Abstract
In this paper, we aimed to evaluate the efficacy and usefulness of three brief, easy-to-administer, and repeatable tests, namely SDMT, Digit Span Forward (DSF), and Digit Span Backward (DSB) in MS patients (MSp), and compared the results with those of healthy volunteers (CONs). We were hoping to identify the most sensitive test that could be used regularly in clinical practice. In addition, we tried to identify the metabolic background of the cognitive setting using the advanced radiological method, Mescher–Garwood (MEGA)-edited 1H Magnetic Resonance Spectroscopy (1H-MRS). A total of 22 relapsing MSp and 22 CONs were enrolled. The SDMT, DSF, and DSB tests were used on all participants. The patients also underwent a 1H-MRS brain examination. In addition to N-Acetyl-Aspartate (tNAA), Myoinositol (mIns), Choline (tCho), and Creatine (tCr) were also evaluated GABA and Glutamate–Glutamine (Glx) ratios. CONs were superior to MSp in the results of all neurocognitive tests. The DSB was found to be the most sensitive test for identifying MSp. The SDMT in MSp correlated with inflammatory and degenerative metabolites in the thalamus, hippocampus, and corpus callosum. A correlation between increased Glx- and GABA-ratios and SDMT was found. Unlike the SDMT, the DSF and DSB showed correlations with inflammatory metabolites in the caudate nucleus and hypothalamus. DSF correlated with GABA ratios in the hippocampus. Our study confirms the efficacy of DSF and DSB tests in evaluating working memory cognitive impairment in MSp, showing an association of the tests with specific brain metabolites.
1. Introduction
Multiple sclerosis (MS), a chronic, immune-mediated neurodegenerative disorder of the central nervous system (CNS), collectively contributes to progressive neurological disabilities [1,2]. While motor and sensory deficits are hallmark clinical features, cognitive dysfunction represents a profound burden, significantly impairing quality of life, vocational capacity, and psychosocial well-being [3]. Cognitive dysfunction may manifest early in the disease course, even without significant physical disability, and msy correlate poorly with conventional magnetic resonance imaging (MRI) markers such as T2 hyperintense lesion load or gadolinium-enhancing activity [4]. This dissociation underscores the limitations of structural imaging in capturing the complex neurobiological substrates of cognitive decline, which are hypothesized to involve microstructural damage, metabolic dysregulation, and network-level disconnection. Emerging evidence suggests that cortical and deep gray matter (GM) atrophy and diffuse white matter (WM) pathology in normal-appearing tissue may drive cognitive impairment. Yet, the molecular mechanisms linking neuroinflammation, neurodegeneration, and neuropsychological deficits remain poorly understood [5,6,7].
One of the advanced MRI methods is 1-proton Magnetic Resonance Spectroscopy (1H-MRS) [8], which detects biochemical alterations in lesioned and normal-appearing brain tissue [4]. In this study, Mescher–Garwood (MEGA)-edited 1H-MRS [9,10] was used, which allows the evaluation of neurotransmitters such as Glutamate with its precursor Glutamine (Glx), and γ-Aminobutyric Acid (GABA), in addition to traditional metabolites reflecting tissue alteration such as N-Acetyl-Aspartate (tNAA), Myoinositol (mIns), and Choline (tCho), and Creatine (tCr)-containing compounds [9,10]. In a summary, tNAA is a marker of neuronal density and mitochondrial function synthesized in neuronal mitochondria; tCr is a compound central to adenosine triphosphate (ATP) buffering and cellular energy homeostasis; tCho is indicative of membrane phospholipid turnover and upregulated in neuroinflammatory states; mIns is a glial-specific osmolyte elevated in astrogliosis and microglial activation; Glx is an excitatory neurotransmitter involved in synaptic signaling and implicated in excitotoxic injury; and GABA has a role as the major inhibitory neurotransmitter referred to as a marker of neuro-plasticity [11,12].
Cognitive function and affective symptoms have been evaluated using a standardized battery of neuropsychological tests and validated self-report measures selected for their established sensitivity to MS-related deficits. The Symbol Digit Modalities Test (SDMT) [13,14,15] is a validated measure of information processing speed and working memory, the primary endpoint for assessing cognitive efficiency in MS. The SDMT emphasizes rapid visual scanning, sustained attention, and mental flexibility, domains disproportionately impaired in MS. It has been identified as the most sensitive cognitive marker of disease progression [14,16,17].
Verbal working memory and attentional capacity could be assessed using the Digit Span subtest from the Wechsler Adult Intelligence Scale (WAIS-IV), administered in both forward (DSF) and backward (DSB) conditions [18]. DSB requires mental manipulation by reversing digit sequences, taxing executive control, and working memory updating. While DSF primarily reflects phonological loop integrity, DSB engages the central executive component of Baddeley’s working memory model, making it sensitive to frontal-subcortical circuit dysfunction common in MS [19].
In this study, we aimed to evaluate the efficacy and usefulness of three brief, easy-to-administer, and repeatable tests, namely SDMT, DSF, and DSB in MS patients, and compare the results with those of healthy volunteers (CONs). We were hoping to identify the most sensitive test that would be used in clinical practice regularly. In addition, we tried to find the metabolic background of the cognitive setting using the advanced radiological method, MEGA-edited 1H-MRS. Selected areas of white and gray matter in the brains of MS patients (MSp) and controls (CONs) were evaluated; data were compared and correlated with the results of the SDMT, DSF, and DSB tests.
2. Results
Table 1 shows differences between MSp and CONs in demographic status (e.g., age, EDSS, disease duration, MRI activity, treatment method) and results of cognitive tests (SDMT, DSF, DSB).

Table 1.
Demographic and basic information about the study groups. The table shows differences between MSp and CONs in demographic status (e.g., age, EDSS, ARR, disease duration, MRI activity, treatment) and cognitive tests (SDMT, DSF, DSB).
The average score of DSF in CONs was 7.86 (0–8) and in MSp 6.08 (0–6). The average score of DSB in CONs was 7.14 (0–9) and in MSp 5.09 (0–10).
The Cochran–Armitage test for trends provided an alternative hypothesis, namely that DSF scores decrease in MSp (Z = 3.2861, dim = 9, p-value = 0.0005079). It revealed that as the DSB series increased, the relative proportion of MSp decreased.
The Cochran–Armitage test for trends provided an alternative hypothesis, namely that DSB scores decrease in MSp (Z = 3.9294, dim = 8, p-value = 0.00004258). It revealed that as the DSB series increased, the relative proportion of MSp decreased.
2.1. Correlations of Brain Metabolites with Cognitive Tests Can Be Found in Table 2, Table 3 and Table 4
Metabolic changes in parts of GM and WM that were independent of the presence of demyelinating lesions were tested and correlated with cognitive tests. Data are in Table 2, Table 3 and Table 4.

Table 2.
Correlation of SDMT with the 1H-MRS metabolite ratios in the brain areas of MSp.
Table 2.
Correlation of SDMT with the 1H-MRS metabolite ratios in the brain areas of MSp.
| The Brain Area | 1H-MRS Metabolite Ratio | Correlation of SDMT with 1H-MRS Metabolite Ratios | |
|---|---|---|---|
| p-Value | Cor | ||
| Thalamus R | tNAA/tCr | 0.040 | 0.430 |
| Hypothalamus L | Glx/tCr | 0.032 | 0.447 |
| Hippocampus L | mIns/tNAA | 0.014 | −0.507 |
| CC_splenium | tCho/tNAA | 0.048 | −0.417 |
| CC_rostral | GABA/tCr | 0.038 | 0.436 |
| CC_genu | tCho/tNAA | 0.021 | −0.479 |
tNAA = N-acetyl aspartate; Glx = glutamate and glutamine; tCho = choline; GABA = γ-amino butyric acid; mIns = myoinositol; tCr = creatine; tCho = choline; CC = corpus callosum; statistics (p-values, correlation coefficient) were evaluated using Welsh two-sample t-test, R = right, L = left.

Table 3.
Correlation of Digit Span Forward series with the 1H-MRS metabolite ratios in the brain areas of MSp.
Table 3.
Correlation of Digit Span Forward series with the 1H-MRS metabolite ratios in the brain areas of MSp.
| The Brain Area | 1H-MRS Metabolite Ratio | Correlation of Digit Span Forward Series with 1H-MRS Metabolite Ratios | |
|---|---|---|---|
| p-Value | Cor | ||
| Caudate R | mIns/tNAA | 0.022 | 0.474 |
| Caudate R | mIns/tCr | 0.021 | 0.477 |
| Hypothalamus R | Glx/tCr | 0.010 | −0.527 |
| Hypothalamus R | tNAA/tCr | 0.040 | −0.432 |
| Hypothalamus L | tCho/tNAA | 0.012 | −0.517 |
| Hippocampus L | GABA/tNAA | 0.007 | 0.545 |
| Hippocampus L | GABA/tCr | 0.026 | 0.464 |
| CC_splenium | mIns/tCr | 0.041 | −0.429 |
| CC_genu | tNAA/tCr | 0.021 | −0.479 |
R = right; L = left; tNAA = N-acetyl aspartate; Glx = glutamate and glutamine; tCho = choline; GABA = γ-amino butyric acid; mIns = myoinositol; tCr = creatine; caudate = nucleus caudatus; CC = corpus callosum; statistics (p-values, correlation coefficient) were evaluated using the Welsh two-sample t-test.

Table 4.
Correlation of Digit Span Backward series with the 1H-MRS metabolite ratios in the brain areas of MSp.
Table 4.
Correlation of Digit Span Backward series with the 1H-MRS metabolite ratios in the brain areas of MSp.
| The Brain Area | 1H-MRS Metabolite Ratio | Correlation of Digit Span Backward Series with 1H-MRS Metabolite Ratios | |
|---|---|---|---|
| p-Value | Cor | ||
| Caudate R | tCho/tNAA | 0.033 | 0.446 |
| Caudate R | mIns/tNAA | 0.014 | 0.505 |
| Caudate R | mIns/tCr | 0.012 | 0.517 |
| Hypothalamus R | tNAA/tCr | 0.03 | −0.453 |
| CC_splenium | tNAA/tCr | 0.048 | −0.417 |
| CC_splenium | mIns/tCr | 0.048 | −0.416 |
R = right, CC = corpus callosum; tNAA = N-acetyl aspartate; tCho = choline; GABA = gaba amino butyric acid; mIns = myoinositol; tCr = creatine; caudate = nucleus caudatus; CC = corpus callosum; statistics (p-values, correlation coefficient) were evaluated using Welsh two-sample t-test.
2.2. Evaluation of the Most Significant Predictors of Multiple Sclerosis
A false positive and false negative rate of approximately 20% should be expected when using the RF machine learning algorithm (trained with the above predictors) for predicting whether a person has MS or is a CON. RF results prediction power was high, and the area under the curve was 86%.
Essentially, the same ranking was obtained by the basic variable importance (VIMP) ranking algorithm via random forest, see Figure 1.
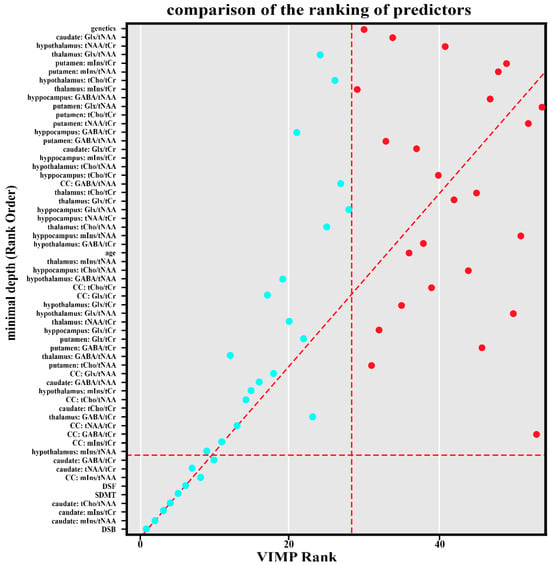
Figure 1.
Comparison of the ranking of predictors by graph depth on the y-axis and by VIMP on the x-axis. The horizontal dashed line represents the cutoff for significance within the graph depth. The predictors below the cutoff are considered necessary according to graph depth. Similarly, the vertical dashed line is for cutoff on VIMP. VIMP = variable importance; Backward = Digit Span Backward; caudate_mIns.tNAA = Caudate Nucleus Myoinositol/tN-Acetyl Aspartate; caudate_mIns.tCr = in Caudate Nucleus Myoinositol/t-Creatine; caudate_tCho.tNAA = Caudate Nucleus tCholin/tN-Acetyl Aspartate; SDMT = Single Digit Modality Test; forward = Digit Span Forward; corpus callosum_mIns.tNAA = Corpus Callosum Cyoinositol/tN-acetyl aspartate; caudate_tNAA.tCr = Caudate Nucleus tN-Acetyl Aspartate/tCreatine; caudate_GABA.tCr = Caudate Nucleus γ-Aminobutyric acid/tCreatine.
Here is an arrangement of predictors according to RF (we show p-values).
- DSB series (Fisher’s exact test p-value < 0.001, decreased in MSp);
- Caudate nucleus mIns/tNAA (Wilcoxon’s p-value < 0.001; increased in MSp);
- Caudate nucleus mIns/tCr (Wilcoxon’s p-value < 0.001; increase in MSp);
- Caudate nucleus tCho/tNAA (Wilcoxon’s p-value < 0.001; increased in MSp);
- SDMT (Wilcoxon’s p-value < 0.001; decreased in MSp);
- DSF series (Fisher’s exact test p-value = 0.001, decreased in MSp);
- Corpus callosum mIns/tNAA (Wilcoxon’s p-value = 0.001; increased in MSp);
- Caudate nucleus tNAA/tCr (Wilcoxon’s p-value = 0.016; decreased in MSp);
- Caudate nucleus GABA/tCr (Wilcoxon’s p-value = 0.001; decreased in MSp).
3. Discussion
Our results confirm that MSp performed significantly worse than healthy controls on neurocognitive tests. The SDMT is currently considered the most sensitive instrument for evaluating processing speed in MS. Impairment in processing speed in MS has been shown to underlie deficits in working memory, executive function, and learning [13,14,15]. However, we also found substantial deficits in working memory and attention in MSp, as assessed by Digit Span tests. The Digit Span task assesses attention, encoding, auditory processing, and working memory capacity. Moreover, DSB was found to be the most sensitive test to identify MSp (See Figure 1 and Figure 2). These results support Beatty’s [20] hypothesis that MSp experience both a generalized difficulty in sustaining concentration and a more specific deficit in attentional resource allocation under multi-task demands.

Figure 2.
Ranking of predictors obtained by the graph depth method via random forest. On the y-axis, predictors are ranked from the most important at the top to the least important one, which is at the significance cutoff at the bottom. The x-axis represents the graph depth, with smaller values indicating greater importance of the predictor. Backward = Digit Span Backward; caudate_mIns.tNAA = Caudate Nucleus Myoinositol/tN-Acetyl Aspartate; caudate_mIns.tCr = Caudate Nucleus Myoinositol/t-Creatine; caudate_tCho.tNAA = Caudate Nucleus tCholin/tN-Acetyl Aspartate; SDMT = Single Digit Modality Test; forward = Digit Span Forward; corpus callosum_mIns.tNAA = Corpus Callosum Myoinositol/tN-Acetyl Aspartate; caudate_tNAA.tCr = Caudate Nucleus tN-Acetyl Aspartate/tCreatine; caudate_GABA.tCr = Caudate Nucleus γ-Aminobutyric acid/tCreatine.
To explore the neural correlates of cognitive dysfunction, we analyzed brain metabolites linked to performance on different cognitive tests.
3.1. SDMT
In our study, lower SDMT scores in GM of MSp correlated with the thalamic neuronal loss (decreased tNAA/tCr ratio reflects diminished neuronal integrity), hippocampal microglial activation (elevated mIns/tNAA was associated with poorer SDMT performance), and hypothalamic glutamate reduction (decreased Glx/tCr correlated with lower SDMT scores).
In the corpus callosum (WM), SDMT impairment was associated with increased choline (tCho/tCr), indicating intensified demyelination, and decreased GABA (GABA/tCr), suggesting reduced inhibitory neurotransmission.
SDMT performance has been previously linked to volumes of the thalamus, cerebellum, putamen, and occipital cortex [21,22]. Although we did not measure thalamic volume directly, metabolite-based findings (i.e., reduced tNAA/tCr) support the association between thalamic integrity and SDMT scores. In early-stage MS, we previously reported correlations between elevated tCho and mIns ratios and lower SDMT scores [23], suggesting that metabolic alterations may anticipate neuronal loss. Additionally, 7 T MRS studies in older versus younger individuals report higher glia-related metabolites (mIns, tCr, tCho), particularly in the hippocampus and thalamus [24]. Our hippocampal mIns/tNAA findings align with those results, supporting a potential role of sustained microglial activity in metabolic dysfunction and neurotoxicity in MS.
Heightened hypothalamic glutamate levels correlated with higher SDMT scores, potentially reflecting increased excitatory neurotransmission to support attention and processing speed via cortico-subcortical pathways.
Higher tCho/tNAA ratios in the corpus callosum corresponded with lower SDMT scores-consistent with increased demyelination and axonal loss [8,25]. Morphometric and diffusion studies have reinforced the role of corpus callosum integrity in cognitive processing speed, including SDMT performance [26]. Moreover, we observed a positive correlation between SDMT and GABA levels in the rostral corpus callosum. GABA is a key inhibitory neurotransmitter crucial for maintaining excitatory-inhibitory balance in cortical circuits. Previous GABA-focused MRS studies in MS did not specifically assess the corpus callosum [27,28,29], highlighting the novelty of our findings.
3.2. Digit Span Forward and Digit Span Backward Series
In contrast to SDMT scores, as the DSB and DSF series increase, the relative proportion of MSp decreases. It was challenging to identify MSp among individuals with high DSB and DSF scores. DSF and DSB scores, indicating better working memory, were positively correlated with increased mIns ratios in the caudate nucleus, suggesting microglial activation. Although this association has not previously been reported in MS, parallel findings in Human Immunodeficiency Virus (HIV) patients show that working memory deficits are linked to elevated glial metabolites, including mIns in the caudate and associated white matter networks [30].
The caudate nucleus plays a vital role in strategic planning, goal-directed behavior, and both visual and verbal working memory via cortico-subcortical circuits [31,32,33,34]. Functional MRI studies show either caudate nucleus or hippocampus activation specifically during spatial working memory tasks [31,32]. Conversely, SDMT performance relies primarily on information processing speed and engages frontoparietal, visual-attention, and cerebellar networks, with minimal basal ganglia involvement [26].
In our cohort, higher DSF scores correlated with increased hippocampal GABA levels. Preclinical and clinical studies indicate that reduced GABAergic inhibition impairs memory and attention [35] and that lower GABA levels in the prefrontal cortex are linked to greater working memory decline under cognitive load [36]. Moreover, reduced hippocampal GABA has been observed in MS patients [27,28] and is associated with cognitive and motor deficits [23,37].
The GABAergic system is critical for synchronizing large neuronal populations. Inhibitory projections, such as those connecting the medial septum, hippocampus, and entorhinal cortex, are crucial for modulating oscillatory activity underlying episodic memory formation [38].
Additionally, both DSF and DSB scores were negatively correlated with hypothalamic tNAA/tCr and positively with tCho/tNAA, indicating neuronal loss and demyelination. Although comparable data are scarce, these findings align with the known role of the hypothalamus in cortico-subcortical pathways that support learning and motivated behavior [39].
Finally, our data extend findings by Llufriu et al. [40], who identified mIns/tNAA in normal-appearing white matter as predictive of disability in MS. We similarly observed correlations of mIns/tCr and tNAA/tCr in the corpus callosum with reduced DSF and DSB scores, reinforcing the use of these metabolite ratios as biomarkers of cognitive decline.
3.3. Limitations of the Study and Suggestions for Further Research
Our study also has several limitations. First, we tested a relatively small number of participants. Second, from a technical perspective, employing absolute metabolite quantification rather than ratio-based analysis could provide more precise biochemical measurements. Additionally, advanced 1H-MRS protocols with higher spatial resolution (7T MRI systems) may improve the detection of localized pathological changes in the brain subregions and also the brain cortex. fMRI studies and evaluation of MRI-tractography could also bring better insights into functional connections between activated brain regions. Correlation with serum biomarkers, such as Neurofilament Light Chain and Glial Fibrillary Acidic Protein, could be another option. These methodological refinements could significantly enhance the future investigations of structure–function relationships in MS-related cognitive pathology.
4. Methods
4.1. Patients
Patients fulfilling the criteria for definite MS according to McDonald 2017 criteria [2] were randomly selected from the Multiple Sclerosis Centre, University Hospital in Martin, Slovakia, and they were entered into the study after providing written consent. The inclusion criteria were as follows: relapsing course of disease, absence of clinical relapse and corticosteroid treatment within at least three months before study entry, Expanded Disability Status Scale (EDSS) score of ≤5.0, age between 18 and 55 years, and the ability and willingness to cooperate in examinations. The exclusion criteria were severe comorbidities and acute depressive symptomatology confirmed by psychological examination.
We recruited 24 MSp (10 males; 14 females) and 23 CONs (7 males; 16 females), who were age-, education-, and gender-matched. Out of all participants, data from 2 MSp (1 male; 1 female) were not complete, and 1H-MRS data from 1 CON subject (1 female) did not meet the technical quality criteria for evaluation, so these subjects were excluded from the analyses. Finally, 22 MSp (9 males, 40.9%; 13 females, 59.1%) and 22 CONs (7 males; 15 females) participated in the study.
In MSp, clinical disability was evaluated by a neurologist certified in EDSS assessment. Cognitive status was tested using the SDMT, DSF, and DSB from a neuropsychological battery, WAIS-IV, by an experienced neuropsychologist.
The SDMT is based on quickly pairing geometric symbols with corresponding numbers. It was performed orally for 90 s. The DSF and DSB tests were carried out as follows: the participant heard a sequence of digits and then immediately verbally repeated the sequence, either as it was heard (forwards) or in reverse order (backwards). If the participant responded correctly, the subsequent trial presented a more extended sequence. The task terminated when participants responded incorrectly on three occasions within a span length. A participant’s span comprised the longest number of sequential digits that the could accurately remember. Test duration was a maximum of 1 to 3 min. Both MSp and CONs were tested under the same laboratory conditions.
4.2. Mescher–Garwood GABA-Edited 1H-MRS
Measurements were performed on a 3 T whole-body MR scanner (TIM Trio®, Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil (SiemensHealthcare, Erlangen, Germany) for signal reception. To ensure accurate and reproducible slice positioning, auto-align and three-dimensional T1-weighted anatomical reference images were used (magnetization-prepared two rapid acquisition gradient echoes—MP2RAGE, TR/TE = 4600/3.2 ms, resolution = 0.8 × 0.8 × 0.8 mm3). MRS- measurements were performed using a three-dimensional MEGA-edited sequence (TR/TE = 1600/68 ms, 32 averages, with a measurement time of ~20 min) with localized adiabatic spin echo refocusing selection and spiral encoding [9,10]. For real-time correction, volumetric, dual-contrast, echo planar imaging-based navigators that update B0 shim, frequency, and motion artifacts were used. All 1H-MRS slices were placed parallel to the anterior commissure–posterior commissure line, covering the centrum semiovale and basal ganglia, with a volume of interest (VOI) of 90 × 80 × 50 mm3, a field of view (FOV) of 160 × 160 × 80 mm3, and ~2 cc nominal resolution. The acquired matrix size of 10 × 10 × 10 voxels was interpolated to the 16 × 16 × 16 matrix of multivoxel (CSI, chemical shift imaging) representation.
Required regions were derived based on T1-weighted images, resulting in the selection of areas without the detectable manifestation of focal WM or GM lesions. All MR-spectra were evaluated in LCModel software (Version 6.3–1, S. Provencher, LC Model, Oakville, ON, Canada) for obtaining the following metabolite ratios tNAA/tCr, tCho/tNAA, tCho/tCr, mIns/tNAA, mIns/tCr, Glx/tNAA, Glx/tCr, GABA/tNAA, and GABA/tCr.
For further analytical processes, all selected voxels had to fulfill quality criteria, including a signal-to-noise ratio of over 10 and a full width at half maximum of less than 0.10 ppm.
We chose brain regions that have connections to pathways responsible for cognition and memory. We examined metabolic changes in parts of GM and WM that were independent of the presence of demyelinating lesions.
4.3. Statistical Analyses
All statistical analyses were performed in NCSS (version 9.0, LLC, Kaysville, UT, USA). Differences between women and men were not significant. One-way ANOVA was used for statistical analyses that found no differences between metabolic values in the left and right voxels. Differences in demographic and clinical parameters (age, SDMT, DSF, DSB, EDSS) and GABA-edited 1H-MRS metabolite ratios between patients and controls were evaluated using Wilcoxon’s rank-sum test, Fisher’s exact test, or one-way ANOVA with Bonferroni’s post hoc test. The Cochrane–Armitage test was applied to compare the trends of the results of the DSF and DSB series. A random forest (RF) classification algorithm was trained with the default settings of the rfsrc function from the R library RandomForestSRC. Both psychological variables and 1H-MRS metabolite ratios concentrations were used as predictors. The performance of the trained classifier was assessed via the Out-Of-Bag ROC curve and AUC. The predictor ranking was obtained using the variable importance (VIMP) and the graph depth.
5. Conclusions
We can conclude that SDMT, DSB, and DSF are the most reliable and sensitive neuropsychological tests, suggesting that they could serve as a day-to-day screening method for neuropsychological impairment in MSp. They are easy-to-perform, non-demanding tests, taking only several minutes. Moreover, the DSB test was found to be the most sensitive in determining MS-related working memory impairments. Additionally, our data show unique metabolic changes in the deep GM and WM of the brain in correlation with cognitive tests. The results confirm the hypothesis that MS is a whole-brain disease.
Author Contributions
E.K. (Ema Kantorová), P.H. and W.B. contributed to the conception and design of the study; J.G., P.H., M.G. and L.K. contributed to the acquisition and analysis of data; J.G., N.K., P.H. and E.K. (Ema Kantorová). contributed to drafting the text or preparing the figures; E.K. (Egon Kurča), P.H. and M.G. revising it critically for important intellectual content. J.G.: writing—original draft, investigation, data curation, conceptualization; M.G.: writing—review and editing, supervision, methodology, funding acquisition, conceptualization; P.H.: writing—review and editing, methodology, funding acquisition; W.B.: supervision, methodology, conceptualization; N.K.: investigation, data curation; L.K.: methodology, investigation, data curation; E.K. (Ema Kantorová): writing—review and editing, supervision, methodology, funding acquisition, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Grant VEGA of The Ministry of Education, Research, Development and Youth of the Slovak Republic, no. 1/0092/22. The funding sources had no involvement in the study design; the collection, analysis, and interpretation of the data; or in the decision to submit this article for publication. The corresponding author had the final responsibility for the decision to submit for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Jessenius Faculty of Medicine in Martin, Comenius University Bratislava, No. EK1849/2016 and No. EK 27/2021, for studies involving humans (approval date: 22 July 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
No new data were created.
Acknowledgments
Generative AI and AI-assisted technologies were used in the writing process to improve the readability and language of the manuscript.
Conflicts of Interest
Ján Grossmann has nothing to declare; Marián Grendár has nothing to declare; Petra Hnilicová has nothing to declare; Nina Kováčiková has nothing to declare; Lucia Kotuľová has nothing to declare; Wolfgang Bogner has nothing to declare; in the last five years, Ema Kantorová and Egon Kurča have had travel expenses or registration fees prepaid or reimbursed to present at international conferences.
Abbreviations
| MS | Multiple Sclerosis |
| MSp | Multiple Sclerosis Patients |
| DSF | Digit Span Forward |
| DSB | Digit Span Backward |
| SDMT | Single Digit Modality Test |
| 1H-MRS | 1-Proton Magnetic Resonance Spectroscopy |
| WM | White Matter |
| GM | Gray Matter |
| Glx | Glutamine and Glutamate |
| GABA | γ-Amino Butyric Acid |
| CONs | Healthy Volunteers |
| MEGA-edited | Mescher–Garwood-edited |
| tNAA | N-Acetyl-Aspartate |
| mIns | Myoinositol |
| tCho | Choline |
| tCr | Creatine |
| ATP | Adenosine TriPhosphate |
| EDSS | Expanded Disability Status State |
| WAIS-IV | Wechsler Adult Intelligence Scale |
| CSI | Chemical Shift Imaging |
| fMRI | Functional Magnetic Resonance Imaging |
| HIV | Human Immunodeficiency Virus |
| 7 T | 7 Tesla |
References
- Bobholz, J.; Rao, S. Cognitive dysfunction in multiple sclerosis: A review of recent developments. Curr. Opin. Neurol. 2003, 16, 283–288. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A.; et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology 2018, 90, 278–288. [Google Scholar] [CrossRef]
- Öz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. MRS Consensus Group. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abreu, C.M.; Gama, L.; Krasemann, S.; Chesnut, M.; Odwin-Dacosta, S.; Hogberg, H.T.; Hartung, T.; Pamies, D. Microglia Increase Inflammatory Responses in iPSC-Derived Human BrainSpheres. Front. Microbiol. 2018, 9, 2766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantero-Fortiz, Y.; Boada, M. The role of inflammation in neurological disorders: A brief overview of multiple sclerosis, Alzheimer’s, and Parkinson’s disease’. Front. Neurol. 2024, 15, 1439125. [Google Scholar] [CrossRef] [PubMed]
- Musella, A.; Gentile, A.; Rizzo, F.R.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; et al. Interplay Between Age and Neuroinflammation in Multiple Sclerosis: Effects on Motor and Cognitive Functions. Front. Aging Neurosci. 2018, 10, 238. [Google Scholar] [CrossRef]
- Hnilicová, P.; Štrbák, O.; Kolisek, M.; Kurča, E.; Zeleňák, K.; Sivák, Š.; Kantorová, E. Current Methods of Magnetic Resonance for Noninvasive Assessment of Molecular Aspects of Pathoetiology in Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 6117. [Google Scholar] [CrossRef]
- Bogner, W.; Gagoski, B.; Hess, A.T.; Bhat, H.; Tisdall, M.D.; van der Kouwe, A.J.; Strasser, B.; Marjańska, M.; Trattnig, S.; Grant, E.; et al. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. Neuroimage 2014, 103, 290–302. [Google Scholar] [CrossRef]
- Hnilicová, P.; Považan, M.; Strasser, B.; Andronesi, O.C.; Gajdošík, M.; Dydak, U.; Ukropec, J.; Dobrota, D.; Trattnig, S.; Bogner, W. Spatial variability and reproducibility of GABA-edited MEGA-LASER 3D-MRSI in the brain at 3 T. NMR Biomed. 2016, 29, 1656–1665. [Google Scholar] [CrossRef]
- Rae, C.D. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 2014, 39, 1–36. [Google Scholar] [CrossRef]
- Hnilicova, P.; Kantorova, E.; Sutovsky, S.; Grofik, M.; Zelenak, K.; Kurca, E.; Zilka, N.; Parvanovova, P.; Kolisek, M. Imaging Methods Applicable in the Diagnostics of Alzheimer’s Disease, Considering the Involvement of Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 3325. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.; Cookfair, D.; Gavett, R.; Gunther, M.; Munschauer, F.; Garg, N.; Weinstock-Guttman, B. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J. Int. Neuropsychol. Soc. 2006, 12, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.; DeLuca, J.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Multiple Sclerosis Outcome Assessments Consortium. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. J. 2017, 23, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Cagna, C.J.; Dobryakova, E.; Weber, E.; Maloku, D.; Chiaravalloti, N.D.; Genova, H.M.; Costa, S.L.; DeLuca, J. Trait fatigue impacts Symbol Digit Modalities Test (SDMT) performance in multiple sclerosis: The role of working memory. Mult. Scler. Relat. Disord. 2025, 96, 106369. [Google Scholar] [CrossRef]
- Dusankova, J.B.; Kalincik, T.; Havrdova, E.; Benedict, R.H. Cross cultural validation of the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS) and the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Clin. Neuropsychol. 2012, 26, 1186–1200. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Müller-Lenke, N.; Naegelin, Y.; Kalt, G.; Bendfeldt, K.; Kuster, P.; Stoecklin, M.; Gass, A.; Sprenger, T.; Radue, E.W.; et al. Contribution of cortical and white matter lesions to cognitive impairment in multiple sclerosis. Mult. Scler. J. 2013, 19, 1290–1296. [Google Scholar] [CrossRef]
- Balsimelli, S.; Mendes, M.F.; Bertolucci, P.H.; Tilbery, C.P. Attention impairment associated with relapsing-remitting multiple sclerosis patients with mild incapacity. Arq. Neuropsiquiatr. 2007, 65, 262–267. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef]
- Beatty, W.W.; Paul, R.H.; Blanco, C.R.; Hames, K.A.; Wilbanks, S.L. Attention in multiple sclerosis: Correlates of impairment on the WAIS-R Digit Span Test. Appl. Neuropsychol. 1995, 2, 139–144. [Google Scholar] [CrossRef]
- Bisecco, A.; Stamenova, S.; Caiazzo, G.; d’Ambrosio, A.; Sacco, R.; Docimo, R.; Esposito, S.; Cirillo, M.; Esposito, F.; Bonavita, S.; et al. Attention and processing speed performance in multiple sclerosis is mostly related to thalamic volume. Brain Imaging Behav. 2018, 12, 20–28. [Google Scholar] [CrossRef]
- Bergsland, N.; Zivadinov, R.; Dwyer, M.G.; Weinstock-Guttman, B.; Benedict, R.H. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult. Scler. J. 2015, 22, 1327–1336. [Google Scholar] [CrossRef]
- Kantorova, E.; Hnilicova, P.; Bogner, W.; Grendar, M.; Grossmann, J.; Kováčová, S.; Hečková, E.; Strasser, B.; Čierny, D.; Zeleňák, K.; et al. Neurocognitive performance in relapsing-remitting multiple sclerosis patients is associated with metabolic abnormalities of the thalamus but not the hippocampus– GABA-edited 1H MRS study. Neurol. Res. 2022, 44, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lind, A.; Boraxbekk, C.J.; Petersen, E.T.; Paulson, O.B.; Siebner, H.R.; Marsman, A. Regional Myo-Inositol, Creatine, and Choline Levels Are Higher at Older Age and Scale Negatively with Visuospatial Working Memory: A Cross-Sectional Proton MR Spectroscopy Study at 7 Tesla on Normal Cognitive Ageing. J. Neurosci. 2020, 40, 8149–8159. [Google Scholar] [CrossRef] [PubMed]
- Swanberg, K.M.; Landheer, K.; Pitt, D.; Juchem, C. Quantifying the Metabolic Signature of Multiple Sclerosis by in vivo Proton Magnetic Resonance Spectroscopy: Current Challenges and Future Outlook in the Translation from Proton Signal to Diagnostic Biomarker. Front. Neurol. 2019, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.H.R.D.; Spedo, C.T.; Baldassarini, C.R.; Benini, C.D.; Ferreira, D.A.; Barreira, A.A.; Leoni, R.F. Brain functional and effective connectivity underlying the information processing speed assessed by the Symbol Digit Modalities Test. Neuroimage 2019, 184, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yin, X.; Edden, R.A.; Evans, A.C.; Xu, J.; Cao, G.; Li, H.; Li, M.; Zhao, B.; Wang, J.; et al. Altered hippocampal GABA and glutamate levels and uncoupling from functional connectivity in multiple sclerosis. Hippocampus 2018, 28, 813–823. [Google Scholar] [CrossRef]
- Cao, G.; Edden, R.A.; Gao, F.; Li, H.; Gong, T.; Chen, W.; Liu, X.; Wang, G.; Zhao, B. Reduced GABA levels correlate with cognitive impairment in patients with relapsing-remitting multiple sclerosis. Eur. Radiol. 2018, 28, 1140–1148. [Google Scholar] [CrossRef]
- Huiskamp, M.; Yaqub, M.; van Lingen, M.R.; Pouwels, P.J.; de Ruiter, L.R.; Killestein, J.; Schwarte, L.A.; Golla, S.S.; van Berckel, B.N.; Boellaard, R.; et al. Cognitive performance in multiple sclerosis: What is the role of the gamma-aminobutyric acid system? Brain Commun. 2023, 5, fcad140. [Google Scholar] [CrossRef]
- Ernst, T.; Chang, L.; Arnold, S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage 2003, 19, 1686–1693. [Google Scholar] [CrossRef]
- Postle, B.R.; D’Esposito, M. Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: An event-related fMRI study. Cogn. Brain Res. 1999, 8, 107–115. [Google Scholar] [CrossRef]
- Iaria, G.; Petrides, M.; Dagher, A.; Pike, B.; Bohbot, V.D. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J. Neurosci. 2003, 23, 5945–5952. [Google Scholar] [CrossRef]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The Cognitive Functions of the Caudate Nucleus. Prog. Neurobiol. 2007, 86, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Monchi, O.; Petrides, M.; Strafella, A.; Worsley, K.; Doyon, J. Functional role of the basal ganglia in the planning and execution of actions. Ann. Neurol. 2006, 59, 257–264. [Google Scholar] [CrossRef] [PubMed]
- McGarrity, S.; Mason, R.; Fone, K.C.; Pezze, M.; Bast, T. Hippocampal Neural Disinhibition Causes Attentional and Memory Deficits. Cereb. Cortex 2017, 27, 4447–4462. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W. Microglia in multiple sclerosis: Protectors turn destroyers. Neuron 2022, 21, 3534–3548. [Google Scholar] [CrossRef]
- Cawley, N.; Solanky, B.S.; Muhlert, N.; Tur, C.; Edden, R.A.; Wheeler-Kingshott, C.A.; Miller, D.H.; Thompson, A.J.; Ciccarelli, O. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 2015, 138, 2584–2595. [Google Scholar] [CrossRef]
- Schmidt-Wilcke, T.; Fuchs, E.; Funke, K.; Vlachos, A.; Müller-Dahlhaus, F.; Puts, N.A.J.; Harris, R.E.; Edden, R.A. GABA—From Inhibition to Cognition: Emerging Concepts. Neuroscientist 2017, 24, 501–515. [Google Scholar] [CrossRef]
- Averbeck, B.B.; Murray, E.A. Hypothalamic Interactions with Large-Scale Neural Circuits Underlying Reinforcement Learning and Motivated Behavior. Trends Neurosci. 2020, 43, 681–694. [Google Scholar] [CrossRef]
- Llufriu, S.; Kornak, J.; Ratiney, H.; Oh, J.; Brenneman, D.; Cree, B.A.; Sampat, M.; Hauser, S.L.; Nelson, S.J.; Pelletier, D. Magnetic resonance spectroscopy markers of disease progression in multiple sclerosis. JAMA Neurol. 2014, 71, 840–847. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).