Synergistic CO2 Cryotherapy and EGF Delivery for Accelerated Wound Healing Through Anti-Inflammatory and Regenerative Pathways
Abstract
1. Introduction
2. Results
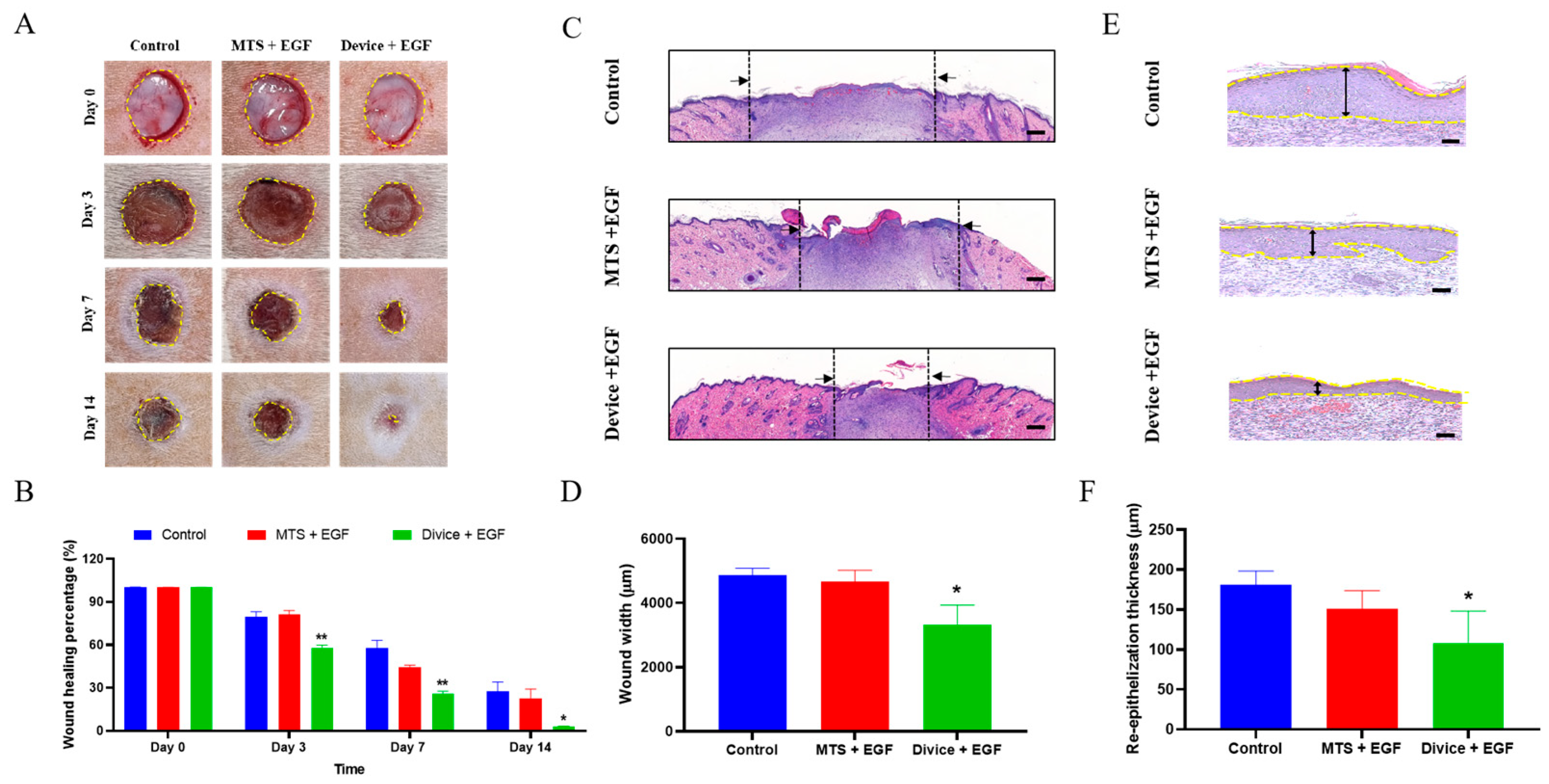
2.1. Macroscopic and Microscopic Observation of the Wound Healing Process
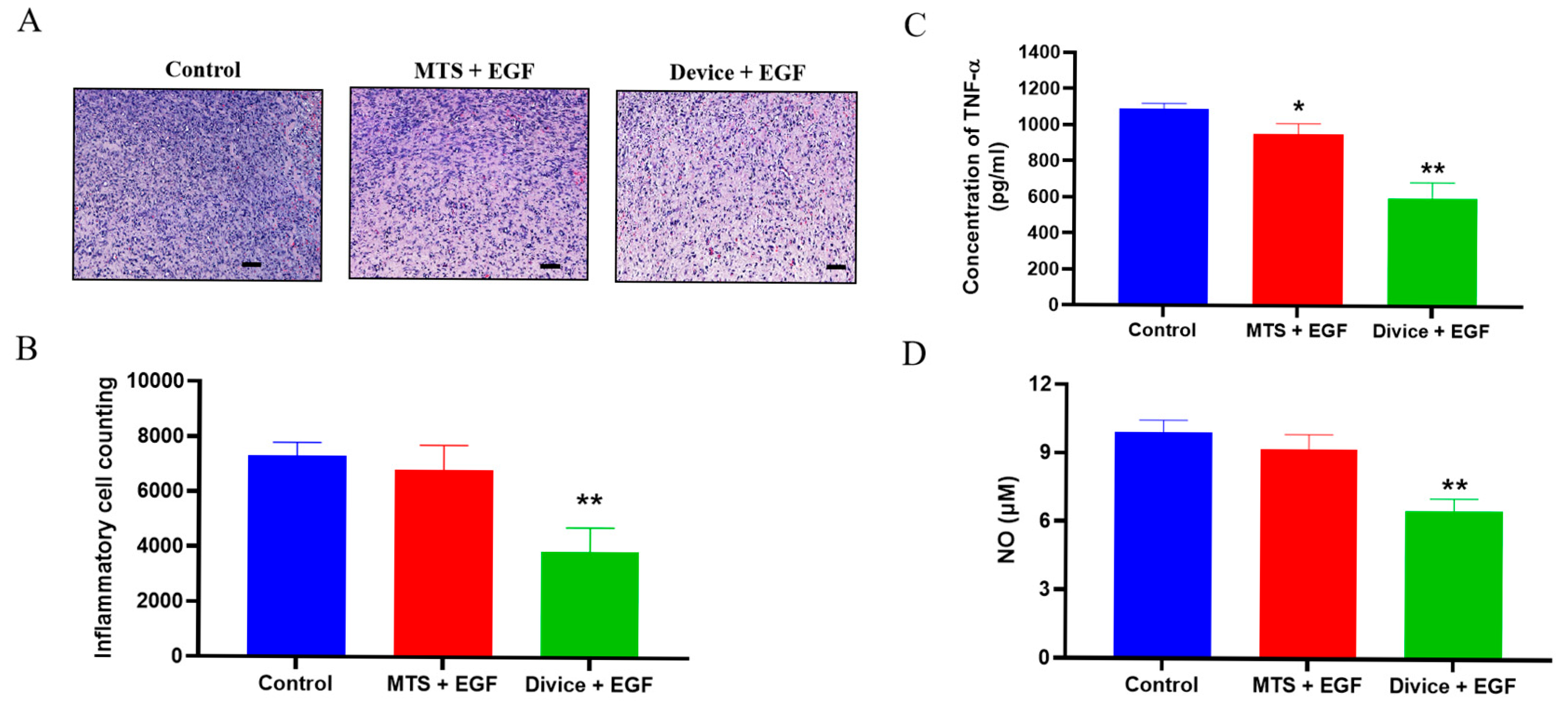
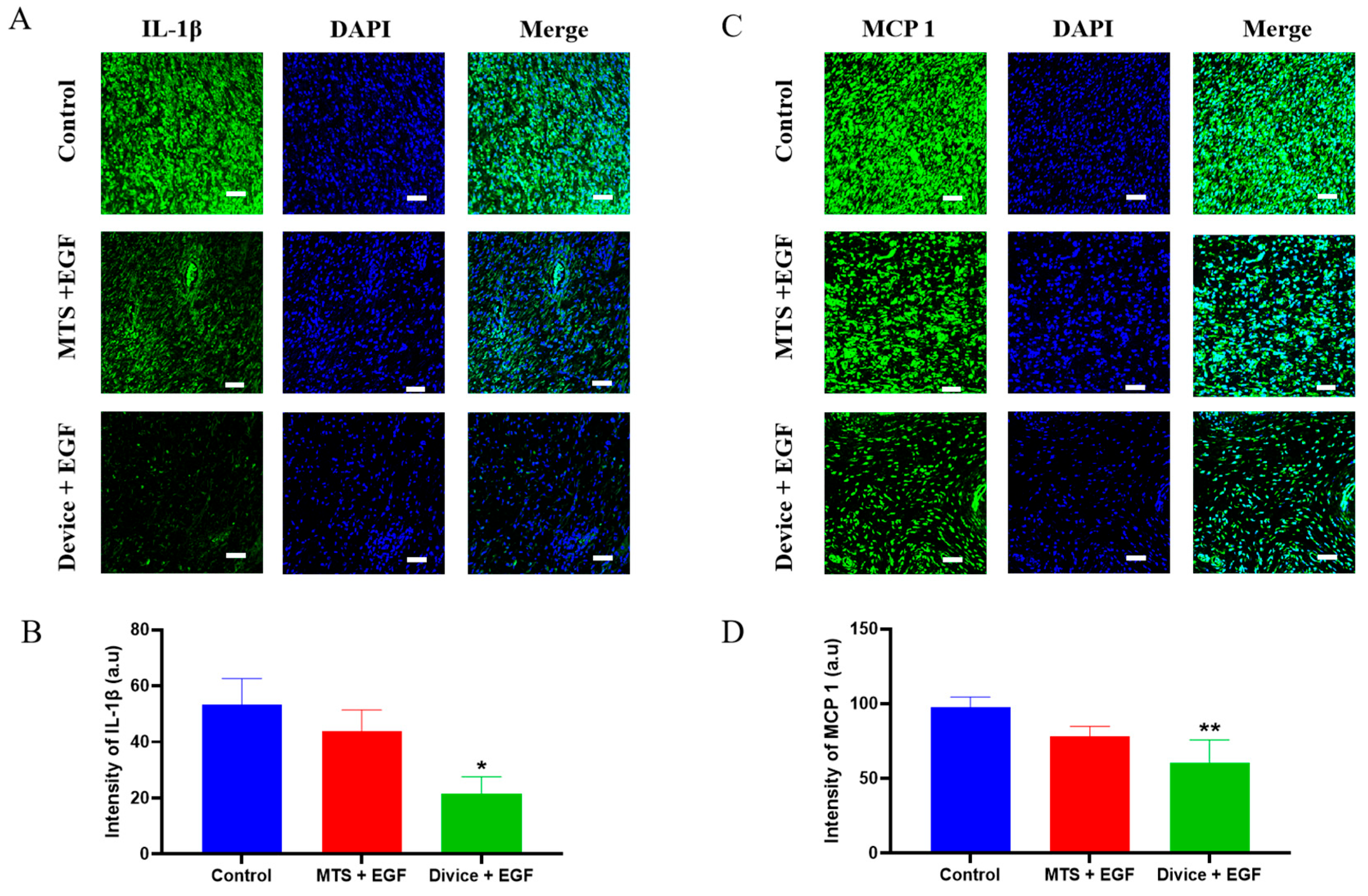
2.2. Effect of the Anti-Inflammation and Antioxidation During the Wound Healing Process
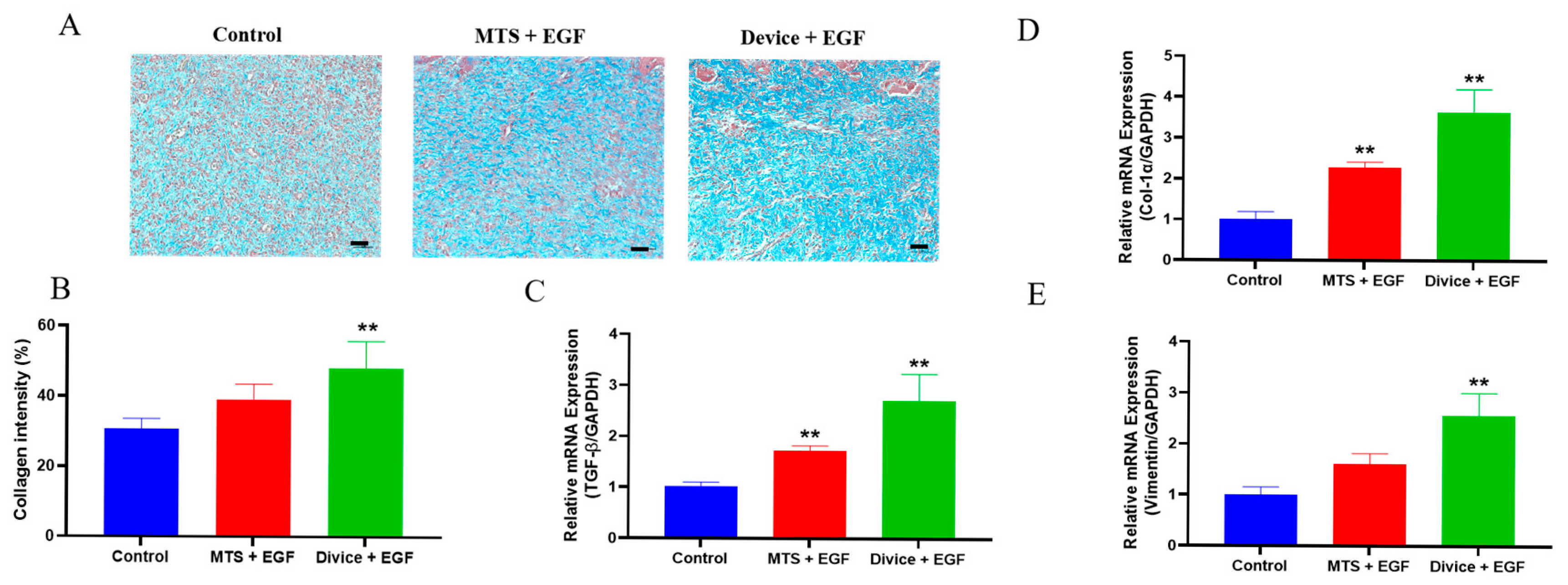
2.3. Acceleration of the Proliferation and Remodeling During the Wound Healing Process
3. Discussion
4. Materials and Methods
4.1. System Architecture and Operational Workflow of the AcuCool™ Device
4.2. Animal Experiment
4.3. Measurement of the Wound Healing Area
4.4. Histological Analysis
4.5. Hematoxylin and Eosin (H&E) Staining
4.6. Masson’s Trichrome (MT) Staining
4.7. Immunofluorescence (If) Staining
4.8. Protein Preparation
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Nitric Oxide Quantification
4.11. RT-PCR
4.12. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGF | epidermal growth factor |
| ELISA | enzyme-linked immunosorbent assay |
| H&E | hematoxylin and eosin |
| IF | immunofluorescence |
| IL-1β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| MTS | microneedle therapy system |
| MCP-1 | monocyte chemoattractant protein 1 |
| MT | Masson’s trichrome |
| NPWT | negative pressure wound therapy |
| NO | nitric oxide |
| PRP | platelet-rich plasma |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| ROS | reactive oxygen species |
| SEM | standard error of the mean |
| TGF-β1 | transforming growth factor-beta 1 |
| TNF-α | tumor necrosis factor alpha |
| VEGF | vascular endothelial growth factor |
References
- Canedo-Dorantes, L.; Canedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Jin, Y.X.; Ngoc Chien, P.; Thi Nga, P.; Zhang, X.R.; Ngan Giang, N.; Thi Thuy Le, L.; Trinh, T.T.; Zhou, S.Y.; Nam, S.Y.; Heo, C.Y. Enhancing wound healing through innovative technologies: Microneedle patches and iontophoresis. Front. Bioeng. Biotechnol. 2024, 12, 1468423. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Serra, M.B.; Barroso, W.A.; da Silva, N.N.; Silva, S.D.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflam. 2017, 2017, 3406215. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Hesseler, M.J.; Shyam, N. Platelet-rich plasma and its utility in medical dermatology: A systematic review. J. Am. Acad. Dermatol. 2019, 81, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Lana, J.F.; Alexander, R.W.; Dallo, I.; Kon, E.; Ambach, M.A.; van Zundert, A.; Podesta, L. Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing. Int. J. Mol. Sci. 2024, 25, 7914. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Garcia-Ojalvo, A.; Fernandez-Montequin, J.; Falcon-Cama, V.; Acosta-Rivero, N.; Guillen-Nieto, G.; Pujol-Ferrer, M.; Limonta-Fernandez, M.; Ayala-Avila, M.; Eriksson, E. Epidermal Growth Factor Intralesional Delivery in Chronic Wounds: The Pioneer and Standalone Technique for Reversing Wound Chronicity and Promoting Sustainable Healing. Int. J. Mol. Sci. 2024, 25, 10883. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Jain, P.; Ajazuddin. Recent advancement in molecular pathways and receptor targeting using natural products for wound healing activity. Inflammopharmacology 2025, 33, 4595–4613. [Google Scholar] [CrossRef]
- Yoon, S.H.; Huh, B.K.; Abdi, S.; Javed, S. The efficacy of high-intensity laser therapy in wound healing: A narrative review. Lasers Med. Sci. 2024, 39, 208. [Google Scholar] [CrossRef]
- Mineroff, J.; Maghfour, J.; Ozog, D.M.; Lim, H.W.; Kohli, I.; Jagdeo, J. Photobiomodulation CME part II: Clinical applications in dermatology. J. Am. Acad. Dermatol. 2024, 91, 805–815. [Google Scholar] [CrossRef]
- Fernandez-Guarino, M.; Bacci, S.; Perez Gonzalez, L.A.; Bermejo-Martinez, M.; Cecilia-Matilla, A.; Hernandez-Bule, M.L. The Role of Physical Therapies in Wound Healing and Assisted Scarring. Int. J. Mol. Sci. 2023, 24, 7487. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, B.; Liu, Q.; Mao, Y.; He, Y.; Liu, X.; Zhao, X.; Chen, Y.; Li, X.; Li, Y.; et al. Dissolvable microneedle-based wound dressing transdermally and continuously delivers anti-inflammatory and pro-angiogenic exosomes for diabetic wound treatment. Bioact. Mater. 2024, 42, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.; Shi, C.; Goh, E.L.; Murphy, E.M.; Reid, A.; Chiverton, L.; Stankiewicz, M.; Dumville, J.C. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst. Rev. 2022, 4, CD009261. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Xu, Y.; Liu, D. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcers: An updated systematic review and meta-analysis. Asian J. Surg. 2022, 45, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, e2100477. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Toriyama, T.; Kumada, Y.; Matsubara, T.; Murata, A.; Ogino, A.; Hayashi, H.; Nakashima, H.; Takahashi, H.; Matsuo, H.; Kawahara, H. Effect of artificial carbon dioxide foot bathing on critical limb ischemia (Fontaine IV) in peripheral arterial disease patients. Int. Angiol. 2002, 21, 367–373. [Google Scholar] [PubMed]
- Brandi, C.; D’Aniello, C.; Grimaldi, L.; Bosi, B.; Dei, I.; Lattarulo, P.; Alessandrini, C. Carbon dioxide therapy in the treatment of localized adiposities: Clinical study and histopathological correlations. Aesthetic Plast. Surg. 2001, 25, 170–174. [Google Scholar] [CrossRef]
- Brandi, C.; D’Aniello, C.; Grimaldi, L.; Caiazzo, E.; Stanghellini, E. Carbon dioxide therapy: Effects on skin irregularity and its use as a complement to liposuction. Aesthetic Plast. Surg. 2004, 28, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.R.; Bassenge, E.; Pittler, M. Effect of carbon dioxide-enriched water and fresh water on the cutaneous microcirculation and oxygen tension in the skin of the foot. Angiology 1997, 48, 337–343. [Google Scholar] [CrossRef]
- Brandi, C.; Grimaldi, L.; Nisi, G.; Brafa, A.; Campa, A.; Calabrò, M.; Campana, M.; D’Aniello, C. The role of carbon dioxide therapy in the treatment of chronic wounds. In Vivo 2010, 24, 223–226. [Google Scholar]
- Tilahun Zewdu, F.; Misganaw Geremew, B.; Gadisa Belachew, E.; Alemu Gelay, K. Effectiveness of carbon dioxide cryotherapy for the treatment of cutaneous leishmaniasis: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2025, 19, e0012741. [Google Scholar] [CrossRef]
- Prazeres, J.; Lima, A.; Ribeiro, G. Effects of Carbon Dioxide Therapy on Skin Wound Healing. Biomedicines 2025, 13, 228. [Google Scholar] [CrossRef]
- Brochado, T.M.M.; de Carvalho Schweich, L.; Di Pietro Simões, N.; Oliveira, R.J.; Antoniolli-Silva, A. Carboxytherapy: Controls the inflammation and enhances the production of fibronectin on wound healing under venous insufficiency. Int. Wound J. 2019, 16, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Sayama, K.; Yuki, K.; Sugata, K.; Fukagawa, S.; Yamamoto, T.; Ikeda, S.; Murase, T. Carbon dioxide inhibits UVB-induced inflammatory response by activating the proton-sensing receptor, GPR65, in human keratinocytes. Sci. Rep. 2021, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Nassar, S.O.; Eltatawy, R.A.R.; Hassan, G.F.R. Safety and efficacy of platelet-rich plasma vs carboxytherapy in the treatment of atrophic scars: A comparative clinical and histopathological study. Dermatol. Ther. 2020, 33, e13942. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef]
- Yuki, K.; Kawano, S.; Mori, S.; Murase, T. Facial application of high-concentration carbon dioxide prevents epidermal impairment associated with environmental changes. Clin. Cosmet. Investig. Dermatol. 2019, 12, 63–69. [Google Scholar] [CrossRef]
- Macura, M.; Ban Frangez, H.; Cankar, K.; Finžgar, M.; Frangez, I. The effect of transcutaneous application of gaseous CO2 on diabetic chronic wound healing-A double-blind randomized clinical trial. Int. Wound J. 2020, 17, 1607–1614. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair. Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Tan, Q.W.; Tang, S.L.; Zhang, Y.; Yang, J.Q.; Wang, Z.L.; Xie, H.Q.; Lv, Q. Hydrogel from Acellular Porcine Adipose Tissue Accelerates Wound Healing by Inducing Intradermal Adipocyte Regeneration. J. Investg. Dermatol. 2019, 139, 455–463. [Google Scholar] [CrossRef]
- Sakai, Y.; Miwa, M.; Oe, K.; Ueha, T.; Koh, A.; Niikura, T.; Iwakura, T.; Lee, S.Y.; Tanaka, M.; Kurosaka, M. A novel system for transcutaneous application of carbon dioxide causing an “artificial Bohr effect” in the human body. PLoS ONE 2011, 6, e24137. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, Y.; Fukui, T.; Takahara, S.; Lee, S.Y.; Oe, K.; Arakura, M.; Kumabe, Y.; Oda, T.; Matsumoto, T.; Matsushita, T.; et al. Topical cutaneous application of CO2 accelerates bone healing in a rat femoral defect model. BMC Musculoskelet. Disord. 2019, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating Inflammatory Markers in Wound Healing: Understanding Implications and Identifying Artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.L.; Lai, C.H.; Chen, P.K.; Cho, C.F.; Hsu, Y.Y.; Wang, K.C.; Lin, W.L.; Chang, B.I.; Liu, S.K.; Wu, Y.T.; et al. Thrombomodulin promotes diabetic wound healing by regulating toll-like receptor 4 expression. J. Investg. Dermatol. 2015, 135, 1668–1675. [Google Scholar] [CrossRef]
- Darby, I.A.; Zakuan, N.; Billet, F.; Desmoulière, A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol. Life Sci. 2016, 73, 1145–1157. [Google Scholar] [CrossRef]
- Coelho-Rato, L.S.; Parvanian, S.; Modi, M.K.; Eriksson, J.E. Vimentin at the core of wound healing. Trends Cell Biol. 2024, 34, 239–254. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmoulière, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef]
- Gardeazabal, L.; Izeta, A. Elastin and collagen fibres in cutaneous wound healing. Exp. Dermatol. 2024, 33, e15052. [Google Scholar] [CrossRef]
- Ramanathan, G.; Muthukumar, T.; Tirichurapalli Sivagnanam, U. In vivo efficiency of the collagen coated nanofibrous scaffold and their effect on growth factors and pro-inflammatory cytokines in wound healing. Eur. J. Pharmacol. 2017, 814, 45–55. [Google Scholar] [CrossRef]
- Oda, T.; Niikura, T.; Fukui, T.; Oe, K.; Kuroiwa, Y.; Kumabe, Y.; Sawauchi, K.; Yoshikawa, R.; Mifune, Y.; Hayashi, S.; et al. Transcutaneous CO2 application accelerates fracture repair in streptozotocin-induced type I diabetic rats. BMJ Open Diabetes Res. Care 2020, 8, e001129. [Google Scholar] [CrossRef]
- Durães, E.F.; Durães Lde, C.; Carneiro, F.P.; Lino, R.d.S., Jr.; Sousa, J.B. The effect of carbon dioxide therapy on composite graft survival. Acta Cir. Bras. 2013, 28, 589–593. [Google Scholar] [CrossRef][Green Version]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burns Trauma. 2019, 7, 10. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Broussard, K.C.; Powers, J.G. Wound dressings: Selecting the most appropriate type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal Delivery of Drugs with Microneedles-Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef]
- Malek-Khatabi, A.; Sadat Razavi, M.; Abdollahi, A.; Rahimzadeghan, M.; Moammeri, F.; Sheikhi, M.; Tavakoli, M.; Rad-Malekshahi, M.; Faraji Rad, Z. Recent progress in PLGA-based microneedle-mediated transdermal drug and vaccine delivery. Biomater. Sci. 2023, 11, 5390–5409. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Lee, Y.-H.; Kim, D.H.; Jin, C.; Zhang, X.; Yoo, J.R.; Kim, G.-H.; Kim, D.H.; Oh, T.-I.; Jung, Y.-S.; et al. Synergistic CO2 Cryotherapy and EGF Delivery for Accelerated Wound Healing Through Anti-Inflammatory and Regenerative Pathways. Int. J. Mol. Sci. 2025, 26, 8796. https://doi.org/10.3390/ijms26188796
Jin Y, Lee Y-H, Kim DH, Jin C, Zhang X, Yoo JR, Kim G-H, Kim DH, Oh T-I, Jung Y-S, et al. Synergistic CO2 Cryotherapy and EGF Delivery for Accelerated Wound Healing Through Anti-Inflammatory and Regenerative Pathways. International Journal of Molecular Sciences. 2025; 26(18):8796. https://doi.org/10.3390/ijms26188796
Chicago/Turabian StyleJin, Yongxun, Yong-Hyun Lee, Do Hwan Kim, Caijun Jin, Xinrui Zhang, Jae Ryeong Yoo, Gun-Ho Kim, Dae Hyun Kim, Taek-In Oh, Yi-Sook Jung, and et al. 2025. "Synergistic CO2 Cryotherapy and EGF Delivery for Accelerated Wound Healing Through Anti-Inflammatory and Regenerative Pathways" International Journal of Molecular Sciences 26, no. 18: 8796. https://doi.org/10.3390/ijms26188796
APA StyleJin, Y., Lee, Y.-H., Kim, D. H., Jin, C., Zhang, X., Yoo, J. R., Kim, G.-H., Kim, D. H., Oh, T.-I., Jung, Y.-S., Chien, P. N., & Heo, C. Y. (2025). Synergistic CO2 Cryotherapy and EGF Delivery for Accelerated Wound Healing Through Anti-Inflammatory and Regenerative Pathways. International Journal of Molecular Sciences, 26(18), 8796. https://doi.org/10.3390/ijms26188796






