P.Y.; Yu, C.T.; Yang, H.; Shan, A.S.; Feng, X.J. Curcumin Alleviated Lipopolysaccharide-Induced Lung Injury via Regulating the Nrf2-ARE and NF-κB Signaling Pathways in Ducks. J. Sci. Food Agric. 2022, 102, 6603–6611. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Bani, S.; Pandey, A.; Ibrahim, M.A.; Thazhathidath, S. Assessment of Safety Profile of Activated Curcumin C3 Complex (AC3®), Enriched Extract of Bisdemethoxycurcumin from the Rhizomes of Curcuma longa. J. Toxicol. 2023, 2023, 3729399. [Google Scholar] [CrossRef] [PubMed]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety Assessment of a Highly Bioavailable Curcumin-Galactomannoside Complex (CurQfen) in Healthy Volunteers, with a Special Reference to the Recent Hepatotoxic Reports of Curcumin Supplements: A 90-Days Prospective Study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and Its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Ippoliti, I.; Menniti-Ippolito, F.; Gallo, E.; Brilli, V.; Lanzi, C.; Mannaioni, G.; Firenzuoli, F.; et al. Acute Liver Injury Following Turmeric Use in Tuscany: An Analysis of the Italian Phytovigilance Database and Systematic Review of Case Reports. Br. J. Clin. Pharmacol. 2021, 87, 741–753. [Google Scholar] [CrossRef]
- Asano, T.; Matsuzaki, H.; Xuan, M.; Yuan, B.; Takayama, J.; Sakamoto, T.; Okazaki, M. Chronic Administration with FAD012 (3,5-Dimethyl-4-Hydroxycinnamic Acid) Maintains Cerebral Blood Flow and Ameliorates Swallowing Dysfunction After Chronic Cerebral Hypoperfusion in Rats. Int. J. Mol. Sci. 2025, 26, 3277. [Google Scholar] [CrossRef]
- Hayashi, Y.; Saeki, A.; Yoshimoto, S.; Yano, E.; Yasukochi, A.; Kimura, S.; Utsunomiya, T.; Minami, K.; Aso, Y.; Hatakeyama, Y.; et al. 4-Octyl Itaconate Attenuates Cell Proliferation by Cellular Senescence via Glutathione Metabolism Disorders and Mitochondrial Dysfunction in Melanoma. Antioxid. Redox Signal. 2025, 42, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Xuan, M.; Iwata, N.; Takayama, J.; Hayashi, K.; Kato, Y.; Aoyama, T.; Sugo, H.; Matsuzaki, H.; Yuan, B.; et al. Involvement of the Restoration of Cerebral Blood Flow and Maintenance of eNOS Expression in the Prophylactic Protective Effect of the Novel Ferulic Acid Derivative FAD012 against Ischemia/Reperfusion Injuries in Rats. Int. J. Mol. Sci. 2023, 24, 9663. [Google Scholar] [CrossRef] [PubMed]
- Mvondo, J.G.M.; Matondo, A.; Mawete, D.T.; Bambi, S.-M.N.; Mbala, B.M.; Lohohola, P.O. In Silico ADME/T Properties of Quinine Derivatives Using SwissADME and pkCSM Webservers. Int. J. Trop. Dis. Health 2021, 42, 1–12. [Google Scholar] [CrossRef]


| Group | Survival Rates (%) | Body Weight Changes (%) |
|---|---|---|
| Control | 100 (18/18) | 101.2 ± 0.4 |
| LPS | 100 (16/16) | 90.1 ± 0.4 ** |
| CUD003 (3 mg/kg) + LPS | 100 (15/15) | 91.2 ± 0.5 ** |
| CUD003 (10 mg/kg) + LPS | 100 (15/15) | 91.4 ± 0.3 ** |
| Cur (3 mg/kg) + LPS | 93.3 (14/15) | 90.5 ± 0.5 ** |
| Cur (10 mg/kg) + LPS | 100 (16/16) | 90.8 ± 0.5 ** |
| LPS | ||||||
|---|---|---|---|---|---|---|
| Control | LPS | 3 mg/kg CUD003 | 10 mg/kg CUD003 | 3 mg/kg Cur | 10 mg/kg Cur | |
| Contraction (g) | ||||||
| 60 mM KCl | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.1 |
| PE | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Emax (%) | ||||||
| PE | 82.1 ± 11.7 | 79.1 ± 9.7 | 81.2 ± 12.5 | 78.4 ± 18.3 | 72.7 ± 5.0 | 80.2 ± 5.6 |
| ACh | 83.8 ± 3.9 | 51.3 ± 2.8 ** | 65.3 ± 7.8 | 76.4 ± 2.4 † | 55.7 ± 4.7 | 67.7 ± 4.6 |
| SNP | 106.4 ± 3.4 | 102.8 ± 6.8 | 107.1 ± 3.9 | 108.4 ± 3.1 | 106.1 ± 4.2 | 108.4 ± 5.7 |
| pD2 | ||||||
| PE | 6.80 ± 0.08 | 6.97 ± 0.17 | 7.12 ± 0.25 | 7.11 ± 0.08 | 6.85 ± 0.14 | 7.10 ± 0.05 |
| ACh | 6.97 ± 0.17 | 6.28 ± 0.20 | 6.93 ± 0.17 | 7.07 ± 0.09 | 6.52 ± 0.25 | 6.48 ± 0.11 |
| SNP | 7.98 ± 0.11 | 7.82 ± 0.11 | 7.99 ± 0.15 | 8.33 ± 0.15 | 7.65 ± 0.12 | 8.14 ± 0.22 |
| Compounds | DPPH Assay (EC50; µM) | TBARS Assay (IC50; µM) |
|---|---|---|
| CUD003 | 81.2 ± 0.7 | 30.0 ± 3.1 |
| Cur | 60.8 ± 1.0 ** | 29.9 ± 4.3 |
| Parameter | CUD003 | Cur |
|---|---|---|
| Physicochemical Properties | ||
| Consensus LogP | 6.70 | 3.03 |
| Water solubility (log mol/L) | −4.41 | −4.07 |
| Topological polar surface area (Å2) | 74.60 | 93.06 |
| Lipinski’s rule | Yes (1 violation) | Yes (0 violation) |
| Absorption | ||
| Caco-2 permeability (log Papp in 10−6 cm/s) | 0.382 | 0.033 |
| Intestinal absorption (% absorbed) | 90.6 | 82.4 |
| P-glycoprotein substrate | Yes | Yes |
| Distribution | ||
| BBB permeability | −0.113 | −0.51 |
| Volume of distribution | −0.65 | −0.26 |
| Metabolism | ||
| CYP1A2 inhibitor | No | No |
| CYP2C9 inhibitor | No | Yes |
| CYP2C19 inhibitor | Yes | Yes |
| CYP2D6 inhibitor | No | No |
| CYP3A4 inhibitor | Yes | Yes |
| Excretion | ||
| Total clearance (log mL/min/kg) | 0.81 | 0.01 |
| Renal OCT2 substrate | No | No |
| Toxicity | ||
| AMES toxicity | No | No |
| Hepatotoxicity | Yes | No |
| Oral rat acute toxicity (LD50; mol/kg) | 1.612 | 1.915 |
| Oral rat chronic toxicity (LOAEL; log mg/kg_bw/day) | 1.683 | 1.586 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, H.; Arai, A.; Xuan, M.; Yuan, B.; Takayama, J.; Sakamoto, T.; Okazaki, M. CUD003, a Novel Curcumin Derivative, Ameliorates LPS-Induced Impairment of Endothelium-Dependent Relaxation and Vascular Inflammation in Mice. Int. J. Mol. Sci. 2025, 26, 8850. https://doi.org/10.3390/ijms26188850
Matsuzaki H, Arai A, Xuan M, Yuan B, Takayama J, Sakamoto T, Okazaki M. CUD003, a Novel Curcumin Derivative, Ameliorates LPS-Induced Impairment of Endothelium-Dependent Relaxation and Vascular Inflammation in Mice. International Journal of Molecular Sciences. 2025; 26(18):8850. https://doi.org/10.3390/ijms26188850
Chicago/Turabian StyleMatsuzaki, Hirokazu, Anna Arai, Meiyan Xuan, Bo Yuan, Jun Takayama, Takeshi Sakamoto, and Mari Okazaki. 2025. "CUD003, a Novel Curcumin Derivative, Ameliorates LPS-Induced Impairment of Endothelium-Dependent Relaxation and Vascular Inflammation in Mice" International Journal of Molecular Sciences 26, no. 18: 8850. https://doi.org/10.3390/ijms26188850
APA StyleMatsuzaki, H., Arai, A., Xuan, M., Yuan, B., Takayama, J., Sakamoto, T., & Okazaki, M. (2025). CUD003, a Novel Curcumin Derivative, Ameliorates LPS-Induced Impairment of Endothelium-Dependent Relaxation and Vascular Inflammation in Mice. International Journal of Molecular Sciences, 26(18), 8850. https://doi.org/10.3390/ijms26188850







