Zebrafish as a Model Organism for Research in Rare Genetic Neuromuscular Diseases
Abstract
1. Introduction
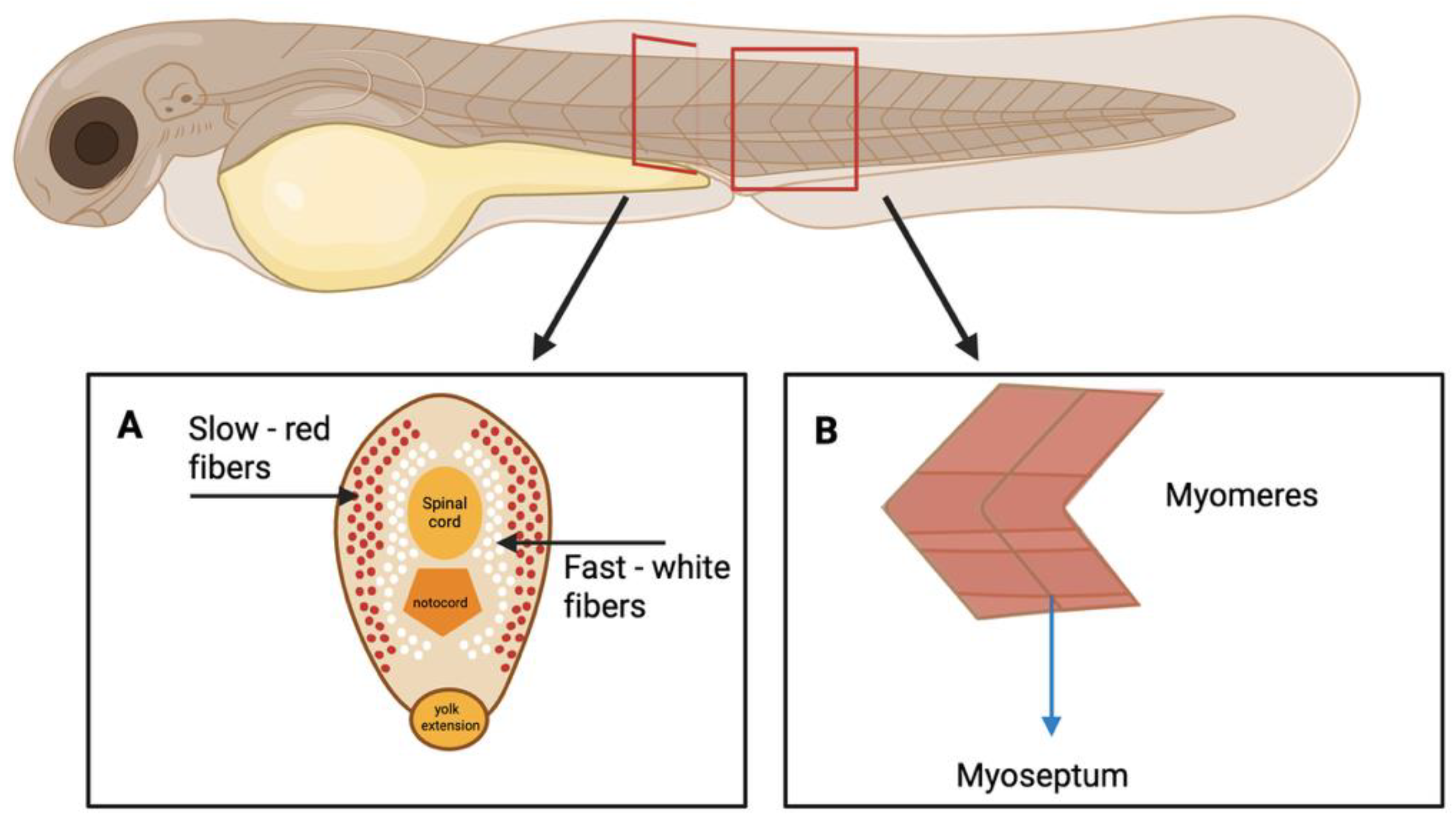
1.1. Zebrafish Neuromuscular System Organization
1.2. Zebrafish Genome Editing
2. Rare Neuromuscular Disease
2.1. Duchenne Muscular Dystrophy (DMD)
2.2. Limb Girdle Muscular Dystrophies (LGMDs)
2.3. Brody Myopathy (BM)
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Teucke, M. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of Clones of Homozygous Diploid Zebra Fish (Brachydanio Rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef]
- Haffter, P.; Odenthal, J.; Mullins, M.C.; Lin, S.; Farrell, M.J.; Vogelsang, E.; Haas, F.; Brand, M.; van Eeden, F.J.; Furutani-Seiki, M.; et al. Mutations Affecting Pigmentation and Shape of the Adult Zebrafish. Dev. Genes Evol. 1996, 206, 260–276. [Google Scholar] [CrossRef]
- Panula, P.; Sallinen, V.; Sundvik, M.; Kolehmainen, J.; Torkko, V.; Tiittula, A.; Moshnyakov, M.; Podlasz, P. Modulatory Neurotransmitter Systems and Behavior: Towards Zebrafish Models of Neurodegenerative Diseases. Zebrafish 2006, 3, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, A.; Guo, S.; Masai, I.; Nicolson, T.; Wu, C.F. Zebrafish: From Genes and Neurons to Circuits, Behavior and Disease. J. Neurogenet. 2017, 31, 59–60. [Google Scholar] [CrossRef]
- Phelan, P.E.; Pressley, M.E.; Witten, P.E.; Mellon, M.T.; Blake, S.; Kim, C.H. Characterization of Snakehead Rhabdovirus Infection in Zebrafish (Danio Rerio). J. Virol. 2005, 79, 1842–1852. [Google Scholar] [CrossRef]
- Ludwig, M.; Palha, N.; Torhy, C.; Briolat, V.; Colucci-Guyon, E.; Bremont, M.; Herbomel, P.; Boudinot, P.; Levraud, J.P. Whole-Body Analysis of a Viral Infection: Vascular Endothelium Is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae. PLoS Pathog. 2011, 7, e1001269. [Google Scholar] [CrossRef] [PubMed]
- Swaim, L.E.; Connolly, L.E.; Volkman, H.E.; Humbert, O.; Born, D.E.; Ramakrishnan, L. Mycobacterium Marinum Infection of Adult Zebrafish Causes Caseating Granulomatous Tuberculosis and Is Moderated by Adaptive Immunity. Infect. Immun. 2006, 74, 6108–6117. [Google Scholar] [CrossRef] [PubMed]
- Levraud, J.P.; Disson, O.; Kissa, K.; Bonne, I.; Cossart, P.; Herbomel, P.; Lecuit, M. Real-Time Observation of Listeria Monocytogenes-Phagocyte Interactions in Living Zebrafish Larvae. Infect. Immun. 2009, 77, 3651–3660. [Google Scholar] [CrossRef]
- Saralahti, A.; Rämet, M. Zebrafish and Streptococcal Infections. Scand. J. Immunol. 2015, 82, 174–183. [Google Scholar] [CrossRef]
- Petrie-Hanson, L.; Hohn, C.; Hanson, L. Characterization of Rag1 Mutant Zebrafish Leukocytes. BMC Immunol. 2009, 10, 8–10. [Google Scholar] [CrossRef]
- Zarkadis, I.K.; Mastellos, D.; Lambris, J.D. Phylogenetic Aspects of the Complement System. Dev. Comp. Immunol. 2001, 25, 745–762. [Google Scholar] [CrossRef]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and Maturation of the Immune System in Zebrafish, Danio Rerio: A Gene Expression Profiling, in Situ Hybridization and Immunological Study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, K.; Sips, P.; Macrae, C.A. Screening Drugs for Myocardial Disease in Vivo with Zebrafish: An Expert Update. Expert Opin. Drug Discov. 2019, 14, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Genge, C.E.; Lin, E.; Lee, L.; Sheng, X.; Rayani, K.; Gunawan, M.; Tibbits, G.F. The Zebrafish Heart as a Model of Mammalian Cardiac Function. Rev. Physiol. Biochem. Pharmacol. 2016, 171, 99–136. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.; Arnaout, R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef]
- Poon, K.L.; Brand, T. The Zebrafish Model System in Cardiovascular Research: A Tiny Fish with Mighty Prospects. Glob. Cardiol. Sci. Pract. 2013, 2013, 9–28. [Google Scholar] [CrossRef]
- Rafferty, S.A.; Quinn, T.A. A Beginner’s Guide to Understanding and Implementing the Genetic Modification of Zebrafish. Prog. Biophys. Mol. Biol. 2018, 138, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Cagan, R.L.; Zon, L.I.; White, R.M. Modeling Cancer with Flies and Fish. Dev. Cell 2019, 49, 317–324. [Google Scholar] [CrossRef]
- Barriuso, J.; Nagaraju, R.; Hurlstone, A. Zebrafish: A New Companion for Translational Research in Oncology. Clin. Cancer Res. 2015, 21, 969–975. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Griffin, A.; Hamling, K.R.; Hong, S.; Anvar, M.; Lee, L.P.; Baraban, S.C. Preclinical Animal Models for Dravet Syndrome: Seizure Phenotypes, Comorbidities and Drug Screening. Front. Pharmacol. 2018, 9, 573. [Google Scholar] [CrossRef]
- Goessling, W.; Sadler, K.C. Zebrafish: An Important Tool for Liver Disease Research. Gastroenterology 2015, 149, 1361–1377. [Google Scholar] [CrossRef] [PubMed]
- Cirio, M.C.; De Caestecker, M.P.; Hukriede, N.A. Zebrafish Models of Kidney Damage and Repair. Curr. Pathobiol. Rep. 2015, 3, 163–170. [Google Scholar] [CrossRef]
- Rissone, A.; Burgess, S.M. Rare Genetic Blood Disease Modeling in Zebrafish. Front. Genet. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Karuppasamy, M.; English, K.G.; Henry, C.A.; Manzini, M.C.; Parant, J.M.; Wright, M.A.; Ruparelia, A.A.; Currie, P.D.; Gupta, V.A.; Dowling, J.J.; et al. Standardization of Zebrafish Drug Testing Parameters for Muscle Diseases. Dis. Model. Mech. 2024, 17, dmm050339. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, A.; Pitto, L.; Fiorillo, C.; Alice Donati, M.; Bruno, C.; Santorelli, F.M. Neuromuscular Disorders in Zebrafish: State of the Art and Future Perspectives. NeuroMolecular Med. 2013, 15, 405–419. [Google Scholar] [CrossRef]
- Singh, J.; Patten, S.A. Modeling Neuromuscular Diseases in Zebrafish. Front. Mol. Neurosci. 2022, 15, 1054573. [Google Scholar] [CrossRef]
- Moreno, R.L.; Ribera, A.B. Zebrafish Motor Neuron Subtypes Differ Electrically Prior to Axonal Outgrowth. J. Neurophysiol. 2009, 102, 2477–2484. [Google Scholar] [CrossRef][Green Version]
- Bone, Q. Locomotor Muscle; Academic Press: Cambridge, MA, USA, 1978; Volume 7, p. 424. [Google Scholar] [CrossRef]
- Devoto, S.H.; Melançon, E.; Eisen, J.S.; Westerfield, M. Identification of Separate Slow and Fast Muscle Precursor Cells in Vivo, Prior to Somite Formation. Development 1996, 122, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Guyon, J.R.; Steffen, L.S.; Howell, M.H.; Pusack, T.J.; Lawrence, C.; Kunkel, L.M. Modeling Human Muscle Disease in Zebrafish. BBA-Mol. Basis Dis. 2007, 1772, 205–215. [Google Scholar] [CrossRef]
- Altringham, J.D.; Ellerby, D.J. Fish Swimming: Patterns in Muscle Function. J. Exp. Biol. 1999, 202 Pt 23, 3397–3403. [Google Scholar] [CrossRef]
- Stickney, H.L.; Barresi, M.J.; Devoto, S.H. Somite Development in Zebrafish. Dev. Dyn. 2000, 219, 287–303. [Google Scholar] [CrossRef]
- Charvet, B.; Malbouyres, M.; Pagnon-Minot, A.; Ruggiero, F.; Le Guellec, D. Development of the Zebrafish Myoseptum with Emphasis on the Myotendinous Junction. Cell Tissue Res. 2011, 346, 439–449. [Google Scholar] [CrossRef]
- Gibbs, E.M.; Horstick, E.J.; Dowling, J.J. Swimming into Prominence: The Zebrafish as a Valuable Tool for Studying Human Myopathies and Muscular Dystrophies. FEBS J. 2013, 280, 4187–4197. [Google Scholar] [CrossRef]
- Kawakami, K.; Shima, A.; Kawakami, N. Identification of a Functional Transposase of the Tol2 Element, an Ac-like Element from the Japanese Medaka Fish, and Its Transposition in the Zebrafish Germ Lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 11403–11408. [Google Scholar] [CrossRef]
- Klem, J.R.; Gray, R.; Lovely, C.B. The Zebrafish Tol2 System: A Modular and Flexible Gateway-Based Transgenesis Approach. J. Vis. Exp. 2022, 189, 10–3791. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Zhang, S.; He, X. Progress in Gene-Editing Technology of Zebrafish. Biomolecules 2021, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Medishetti, R.; Balamurugan, K.; Yadavalli, K.; Rani, R.; Sevilimedu, A.; Challa, A.K.; Chatti, K. CRISPR-Cas9-Induced Gene Knockout in Zebrafish. STAR Protoc. 2022, 3, 101779. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M. The Genome Editing Revolution. J. Genet. Eng. Biotechnol. 2020, 18, 396–409. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Platt, R.J.; Zhang, F. Therapeutic Genome Editing: Prospects and Challenges. Nat. Med. 2015, 21, 121–131. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Bhatt, J.M. The Epidemiology of Neuromuscular Diseases. Neurol. Clin. 2016, 34, 999–1021. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish Disease Models in Drug Discovery: From Preclinical Modelling to Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Ricci, G.; Torri, F.; Bianchi, F.; Fontanelli, L.; Schirinzi, E.; Gualdani, E.; Francesconi, P.; Gagliardi, D.; Serra, G.; Mongini, T.; et al. Frailties and Critical Issues in Neuromuscular Diseases Highlighted by SARS-CoV-2 Pandemic: How Many Patients Are Still “Invisible”? Acta Myol. 2022, 41, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Shilling, C.; Leslie, N.D.; Flanigan, K.M.; al-Dahhak, R.; Gastier-Foster, J.; Weiss, R.B. Evidence-based Path to Newborn Screening for Duchenne Muscular Dystrophy. Ann. Neurol. 2012, 71, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.P.; Neve, R.L.; Colletti-Feener, C.; Bertelson, C.J.; Kurnit, D.M.; Kunkel, L.M. Isolation of Candidate cDNAs for Portions of the Duchenne Muscular Dystrophy Gene. Nature 1986, 323, 646–650. [Google Scholar] [CrossRef]
- Crone, M.; Mah, J.K. Current and Emerging Therapies for Duchenne Muscular Dystrophy. Curr. Treat. Options Neurol. 2018, 20, 31. [Google Scholar] [CrossRef]
- Farr, G.H.; Morris, M.; Gomez, A.; Pham, T.; Kilroy, E.; Parker, E.U.; Maves, L. A Novel Chemical-Combination Screen in Zebrafish Identifies Epigenetic Small Molecule Candidates for the Treatment of Duchenne Muscular Dystrophy. Skelet. Muscle 2020, 10, 29. [Google Scholar] [CrossRef]
- Cui, Y.; Shao, S.; Zhang, L.; Wu, J.; Ma, F.; Cai, X.; Wang, C. The Effects of Glucocorticoids on Cardiac Function of Patients with Duchenne Muscular Dystrophy: Benefit or Not? Eur. J. Pediatr. 2025, 184, 313. [Google Scholar] [CrossRef]
- Patterson, G.; Conner, H.; Groneman, M.; Blavo, C.; Parmar, M.S. Duchenne Muscular Dystrophy: Current Treatment and Emerging Exon Skipping and Gene Therapy Approach. Eur. J. Pharmacol. 2023, 947, 175675. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal Models of Duchenne Muscular Dystrophy: From Basic Mechanisms to Gene Therapy. Dis. Models Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef]
- Kawahara, G.; Karpf, J.A.; Myers, J.A.; Alexander, M.S.; Guyon, J.R.; Kunkel, L.M. Drug Screening in a Zebrafish Model of Duchenne Muscular Dystrophy. Proc. Natl. Acad. Sci. USA 2011, 108, 5331–5336. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.R.; Spinazzola, J.M.; Widrick, J.J.; Pakula, A.; Conner, J.R.; Chin, J.E.; Kunkel, L.M. PDE10A Inhibition Reduces the Manifestation of Pathology in DMD Zebrafish and Represses the Genetic Modifier PITPNA. Mol. Ther. 2021, 29, 1086–1101. [Google Scholar] [CrossRef]
- Stocco, A.; Smolina, N.; Sabatelli, P.; Šileikytė, J.; Artusi, E.; Mouly, V.; Bernardi, P. Treatment with a Triazole Inhibitor of the Mitochondrial Permeability Transition Pore Fully Corrects the Pathology of Sapje Zebrafish Lacking Dystrophin. Pharmacol. Res. 2021, 165, 105421. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Eeden, F.J.V.; Schach, U.; Trowe, T.; Brand, M.; Furutani-Seiki, M.; Nüsslein-Volhard, C. Genes Controlling and Mediating Locomotion Behavior of the Zebrafish Embryo and Larva. Development 1996, 123, 399–413. [Google Scholar] [CrossRef]
- Guyon, J.R.; Goswami, J.; Jun, S.J.; Thorne, M.; Howell, M.; Pusack, T.; Kunkel, L.M. Genetic Isolation and Characterization of a Splicing Mutant of Zebrafish Dystrophin. Hum. Mol. Genet. 2009, 18, 202–211. [Google Scholar] [CrossRef]
- Bassett, D.I.; Bryson-Richardson, R.J.; Daggett, D.F.; Gautier, P.; Keenan, D.G.; Currie, P.D. Dystrophin Is Required for the Formation of Stable Muscle Attachments in the Zebrafish Embryo. Development 2003, 130, 5851–5860. [Google Scholar] [CrossRef]
- Berger, J.; Berger, S.; Hall, T.E.; Lieschke, G.J.; Currie, P.D. Dystrophin-Deficient Zebrafish Feature Aspects of the Duchenne Muscular Dystrophy Pathology. J. Neuromuscul. Dis. 2010, 20, 826–832. [Google Scholar] [CrossRef]
- Kawahara, G.; Kunkel, L.M. Zebrafish Based Small Molecule Screens for Novel DMD Drugs. Drug Discov. Today Technol. 2013, 10, e91–e96. [Google Scholar] [CrossRef]
- Widrick, J.J.; Alexander, M.S.; Sanchez, B.; Gibbs, D.E.; Kawahara, G.; Beggs, A.H.; Kunkel, L.M. Muscle Dysfunction in a Zebrafish Model of Duchenne Muscular Dystrophy. Physiol. Genom. 2016, 48, 850–860. [Google Scholar] [CrossRef]
- Zulian, A.; Menazza, S.; Petronilli, V.; Argenton, F.; Merlini, L.; Sabatelli, P.; Bernardi, P. Alisporivir Rescues Defective Mitochondrial Respiration in Duchenne Muscular Dystrophy. Pharmacol. Res. 2017, 125, 122–131. [Google Scholar] [CrossRef]
- Widrick, J.J.; Kawahara, G.; Alexander, M.S.; Beggs, A.H.; Kunkel, L.M. Discovery of Novel Therapeutics for Muscular Dystrophies Using Zebrafish Phenotypic Screens. J. Neuromuscul. Dis. 2019, 6, 271–287. [Google Scholar] [CrossRef]
- Nesari, V.; Balakrishnan, S.; Nongthomba, U. JAG1 Overexpression Partially Rescues Muscle Function in a Zebrafish Model of Duchenne Muscular Dystrophy. J. Genet. 2025, 104, 2. [Google Scholar] [CrossRef]
- Lerma, G.; Ryhlick, K.R.; Carraher, O.M.; Beljan, J.C.; Amacher, S.L. Validation of Duchenne Muscular Dystrophy Candidate Modifiers Using a CRISPR-Cas9-Based Approach in Zebrafish. bioRxiv 2025. Preprint. [Google Scholar] [CrossRef]
- Nigro, V.; Savarese, M. Genetic Basis of Limb-Girdle Muscular Dystrophies: The 2014 Update. Acta Myol. 2014, 33, 1–12. [Google Scholar] [PubMed]
- Straub, V.; Murphy, A.; Udd, B.; Corrado, A.; Aymé, S.; Bönneman, C.; Walter, M. 229th ENMC International Workshop: Limb Girdle Muscular Dystrophies--Nomenclature and Reformed Classification Naarden, the Netherlands, 17--19 March 2017. J. Neuromuscul. Dis. 2018, 28, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Aurino, S.; Piluso, G. Limb Girdle Muscular Dystrophies: Update on Genetic Diagnosis and Therapeutic Approaches. Curr. Opin. Neurol. 2011, 24, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C. LGMD. Identification, Description and Classification. Acta Myol. 2020, 39, 207. [Google Scholar] [CrossRef]
- Georganopoulou, D.G.; Moisiadis, V.G.; Malik, F.A.; Mohajer, A.; Dashevsky, T.M.; Wuu, S.T.; Hu, C.K. A Journey with LGMD: From Protein Abnormalities to Patient Impact. Protein J. 2021, 40, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.B.; Sommerville, R.B.; Allred, P.; Bell, S.; Ma, D.; Cooper, P.; Baloh, R.H. Exome Sequencing Reveals DNAJB6 Mutations in Dominantly-inherited Myopathy. Ann. Neurol. 2012, 71, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hayashi, Y.K.; Oya, Y.; Kondo, T.; Sugie, K.; Kaneda, D.; Nishino, I. DNAJB6 Myopathy in an Asian Cohort and Cytoplasmic/Nuclear Inclusions. Neuromuscul. Disord. 2013, 23, 269–276. [Google Scholar] [CrossRef]
- Sarparanta, J.; Jonson, P.H.; Golzio, C.; Sandell, S.; Luque, H.; Screen, M.; Udd, B. Mutations Affecting the Cytoplasmic Functions of the Co-Chaperone DNAJB6 Cause Limb-Girdle Muscular Dystrophy. Nat. Genet. 2012, 44, 450–455. [Google Scholar] [CrossRef]
- Nam, T.S.; Li, W.; Heo, S.H.; Lee, K.H.; Cho, A.; Shin, J.H.; Choi, S.Y. A Novel Mutation in DNAJB6, p.(Phe91Leu), in Childhood-Onset LGMD1D with a Severe Phenotype. Neuromuscul. Disord. 2015, 25, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Melia, M.J.; Kubota, A.; Ortolano, S.; Vilchez, J.J.; Gamez, J.; Tanji, K.; Bonilla, E.; Palenzuela, L.; Fernandez-Cadenas, I.; Pristoupilova, A.; et al. Limb-Girdle Muscular Dystrophy 1F Is Caused by a Microdeletion in the Transportin 3 Gene. Brain 2013, 136, 1508–1517. [Google Scholar] [CrossRef]
- Torella, A.; Fanin, M.; Mutarelli, M.; Peterle, E.; Del Vecchio Blanco, F.; Rispoli, R.; Savarese, M.; Garofalo, A.; Piluso, G.; Morandi, L.; et al. Next-Generation Sequencing Identifies Transportin 3 as the Causative Gene for LGMD1F. PLoS ONE 2013, 8, e63536. [Google Scholar] [CrossRef]
- Vieira, N.M.; Naslavsky, M.S.; Licinio, L.; Kok, F.; Schlesinger, D.; Vainzof, M.; Sanchez, N.; Kitajima, J.P.; Gal, L.; Cavacana, N.; et al. A Defect in the RNA-Processing Protein HNRPDL Causes Limb-Girdle Muscular Dystrophy 1G (LGMD1G). Hum. Molec. Genet. 2014, 23, 4103–4110. [Google Scholar] [CrossRef]
- Martinez-Thompson, J.M.; Niu, Z.; Tracy, J.A.; Moore, S.A.; Swenson, A.; Wieben, E.D.; Milone, M. Autosomal dominant calpainopathy due to heterozygous CAPN3 c.643_663del21. Muscle Nerve 2018, 57, 679–683. [Google Scholar] [CrossRef]
- Vissing, J.; Barresi, R.; Witting, N.; Van Ghelue, M.; Gammelgaard, L.; Bindoff, L.A.; Straub, V.; Lochmuller, H.; Hudson, J.; Wahl, C.M.; et al. A Heterozygous 21-Bp Deletion in CAPN3 Causes Dominantly Inherited Limb Girdle Muscular Dystrophy. Brain 2016, 139, 2154–2163. [Google Scholar] [CrossRef]
- Jobsis, G.J.; Keizers, H.; Vreijling, J.P.; de Visser, M.; Speer, M.C.; Wolterman, R.A.; Baas, F.; Bohlhuis, P.A. Type VI Collagen Mutations in Bethlem Myopathy, an Autosomal Dominant Myopathy with Contractures. Nat. Genet. 1996, 14, 113–115. [Google Scholar] [CrossRef]
- Tonelotto, V.; Consorti, C.; Facchinello, N.; Trapani, V.; Sabatelli, P.; Giraudo, C.; Spizzotin, M.; Cescon, M.; Bertolucci, C.; Bonaldo, P. Collagen VI Ablation in Zebrafish Causes Neuromuscular Defects during Developmental and Adult Stages. Matrix Biol. 2022, 112, 39–61. [Google Scholar] [CrossRef]
- Radev, Z.; Hermel, J.M.; Elipot, Y.; Bretaud, S.; Arnould, S.; Duchateau, P.; Ruggiero, F.; Joly, J.S.; Sohm, F. A TALEN-Exon Skipping Design for a Bethlem Myopathy Model in Zebrafish. PLoS ONE 2015, 10, e0133986. [Google Scholar] [CrossRef] [PubMed]
- Telfer, W.R.; Busta, A.S.; Bonnemann, C.G.; Feldman, E.L.; Dowling, J.J. Zebrafish Models of Collagen VI-Related Myopathies. Hum. Mol. Genet. 2010, 19, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Richard, I.; Broux, O.; Allamand, V.; Fougerousse, F.; Chiannilkulchai, N.; Bourg, N.; Brenguier, L.; Devaud, C.; Pasturaud, P.; Roudaut, C.; et al. Mutations in the Proteolytic Enzyme Calpain 3 Cause Limb-Girdle Muscular Dystrophy Type 2A. Cell 1995, 81, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Prykhozhij, S.V.; Caceres, L.; Ban, K. Loss of Calpain3b in Zebrafish, a Model of Limb-Girdle Muscular Dystrophy, Increases Susceptibility to Muscle Defects Due to Elevated Muscle Activity. Genes 2023, 14, 492. [Google Scholar] [CrossRef]
- Chen, F.; Huang, D.; Shi, H. Capn3 Depletion Causes Chk1 and Wee1 Accumulation and Disrupts Synchronization of Cell Cycle Reentry during Liver Regeneration after Partial Hepatectomy. Cell Regen 2020, 9, 8. [Google Scholar] [CrossRef]
- Bashir, R.; Britton, S.; Strachan, T.; Keers, S.; Vafiadaki, E.; Lako, M.; Richard, I.; Marchand, S.; Bourg, N.; Argov, Z.; et al. A Gene Related to Caenorhabditis Elegans Spermatogenesis Factor Fer-1 Is Mutated in Limb-Girdle Muscular Dystrophy Type 2B. Nat. Genet. 1998, 20, 37–42. [Google Scholar] [CrossRef]
- Kawahara, G.; Serafini, P.R.; Myers, J.A.; Alexander, M.S.; Kunkel, L.M. Characterization of Zebrafish Dysferlin by Morpholino Knockdown. Biochem. Biophys. Res. Commun. 2011, 413, 358–363. [Google Scholar] [CrossRef][Green Version]
- Roostalu, U.; Strähle, U. In Vivo Imaging of Molecular Interactions at Damaged Sarcolemma. Develop. Cell 2012, 22, 515–529. [Google Scholar] [CrossRef]
- Roberds, S.L.; Leturcq, F.; Allamand, V.; Piccolo, F.; Jeanpierre, M.; Anderson, R.D.; Lim, L.E.; Lee, J.C.; Tomé, F.M.; Romero, N.B. Missense Mutations in the Adhalin Gene Linked to Autosomal Recessive Muscular Dystrophy. Cell 1994, 78, 625–633. [Google Scholar] [CrossRef]
- Romero, N.B.; Tome, F.M.S.; Leturcq, F.; El Kerch, F.; Azibi, K.; Bachner, L.; Anderson, R.D.; Roberds, S.L.; Campbell, K.P.; Fardeau, M.; et al. Genetic Heterogeneity of Severe Childhood Autosomal Recessive Muscular Dystrophy with Adhalin (50 kDa Dystrophin-Associated Glycoprotein) Deficiency. C. R. Acad. Sci. III 1994, 317, 70–76. [Google Scholar]
- Bönnemann, C.G.; Modi, R.; Noguchi, S.; Mizuno, Y.; Yoshida, M.; Gussoni, E.; McNally, E.M.; Duggan, D.J.; Angelini, C.; Hoffman, E.P.; et al. β-Sarcoglycan (A3b) Mutations Cause Autosomal Recessive Muscular Dystrophy with Loss of the Sarcoglycan Complex. Nat. Genet. 1995, 11, 266–273, Erratum in Nat. Genet. 1996, 12, 110. [Google Scholar] [CrossRef]
- Lim, L.E.; Duclos, F.; Broux, O.; Bourg, N.; Sunada, Y.; Allamand, V.; Meyer, J.; Richard, I.; Moomaw, C.; Slaughter, C. Beta-Sarcoglycan: Characterization and Role in Limb Girdle Muscular Dystrophy Linked to 4q12. Nat. Genet. 1995, 11, 257–265. [Google Scholar] [CrossRef]
- Dalla Barba, F.; Soardi, M.; Mouhib, L.; Risato, G.; Akyürek, E.E.; Lucon-Xiccato, T.; Sandonà, D. Modeling Sarcoglycanopathy in Danio Rerio. Int. J. Mol. Sci. 2023, 24, 12707. [Google Scholar] [CrossRef]
- Ben Othmane, K.; Ben Hamida, M.; Pericak-Vance, M.A.; Ben Hamida, C.; Blel, S.; Carter, S.C.; Bowcock, A.M.; Petruhkin, K.; Gilliam, T.C.; Roses, A.D.; et al. Linkage of Tunisian Autosomal Recessive Duchenne-like Muscular Dystrophy to the Pericentromeric Region of Chromosome 13q. Nat. Genet. 1992, 2, 315–317. [Google Scholar] [CrossRef]
- Noguchi, S.; MacNally, E.M.; Ben Othmane, K.; Hagiwara, Y.; Mizuno, Y.; Yoshida, M.; Yamamoto, H.; Bönneman, C.G.; Gussoni, E.; Denton, P.; et al. Mutations in the Dystrophin-Associated Protein γ-Sarcoglycan in Chromosome 13 Muscular Dystrophy. Science 1995, 270, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; De Sa’ Moreira, E.; Piluso, G.; Vainzof, M.; Belsito, A.; Politano, L. Autosomal Recessive Limb- Girdle Muscular Dystrophy, LGMD 2F, Is Caused by a Mutation in the Sarcoglycan Gene. Nat. Genet. 1996, 14, 195–198. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, X.F.; Yang, X.Y.; Chong, M.; Cheng, J.; Li, G.; Lu, D.R. δ-Sarcoglycan Is Necessary for Early Heart and Muscle Development in Zebrafish. Biochem. Biophys. Res. Commun. 2006, 344, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Guyon, J.R.; Mosley, A.N.; Jun, S.J.; Montanaro, F.; Steffen, L.S.; Zhou, Y.; Kunkel, L.M. δ-Sarcoglycan Is Required for Early Zebrafish Muscle Organization. Exp. Cell Res. 2005, 304, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.S.; Vainzof, M.; Marie, S.K.; Sertie, A.L.; Zatz, M.; Passos-Bueno, M.R. The Seventh Form of Autosomal Recessive Limb-Girdle Muscular Dystrophy Is Mapped to 17q11-12. Am. J. Hum. Genet. 1997, 61, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.S.; Wiltshire, T.J.; Faulkner, G.; Nilforoushan, A.; Vainzof, M.; Suzuki, O.T.; Valle, G.; Reeves, R.; Zatz, M.; Passos-Bueno, M.R.; et al. Limb-Girdle Muscular Dystrophy Type 2G Is Caused by Mutations in the Gene Encoding the Sarcomeric Protein Telethonin. Nat. Genet. 2000, 24, 163–166. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Zhu, J.; Xu, X. Depletion of Zebrafish Tcap Leads to Muscular Dystrophy via Disrupting Sarcomere-Membrane Interaction, Not Sarcomere Assembly. Hum. Molec. Genet. 2009, 18, 4130–4140. [Google Scholar] [CrossRef]
- Frosk, P.; Weiler, T.; Nylen, E.; Sudha, T.; Greenberg, C.R.; Morgan, K.; Fujiwara, T.M.; Wrogemann, K. Limb-Girdle Muscular Dystrophy Type 2H Associated with Mutation in TRIM32, a Putative E3-Ubiquitin-Ligase Gene. Am. J. Hum. Genet. 2002, 70, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Brockington, M.; Blake, D.J.; Prandini, P.; Brown, S.C.; Torelli, S.; Benson, M.A.; Ponting, C.P.; Estournet, B.; Romero, N.B.; Mercuri, E.; et al. Mutations in the Fukutin-Related Protein Gene (FKRP) Cause a Form of Congenital Muscular Dystrophy with Secondary Laminin A2 Deficiency and Abnormal Glycosylation of α-Dystroglycan. Am. J. Hum. Genet. 2001, 69, 1198–1209. [Google Scholar] [CrossRef]
- Serafini, P.R.; Feyder, M.J.; Hightower, R.M.; Garcia-Perez, D.; Vieira, N.M.; Lek, A.; Gibbs, D.E.; Moukha-Chafiq, O.; Augelli-Szafran, C.E.; Kawahara, G. A Limb-Girdle Muscular Dystrophy 2I Model of Muscular Dystrophy Identifies Corrective Drug Compounds for Dystroglycanopathies. JCI Insight 2018, 3, e120493. [Google Scholar] [CrossRef]
- Wood, A.J.; Lin, C.H.; Li, M.; Nishtala, K.; Alaei, S.; Rossello, F.; Sonntag, C.; Hersey, L.; Miles, L.B.; Krisp, C. FKRP-Dependent Glycosylation of Fibronectin Regulates Muscle Pathology in Muscular Dystrophy. Nat. Commun. 2021, 12, 2951. [Google Scholar] [CrossRef]
- Lin, Y.Y.; White, R.J.; Torelli, S.; Cirak, S.; Muntoni, F.; Stemple, D.L. Zebrafish Fukutin Family Proteins Link the Unfolded Protein Response with Dystroglycanopathies. Hum. Mol. Genet. 2011, 20, 1763–1775. [Google Scholar] [CrossRef]
- Wood, A.J.; Muller, J.S.; Jepson, C.D.; Laval, S.H.; Lochmuller, H.; Bushby, K.; Barresi, R.; Straub, V. Abnormal Vascular Development in Zebrafish Models for Fukutin and FKRP Deficiency. Hum. Mol. Genet. 2011, 20, 4879–4890. [Google Scholar] [CrossRef]
- Thornhill, P.; Bassett, D.; Lochmuller, H.; Bushby, K.; Straub, V. Developmental Defects in a Zebrafish Model for Muscular Dystrophies Associated with the Loss of Fukutin-Related Protein (FKRP). Brain 2008, 131 Pt 6, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, G.; Guyon, J.R.; Nakamura, Y.; Kunkel, L.M. Zebrafish Models for Human FKRP Muscular Dystrophies. Hum. Mol. Genet. 2010, 19, 623–633. [Google Scholar] [CrossRef]
- Udd, B.; Rapola, J.; Nokelainen, P.; Arikawa, E.; Somer, H. Nonvacuolar Myopathy in a Large Family with Both Late Adult Onset Distal Myopathy and Severe Proximal Muscular Dystrophy. J. Neurol. Sci. 1992, 113, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hackman, P.; Vihola, A.; Haravuori, H.; Marchand, S.; Sarparanta, J.; de Seze, J.; Labeit, S.; Witt, C.; Peltonen, L.; Richard, I.; et al. Tibial Muscular Dystrophy Is a Titinopathy Caused by Mutations in TTN, the Gene Encoding the Giant Skeletal-Muscle Protein Titin. Am. J. Hum. Genet. 2002, 71, 492–500. [Google Scholar] [CrossRef]
- Zou, J.; Tran, D.; Baalbaki, M.; Tang, L.F.; Poon, A.; Pelonero, A.; Titus, E.W.; Yuan, C.; Shi, C.; Patchava, S.; et al. An Internal Promoter Underlies the Difference in Disease Severity between N- and C-Terminal Truncation Mutations of Titin in Zebrafish. eLife 2015, 4, e09406. [Google Scholar] [CrossRef] [PubMed]
- Steffen, L.S.; Guyon, J.R.; Vogel, E.D.; Howell, M.H.; Zhou, Y.; Weber, G.J.; Zon, L.I.; Kunkel, L.M. The Zebrafish Runzel Muscular Dystrophy Is Linked to the Titin Gene. Dev. Biol. 2007, 309, 180–192. [Google Scholar] [CrossRef]
- Dincer, P.; Balci, B.; Yuva, Y.; Talim, B.; Brockington, M.; Dincel, D.; Torelli, S.; Brown, S.; Kale, G.; Haliloglu, G.; et al. A Novel Form of Recessive Limb Girdle Muscular Dystrophy with Mental Retardation and Abnormal Expression of Alpha-Dystroglycan. Neuromusc. Disord. 2003, 13, 771–778. [Google Scholar] [CrossRef]
- Balci, B.; Uyanik, G.; Dincer, P.; Gross, C.; Willer, T.; Talim, B.; Haliloglu, G.; Kale, G.; Hehr, U.; Winkler, J.; et al. An Autosomal Recessive Limb Girdle Muscular Dystrophy (LGMD2) with Mild Mental Retardation Is Allelic to Walker-Warburg Syndrome (WWS) Caused by a Mutation in the POMT1 Gene. Neuromusc. Disord. 2005, 15, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Avsar-Ban, E.; Ishikawa, H.; Manya, H.; Watanabe, M.; Akiyama, S.; Miyake, H.; Endo, T.; Tamaru, Y. Protein O-Mannosylation Is Necessary for Normal Embryonic Development in Zebrafish. Glycobiology 2010, 20, 1089–1102. [Google Scholar] [CrossRef]
- Bolduc, V.; Marlow, G.; Boycott, K.M.; Saleki, K.; Inoue, H.; Kroon, J.; Itakura, M.; Robitaille, Y.; Parent, L.; Baas, F.; et al. Recessive Mutations in the Putative Calcium-Activated Chloride Channel Anoctamin 5 Cause Proximal LGMD2L and Distal MMD3 Muscular Dystrophies. Am. J. Hum. Genet. 2010, 86, 213–221. [Google Scholar] [CrossRef]
- Godfrey, C.; Escolar, D.; Brockington, M.; Clement, E.M.; Mein, R.; Jimenez-Mallebrera, C.; Torelli, S.; Feng, L.; Brown, S.C.; Sewry, C.A.; et al. Fukutin gene mutations in steroid-responsive limb girdle muscular dystrophy. Ann. Neurol. 2006, 60, 603–610. [Google Scholar] [CrossRef]
- Godfrey, C.; Clement, E.; Mein, R.; Brockington, M.; Smith, J.; Talim, B.; Straub, V.; Robb, S.; Quinlivan, R.; Feng, L.; et al. Refining Genotype-Phenotype Correlations in Muscular Dystrophies with Defective Glycosylation of Dystroglycan. Brain 2007, 130, 2725–2735. [Google Scholar] [CrossRef] [PubMed]
- Clement, E.M.; Godfrey, C.; Tan, J.; Brockington, M.; Torelli, S.; Feng, L.; Brown, S.C.; Jimenez-Mallebrera, C.; Sewry, C.A.; Longman, C.; et al. Mild POMGnT1 Mutations Underlie a Novel Limb-Girdle Muscular Dystrophy Variant. Arch. Neurol. 2008, 65, 137–141. [Google Scholar] [CrossRef]
- Raducu, M.; Baets, J.; Fano, O.; Van Coster, R.; Cruces, J. Promoter alteration causes transcriptional repression of the POMGNT1 gene in limb-girdle muscular dystrophy type 2O. Europ. J. Hum. Genet. 2012, 20, 945–952. [Google Scholar] [CrossRef]
- Hara, Y.; Balci-Hayta, B.; Yoshida-Moriguchi, T.; Kanagawa, M.; Beltran-Valero de Bernabe, D.; Gundesli, H.; Willer, T.; Satz, J.S.; Crawford, R.W.; Burden, S.J.; et al. A Dystroglycan Mutation Associated with Limb-Girdle Muscular Dystrophy. New Eng. J. Med. 2011, 364, 939–946. [Google Scholar] [CrossRef]
- Parsons, M.J.; Campos, I.; Hirst, E.M.; Stemple, D.L. Removal of Dystroglycan Causes Severe Muscular Dystrophy in Zebrafish Embryos. Development 2002, 129, 3505–3512. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kawahara, G.; Gundry, S.R.; Chen, A.T.; Lencer, W.I.; Zhou, Y.; Zon, L.I.; Kunkel, L.M.; Beggs, A.H. The Zebrafish Dag1 Mutant: A Novel Genetic Model for Dystroglycanopathies. Hum. Mol. Genet. 2011, 20, 1712–1725. [Google Scholar] [CrossRef] [PubMed]
- Gundesli, H.; Talim, B.; Korkusuz, P.; Balci-Hayta, B.; Cirak, S.; Akarsu, N.A.; Topaloglu, H.; Dincer, P. Mutation in Exon 1f of PLEC, Leading to Disruption of Plectin Isoform 1f, Causes Autosomal-Recessive Limb-Girdle Muscular Dystrophy. Am. J. Hum. Genet. 2010, 87, 834–841. [Google Scholar] [CrossRef]
- Bogershausen, N.; Shahrzad, N.; Chong, J.X.; von Kleist-Retzow, J.-C.; Stanga, D.; Li, Y.; Bernier, F.P.; Loucks, C.M.; Wirth, R.; Puffenberger, E.G.; et al. Recessive TRAPPC11 Mutations Cause a Disease Spectrum of Limb Girdle Muscular Dystrophy and Myopathy with Movement Disorder and Intellectual Disability. Am. J. Hum. Genet. 2013, 93, 181–190. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Ogino, Y.; Tse, W.K.F. FGF8 Rescues Motor Deficits in Zebrafish Model of Limb-Girdle Muscular Dystrophy R18. Biochem. Biophys. Res. Commun. 2023, 652, 76–83. [Google Scholar] [CrossRef]
- Carss, K.J.; Stevens, E.; Foley, A.R.; Cirak, S.; Riemersma, M.; Torelli, S.; Hoischen, A.; Willer, T.; van Scherpenzeel, M.; Moore, S.A.; et al. Mutations in GDP-Mannose Pyrophosphorylase B Cause Congenital and Limb-Girdle Muscular Dystrophies Associated with Hypoglycosylation of Alpha-Dystroglycan. Am. J. Hum. Genet. 2013, 93, 29–41. [Google Scholar] [CrossRef]
- Tasca, G.; Moro, F.; Aiello, C.; Cassandrini, D.; Fiorillo, C.; Bertini, E.; Bruno, C.; Santorelli, F.M.; Ricci, E. Limb-Girdle Muscular Dystrophy with Alpha-Dystroglycan Deficiency and Mutations in the ISPD Gene. Neurology 2013, 80, 963–965. [Google Scholar] [CrossRef]
- Servian-Morilla, E.; Takeuchi, H.; Lee, T.V.; Clarimon, J.; Mavillard, F.; Area-Gomez, E.; Rivas, E.; Nieto-Gonzalez, J.L.; Rivero, M.C.; Cabrera-Serrano, M.; et al. A POGLUT1 Mutation Causes a Muscular Dystrophy with Reduced Notch Signaling and Satellite Cell Loss. EMBO Molec. Med. 2016, 8, 1289–1309. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, F.; Urciuolo, A.; Martoni, E.; Sabatelli, P.; Squarzoni, S.; Bovolenta, M.; Messina, S.; Mercuri, E.; Franchella, A.; Ferlini, A.; et al. Autosomal Recessive Bethlem Myopathy. Neurology 2009, 73, 1883–1891. [Google Scholar] [CrossRef]
- Gavassini, B.F.; Carboni, N.; Nielsen, J.E.; Danielsen, E.R.; Thomsen, C.; Svenstrup, K.; Bello, L.; Maioli, M.A.; Marrosu, G.; Ticca, A.F.; et al. Clinical and Molecular Characterization of Limb-Girdle Muscular Dystrophy Due to LAMA2 Mutations. Muscle Nerve 2011, 44, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.E.; Bryson-Richardson, R.J.; Berger, S.; Jacoby, A.S.; Cole, N.J.; Hollway, G.E.; Berger, J.; Currie, P.D. The Zebrafish Candyfloss Mutant Implicates Extracellular Matrix Adhesion Failure in Laminin Alpha2-Deficient Congenital Muscular Dystrophy. Proc. Natl. Acad. Sci. USA 2007, 104, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.A.; Kawahara, G.; Myers, J.A.; Chen, A.T.; Hall, T.E.; Manzini, M.C.; Currie, P.D.; Zhou, Y.; Zon, L.I.; Kunkel, L.M. A Splice Site Mutation in Laminin-Alpha2 Results in a Severe Muscular Dystrophy and Growth Abnormalities in Zebrafish. PLoS ONE 2012, 7, e43794. [Google Scholar] [CrossRef]
- Endo, Y.; Dong, M.; Noguchi, S.; Ogawa, M.; Hayashi, Y.K.; Kuru, S.; Sugiyama, K.; Nagai, S.; Ozasa, S.; Nonaka, I.; et al. Milder Forms of Muscular Dystrophy Associated with POMGNT2 Mutations. Neurol. Genet. 2015, 1, e33. [Google Scholar] [CrossRef]
- Schindler, R.F.R.; Scotton, C.; Zhang, J.; Passarelli, C.; Ortiz-Bonnin, B.; Simrick, S.; Schwerte, T.; Poon, K.-L.; Fang, M.; Rinne, S.; et al. POPDC1-S201F Causes Muscular Dystrophy and Arrhythmia by Affecting Protein Trafficking. J. Clin. Investig. 2016, 126, 239–253. [Google Scholar] [CrossRef]
- Vissing, J.; Johnson, K.; Topf, A.; Nafissi, S.; Diaz-Manera, J.; French, V.M.; Schindler, R.F.; Sarathchandra, P.; Lokken, N.; Rinne, S.; et al. POPDC3 Gene Variants Associate with a New Form of Limb Girdle Muscular Dystrophy. Ann. Neurol. 2019, 86, 832–843. [Google Scholar] [CrossRef]
- Coppens, S.; Barnard, A.M.; Puusepp, S.; Pajusalu, S.; Ounap, K.; Vargas-Franco, D.; Bruels, C.C.; Donkervoort, S.; Pais, L.; Chao, K.R.; et al. A Form of Muscular Dystrophy Associated with Pathogenic Variants in JAG2. Am. J. Hum. Genet. 2021, 108, 840–856. [Google Scholar] [CrossRef]
- Yogev, Y.; Shorer, Z.; Koifman, A.; Wormser, O.; Drabkin, M.; Halperin, D.; Dolgin, V.; Proskorovski-Ohayon, R.; Hadar, N.; Davidov, G.; et al. Limb Girdle Muscular Disease Caused by HMGCR Mutation and Statin Myopathy Treatable with Mevalonolactone. Proc. Nat. Acad. Sci. USA 2023, 120, e2217831120. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Tremblay, J.P. Limb–Girdle Muscular Dystrophies Classification and Therapies. J. Clin. Med. 2023, 12, 4769. [Google Scholar] [CrossRef] [PubMed]
- Zelikovich, A.S.; Joslin, B.C.; Casey, P.; McNally, E.M.; Ajroud-Driss, S. An Open Label Exploratory Clinical Trial Evaluating Safety and Tolerability of Once-Weekly Prednisone in Becker and Limb-Girdle Muscular Dystrophy. J. Neuromuscul. Dis. 2022, 9, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Ganaraja, V.H.; Polavarapu, K.; Bardhan, M.; Preethish-Kumar, V.; Leena, S.; Anjanappa, R.M.; Vengalil, S.; Nashi, S.; Arunachal, G.; Gunasekaran, S.; et al. Disease Progression and Mutation Pattern in a Large Cohort of LGMD R1/LGMD 2A Patients from India. Glob Med. Genet. 2021, 9, 34–41. [Google Scholar] [CrossRef]
- Angelini, C. Treatabolome for Finely Targeting Muscle Pathology in LGMD. Acta Myol. 2025, 44, 37–41. [Google Scholar] [CrossRef]
- Bardakov, S.N.; Sorochanu, I.; Mkrtchyan, L.A. Calpainopathy (Limb-Girdle Muscular Dystrophy Type R1): Clinical Features, Diagnostic Approaches, and Biotechnological Treatment Methods. J. Neuromuscul. Dis. 2025, 12, 594–618. [Google Scholar] [CrossRef]
- Anwar, S.; Yokota, T. The Dysferlinopathies Conundrum: Clinical Spectra, Disease Mechanism and Genetic Approaches for Treatments. Biomolecules 2024, 14, 256. [Google Scholar] [CrossRef]
- Carotti, M.; Fecchio, C.; Sandonà, D. Emerging Therapeutic Strategies for Sarcoglycanopathy. Expert Opin. Orphan Drugs 2017, 5, 381–396. [Google Scholar] [CrossRef]
- Scano, M.; Benetollo, A.; Dalla Barba, F.; Sandonà, D. Advanced Therapeutic Approaches in Sarcoglycanopathies. Curr. Opin. Pharmacol. 2024, 76, 102459. [Google Scholar] [CrossRef]
- Nigro, V. Molecular Bases of Autosomal Recessive Limb-Girdle Muscular Dystrophies. Acta Myol. 2003, 22, 35–42. [Google Scholar] [PubMed]
- Fanin, M.; Nascimbeni, A.C.; Aurino, S.; Tasca, E.; Pegoraro, E.; Nigro, V.; Angelini, C. Frequency of LGMD Gene Mutations in Italian Patients with Distinct Clinical Phenotypes. J. Neurol. 2009, 72, 1432–1435. [Google Scholar] [CrossRef]
- Gaina, G.; Popa, A. Muscular Dystrophy: Experimental Animal Models and Therapeutic Approaches. Exp. Ther. Med. 2021, 21, 610. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Pozsgai, E.R.; Lewis, S.; Griffin, D.A.; Lowes, L.P.; Alfano, L.N.; Lehman, K.J.; Church, K.; Reash, N.F.; Iammarino, M.A.; et al. Gene Therapy with Bidridistrogene Xeboparvovec for Limb-Girdle Muscular Dystrophy Type 2E/R4: Phase 1/2 Trial Results. Nat. Med. 2024, 30, 199–206. [Google Scholar] [CrossRef]
- Sharma, A.; Sane, H.; Gokulchandran, N.; Gandhi, S.; Bhovad, P.; Khopkar, D.; Paranjape, A.; Bhagwanani, K.; Badhe, P. The Role of Cell Transplantation in Modifying the Course of Limb Girdle Muscular Dystrophy: A Longitudinal 5-Year Study. Degener. Neurol. Neuromuscul. Dis. 2015, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Carotti, M.; Scano, M.; Fancello, I.; Richard, I.; Risato, G.; Bensalah, M.; Soardi, M.; Sandonà, D. Combined Use of CFTR Correctors in LGMD2D Myotubes Improves Sarcoglycan Complex Recovery. Int. J. Mol. Sci. 2020, 21, 1813. [Google Scholar] [CrossRef]
- Scano, M.; Benetollo, A.; Nogara, L.; Bondì, M.; Dalla Barba, F.; Soardi, M.; Furlan, S.; Akyurek, E.E.; Caccin, P.; Carotti, M.; et al. CFTR Corrector C17 Is Effective in Muscular Dystrophy, in Vivo Proof of Concept in LGMDR3. Hum. Mol. Genet. 2022, 31, 499–509. [Google Scholar] [CrossRef]
- Benetollo, A.; Parrasia, S.; Scano, M.; Biasutto, L.; Rossa, A.; Nogara, L.; Blaauw, B.; Dalla Barba, F.; Caccin, P.; Carotti, M.; et al. The Novel Use of the CFTR Corrector C17 in Muscular Dystrophy: Pharmacological Profile and in Vivo Efficacy. Biochem. Pharmacol. 2025, 233, 116779. [Google Scholar] [CrossRef]
- Odermatt, A.; Taschner, P.E.M.; Khanna, V.K.; Busch, H.F.; Karpati, G.; Jablecki, C.K. Mutations in the Gene—Encoding SERCA1, the Fast--Twitch Skeletal Muscle Sarcoplasmic Reticulum Ca2+ ATPase, Are Associated with Brody Disease. Nat. Genet. 1996, 14, 191–194. [Google Scholar] [CrossRef]
- Brody, I.A. Muscle Contracture Induced by Exercise: A Syndrome Attributable to Decreased Relaxing Factor. New Eng. J. Med. 1996, 281, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Karpati, G.; Charuk, J.; Carpenter, S.; Jablecki, C.; Holland, P. Myopathy Caused by a Deficiency of Ca2+-Adenosine Triphosphatase in Sarcoplasmic Reticulum (Brody’s Disease). Ann. Neurol. 1986, 20, 38–49. [Google Scholar] [CrossRef]
- Taylor, D.J.; Brosnan, M.J.; Arnold, D.L.; Bore, P.J.; Styles, P.; Walton, J.; Radda, G.K. Ca2+-ATPase Deficiency in a Patient with an Exertional Muscle Pain Syndrome. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1425–1433. [Google Scholar] [CrossRef]
- Wevers, R.A.; Poels, P.J.; Joosten, E.M.; Steenbergen, G.G.; Benders, A.A.; Veerkamp, J.H. Ischaemic Forearm Testing in a Patient with Ca2+-ATPase Deficiency. J. Inherit. Metab. Dis. 1992, 15, 423–425. [Google Scholar] [CrossRef]
- Poels, P.J.; Wevers, R.A.; Braakhekke, J.P.; Benders, A.A.; Veerkamp, J.H.; Joosten, E.M. Exertional Rhabdomyolysis in a Patient with Calcium Adenosine Triphosphatase Deficiency. J. Neurol. Neurosurg. Psychiatry. 1993, 56, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Benders, A.A.; Veerkamp, J.H.; Oosterhof, A.; Jongen, P.J.; Bindels, R.J.; Smitn, L.M. Ca2+ Homeostasis in Brody’s Disease. A Study in Skeletal Muscle and Cultured Muscle Cells and the Effects of Dantrolene an Verapamil. J. Clin. Investig. 1994, 94, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, V.; Vattemi, G.; Gualandi, F.; Voermans, N.C.; Marini, M.; Scotton, C.; Tomelleri, G. SERCA1 Protein Expression in Muscle of Patients with Brody Disease and Brody Syndrome and in Cultured Human Muscle Fibers. Mol. Genet. Metab. 2013, 110, 162–169. [Google Scholar] [CrossRef]
- Pan, Y.; Zvaritch, E.; Tupling, A.R.; Rice, W.J.; de Leon, S.; Rudnicki, M.; McKerlie, C.; Banwell, B.L.; MacLennan, D.H. Targeted Disruption of the ATP2A1 Gene Encoding the Sarco(Endo)Plasmic Reticulum Ca2+ ATPase Isoform 1 (SERCA1) Impairs Diaphragm Function and Is Lethal in Neonatal Mice. J. Biol. Chem. 2003, 278, 13367–13375. [Google Scholar] [CrossRef] [PubMed]
- Sacchetto, R.; Testoni, S.; Gentile, A.; Damiano, E.; Rossi, M.; Liguori, R.; Drögemüller, C.; Mascarello, F. A Defective SERCA1 Protein Is Responsible for Congenital Pseudomyotonia in Chianina Cattle. Am. J. Pathol. 2009, 174, 565–573. [Google Scholar] [CrossRef]
- Bianchini, E.; Testoni, S.; Gentile, A.; Calì, T.; Ottolini, D.; Villa, A.; Brini, M.; Betto, R.; Mascarello, F.; Nissen, P.; et al. Inhibition of Ubiquitin Proteasome System Rescues the Defective Sarco(Endo)Plasmic Reticulum Ca2+-ATPase (SERCA1. Protein Causing Chianina Cattle Pseudomyotonia. J. Biol. Chem. 2014, 289, 33073–33082. [Google Scholar] [CrossRef]
- Akyürek, E.E.; Busato, F.; Murgiano, L.; Bianchini, E.; Carotti, M.; Sandonà, D.; Sacchetto, R. Differential Analysis of Gly211Val and Gly286Val Mutations Affecting Sarco (Endo) Plasmic Reticulum Ca2+-ATPase (SERCA1) in Congenital Pseudomyotonia Romagnola Cattle. Int. J. Mol. Sci. 2022, 23, 12364. [Google Scholar] [CrossRef]
- Hirata, H.; Saint-Amant, L.; Waterbury, J.; Cui, W.; Zhou, W.; Li, Q.; Goldman, D.; Granato, M.; Kuwada, J.Y. Accordion, a Zebrafish Behavioral Mutant, Has a Muscle Relaxation Defect Due to a Mutation in the ATPase Ca2+ Pump SERCA1. Development 2004, 131, 5457–5468. [Google Scholar] [CrossRef]
- Gleason, M.R.; Armisen, R.; Verdecia, M.A.; Sirotkin, H.; Brehm, P.; Mandel, G. A Mutation in Serca Underlies Motility Dysfunction in Accordion Zebrafish. Dev. Biol. 2004, 276, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, C.; Inesi, G. Structural Basis of Ion Pumping by Ca2+-ATPase of the Sarcoplasmic Reticulum. Annu. Rev. Biochem. 2004, 73, 269–292. [Google Scholar] [CrossRef]
- Akyürek, E.E.; Greco, F.; Tesoriero, C.; Dalla Barba, F.; Carotti, M.; Gorni, G.; Sacchetto, R. The Accordion Zebrafish Tq206 Mutant in the Assessment of a Novel Pharmaceutical Approach to Brody Myopathy. Int. J. Mol. Sci. 2024, 25, 9229. [Google Scholar] [CrossRef]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative human three-dimensional tissue-engineered models as an alternative to animal testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Veinotte, C.J.; Dellaire, G.; Berman, J.N. Hooking the big one: The potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis. Models Mech. 2014, 7, 745–754. [Google Scholar] [CrossRef]
- Bartoli, M.; Gicquel, E.; Barrault, L.; Soheili, T.; Malissen, M.; Malissen, B.; Vincent-Lacaze, N.; Perez, N.; Udd, B.; Danos, O.; et al. Mannosidase I inhibition rescues the human alpha-sarcoglycan R77C recurrent mutation. Hum. Mol. Genet. 2008, 17, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Kobuke, K.; Piccolo, F.; Garringer, K.W.; Moore, S.A.; Sweezer, E.; Yang, B.; Campbell, K.P. A common disease-associated missense mutation in alpha-sarcoglycan fails to cause muscular dystrophy in mice. Hum. Mol. Genet. 2008, 17, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Henriques, S.F.; Patissier, C.; Bourg, N.; Fecchio, C.; Sandona, D.; Marsolier, J.; Richard, I. Different outcome of sarcoglycan missense mutation between human and mouse. PLoS ONE 2018, 13, e0191274. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.L.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Directive, E. 63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- MacArthur Clark, J. The 3Rs in research: A contemporary approach to replacement, reduction and refinement. Br. J. Nutr. 2018, 120, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Bailone, R.L.; Fukushima, H.C.S.; Ventura Fernandes, B.H.; De Aguiar, L.K.; Corrêa, T.; Janke, H.; Borra, R.C. Zebrafish as an Alternative Animal Model in Human and Animal Vaccination Research. Lab. Anim. Res. 2020, 36, 13. [Google Scholar] [CrossRef]
| Inheritance | Location | Gene | Phenotype | Abbreviation | Reference for Gene | Zebrafish Model | Reference For ZF |
|---|---|---|---|---|---|---|---|
| AD | 7q36.3 | DNAJB6 | DNAJB6-related muscular dystrophy | LGMDD1 | [73,74] | MO mRNA injection | [75,76] |
| AD | 7q32.1 | TNPO3 | TNP03-related muscular dystrophy | LGMDD2 | [77,78] | - | - |
| AD | 4q21.22 | HNRNPDL | HNRNPDL-related muscular dystrophy | LGMDD3 | [79] | MO | [79] |
| AD | 15q15.1 | CAPN3 | Calpain3-related muscular dystrophy | LGMDD4 | [80,81] | - | - |
| AD | 21q22.3 | COL6A | Bethlem myopathy | LGMDD5 | [82] | CRISPR/Cas9 TALEN MO | [83,84,85] |
| AR | 15q15.1 | CAPN3 | Calpainopathies | LGMDR1 | [86] | CRISPR/Cas9 TALEN | [87,88] |
| AR | 2p13.2 | DYSF | Dysferlinopathy | LGMDR2 | [89] | MO | [90,91] |
| AR | 17q21.33 | SGCA | α-sarcoglycanopathy | LGMDR3 | [92,93] | - | - |
| AR | 4q12 | SGCB | β-sarcoglycanopathy | LGMDR4 | [94,95] | CRISPR/Cas9 | [96] |
| AR | 13q12.12 | SGCG | γ-sarcoglycanopathy | LGMDR5 | [97,98] | - | - |
| AR | 5q33.2-q33.3 | SGCD | δ-sarcoglycanopathy | LGMDR6 | [99] | CRISPR/Cas9 MO | [96,100,101] |
| AR | 17q12 | TCAP | Telethonin-related muscular dystrophy | LGMDR7 | [102,103] | MO | [104] |
| AR | 9q33.1 | TRIM32 | Sarcotubular myopathy | LGMDR8 | [105] | - | - |
| AR | 19q13.32 | FKRP | FKRP related muscular dystrophy | LGMDR9 | [106] | TALEN, Tol2 transposon system, CRISPR/Cas9, MO | [107,108,109,110,111,112] |
| AR | 2q31.2 | TTN | Tinin-related muscular dystrophy | LGMDR10 | [113,114] | CRISPR/Cas9 MO | [115,116] |
| AR | 9q34.13 | POMT1 | POMT1-related muscular dystrophy | LGMDR11 | [117,118] | MO | [119] |
| AR | 11p14.3 | ANO5 | Anoctamin5-related muscular dystrophy | LGMDR12 | [120] | - | - |
| AR | 9q31.2 | FKTN | Fucutin-related muscular dystrophy | LGMDR13 | [121] | - | - |
| AR | 14q24.3 | POMT2 | POMT2-related muscular dystrophy | LGMDR14 | [122] | MO | [119] |
| AR | 1p34.1 | POMGnT1 | POMGnT1-related muscular dystrophy | LGMDR15 | [123,124] | - | - |
| AR | 3p21.31 | DAG1 | α-dystroglycan-related muscular dystrophy | LGMDR16 | [117,125] | MO ENU screening | [126,127] |
| AR | 8q24.3 | PLEC1 | Plectin-related muscular dystrophy | LGMDR17 | [128] | - | - |
| AR | 4q35.1 | TRAPPC11 | TRAPPC11-related muscular dystrophy | LGMDR18 | [129] | MO | [130] |
| AR | 3p21.31 | GMPPB | GMPPB-related muscular dystrophy | LGMDR19 | [131] | MO | [131] |
| AR | 7p21.2 | ISPD (CRPPA) | ISPD-related muscular dystrophy | LGMDR20 | [132] | - | - |
| AR | 3q13.33 | POGLUT1 | POGLUT1-related muscular dystrophy | LGMDR21 | [133] | - | - |
| AR | 21q22.3 | COL6A | Autosomal recessive Bethlem myopathy | LGMDR22 | [134] | CRISPR/Cas9 TALEN MO | [83,84,85] |
| AR | 6q22.33 | LAMA2 | Laminin α2-related muscular dystrophy | LGMDR23 | [135] | MO ENU screening | [136,137] |
| AR | 3p22.1 | POMGNT2 | PMGNT2-related muscular dystrophy | LGMDR24 | [138] | - | - |
| AR | 6q21 | BVES (POPDC1) | Muscular dystrophy | LGMDR25 | [139] | MO | [139] |
| AR | 6q21 | POPDC3 | Muscular dystrophy | LGMDR26 | [140] | MO | [140] |
| AR | 14q32.33 | JAG2 | Muscular dystrophy | LGMDR27 | [141] | - | - |
| AR | 5q13.3 | HMGCR | Muscular dystrophy | LGMDR28 | [142] | - | - |
| Zebrafish | Rodents |
|---|---|
| 70% of homologous gene conservation with humans [2] | 85% of homologous gene conservation with humans [2] |
| Maintenance cost is low [176] | Maintenance cost is high [176] |
| Possibility of genetic modification [175] | Possibility of genetic modification [175] |
| Rapid development and transparency of eggs [1] | Development takes time [176] |
| Suitable for embryonic developmental studies [1] | Embryonic studies require maternal sacrifice [176] |
| Rapid phenotyping [176] | Slow phenotyping [176] |
| Back-crossing is rapid compared to mice [176] | Back-crossing is slower compared to zebrafish [176] |
| Genes, molecular pathways, and organ systems are conserved with humans’ less than those of mice [176] | Genes, molecular pathways, and organ systems are highly conserved with humans’ [176] |
| Compound screening is easy [176,177,178,179,180] | Compound screening is laborious |
| High number of offspring [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176] | Small number of offspring [176] |
| The permeability of embryos facilitates compound screening in fish water [176] | In some cases, mouse models do not mimic human phenotype [167,177,178,179] |
| Low experimental reagent availability [180] | High experimental reagent availability [180] |
| Less strict regulations for zebrafish use until 5dpf [181,182,183,184] | Very strict regulations [183,184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akyürek, E.E.; Erba, M.; Dalla Barba, F.; Sandonà, D.; Sacchetto, R. Zebrafish as a Model Organism for Research in Rare Genetic Neuromuscular Diseases. Int. J. Mol. Sci. 2025, 26, 8832. https://doi.org/10.3390/ijms26188832
Akyürek EE, Erba M, Dalla Barba F, Sandonà D, Sacchetto R. Zebrafish as a Model Organism for Research in Rare Genetic Neuromuscular Diseases. International Journal of Molecular Sciences. 2025; 26(18):8832. https://doi.org/10.3390/ijms26188832
Chicago/Turabian StyleAkyürek, Eylem Emek, Martina Erba, Francesco Dalla Barba, Dorianna Sandonà, and Roberta Sacchetto. 2025. "Zebrafish as a Model Organism for Research in Rare Genetic Neuromuscular Diseases" International Journal of Molecular Sciences 26, no. 18: 8832. https://doi.org/10.3390/ijms26188832
APA StyleAkyürek, E. E., Erba, M., Dalla Barba, F., Sandonà, D., & Sacchetto, R. (2025). Zebrafish as a Model Organism for Research in Rare Genetic Neuromuscular Diseases. International Journal of Molecular Sciences, 26(18), 8832. https://doi.org/10.3390/ijms26188832





