Kinetic Model with Feedback Cycle for Age-Dependent Amyloid Beta Accumulation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. System and Kinetic Data
2.2. Kinetic Model
2.2.1. Considerations on Kinetics and Modeling of Mouse Data
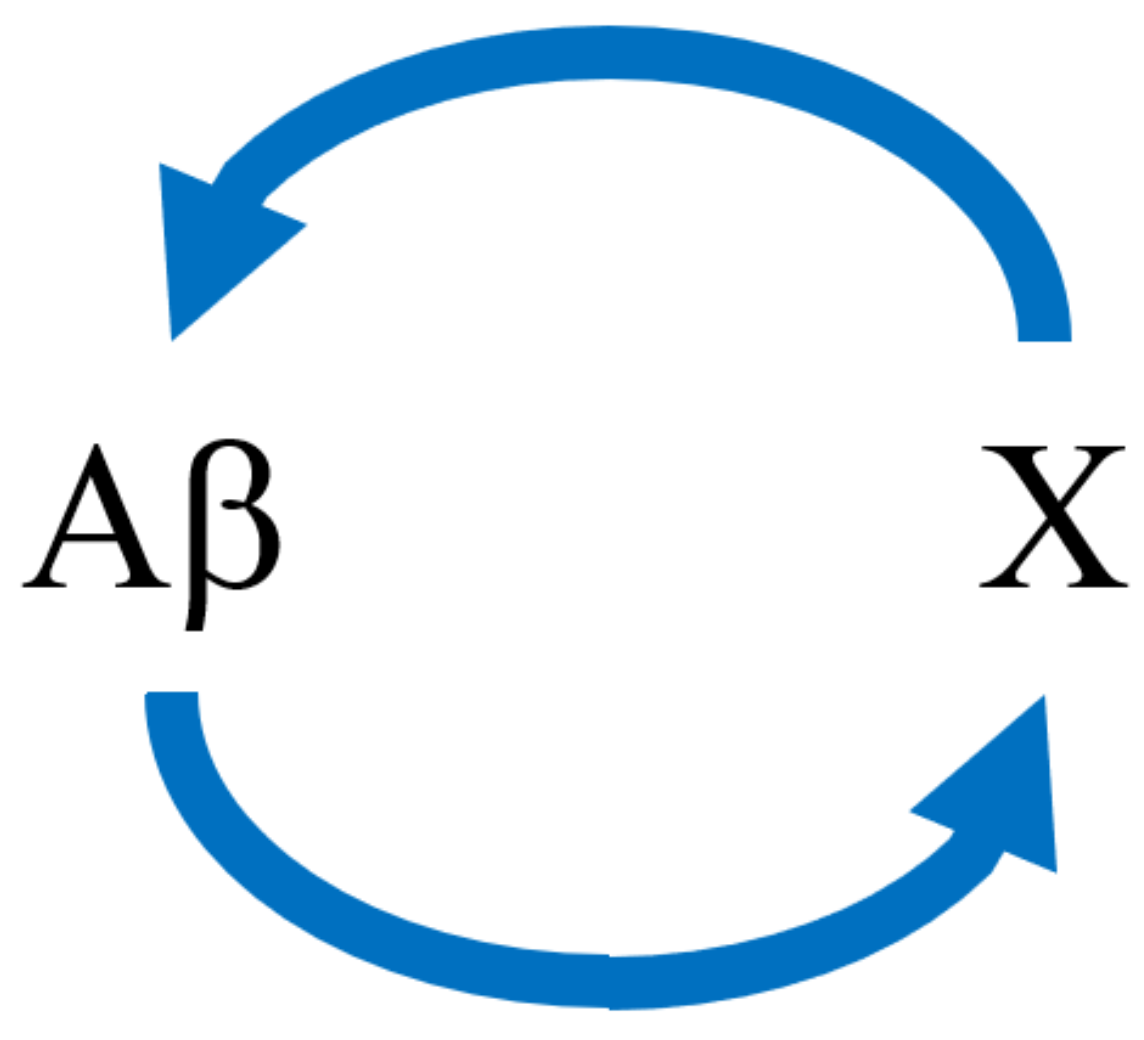
2.2.2. Kinetic Model with Nonlinear Feedback, and Fitting to the Model
2.3. The Question of Bistability
3. Results and Discussion
3.1. Intervention in the Disease Process
3.2. Clinical Strategies of Intervention
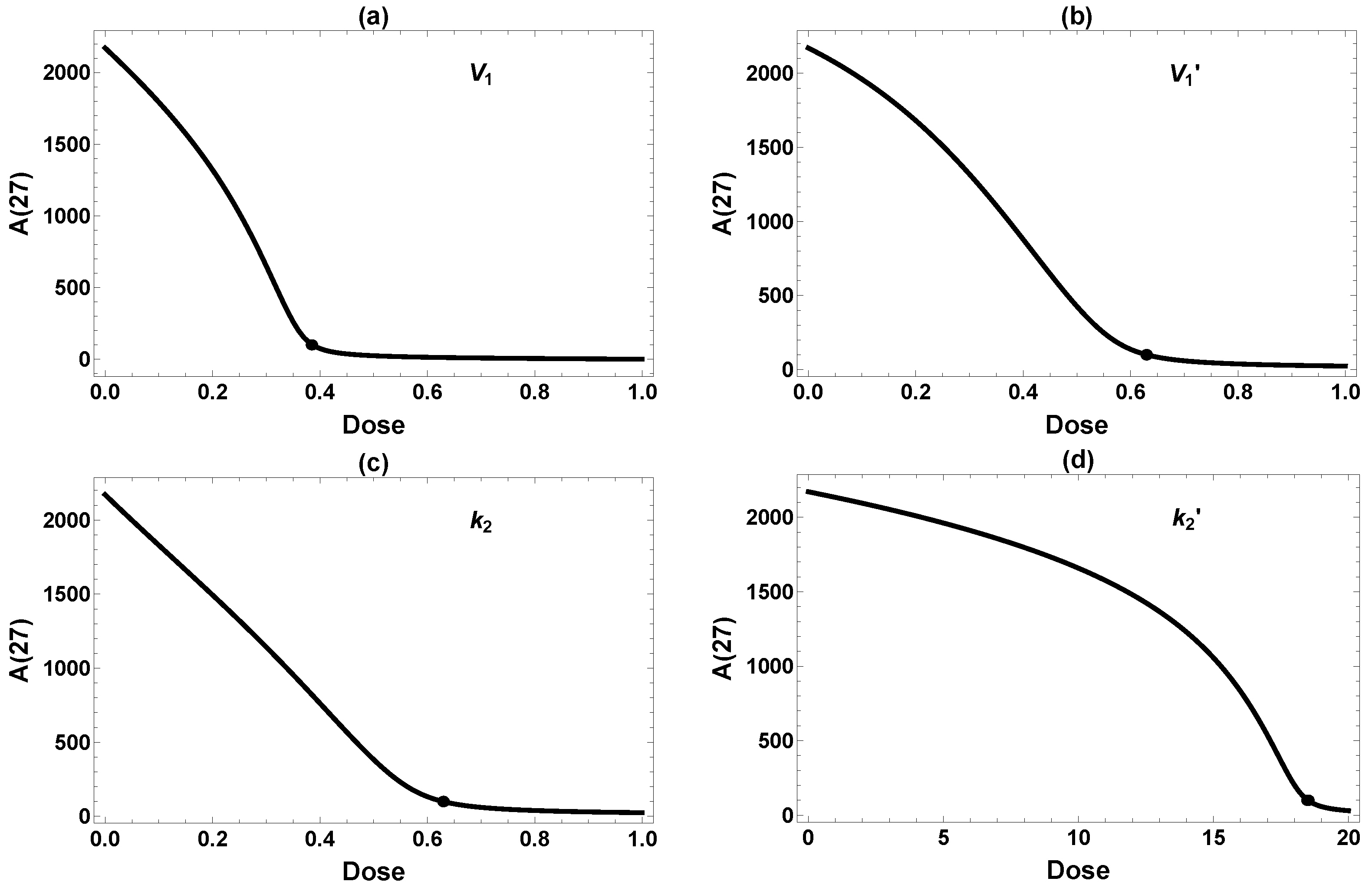
3.3. Effects of Single Interventions
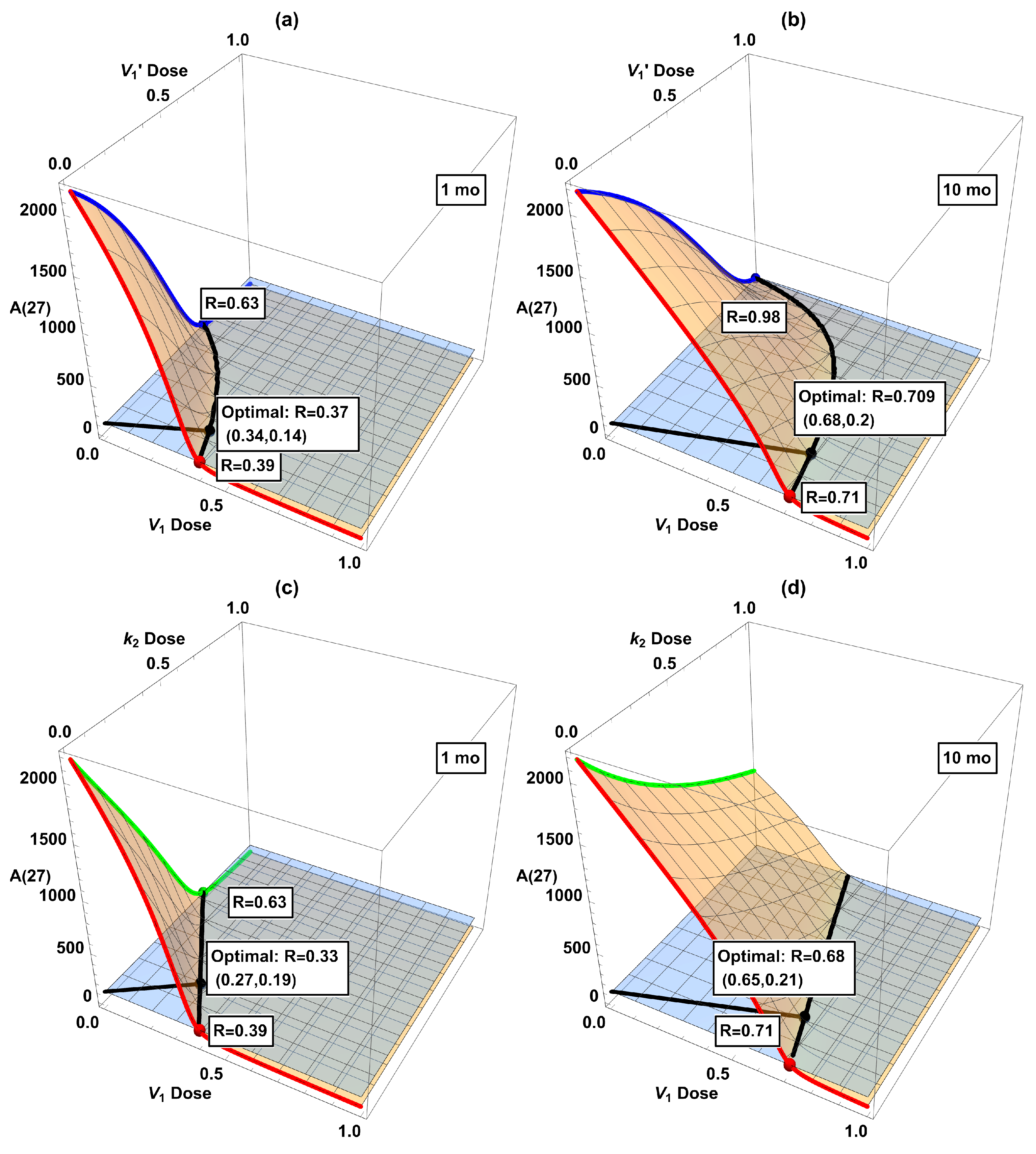
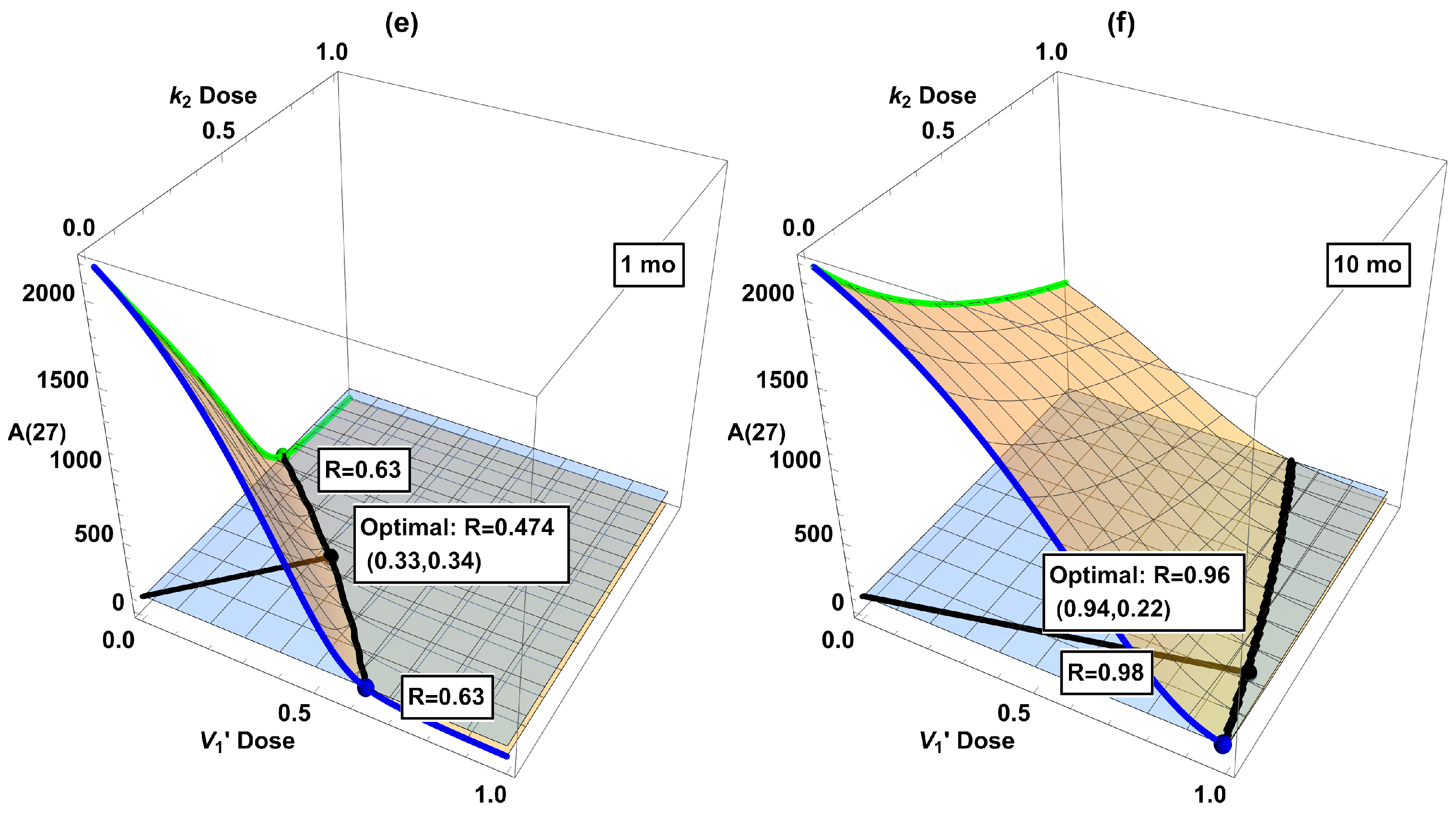
3.4. Combination Interventions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masters, C.L.; Selkoe, D.J. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006262. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; De Strooper, B. The amyloid cascade hypothesis: Are we poised for success or failure? J. Neurochem. 2016, 139, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2024. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2024, 10, e12465. [Google Scholar] [CrossRef]
- Chami, L.; Checler, F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Mol. Neurodegener. 2012, 7, 52. [Google Scholar] [CrossRef]
- Standridge, J.B. Vicious cycles within the neuropathophysiologic mechanisms of Alzheimer’s disease. Curr. Alzheimer Res. 2006, 3, 95–107. [Google Scholar] [CrossRef]
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar]
- Arnsten, A.F.T.; Datta, D.; Del Tredici, K.; Braak, H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Alavi Naini, S.M.; Soussi-Yanicostas, N. Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxid. Med. Cell. Longev. 2015, 2015, 151979. [Google Scholar] [CrossRef]
- Minter, M.R.; Taylor, J.M.; Crack, P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 2016, 136, 457–474. [Google Scholar] [CrossRef]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef]
- Zilberter, Y.; Zilberter, M. The vicious circle of hypometabolism in neurodegenerative diseases: Ways and mechanisms of metabolic correction. J. Neurosci. Res. 2017, 95, 2217–2235. [Google Scholar] [CrossRef]
- Parkinson, J.; Ploeger, B.; Appelkvist, P.; Bogstedt, A.; Dillner Bergstedt, K.; Eketjäll, S.; Visser, S.A. Modeling of age-dependent amyloid accumulation and γ-secretase inhibition of soluble and insoluble Aβ in a transgenic mouse model of amyloid deposition. Pharmacol. Res. Perspect. 2013, 1, e00012. [Google Scholar] [CrossRef]
- Tyng, V.; Kellman, M.E. Kinetic model of translational autoregulation. J. Phys. Chem. B 2018, 123, 369–378. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Update on hypothetical model of Alzheimer’s disease biomarkers. Lancet Neurol. 2013, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropath. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid β-protein. J. Alzheimer’s Dis. 2001, 3, 75–80. [Google Scholar] [CrossRef]
- Bateman, R.J.; Munsell, L.Y.; Morris, J.C.; Swarm, R.; Yarasheski, K.E.; Holtzman, D.M. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat. Med. 2006, 12, 856. [Google Scholar] [CrossRef] [PubMed]
- Doig, A.J. Positive feedback loops in Alzheimer’s disease: The Alzheimer’s feedback hypothesis. J. Alzheimer’s Dis. 2018, 66, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Amyloid is essential but insufficient for Alzheimer causation: Addition of subcellular cofactors is required for dementia. Int. J. Geriatr. Psychiatry 2018, 33, e14–e21. [Google Scholar] [CrossRef]
- Elliott, C.; Rojo, A.I.; Ribe, E.; Broadstock, M.; Xia, W.; Morin, P.; Semenov, M.; Baillie, G.; Cuadrado, A.; Al-Shawi, R.; et al. A role for APP in Wnt signalling links synapse loss with β-amyloid production. Transl. Psychiatry 2018, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Ingalls, B.P. Mathematical Modeling in Systems Biology: An Introduction; The MIT Press: Cambridge, MA, USA, 2013. [Google Scholar]
- De Caluwé, J.; Dupont, G. The progression towards Alzheimer’s disease described as a bistable switch arising from the positive loop between amyloids and Ca2+. J. Theor. Biol. 2013, 331, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Burlando, B.; Losacco, S.; Villa, V.; Fedele, E.; Ricciarelli, R. A new bistable switch model of Alzheimer’s Disease pathogenesis. Int. J. Mol. Sci. 2022, 23, 7061. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Pontecorvo, M.J.; Beach, T.G.; Bedell, B.J.; Coleman, R.E.; Doraiswamy, P.M.; Fleisher, A.S.; Reiman, E.M.; Sabbagh, M.N.; Sadowsky, C.H.; et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: A prospective cohort study. Lancet Neurol. 2012, 11, 669–678. [Google Scholar] [CrossRef]
- Jack, C.R.; Therneau, T.M.; Wiste, H.J.; Weigand, S.D.; Knopman, D.S.; Lowe, V.J.; Mielke, M.M.; Vemuri, P.; Roberts, R.O.; Machulda, M.M.; et al. Transition rates between amyloid and neurodegeneration biomarker states and to dementia: A population-based, longitudinal cohort study. Lancet Neurol. 2016, 15, 56–64. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Altomare, D.; Thal, D.R.; Ribaldi, F.; van der Kant, R.; Ossenkoppele, R.; Blennow, K.; Cummings, J.; van Duijn, C.; Nilsson, P.M.; et al. The probabilistic model of Alzheimer disease: The amyloid hypothesis revised. Nat. Rev. Neurosci. 2022, 23, 53–66. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer’s disease: A focus on aducanumab and lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Kepp, K.P.; Sensi, S.L.; Johnsen, K.B.; Barrio, J.R.; Høilund-Carlsen, P.F.; Neve, R.L.; Alavi, A.; Herrup, K.; Perry, G.; Robakis, N.K.; et al. The anti-amyloid monoclonal antibody lecanemab: 16 cautionary notes. J. Alzheimer’s Dis. 2023, 94, 497–507. [Google Scholar] [CrossRef]
- Kaur, U.; Reddy, J.; Tiwari, A.; Chakrabarti, S.; Chakrabarti, S.S. Lecanemab: More questions than answers! Clin. Drug Investig. 2024, 44, 1–10. [Google Scholar] [CrossRef]
- Ang, J.; Harris, E.; Hussey, B.J.; Kil, R.; McMillen, D.R. Tuning response curves for synthetic biology. ACS Synth. Biol. 2013, 2, 547–567. [Google Scholar] [CrossRef]
- Salloway, S.P.; Sevingy, J.; Budur, K.; Pederson, J.T.; DeMattos, R.B.; Von Rosenstiel, P.; Paez, A.; Evans, R.; Weber, C.J.; Hendrix, J.A.; et al. Advancing combination therapy for Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12073. [Google Scholar] [CrossRef] [PubMed]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149, Correction in Pharmacol. Res. Perspect. 2019, 7, e00549. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.T.; Wooten, D.J.; Lopez, C.F.; Quaranta, V. Charting the fragmented landscape of drug synergy. Trends Pharmacol. Sci. 2020, 41, 266–280. [Google Scholar] [CrossRef]
- Götz, J.; Bodea, L.G.; Goedert, M. Rodent models for Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 583–598. [Google Scholar] [CrossRef]
- Bateman, R.J.; Benzinger, T.L.; Berry, S.; Clifford, D.B.; Duggan, C.; Fagan, A.M.; Fanning, K.; Farlow, M.R.; Hassenstab, J.; McDade, E.M.; et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimer’s Dement. 2017, 13, 8–19. [Google Scholar] [CrossRef] [PubMed]



| mo−1 | 450 * | |
| pg mg−1 mo−1 | 1.36 × 106 | |
| – | 10.7 | |
| pg mg−1 | 34 * | |
| mo−1 | 0.00168 | |
| mo−1 | 0.998 | |
| pg mg−1 | 134.2 | |
| – | 1 * | |
| LogErr | – | 0.540 |
| 0.63 | 0.24 | 0.34 | |
| - | 0.39 | 0.34 | |
| - | - | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyng, V.; Kellman, M.E. Kinetic Model with Feedback Cycle for Age-Dependent Amyloid Beta Accumulation in Mice. Int. J. Mol. Sci. 2025, 26, 8803. https://doi.org/10.3390/ijms26188803
Tyng V, Kellman ME. Kinetic Model with Feedback Cycle for Age-Dependent Amyloid Beta Accumulation in Mice. International Journal of Molecular Sciences. 2025; 26(18):8803. https://doi.org/10.3390/ijms26188803
Chicago/Turabian StyleTyng, Vivian, and Michael E. Kellman. 2025. "Kinetic Model with Feedback Cycle for Age-Dependent Amyloid Beta Accumulation in Mice" International Journal of Molecular Sciences 26, no. 18: 8803. https://doi.org/10.3390/ijms26188803
APA StyleTyng, V., & Kellman, M. E. (2025). Kinetic Model with Feedback Cycle for Age-Dependent Amyloid Beta Accumulation in Mice. International Journal of Molecular Sciences, 26(18), 8803. https://doi.org/10.3390/ijms26188803







