SARS-CoV-2 Spike Protein and Molecular Mimicry: An Immunoinformatic Screen for Cross-Reactive Autoantigen Candidates
Abstract
1. Introduction
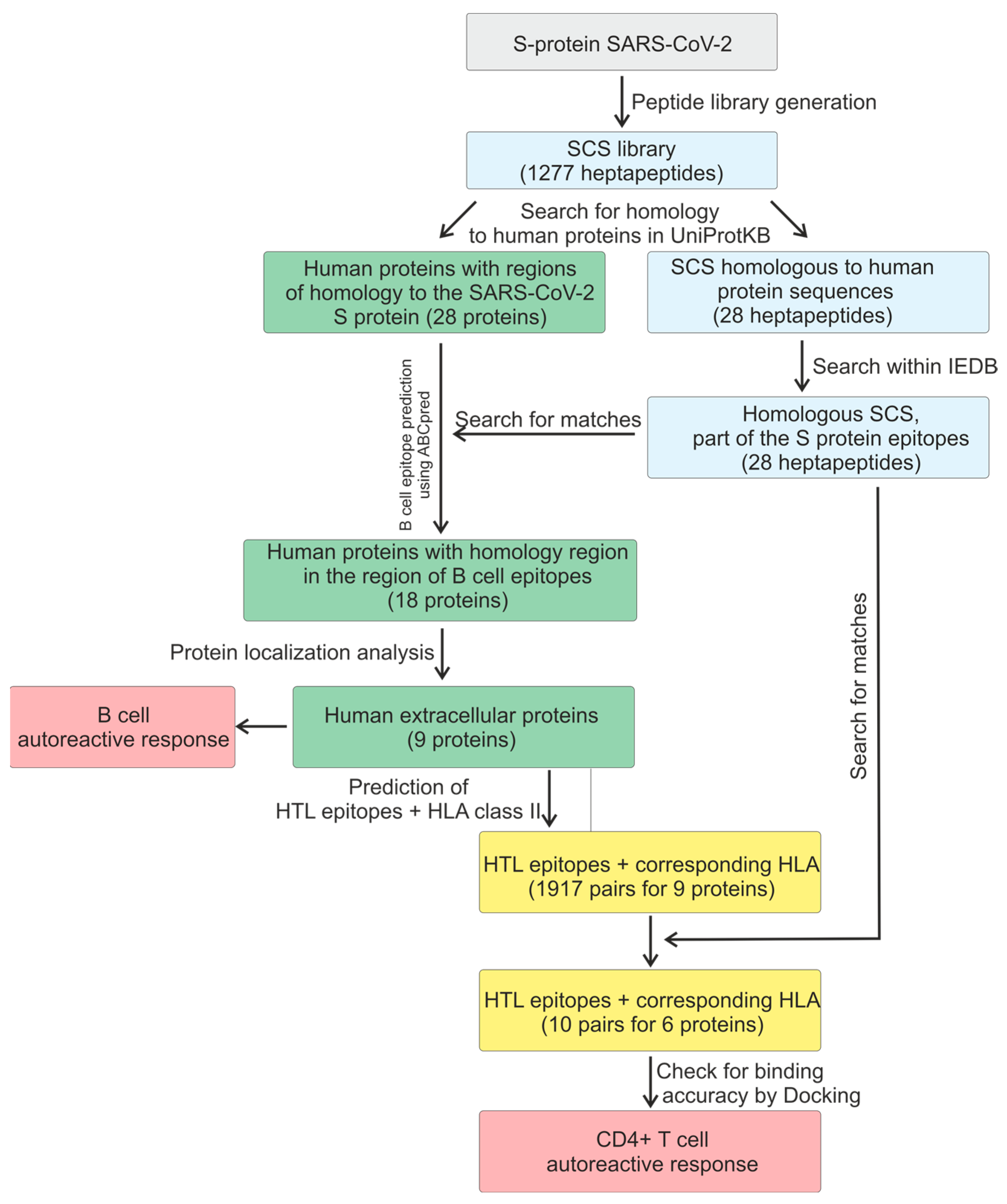
2. Results
2.1. Predicting B Antigen Recognition by B Cells
2.2. T-Cell-Dependent Antibody Response
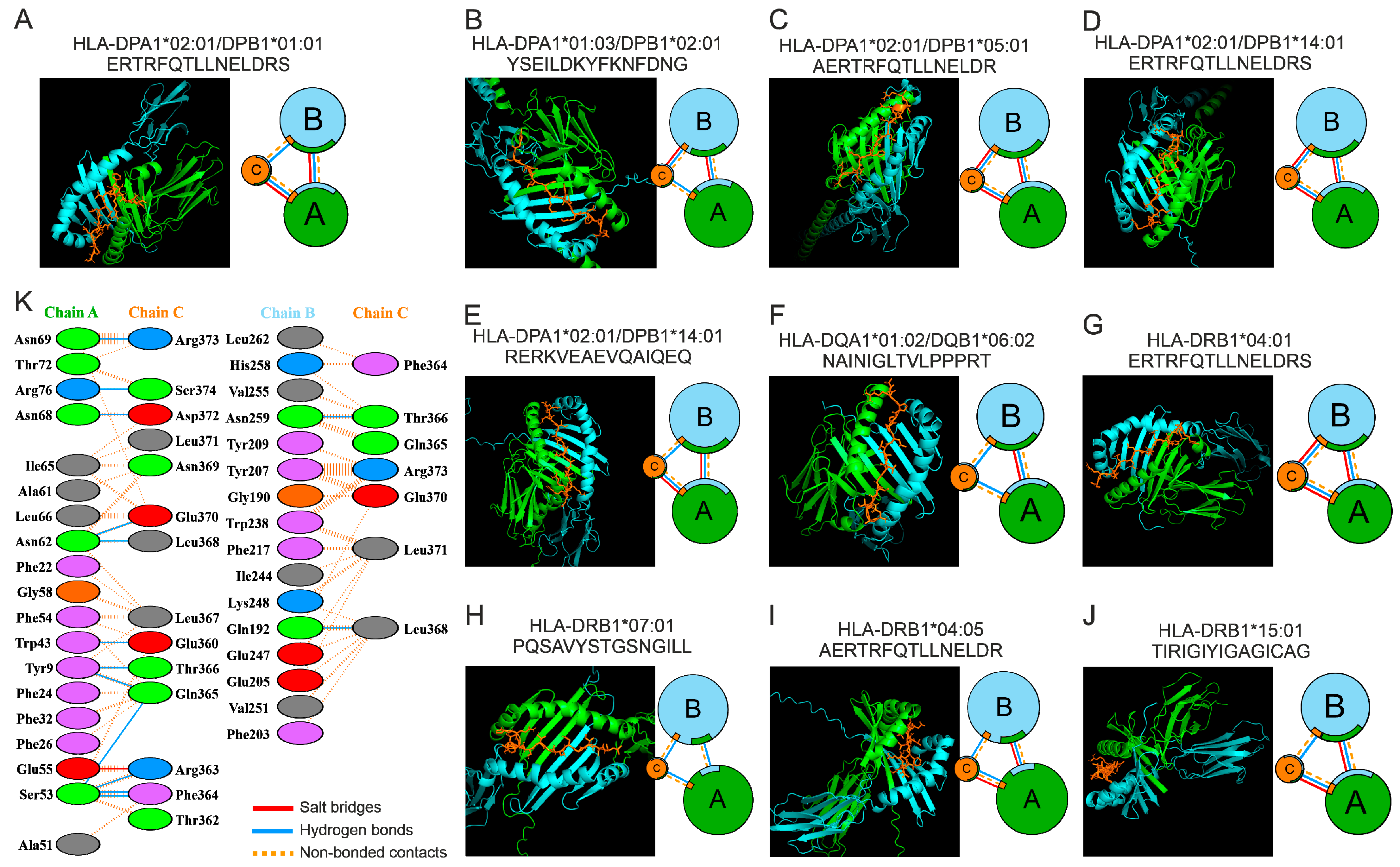
2.3. Molecular Docking of HTL Epitopes with HLA-II
2.4. Molecular Dynamics Simulations of the HTL–Epitope Complex and HLA-II
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Obtaining the S Protein Sequence of SARS-CoV-2 and Predicting B-Cell Epitopes Using IEDB
4.2. Obtaining Human Protein Sequences and Predicting B-Cell Epitopes Using ABCpred
4.3. Predicting HTL (Helper T Lymphocyte) Epitopes
4.4. Molecular Docking
4.5. Molecular Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldstone, M.B.A. Molecular mimicry and immune-mediated diseases. FASEB J. 1998, 12, 1255–1265. [Google Scholar] [CrossRef]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular Mimicry as a Mechanism of Autoimmune Disease. Clin. Rev. Allergy Immunol. 2011, 42, 102–111. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Shevach, E.M. Regulatory T Cells in Autoimmmunity. Annu. Rev. Immunol. 2000, 18, 423–449. [Google Scholar] [CrossRef]
- Sakaguchi, S. Naturally Arising CD4+ Regulatory T Cells for Immunologic Self-Tolerance and Negative Control of Immune Responses. Annu. Rev. Immunol. 2004, 22, 531–562. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.A. The historical postulate: Is it the basis, at the level of the system, for self-nonself discrimination? Scand. J. Immunol. 2021, 94, e13033. [Google Scholar] [CrossRef]
- Zemková, M.; Zahradník, D.; Mokrejš, M.; Flegr, J. Parasitism as the main factor shaping peptide vocabularies in current organisms. Parasitology 2017, 144, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D.; Stufano, A.; Lucchese, G.; Kusalik, A. Massive peptide sharing between viral and human proteomes. Peptides 2008, 29, 1755–1766. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F.; Giovannoni, G.; Salvetti, M. Epstein-Barr virus as a cause of multiple sclerosis: Opportunities for prevention and therapy. Lancet Neurol. 2023, 22, 338–349. [Google Scholar] [CrossRef]
- Mechelli, R.; Manzari, C.; Policano, C.; Annese, A.; Picardi, E.; Umeton, R.; Fornasiero, A.; D’Erchia, A.M.; Buscarinu, M.C.; Agliardi, C.; et al. Epstein-Barr virus genetic variants are associated with multiple sclerosis. Neurology 2015, 84, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Münz, C.; Cohen, J.I.; Ascherio, A. Epstein–Barr virus as a leading cause of multiple sclerosis: Mechanisms and implications. Nat. Rev. Neurol. 2023, 19, 160–171. [Google Scholar] [CrossRef]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Hou, J.; Said, C.; Franchi, D.; Dockstader, P.; Chatterjee, N.K. Antibodies to Glutamic Acid Decarboxylase and P2-C Peptides in Sera from Coxsackie Virus B4-Infected Mice and IDDM Patients. Diabetes 1994, 43, 1260–1266. [Google Scholar] [CrossRef]
- Yokota, I.; Shima, K. GAD Antibody in IDDM. Rinsho Byori. 1998, 46, 331–337. [Google Scholar] [PubMed]
- Lönnrot, M.; Hyöty, H.; Knip, M.; Roivainen, M.; Kulmala, P.; Leinikki, P.; Åkerblom, H.K. Childhood Diabetes in Finland Study Group Antibody cross-reactivity induced by the homologous regions in glutamic acid decarboxylase (GAD65) and 2C protein of coxsackievirus B4. Clin. Exp. Immunol. 1996, 104, 398–405. [Google Scholar] [CrossRef]
- Kanduc, D. The comparative biochemistry of viruses and humans: An evolutionary path towards autoimmunity. Biol. Chem. 2018, 400, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Weaver, D.F. COVID-19 as a Trigger of Brain Autoimmunity. ACS Chem. Neurosci. 2021, 12, 2558–2561. [Google Scholar] [CrossRef]
- Sher, E.K.; Ćosović, A.; Džidić-Krivić, A.; Farhat, E.K.; Pinjić, E.; Sher, F. COVID-19 a triggering factor of autoimmune and multi-inflammatory diseases. Life Sci. 2023, 319, 121531. [Google Scholar] [CrossRef]
- Votto, M.; Castagnoli, R.; Marseglia, G.L.; Licari, A.; Brambilla, I. COVID-19 and autoimmune diseases: Is there a connection? Curr. Opin. Allergy Clin. Immunol. 2023, 23, 185–192. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Sedykh, S.E.; Nevinsky, G.A. SARS-CoV-2 infection as a risk factor for the development of autoimmune pathology. Mol. Meditsina (Mol. Med.) 2022, 20, 3–10. [Google Scholar] [CrossRef]
- Bennion, B.G.; Ingle, H.; Ai, T.L.; Miner, C.A.; Platt, D.J.; Smith, A.M.; Baldridge, M.T.; Miner, J.J.; Longnecker, R.M. A Human Gain-of-Function STING Mutation Causes Immunodeficiency and Gammaherpesvirus-Induced Pulmonary Fibrosis in Mice. J. Virol. 2019, 93, e01806-18. [Google Scholar] [CrossRef]
- Marino Gammazza, A.; Légaré, S.; Lo Bosco, G.; Fucarino, A.; Angileri, F.; Conway de Macario, E.; Macario, A.J.L.; Cappello, F. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: Possible role of molecular mimicry in COVID-19. Cell Stress Chaperon. 2020, 25, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, G.; Flöel, A. SARS-CoV-2 and Guillain-Barré syndrome: Molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperon. 2020, 25, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D. From Anti-SARS-CoV-2 Immune Responses to COVID-19 via Molecular Mimicry. Antibodies 2020, 9, 33. [Google Scholar] [CrossRef]
- Cuspoca, A.F.; Estrada, P.I.; Velez-Van-Meerbeke, A. Molecular Mimicry of SARS-CoV-2 Spike Protein in the Nervous System: A Bioinformatics Approach. Comput. Struct. Biotechnol. J. 2022, 20, 6041–6054. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, N.Y.; Normatov, M.G.; Soprun, L.A.; Utekhin, V.J.; Fedotkina, T.V.; Churilov, L.P. Autoantigens of Small Nerve Fibers and Human Coronavirus Antigens: Is There a Possibility for Molecular Mimicry? Curr. Microbiol. 2024, 81, 366. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Sedykh, S.E.; Litvinova, E.A.; Dolgushin, S.A.; Matveev, A.L.; Tikunova, N.V.; Nevinsky, G.A. Binding of Natural Antibodies Generated after COVID-19 and Vaccination with Individual Peptides Corresponding to the SARS-CoV-2 S-Protein. Vaccines 2024, 12, 426. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Sedykh, S.E.; Dmitrenok, P.S.; Nevinsky, G.A. Identification of Antibody-Mediated Hydrolysis Sites of Oligopeptides Corresponding to the SARS-CoV-2 S-Protein by MALDI-TOF Mass Spectrometry. Int. J. Mol. Sci. 2023, 24, 14342. [Google Scholar] [CrossRef]
- Dotan, A.; Kanduc, D.; Muller, S.; Makatsariya, A.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 and the female reproductive system. Am. J. Reprod. Immunol. 2021, 86, e13494. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2019, 20, 173–185. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of Tissue Inflammation by the Immune Receptor Tim-3 Expressed on Innate Immune Cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Shou, S.-T.; Chai, Y.-F. Immune checkpoints in sepsis: New hopes and challenges. Int. Rev. Immunol. 2021, 41, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.V.; Colgan, J.D. Regulation of T cell responses by the receptor molecule Tim-3. Immunol. Res. 2014, 59, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fueyo, A.; Tian, J.; Picarella, D.; Domenig, C.; Zheng, X.X.; Sabatos, C.A.; Manlongat, N.; Bender, O.; Kamradt, T.; Kuchroo, V.K.; et al. Tim-3 inhibits T helper type 1–mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003, 4, 1093–1101. [Google Scholar] [CrossRef]
- Lu, C.; Tan, Y. Promising immunotherapy targets: TIM3, LAG3, and TIGIT joined the party. Mol. Ther. Oncol. 2024, 32, 200773. [Google Scholar] [CrossRef]
- Schnell, A.; Bod, L.; Madi, A.; Kuchroo, V.K. The yin and yang of co-inhibitory receptors: Toward anti-tumor immunity without autoimmunity. Cell Res. 2020, 30, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Sever-Chroneos, Z.; Krupa, A.; Davis, J.; Hasan, M.; Yang, C.-H.; Szeliga, J.; Herrmann, M.; Hussain, M.; Geisbrecht, B.V.; Kobzik, L.; et al. Surfactant Protein A (SP-A)-mediated Clearance of Staphylococcus aureus Involves Binding of SP-A to the Staphylococcal Adhesin Eap and the Macrophage Receptors SP-A Receptor 210 and Scavenger Receptor Class A. J. Biol. Chem. 2011, 286, 4854–4870. [Google Scholar] [CrossRef]
- Yang, L.; Carrillo, M.; Wu, Y.M.; DiAngelo, S.L.; Silveyra, P.; Umstead, T.M.; Halstead, E.S.; Davies, M.L.; Hu, S.; Floros, J.; et al. SP-R210 (Myo18A) Isoforms as Intrinsic Modulators of Macrophage Priming and Activation. PLoS ONE 2015, 10, e0126576. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.B.; Enyindah-Asonye, G.; Matsui, K.; Kosik, I.; Dvorina, N.; Baldwin, W.M.; Yewdell, J.W.; Gupta, N. Cutting Edge: Myosin 18A Is a Novel Checkpoint Regulator in B Cell Differentiation and Antibody-Mediated Immunity. J. Immunol. 2021, 206, 2521–2526. [Google Scholar] [CrossRef]
- Kremlev, S.G.; Janic, B.; Umstead, T.M.; Phelps, D.S.; Floros, J.; Mikerov, A.N.; Huang, W.; Liu, W.; Wang, G.; Al-Mondhiry, H.; et al. Surfactant protein A regulates cytokine production in the monocytic cell line THP-1. Am. J. Physiol. Cell. Mol. Physiol. 1997, 272, L996–L1004. [Google Scholar] [CrossRef] [PubMed]
- Kremlev, S.G.; Janic, B.; Umstead, T.M.; Phelps, D.S.; Floros, J.; Huang, W.; Wang, G.; Al-Mondhiry, H.; De Lara, L.V.; Becerril, C.; et al. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. Am. J. Physiol. Cell. Mol. Physiol. 1997, 272, L1070–L1077. [Google Scholar] [CrossRef]
- Koptides, M.; Umstead, T.M.; Floros, J.; Phelps, D.S. Surfactant protein A activates NF-kappa B in the THP-1 monocytic cell line. Am. J. Physiol. Cell. Mol. Physiol. 1997, 273, L382–L388. [Google Scholar] [CrossRef]
- Murakami, K.; Tanaka, M.; Usui, T.; Kawabata, D.; Shiomi, A.; Iguchi-Hashimoto, M.; Shimizu, M.; Yukawa, N.; Yoshifuji, H.; Nojima, T.; et al. Follistatin-related protein/follistatin-like 1 evokes an innate immune response via CD14 and toll-like receptor 4. FEBS Lett. 2012, 586, 319–324. [Google Scholar] [CrossRef]
- Jin, X.; Nie, E.; Zhou, X.; Zeng, A.; Yu, T.; Zhi, T.; Jiang, K.; Wang, Y.; Zhang, J.; You, Y. Fstl1 Promotes Glioma Growth Through the BMP4/Smad1/5/8 Signaling Pathway. Cell. Physiol. Biochem. 2017, 44, 1616–1628. [Google Scholar] [CrossRef]
- Le Luduec, J.B.; Condamine, T.; Louvet, C.; Thebault, P.; Heslan, J.; Heslan, M.; Chiffoleau, E.; Cuturi, M. An Immunomodulatory Role for Follistatin-Like 1 in Heart Allograft Transplantation. Am. J. Transplant. 2008, 8, 2297–2306. [Google Scholar] [CrossRef]
- Staruschenko, A.; Medina, J.L.; Patel, P.; Shapiro, M.S.; Booth, R.E.; Stockand, J.D. Fluorescence Resonance Energy Transfer Analysis of Subunit Stoichiometry of the Epithelial Na+ Channel. J. Biol. Chem. 2004, 279, 27729–27734. [Google Scholar] [CrossRef]
- Mutchler, S.M.; Kirabo, A.; Kleyman, T.R. Epithelial Sodium Channel and Salt-Sensitive Hypertension. Hypertension 2021, 77, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Hummler, E.; Barker, P.; Gatzy, J.; Beermann, F.; Verdumo, C.; Schmidt, A.; Boucher, R.; Rossier, B.C. Early death due to defective neonatal lung liquid clearance in αENaC-deficient mice. Nat. Genet. 1996, 12, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Althaus, M.; Clauss, W.G.; Fronius, M. Amiloride-Sensitive Sodium Channels and Pulmonary Edema. Pulm. Med. 2010, 2011, 830320. [Google Scholar] [CrossRef] [PubMed]
- Sternak, M.; Bar, A.; Adamski, M.G.; Mohaissen, T.; Marczyk, B.; Kieronska, A.; Stojak, M.; Kus, K.; Tarjus, A.; Jaisser, F.; et al. The Deletion of Endothelial Sodium Channel α (αENaC) Impairs Endothelium-Dependent Vasodilation and Endothelial Barrier Integrity in Endotoxemia in Vivo. Front. Pharmacol. 2018, 9, 178. [Google Scholar] [CrossRef]
- Romero, M.J.; Yue, Q.; Singla, B.; Hamacher, J.; Sridhar, S.; Moseley, A.S.; Song, C.; Mraheil, M.A.; Fischer, B.; Zeitlinger, M.; et al. Direct endothelial ENaC activation mitigates vasculopathy induced by SARS-CoV2 spike protein. Front. Immunol. 2023, 14, 1241448. [Google Scholar] [CrossRef]
- Nomura, K.; Nakanishi, M.; Ishidate, F.; Iwata, K.; Taruno, A. All-Electrical Ca2+-Independent Signal Transduction Mediates Attractive Sodium Taste in Taste Buds. Neuron 2020, 106, 816–829.e6. [Google Scholar] [CrossRef]
- Barrachina-Esteve, O.; Anguita, A.; Reverter, A.; Espinosa, J.; Lafuente, C.; Rubio-Roy, M.; Crosas, M.; Vila-Sala, C.; Acero, C.; Navarro, M.; et al. Neurologic features in hospitalized patients with COVID-19: A prospective cohort in a catalan hospital. Neurol. Sci. 2025, 46, 1477–1488. [Google Scholar] [CrossRef]
- Martínez, Y.B.; Oriani, G.A.B.; Montes, P.V.; Moreno, E.M.; Pérez, A.P.; Pérez, R.J.C.; Cobos, F.M. Long-term persistence of post-COVID-19 symptoms: A two-year follow-up of a Primary Care cohort. An. Sist. Sanit. Navar. 2025, 48, e1101. [Google Scholar] [CrossRef]
- Anand, P.; Puranik, A.; Aravamudan, M.; Venkatakrishnan, A.; Soundararajan, V. SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. eLife 2020, 9, e58603. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.-F.; Strazielle, N.; Catala, M.; Silva-Vargas, V.; Doetsch, F.; Engelhardt, B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018, 135, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Owens, T.; Bechmann, I.; Engelhardt, B. Perivascular Spaces and the Two Steps to Neuroinflammation. J. Neuropathol. Exp. Neurol. 2008, 67, 1113–1121. [Google Scholar] [CrossRef]
- Schwartz, M.; Kipnis, J.; Rivest, S.; Prat, A. How Do Immune Cells Support and Shape the Brain in Health, Disease, and Aging? J. Neurosci. 2013, 33, 17587–17596. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.H.; Weninger, W.; Hunter, C.A. Trafficking of immune cells in the central nervous system. J. Clin. Investig. 2010, 120, 1368–1379. [Google Scholar] [CrossRef]
- Frebel, H.; Richter, K.; Oxenius, A. How chronic viral infections impact on antigen-specific T-cell responses. Eur. J. Immunol. 2010, 40, 654–663. [Google Scholar] [CrossRef]
- Jain, R.W.; Yong, V.W. B cells in central nervous system disease: Diversity, locations and pathophysiology. Nat. Rev. Immunol. 2021, 22, 513–524. [Google Scholar] [CrossRef]
- Meinl, E.; Krumbholz, M.; Hohlfeld, R. B lineage cells in the inflammatory central nervous system environment: Migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 2006, 59, 880–892. [Google Scholar] [CrossRef]
- Barr, T.A.; Shen, P.; Brown, S.; Lampropoulou, V.; Roch, T.; Lawrie, S.; Fan, B.; O’cOnnor, R.A.; Anderton, S.M.; Bar-Or, A.; et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J. Exp. Med. 2012, 209, 1001–1010. [Google Scholar] [CrossRef]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.J., Jr.; Pröbstel, A.-K.; Zamvil, S.S. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 2019, 20, 728–745. [Google Scholar] [CrossRef]
- Brilot, F.; Dale, R.C.; Selter, R.C.; Grummel, V.; Kalluri, S.R.; Aslam, M.; Busch, V.; Zhou, D.; Cepok, S.; Hemmer, B. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann. Neurol. 2009, 66, 833–842. [Google Scholar] [CrossRef]
- Aulova, K.S.; Urusov, A.E.; Toporkova, L.B.; Sedykh, S.E.; Shevchenko, Y.A.; Tereshchenko, V.P.; Sennikov, S.V.; Budde, T.; Meuth, S.G.; Orlovskaya, I.A.; et al. Catalytic antibodies in the bone marrow and other organs of Th mice during spontaneous development of experimental autoimmune encephalomyelitis associated with cell differentiation. Mol. Biol. Rep. 2021, 48, 1055–1068. [Google Scholar] [CrossRef]
- Aulova, K.S.; Urusov, A.E.; Toporkova, L.B.; Sedykh, S.E.; Shevchenko, Y.A.; Tereshchenko, V.P.; Sennikov, S.V.; Budde, T.; Meuth, S.G.; Popova, N.A.; et al. Production of Abzymes in Th, CBA, and C57BL/6 Mice before and after MOG Treatment: Comparing Changes in Cell Differentiation and Proliferation. Biomolecules 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Almulla, A.F.; Vojdani, E.; Li, J.; Zhang, Y.; Maes, M. Autoimmune responses to myelin-associated proteins as diagnostic and prognostic biomarkers of relapsing-remitting multiple sclerosis: Associations with human herpesvirus-6 and Epstein-Barr virus reactivation. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Bezuglova, A.M.; Konenkova, L.P.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity and metal-dependence of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with systemic lupus erythematosus. J. Mol. Recognit. 2011, 24, 960–974. [Google Scholar] [CrossRef]
- Legostaeva, G.A.; Polosukhina, D.I.; Bezuglova, A.M.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with multiple sclerosis. J. Cell. Mol. Med. 2010, 14, 699–709. [Google Scholar] [CrossRef]
- Boronat, A.; Gelfand, J.M.; Gresa-Arribas, N.; Jeong, H.; Walsh, M.; Roberts, K.; Martinez-Hernandez, E.; Rosenfeld, M.R.; Balice-Gordon, R.; Graus, F.; et al. Encephalitis and antibodies to dipeptidyl-peptidase–like protein-6, a subunit of Kv4.2 potassium channels. Ann. Neurol. 2012, 73, 120–128. [Google Scholar] [CrossRef]
- Li, E.-C.; Zhang, T.-Y.; Cai, M.-T.; Su, S.-Y.; Shen, C.-H.; Lai, Q.-L.; Zhang, Y.-X. Clinical and Paraclinical Characterizations, Management, and Prognosis in DPPX Antibody-Associated Encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200350. [Google Scholar] [CrossRef]
- Alisch, M.; Foersterling, F.; Zocholl, D.; Muinjonov, B.; Schindler, P.; Duchnow, A.; Otto, C.; Ruprecht, K.; Schmitz-Hübsch, T.; Jarius, S.; et al. Distinguishing Neuromyelitis Optica Spectrum Disorders Subtypes: A Study on AQP4 and C3d Epitope Expression in Cytokine-Primed Human Astrocytes. Glia 2025, 73, 1090–1106. [Google Scholar] [CrossRef]
- Siriratnam, P.; Rocchi, C.; Gibbons, E.; Kelly, P.; Linaker, S.; Huda, S. Is a Benign Disease Course Possible in Untreated AQP4-IgG NMOSD? Eur. J. Neurol. 2025, 32, e70049. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Alexopoulos, H.; Spaeth, P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020, 16, 601–617. [Google Scholar] [CrossRef]
- Prüss, H. Autoantibodies in neurological disease. Nat. Rev. Immunol. 2021, 21, 798–813. [Google Scholar] [CrossRef]
- Pinto, A.A.; Carroll, L.S.; Nar, V.; Varatharaj, A.; Galea, I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e813. [Google Scholar] [CrossRef]
- Guilmot, A.; Slootjes, S.M.; Sellimi, A.; Bronchain, M.; Hanseeuw, B.; Belkhir, L.; Yombi, J.C.; De Greef, J.; Pothen, L.; Yildiz, H.; et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2020, 268, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Klyaus, N.A.; Sedykh, S.E.; Nevinsky, G.A. Antibodies Specific to Rheumatologic and Neurologic Pathologies Found in Patient with Long COVID. Rheumato 2025, 5, 1. [Google Scholar] [CrossRef]
- Almulla, A.F.; Maes, M.; Zhou, B.; Al-Hakeim, H.K.; Vojdani, A. Brain-targeted autoimmunity is strongly associated with Long COVID and its chronic fatigue syndrome as well as its affective symptoms. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Goode, B.L.; Eck, M.J. Mechanism and Function of Formins in the Control of Actin Assembly. Annu. Rev. Biochem. 2007, 76, 593–627. [Google Scholar] [CrossRef]
- Cristobal, C.D.; Wang, C.-Y.; Zuo, Z.; Smith, J.A.; Lindeke-Myers, A.; Bellen, H.J.; Lee, H.K. Daam2 Regulates Myelin Structure and the Oligodendrocyte Actin Cytoskeleton through Rac1 and Gelsolin. J. Neurosci. 2022, 42, 1679–1691. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zuo, Z.; Jo, J.; Kim, K.I.; Madamba, C.; Ye, Q.; Jung, S.Y.; Bellen, H.J.; Lee, H.K. Daam2 phosphorylation by CK2α negatively regulates Wnt activity during white matter development and injury. Proc. Natl. Acad. Sci. USA 2023, 120, e2304112120. [Google Scholar] [CrossRef]
- Fernandes, L.; Kleene, R.; Congiu, L.; Freitag, S.; Kneussel, M.; Loers, G.; Schachner, M. CHL1 depletion affects dopamine receptor D2-dependent modulation of mouse behavior. Front. Behav. Neurosci. 2023, 17, 1288509. [Google Scholar] [CrossRef] [PubMed]
- Montag-Sallaz, M.; Baarke, A.; Montag, D. Aberrant neuronal connectivity in CHL1-deficient mice is associated with altered information processing-related immediate early gene expression. J. Neurobiol. 2003, 57, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Schmalbach, B.; Lepsveridze, E.; Djogo, N.; Papashvili, G.; Kuang, F.; Leshchyns’ka, I.; Sytnyk, V.; Nikonenko, A.G.; Dityatev, A.; Jakovcevski, I.; et al. Age-dependent loss of parvalbumin-expressing hippocampal interneurons in mice deficient in CHL1, a mental retardation and schizophrenia susceptibility gene. J. Neurochem. 2015, 135, 830–844. [Google Scholar] [CrossRef]
- Irintchev, A.; Koch, M.; Needham, L.K.; Maness, P.; Schachner, M. Impairment of sensorimotor gating in mice deficient in the cell adhesion molecule L1 or its close homologue, CHL1. Brain Res. 2004, 1029, 131–134. [Google Scholar] [CrossRef]
- Nikonenko, A.G.; Sun, M.; Lepsveridze, E.; Apostolova, I.; Petrova, I.; Irintchev, A.; Dityatev, A.; Schachner, M. Enhanced perisomatic inhibition and impaired long-term potentiation in the CA1 region of juvenile CHL1-deficient mice. Eur. J. Neurosci. 2006, 23, 1839–1852. [Google Scholar] [CrossRef]
- Taniguchi, K.; Takeya, R.; Suetsugu, S.; Kan-O, M.; Narusawa, M.; Shiose, A.; Tominaga, R.; Sumimoto, H. Mammalian Formin Fhod3 Regulates Actin Assembly and Sarcomere Organization in Striated Muscles. J. Biol. Chem. 2009, 284, 29873–29881. [Google Scholar] [CrossRef]
- Iskratsch, T.; Lange, S.; Dwyer, J.; Kho, A.L.; dos Remedios, C.; Ehler, E. Formin follows function: A muscle-specific isoform of FHOD3 is regulated by CK2 phosphorylation and promotes myofibril maintenance. J. Cell Biol. 2010, 191, 1159–1172. [Google Scholar] [CrossRef]
- Kan-O, M.; Takeya, R.; Taniguchi, K.; Tanoue, Y.; Tominaga, R.; Sumimoto, H.; Weed, S.A. Expression and Subcellular Localization of Mammalian Formin Fhod3 in the Embryonic and Adult Heart. PLoS ONE 2012, 7, e34765. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, M.; Arnaud, C.-A.; Hanley, P.J. Are the class 18 myosins Myo18A and Myo18B specialist sarcomeric proteins? Front. Physiol. 2024, 15, 1401717. [Google Scholar] [CrossRef]
- Schiavinato, A.; Becker, A.-K.A.; Zanetti, M.; Corallo, D.; Milanetto, M.; Bizzotto, D.; Bressan, G.; Guljelmovic, M.; Paulsson, M.; Wagener, R.; et al. EMILIN-3, Peculiar Member of Elastin Microfibril Interface-located Protein (EMILIN) Family, Has Distinct Expression Pattern, Forms Oligomeric Assemblies, and Serves as Transforming Growth Factor β (TGF-β) Antagonist. J. Biol. Chem. 2012, 287, 11498–11515. [Google Scholar] [CrossRef] [PubMed]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef]
- Yogalingam, G.; Bonten, E.J.; van de Vlekkert, D.; Hu, H.; Moshiach, S.; Connell, S.A.; D’AZzo, A. Neuraminidase 1 Is a Negative Regulator of Lysosomal Exocytosis. Dev. Cell 2008, 15, 74–86. [Google Scholar] [CrossRef]
- Kima, P.E.; Burleigh, B.; Andrews, N.W. Surface-targeted lysosomal membrane glycoprotein-1 (Lamp-1) enhances lysosome exocytosis and cell invasion by Trypanosoma cruzi. Cell. Microbiol. 2000, 2, 477–486. [Google Scholar] [CrossRef]
- Reddy, A.; Caler, E.V.; Andrews, N.W. Plasma Membrane Repair Is Mediated by Ca2+-Regulated Exocytosis of Lysosomes. Cell 2001, 106, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.T.A.; Chikhlikar, P.; de Arruda, L.B.; Leao, I.C.; Lu, Y.; Wong, J.; Chen, J.-S.; Byrne, B.; August, J.T. HIV-1 p55Gag Encoded in the Lysosome-associated Membrane Protein-1 as a DNA Plasmid Vaccine Chimera Is Highly Expressed, Traffics to the Major Histocompatibility Class II Compartment, and Elicits Enhanced Immune Responses. J. Biol. Chem. 2003, 278, 37926–37936. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Zhang, L.-Q.; Liu, X.-Q.; Ye, W.; Zhao, Y.-X.; Qiang, Z.-X.; Zhang, L.-X.; Lei, Y.-F.; Jiang, D.-B.; Cheng, L.-F.; et al. Construction and evaluation of DNA vaccine encoding Crimean Congo hemorrhagic fever virus nucleocapsid protein, glycoprotein N-terminal and C-terminal fused with LAMP1. Front. Cell. Infect. Microbiol. 2023, 13, 1121163. [Google Scholar] [CrossRef]
- Jiang, D.-B.; Zhang, J.-P.; Cheng, L.-F.; Zhang, G.-W.; Li, Y.; Li, Z.-C.; Lu, Z.-H.; Zhang, Z.-X.; Lu, Y.-C.; Zheng, L.-H.; et al. Hantavirus Gc induces long-term immune protection via LAMP-targeting DNA vaccine strategy. Antivir. Res. 2017, 150, 174–182. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, B.; Pan, J.; Feng, Y.; Ye, W.; Xu, J.; Lan, M.; Sun, H.; Zhang, X.; Sun, Y.; et al. Construction and evaluation of DNA vaccine encoding Ebola virus glycoprotein fused with lysosome-associated membrane protein. Antivir. Res. 2021, 193, 105141. [Google Scholar] [CrossRef]
- Zheng, Y.; Shang, J.; Yang, Y.; Liu, C.; Wan, Y.; Geng, Q.; Wang, M.; Baric, R.; Li, F.; Gallagher, T. Lysosomal Proteases Are a Determinant of Coronavirus Tropism. J. Virol. 2018, 92, e01504-18. [Google Scholar] [CrossRef] [PubMed]
- Hulseberg, C.E.; Fénéant, L.; Szymańska, K.M.; White, J.M.; Moscona, A. Lamp1 Increases the Efficiency of Lassa Virus Infection by Promoting Fusion in Less Acidic Endosomal Compartments. mBio 2018, 9, e01818-17. [Google Scholar] [CrossRef]
- Jiang, D.-B.; Sun, Y.-J.; Cheng, L.-F.; Zhang, G.-F.; Dong, C.; Jin, B.-Q.; Song, C.-J.; Ma, Y.; Zhang, F.-L.; Yang, K. Construction and evaluation of DNA vaccine encoding Hantavirus glycoprotein N-terminal fused with lysosome-associated membrane protein. Vaccine 2015, 33, 3367–3376. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.T.; Craft, J. The pathogenesis of systemic lupus erythematosus—An update. Curr. Opin. Immunol. 2012, 24, 651–657. [Google Scholar] [CrossRef]
- Ruiz-Pablos, M. CD4+ Cytotoxic T Cells Involved in the Development of EBV-Associated Diseases. Pathogens 2022, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.M. B-cell acquisition of antigen: Sensing the surface. Eur. J. Immunol. 2015, 45, 1600–1604. [Google Scholar] [CrossRef]
- Baumgarth, N. A two-phase model of B-cell activation. Immunol. Rev. 2000, 176, 171–180. [Google Scholar] [CrossRef]
- Lopes-Carvalho, T.; Foote, J.; Kearney, J.F. Marginal zone B cells in lymphocyte activation and regulation. Curr. Opin. Immunol. 2005, 17, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Knight, A.M. Lowering the affinity between antigen and the B cell receptor can enhance antigen presentation. Eur. J. Immunol. 2004, 34, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; MacLennan, I.C. Follicular Helper T Cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar] [CrossRef]
- Barker, D.J.; Maccari, G.; Georgiou, X.; A Cooper, M.; Flicek, P.; Robinson, J.; E Marsh, S.G. The IPD-IMGT/HLA Database. Nucleic Acids Res. 2022, 51, D1053–D1060. [Google Scholar] [CrossRef] [PubMed]
- Shawan, M.M.A.K.; Sharma, A.R.; Halder, S.K.; Al Arian, T.; Shuvo, N.; Sarker, S.R.; Hasan, A. Advances in Computational and Bioinformatics Tools and Databases for Designing and Developing a Multi-Epitope-Based Peptide Vaccine. Int. J. Pept. Res. Ther. 2023, 29, 60. [Google Scholar] [CrossRef]
- Wang, P.; Sidney, J.; Kim, Y.; Sette, A.; Lund, O.; Nielsen, M.; Peters, B. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinform. 2010, 11, 568. [Google Scholar] [CrossRef]
- Paul, S.; Arlehamn, C.S.L.; Scriba, T.J.; Dillon, M.B.; Oseroff, C.; Hinz, D.; McKinney, D.M.; Pro, S.C.; Sidney, J.; Peters, B.; et al. Development and validation of a broad scheme for prediction of HLA class II restricted T cell epitopes. J. Immunol. Methods 2015, 422, 28–34. [Google Scholar] [CrossRef]
- Greenbaum, J.; Sidney, J.; Chung, J.; Brander, C.; Peters, B.; Sette, A. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics 2011, 63, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lund, O.; Nielsen, M.; Kesmir, C.; Petersen, A.G.; Justesen, S.; Lundegaard, C.; Worning, P.; Sylvester-Hvid, C.; Lamberth, K.; Røder, G.; et al. Definition of supertypes for HLA molecules using clustering of specificity matrices. Immunogenetics 2004, 55, 797–810. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Blicher, T.; Peters, B.; Sette, A.; Justesen, S.; Buus, S.; Lund, O.; Nussinov, R. Quantitative Predictions of Peptide Binding to Any HLA-DR Molecule of Known Sequence: NetMHCIIpan. PLOS Comput. Biol. 2008, 4, e1000107. [Google Scholar] [CrossRef] [PubMed]
- Sturniolo, T.; Bono, E.; Ding, J.; Raddrizzani, L.; Tuereci, O.; Sahin, U.; Braxenthaler, M.; Gallazzi, F.; Protti, M.P.; Sinigaglia, F.; et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat. Biotechnol. 1999, 17, 555–561. [Google Scholar] [CrossRef]
- Racle, J.; Guillaume, P.; Schmidt, J.; Michaux, J.; Larabi, A.; Lau, K.; Perez, M.A.; Croce, G.; Genolet, R.; Coukos, G.; et al. Machine learning predictions of MHC-II specificities reveal alternative binding mode of class II epitopes. Immunity 2023, 56, 1359–1375.e13. [Google Scholar] [CrossRef]
- Fatima, T.; Mubasher, M.M.; Rehman, H.M.; Niyazi, S.; Alanzi, A.R.; Kalsoom, M.; Khalid, S.; Bashir, H. Computational modeling study of IL-15-NGR peptide fusion protein: A targeted therapeutics for hepatocellular carcinoma. AMB Express 2024, 14, 91. [Google Scholar] [CrossRef]
- Vangone, A.; Bonvin, A.M. Contacts-based prediction of binding affinity in protein–protein complexes. eLife 2015, 4, e07454. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein–protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef]
- Tan, C.; Zhu, F.; Pan, P.; Wu, A.; Li, C. Development of multi-epitope vaccines against the monkeypox virus based on envelope proteins using immunoinformatics approaches. Front. Immunol. 2023, 14, 1112816. [Google Scholar] [CrossRef]
- Smatti, M.K.; Cyprian, F.S.; Nasrallah, G.K.; Al Thani, A.A.; Almishal, R.O.; Yassine, H.M. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 2019, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, R.S.; von Herrath, M.G.; Christen, U.; Whitton, J.L. Molecular Mimicry, Bystander Activation, or Viral Persistence: Infections and Autoimmune Disease. Clin. Microbiol. Rev. 2006, 19, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Ras-Carmona, A.; Lehmann, A.; Reche, P.A. Similarity to Self-Antigens Shapes Epitope Recognition from Viruses Under Autoimmune and Infectious Disease. Int. J. Mol. Sci. 2025, 26, 6041. [Google Scholar] [CrossRef]
- Gowthaman, U.; Eswarakumar, V.P. Molecular mimicry. Virulence 2013, 4, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Maguire, C.; Wang, C.; Ramasamy, A.; Fonken, C.; Morse, B.; Lopez, N.; Wylie, D.; Melamed, E. Molecular mimicry as a mechanism of viral immune evasion and autoimmunity. Nat. Commun. 2024, 15, 9403. [Google Scholar] [CrossRef]
- Moody, R.; Wilson, K.L.; Boer, J.C.; Holien, J.K.; Flanagan, K.L.; Jaworowski, A.; Plebanski, M. Predicted B Cell Epitopes Highlight the Potential for COVID-19 to Drive Self-Reactive Immunity. Front. Bioinform. 2021, 1, 709533. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S. COVID-19 disease and autoimmune disorders: A mutual pathway. World J. Methodol. 2022, 12, 200–223. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Sedykh, S.E.; Ermakov, E.A.; Matveev, A.L.; Odegova, E.I.; Sedykh, T.A.; Shcherbakov, D.N.; Merkuleva, I.A.; Volosnikova, E.A.; Nesmeyanova, V.S.; et al. Natural IgG against S-Protein and RBD of SARS-CoV-2 Do Not Bind and Hydrolyze DNA and Are Not Autoimmune. Int. J. Mol. Sci. 2022, 23, 13681. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K. Guillain-Barre syndrome during COVID-19 pandemic: An overview of the reports. Neurol. Sci. 2020, 41, 3149–3156. [Google Scholar] [CrossRef]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for COVID-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Lopez, C.; Kim, J.; Pandey, A.; Huang, T.; De Loughery, T.G. Simultaneous onset of COVID-19 and autoimmune hemolytic anemia. Br. J. Haematol. 2020, 190, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Avenell, A.; Aucott, L.; A Vickers, M. Systematic review and meta-analysis of the sero-epidemiological association between Epstein-Barr virus and systemic lupus erythematosus. Arthritis Res. Ther. 2014, 16, R3. [Google Scholar] [CrossRef]
- Yeung, W.-C.G.; Rawlinson, W.D.; Craig, M.E. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. BMJ 2011, 342, d35. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Talathi, S.; A Lopez-Olivo, M.; E Suarez-Almazor, M. Risk of developing antiphospholipid antibodies following viral infection: A systematic review and meta-analysis. Lupus 2017, 27, 572–583. [Google Scholar] [CrossRef]
- Thomas, O.G.; Bronge, M.; Tengvall, K.; Akpinar, B.; Nilsson, O.B.; Holmgren, E.; Hessa, T.; Gafvelin, G.; Khademi, M.; Alfredsson, L.; et al. Cross-reactive EBNA1 immunity targets alpha-crystallin B and is associated with multiple sclerosis. Sci. Adv. 2023, 9, eadg3032. [Google Scholar] [CrossRef]
- Lunardi, C.; Bason, C.; Navone, R.; Millo, E.; Damonte, G.; Corrocher, R.; Puccetti, A. Systemic sclerosis immunoglobulin G autoantibodies bind the human cytomegalovirus late protein UL94 and induce apoptosis in human endothelial cells. Nat. Med. 2000, 6, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, R.S.; Oldstone, M.B.A. Amino Acid Homology Between the Encephalitogenic Site of Myelin Basic Protein and Virus: Mechanism for Autoimmunity. Science 1985, 230, 1043–1045. [Google Scholar] [CrossRef]
- Jog, N.R.; McClain, M.T.; Heinlen, L.D.; Gross, T.; Towner, R.; Guthridge, J.M.; Axtell, R.C.; Pardo, G.; Harley, J.B.; James, J.A. Epstein Barr virus nuclear antigen 1 (EBNA-1) peptides recognized by adult multiple sclerosis patient sera induce neurologic symptoms in a murine model. J. Autoimmun. 2020, 106, 102332. [Google Scholar] [CrossRef]
- Vlachoyiannopoulos, P.G.; Magira, E.; Alexopoulos, H.; Jahaj, E.; Theophilopoulou, K.; Kotanidou, A.; Tzioufas, A.G. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann. Rheum. Dis. 2020, 79, 1661–1663. [Google Scholar] [CrossRef]
- Pascolini, S.; Vannini, A.; Deleonardi, G.; Ciordinik, M.; Sensoli, A.; Carletti, I.; Veronesi, L.; Ricci, C.; Pronesti, A.; Mazzanti, L.; et al. COVID-19 and Immunological Dysregulation: Can Autoantibodies be Useful? Clin. Transl. Sci. 2020, 14, 502–508. [Google Scholar] [CrossRef]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.E.; Wilson, S.; Thombare, A.; Weiss, S.; Ma, A. Cold agglutinin syndrome as a complication of COVID-19 in two cases. Clin. Infect. Pract. 2020, 7–8, 100041. [Google Scholar] [CrossRef]
- Gao, Z.-W.; Wang, X.; Lin, F.; Dong, K. The correlation between SARS-CoV-2 infection and rheumatic disease. Autoimmun. Rev. 2020, 19, 102557. [Google Scholar] [CrossRef]
- Angileri, F.; Légaré, S.; Gammazza, A.M.; De Macario, E.C.; Macario, A.J.L.; Cappello, F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br. J. Haematol. 2020, 190, e92–e93. [Google Scholar] [CrossRef] [PubMed]
- Angileri, F.; Legare, S.; Gammazza, A.M.; de Macario, E.C.; Macario, A.J.; Cappello, F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun. Rev. 2020, 19, 102591. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Kanduc, D.; Shoenfeld, Y. On the molecular determinants of the SARS-CoV-2 attack. Clin. Immunol. 2020, 215, 108426. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, G.; Flöel, A. Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmun. Rev. 2020, 19, 102556. [Google Scholar] [CrossRef]
- Lyons-Weiler, J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J. Transl. Autoimmun. 2020, 3, 100051. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M.; Ienaka, S.; Gotoh, T.; Yamamoto, H. Availability of short amino acid sequences in proteins. Protein Sci. 2005, 14, 617–625. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Otaki, J.M. Parallel and Antiparallel β-Strands Differ in Amino Acid Composition and Availability of Short Constituent Sequences. J. Chem. Inf. Model. 2011, 51, 1457–1464. [Google Scholar] [CrossRef]
- Motomura, K.; Nakamura, M.; Otaki, J.M. A Frequency-Based Linguistic Approach to Protein Decoding and Design: Simple Concepts, Diverse Applications, and The SCS Package. Comput. Struct. Biotechnol. J. 2013, 5, e201302010. [Google Scholar] [CrossRef]
- Otaki, J.M.; Gotoh, T.; Yamamoto, H. Potential Implications of Availability of Short Amino Acid Sequences in Proteins: An Old and New Approach to Protein Decoding and Design. Biotechnol. Annu. Rev. 2008, 14, 109–141. [Google Scholar] [CrossRef]
- Endo, S.; Motomura, K.; Tsuhako, M.; Kakazu, Y.; Nakamura, M.; Otaki, J.M. Search for Human-Specific Proteins Based on Availability Scores of Short Constituent Sequences: Identification of a WRWSH Protein in Human Testis. In Computational Biology and Chemistry; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Kanduc, D. Pentapeptides as Minimal Functional Units in Cell Biology and Immunology. Curr. Protein Pept. Sci. 2013, 14, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Pieczenik, G. Are the universes of antibodies and antigens symmetrical? Reprod. Biomed. Online 2003, 6, 154–156. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Z.; Wang, H.; Li, Y.; Liu, Y.; He, Y.; Liu, Q.; Chen, Z.; Ji, Y.; Al-Shammari, A.M. Screening of Diabetes-Associated Autoantigens and Serum Antibody Profiles Using a Phage Display System. Int. J. Microbiol. 2024, 2024, 1220644. [Google Scholar] [CrossRef]
- Itoh, K.; Inoue, K.; Hayashi, H.; Suzuki, T.; Masuko, T. Identification of cell proliferation-associated epitope on CD98 oncoprotein using phage display random peptide library. Cancer Sci. 2007, 98, 1696–1700. [Google Scholar] [CrossRef]
- Kaikkonen, L.; Lankinen, H.; Harjunpää, I.; Hokynar, K.; Söderlund-Venermo, M.; Oker-Blom, C.; Hedman, L.; Hedman, K. Acute-Phase-Specific Heptapeptide Epitope for Diagnosis of Parvovirus B19 Infection. J. Clin. Microbiol. 1999, 37, 3952–3956, Erratum in J. Clin. Microbiol. 2000, 38, 944. [Google Scholar] [CrossRef]
- A Osman, A.; Uhlig, H.; Thamm, B.; Schneider-Mergener, J.; Mothes, T. Use of the phage display technique for detection of epitopes recognized by polyclonal rabbit gliadin antibodies. FEBS Lett. 1998, 433, 103–107. [Google Scholar] [CrossRef]
- Geysen, H.M.; Meloen, R.H.; Barteling, S.J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 1984, 81, 3998–4002. [Google Scholar] [CrossRef] [PubMed]
- Jernbom, A.F.; Skoglund, L.; Pin, E.; Sjöberg, R.; Tegel, H.; Hober, S.; Rostami, E.; Rasmusson, A.; Cunningham, J.L.; Havervall, S.; et al. Prevalent and persistent new-onset autoantibodies in mild to severe COVID-19. Nat. Commun. 2024, 15, 8941. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, Y.; Miyakawa, K.; Kimura, Y.; Horikawa, K.; Hirahata, K.; Kimura, H.; Kato, H.; Goto, A.; Ryo, A. Identification of Putative Serum Autoantibodies Associated with Post-Acute Sequelae of COVID-19 via Comprehensive Protein Array Analysis. Int. J. Mol. Sci. 2025, 26, 1751. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Sang, A.; Danhorn, T.; Peterson, J.N.; Rankin, A.L.; O’cOnnor, B.P.; Leach, S.M.; Torres, R.M.; Pelanda, R. Innate and adaptive signals enhance differentiation and expansion of dual-antibody autoreactive B cells in lupus. Nat. Commun. 2018, 9, 3973. [Google Scholar] [CrossRef] [PubMed]
- Ameer, M.A.; Chaudhry, H.; Mushtaq, J.; Khan, O.S.; Babar, M.; Hashim, T.; Zeb, S.; Tariq, M.A.; Patlolla, S.R.; Ali, J.; et al. An Overview of Systemic Lupus Erythematosus (SLE) Pathogenesis, Classification, and Management. Cureus 2022, 14, e30330. [Google Scholar] [CrossRef]
- Iwata, S.; Sumikawa, M.H.; Tanaka, Y. B cell activation via immunometabolism in systemic lupus erythematosus. Front. Immunol. 2023, 14, 1155421. [Google Scholar] [CrossRef]
- Cambier, J.C.; Gauld, S.B.; Merrell, K.T.; Vilen, B.J. B-cell anergy: From transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 2007, 7, 633–643. [Google Scholar] [CrossRef]
- Johnson, J.L.; Scholz, J.L.; Marshak-Rothstein, A.; Cancro, M.P. Molecular pattern recognition in peripheral B cell tolerance: Lessons from age-associated B cells. Curr. Opin. Immunol. 2019, 61, 33–38. [Google Scholar] [CrossRef]
- Wardemann, H.; Yurasov, S.; Schaefer, A.; Young, J.W.; Meffre, E.; Nussenzweig, M.C. Predominant Autoantibody Production by Early Human B Cell Precursors. Science 2003, 301, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Su, K.-Y.; Kuraoka, M.; Yang, G.; Reynolds, A.E.; Schmidt, A.G.; Harrison, S.C.; Haynes, B.F.; Clair, E.W.S.; Kelsoe, G. Self-tolerance curtails the B cell repertoire to microbial epitopes. J. Clin. Investig. 2019, 4, e122551. [Google Scholar] [CrossRef] [PubMed]
- Havenar-Daughton, C.; Abbott, R.K.; Schief, W.R.; Crotty, S. When designing vaccines, consider the starting material: The human B cell repertoire. Curr. Opin. Immunol. 2018, 53, 209–216. [Google Scholar] [CrossRef]
- Steach, H.R.; DeBuysscher, B.L.; Schwartz, A.; Boonyaratanakornkit, J.; Baker, M.L.; Tooley, M.R.; Pease, N.A.; Taylor, J.J. Cross-Reactivity with Self-Antigen Tunes the Functional Potential of Naive B Cells Specific for Foreign Antigens. J. Immunol. 2020, 204, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, R.; Liang, Q.; Ni, S.; Yang, M.; Qiu, L.; Ji, J.; Gu, Z.; Dong, C. Organ-based characterization of B cells in patients with systemic lupus erythematosus. Front. Immunol. 2025, 16, 1509033. [Google Scholar] [CrossRef]
- Ishigaki, K.; Lagattuta, K.A.; Luo, Y.; James, E.A.; Buckner, J.H.; Raychaudhuri, S. HLA autoimmune risk alleles restrict the hypervariable region of T cell receptors. Nat. Genet. 2022, 54, 393–402. [Google Scholar] [CrossRef]
- Scally, S.W.; Petersen, J.; Law, S.C.; Dudek, N.L.; Nel, H.J.; Loh, K.L.; Wijeyewickrema, L.C.; Eckle, S.B.; van Heemst, J.; Pike, R.N.; et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J. Exp. Med. 2013, 210, 2569–2582. [Google Scholar] [CrossRef]
- Eyre, S.; Bowes, J.; Diogo, D.; Lee, A.; Barton, A.; Martin, P.; Zhernakova, A.; Stahl, E.; Viatte, S.; McAllister, K.; et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat. Genet. 2012, 44, 1336–1340. [Google Scholar] [CrossRef]
- Inoue, M.; Nagafuchi, Y.; Ota, M.; Tsuchiya, H.; Tateishi, S.; Kanda, H.; Fujio, K. Carriers of HLA-DRB1*04:05 have a better clinical response to abatacept in rheumatoid arthritis. Sci. Rep. 2023, 13, 15250. [Google Scholar] [CrossRef]
- Turesson, C.; Schaid, D.J.; Weyand, C.M.; Jacobsson, L.T.; Goronzy, J.J.; Petersson, I.F.; Sturfelt, G.; Nyhäll-Wåhlin, B.-M.; Truedsson, L.; A Dechant, S.; et al. The impact of HLA-DRB1 genes on extra-articular disease manifestations in rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R1386-93. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Oryoji, D.; Yamamoto, K.; Noh, J.Y.; Okamura, K.; Noda, M.; Kashiwase, K.; Kosuga, Y.; Sekiya, K.; Inoue, K.; et al. Identification of Independent Susceptible and Protective HLA Alleles in Japanese Autoimmune Thyroid Disease and Their Epistasis. J. Clin. Endocrinol. Metab. 2014, 99, E379–E383. [Google Scholar] [CrossRef]
- Prapas, P.; Anagnostouli, M. Macrophages and HLA-Class II Alleles in Multiple Sclerosis: Insights in Therapeutic Dynamics. Int. J. Mol. Sci. 2024, 25, 7354. [Google Scholar] [CrossRef]
- Maniaol, A.H.; Elsais, A.; Lorentzen, Å.R.; Owe, J.F.; Viken, M.K.; Sæther, H.; Flåm, S.T.; Bråthen, G.; Kampman, M.T.; Midgard, R.; et al. Late Onset Myasthenia Gravis Is Associated with HLA DRB1*15:01 in the Norwegian Population. PLoS ONE 2012, 7, e36603. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Radwan, J.; Babik, W.; Kaufman, J.; Lenz, T.L.; Winternitz, J. Advances in the Evolutionary Understanding of MHC Polymorphism. Trends Genet. 2020, 36, 298–311. [Google Scholar] [CrossRef]
- Zaru, R.; Orchard, S. The UniProt Consortium UniProt Tools: BLAST, Align, Peptide Search, and ID Mapping. Curr. Protoc. 2023, 3, e697. [Google Scholar] [CrossRef]
- Pundir, S.; Martin, M.J.; O’DOnovan, C. The UniProt Consortium UniProt Tools. Curr. Protoc. Bioinform. 2016, 53, 1.29.1–1.29.15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, P.; Kim, Y.; Haste-Andersen, P.; Beaver, J.; Bourne, P.E.; Bui, H.-H.; Buus, S.; Frankild, S.; Greenbaum, J.; et al. Immune epitope database analysis resource (IEDB-AR). Nucleic Acids Res. 2008, 36, W513–W518. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Raghava, G.P.S. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins Struct. Funct. Bioinform. 2006, 65, 40–48. [Google Scholar] [CrossRef]
- Saha, S.; Raghava, G.P. Prediction methods for B-cell epitopes. Methods Mol. Biol. 2007, 409, 387–394. [Google Scholar] [CrossRef]
- Nilsson, J.B.; Kaabinejadian, S.; Yari, H.; Kester, M.G.D.; van Balen, P.; Hildebrand, W.H.; Nielsen, M. Accurate prediction of HLA class II antigen presentation across all loci using tailored data acquisition and refined machine learning. Sci. Adv. 2023, 9, eadj6367. [Google Scholar] [CrossRef]
- Nilsson, J.B.; Kaabinejadian, S.; Yari, H.; Peters, B.; Barra, C.; Gragert, L.; Hildebrand, W.; Nielsen, M. Machine learning reveals limited contribution of trans-only encoded variants to the HLA-DQ immunopeptidome. Commun. Biol. 2023, 6, 442. [Google Scholar] [CrossRef]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A. PDBsum: Summaries and analyses of PDB structures. Nucleic Acids Res. 2001, 29, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Aripov, V.S.; Zaykovskaya, A.V.; Mechetina, L.V.; Najakshin, A.M.; Bondar, A.A.; Arkhipov, S.G.; Mustaev, E.A.; Ilyina, M.G.; Borisevich, S.S.; Ilyichev, A.A.; et al. The Use of Heterologous Antigens for Biopanning Enables the Selection of Broadly Neutralizing Nanobodies Against SARS-CoV-2. Antibodies 2025, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Shanshin, D.V.; Borisevich, S.S.; Bondar, A.A.; Porozov, Y.B.; Rukhlova, E.A.; Protopopova, E.V.; Ushkalenko, N.D.; Loktev, V.B.; Chapoval, A.I.; Ilyichev, A.A.; et al. Can Modern Molecular Modeling Methods Help Find the Area of Potential Vulnerability of Flaviviruses? Int. J. Mol. Sci. 2022, 23, 7721. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
| Gene Names | ID UniprotKB | Heptapeptides | Autoepitope Containing the Region of Homology | Score ABCpred | Protein Localization |
|---|---|---|---|---|---|
| ZNF528 | Q3MIS6 | DKVFRSS | CHECDKVFRSSSKLAQ | 0.71 | Nucleus |
| OTUD6A | Q7L8S5 | FLPFFSN | HVDEFLPFFSNPETSD | 0.71 | Cellular |
| LAMP1 | P11279 | VSGTNGT | SPSVDKYNVSGTNGTC | 0.86 | Transmembrane |
| ABCA10 | Q8WWZ4 | SLLIVNN | EQIPKTPLTSLLIVNN | 0.91 | Transmembrane |
| DAAM2 | Q86T65 | TRFQTLL | QVYAAERTRFQTLLNE | 0.84 | Extracellular |
| BLTP1 | Q2LD37 | SSSGWTA | SSSGWTAVGMENDKKE | 0.90 | Cellular |
| CHL1 | NCHL1 | YSTGSNV | QSAVYSTGSNGILLCE | 0.77 | Extracellular |
| HAVCR2 | Q8TDQ0 | IGAGICA | GIYIGAGICAGLALAL | 0.84 | Extracellular |
| HPS1 | A0A0S2Z3U9 | SPRRARS | DDIQPSPRRARSSQNI | 0.89 | Cellular |
| SCNN1A | C5HTY8 | RRARSVA | HGARRARSVASSLRDN | 0.84 | Transmembrane |
| TNK1 | Q13470 | VTLADAG | SFPASAVTLADAGGLP | 0.85 | Cellular |
| FHOD3 | A0A0A0MTS9 | GLTVLPP | AINIGLTVLPPPRTIK | 0.74 | Extracellular |
| EMILIN3 | Q9NT22 | KVEAEVQ | ERKVEAEVQAIQEQVS | 0.73 | Extracellular |
| MYO18A | A0A994J771 | LIRAAEI | ARLIRAAEINGEVDDD | 0.90 | Extracellular |
| FSTL1 | Q12841 | LDKYFKN | YSEILDKYFKNFDNGD | 0.84 | Extracellular |
| THADA | H0Y3V5 | NASVVNI | HDSFDMKDLNASVVNI | 0.72 | Cellular |
| SET | A0A0C4DFV9 | EIDRLNE | IEHIDEVQNEIDRLNE | 0.80 | Nucleus |
| MYO16 | F8W883 | EDDSEPV | GDEDDSEPVYIEMLGH | 0.70 | Cellular |
| HLA Class II | Start Position | SCS | HTL Epitope | Score | Rank |
|---|---|---|---|---|---|
| DAAM2 | |||||
| HLA-DRB1*04:05 | 248 | TRFQTLL | AERTRFQTLLNELDR | 0.9254 | 0.2 |
| HLA-DPA1*02:01/DPB1*05:01 | 248 | TRFQTLL | AERTRFQTLLNELDR | 0.1115 | 1.8 |
| HLA-DPA1*02:01/DPB1*01:01 | 249 | TRFQTLL | ERTRFQTLLNELDRS | 0.1398 | 1.8 |
| HLA-DRB1*04:01 | 249 | TRFQTLL | ERTRFQTLLNELDRS | 0.6160 | 1.9 |
| HLA-DPA1*02:01/DPB1*14:01 | 249 | TRFQTLL | ERTRFQTLLNELDRS | 0.1356 | 2.0 |
| HAVCR2 | |||||
| HLA-DRB1*15:01 | 198 | IGAGICA | TIRIGIYIGAGICAG | 0.7440 | 0.7 |
| FHOD3 | |||||
| HLA-DQA1*01:02/DQB1*06:02 | 73 | GLTVLPP | NAINIGLTVLPPPRT | 0.4816 | 1.6 |
| CHL1 | |||||
| HLA-DRB1*07:01 | 337 | YSTGSNV | PQSAVYSTGSNGILL | 0.7595 | 0.7 |
| EMILIN3 | |||||
| HLA-DPA1*02:01/DPB1*14:01 | 622 | KVEAEVQ | RERKVEAEVQAIQEQ | 0.2145 | 0.7 |
| FSTL1 | |||||
| HLA-DPA1*01:03/DPB1*02:01 | 145 | LDKYFKN | YSEILDKYFKNFDNG | 0.6819 | 0.5 |
| HLA (Chain A, B) + Epitope (Chain C) | Chains | No. of InterfaceResidues | Interface Area (Å2) | No. of Salt Bridges | No. of Hydrogen Bonds | No. of Non-Bonded Contacts |
|---|---|---|---|---|---|---|
| HLA-DRB1*04:05 AERTRFQTLLNELDR (DAAM2) | A C | 23:13 | 702:935 | 1 | 12 | 124 |
| B C | 18:10 | 662:839 | - | 7 | 92 | |
| HLA-DPA1*02:01/DPB1*05:01 AERTRFQTLLNELDR (DAAM2) | A C | 18:13 | 755:820 | 2 | 13 | 105 |
| B C | 19:13 | 682:814 | 1 | 6 | 79 | |
| HLA-DPA1*01:03/DPB1*02:01 YSEILDKYFKNFDNG (FSTL1) | A C | 16:13 | 729:790 | - | 9 | 92 |
| B C | 19:11 | 615:762 | 4 | 9 | 114 | |
| HLA-DRB1*04:01 ERTRFQTLLNELDRS (DAAM2) | A C | 22:13 | 754:903 | 2 | 11 | 104 |
| B C | 16:12 | 731:784 | 1 | 7 | 86 | |
| HLA-DPA1*02:01/DPB1*01:01 ERTRFQTLLNELDRS (DAAM2) | A C | 19:14 | 746:813 | 1 | 12 | 120 |
| B C | 16:7 | 528:643 | - | 2 | 69 | |
| HLA-DQA1*01:02/DQB1*06:02 NAINIGLTVLPPPRT (FHOD3) | A C | 18:13 | 665:759 | - | 7 | 83 |
| B C | 17:12 | 601:718 | - | 7 | 69 | |
| HLA-DPA1*02:01/DPB1*14:01 RERKVEAEVQAIQEQ (EMILIN3) | A C | 19:12 | 701:771 | 2 | 8 | 95 |
| B C | 14:11 | 542:629 | - | 4 | 53 | |
| HLA-DRB1*07:01 PQSAVYSTGSNGILL (CHL1) | A C | 15:10 | 614:654 | - | 4 | 57 |
| B C | 17:13 | 566:718 | - | 8 | 81 | |
| HLA-DRB1*15:01 TIRIGIYIGAGICAG (HAVCR2) | A C | 13:12 | 622:666 | 1 | 6 | 67 |
| B C | 17:13 | 629:698 | - | 5 | 79 | |
| HLA-DPA1*02:01/DPB1*14:01 ERTRFQTLLNELDRS (DAAM2) | A C | 17:12 | 608:727 | 1 | 4 | 88 |
| B C | 17:9 | 604:731 | 1 | 5 | 69 |
| Complex HLA-II and HTL Epitope | ΔG (kcal mol−1) | Kd (M) at 37 °C |
|---|---|---|
| HLA-DRB1*04:01 + ERTRFQTLLNELDRS (DAAM2) | −12.9 | 8.4 × 10−10 |
| HLA-DPA1*01:03/DPB1*02:01 + YSEILDKYFKNFDNG (FSTL1) | −12.7 | 1.1 × 10−9 |
| HLA-DRB1*04:05 + AERTRFQTLLNELDR (DAAM2) | −11.9 | 4.0 × 10−9 |
| HLA-DQA1*01:02/DQB1*06:02 + NAINIGLTVLPPPRT (FHOD3) | −11.8 | 4.6 × 10−9 |
| HLA-DPA1*02:01/DPB1*05:01 + AERTRFQTLLNELDR (DAAM2) | −11.8 | 4.7 × 10−9 |
| HLA-DPA1*02:01/DPB1*14:01 + ERTRFQTLLNELDRS (DAAM2) | −11.5 | 7.8 × 10−9 |
| HLA-DPA1*02:01/DPB1*14:01 + RERKVEAEVQAIQEQ (EMILIN3) | −11.2 | 1.3 × 10−8 |
| HLA-DPA1*02:01/DPB1*01:01 + ERTRFQTLLNELDRS (DAAM2) | −10.9 | 1.9 × 10−8 |
| HLA-DRB1*15:01 + TIRIGIYIGAGICAG (HAVCR2) | −10.8 | 2.6 × 10−8 |
| HLA-DRB1*07:01 + PQSAVYSTGSNGILL (CHL1) | −9.3 | 2.6 × 10−7 |
| Complex HLA-II and HTL Epitope | Charged | Hydrophobic | Polar | Other Interactions |
|---|---|---|---|---|
| HLA-DRB1*04:01 + ERTRFQTLLNELDRS | B: ASP 237 (97%) B: ASP 237 (80%) * B: GLU 189 (68%) A: ARG 75 (62%) A: GLU 54 (48%) B: ASP 208 (34%) A: ASP 65 (33%) A: ARG 75 (31%) B: LYS 251 (30%) | B: TRP 241 (75%) B: TYR 210 (71%) B: TYR 212 (33%) | A: ASN 68 (99%) A: ASN 68 (96%) A: ASN 61 (72%) A: ASN 61 (67%) B: HIS 193 (59%) A: ASN 61 (39%) | |
| HLA-DPA1*01:03/DPB1*02:01 + YSEILDKYFKNFDNG | A: ARG 107 (88%) B: GLU 55 (87%) B: GLU 98 (85%) B: ASP 84 (73%) B: GLU 98 (56%) B: ARG 104 (51%) B: GLU 98 (47%) B: GLU 55 (47%) A: GLU 86 (40%) A: GLU 86 (40%) B: ARG 104 (33%) A:ARG 107 (32%) | B: TRP 88 (90%) B: TRP 88 (63%) | A: ASN 100 (99%) A: ASN 100 (98%) B: HIS 108 (48%) A: ASN 99 (31%) A: ASN 99 (30%) | |
| HLA-DRB1*04:05 + AERTRFQTLLNELDR | A:GLU 38 (95%) B:ARG 100 (82%) A:GLU 38 (78%) A:ARG 100 (50%) B:ARG 100 (30%) | B: TRP 90 (64%) B: TYR 59 (47%) B: TYR 76 (45%) B: TRP 90 (40%) A: TYR 89 (36%) A: TYR 89 (33%) | B:ASN 111 (64%) | |
| HLA-DQA1*01:02/DQB1*06:02 + NAINIGLTVLPPPRT | A: ASN 57 (95%) B: GLU 255 (39%) A: ASN 57 (34%) B: ARG 269 (33%) | B: TRP 65 (95%) B: TYR 211 (62%) B: TYR 211 (44%) B: TYR 211 (39%) | A: ASN 64 (88%) A: ASN 71 (56%) A: ASN 64 (55%) | A: GLY 55 (93%) A: GLY 55 (54%) |
| HLA-DPA1*02:01/DPB1*05:01 + AERTRFQTLLNELDR | B: GLU 315 (98%) B: LYS 358 (82%) B: GLU 357 (77%) B: GLU 357 (70%) A: GLU 86 (65%) B: GLU 357 (65%) B: LYS 358 (47%) B: LYS 358 (43%) A: GLU 86 (41%) | B: TYR 317 (99%) B: TRP 348 (82%) | A: ASN 93 (99%) A: ASN 100 (89%) B: GLN 302 (83%) A: ASN 100 (82%) B: GLN 302 (61%) | B: TYR 317 (73%)—Pi-Pi stacking |
| HLA-DPA1*02:01/DPB1*14:01 + ERTRFQTLLNELDRS | B: GLU 357 (72%) B: LYS 358 (70%) B: ARG 364 (54%) B: ARG 364 (50%) B: GLU 357 (48%) | B: TYR 317 (62%) B: LEU 354 (59%) | B: GLN 351 (79%) B: GLN 302 (46%) B: GLN 351 (43%) A: ASN 93 (37%) | A: GLY 98 (31%) |
| HLA-DPA1*02:01/DPB1*14:01 + RERKVEAEVQAIQEQ | A: GLU 86 (53%) B: GLU 357 (34%) | A: TYR 40 (80%) A: ALA 82 (80%) A: PHE 83 (48%) | A: SER 84 (99%) A: SER 84 (99%) B: ASN 369 (98%) A: ASN 93 (45%) B: GLN 302 (42%) | A: GLU 80 (42%) |
| HLA-DPA1*02:01/DPB1*01:01 + ERTRFQTLLNELDRS | B: TYR 207 (88%) A: TYR 9 (84%) B: TYR 209 (79%) B: TYR 209 (79%) B: TYR 214 (38%) B: TRP 238 (37%) B: TRP 238 (34%) B: TYR 214 (32%) | A: SER 53 (97%) A: ASN 62 (96%) B: HIS 258 (88%) A: SER 53 (82%) A: ASN 69 (77%) A: ASN 69 (73%) A: ASN 62 (49%) B: GLN 192 (49%) A: SER 53 (40%) B: ASN 259 (36%) | ||
| HLA-DRB1*15:01 + TIRIGIYIGAGICAG | A: ASP 430 (97%) B: ARG 573 (59%) B: ARG 557 (50%) B: LYS 683 (48%) B: ASP 572 (38%) B: ARG 557 (37%) A: GLU 419 (32%) | A: PHE 415 (50%) B: PRO 555 (39%) B: TYR 574 (38%) B: PRO 555 (33%) | B: ASN 626 (100%) A: SER 417 (96%) A: SER 417 (95%) A: ASN 426 (82%) A: ASN 426 (54%) B: ASN 626 (44%) A: GLN 373 (41%) B: GLN 614 (33%) A: ASN 433 (31%) A: GLN 373 (31%) A: GLN 373 (30%) B: ASN 626 (30%) | B: HIS 625 (35%)—Pi-Pi stacking |
| HLA-DRB1*07:01 + PQSAVYSTGSNGILL | A: GLU 330 (52%) A: ASP 99 (50%) A: ASP 99 (47%) | B: TYR 327 (91%) A: TYR 42 (78%) B: LEU 348 (47%) A: TYR 152 (44%) B: TRP 304 (30%) | B: ASN 328 (46%) B: THR 372 (46%) B: ASN 377 (45%) B: ASN 377 (40%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeeva, A.M.; Aulova, K.S.; Mustaev, E.A.; Nevinsky, G.A. SARS-CoV-2 Spike Protein and Molecular Mimicry: An Immunoinformatic Screen for Cross-Reactive Autoantigen Candidates. Int. J. Mol. Sci. 2025, 26, 8793. https://doi.org/10.3390/ijms26188793
Timofeeva AM, Aulova KS, Mustaev EA, Nevinsky GA. SARS-CoV-2 Spike Protein and Molecular Mimicry: An Immunoinformatic Screen for Cross-Reactive Autoantigen Candidates. International Journal of Molecular Sciences. 2025; 26(18):8793. https://doi.org/10.3390/ijms26188793
Chicago/Turabian StyleTimofeeva, Anna M., Kseniya S. Aulova, Egor A. Mustaev, and Georgy A. Nevinsky. 2025. "SARS-CoV-2 Spike Protein and Molecular Mimicry: An Immunoinformatic Screen for Cross-Reactive Autoantigen Candidates" International Journal of Molecular Sciences 26, no. 18: 8793. https://doi.org/10.3390/ijms26188793
APA StyleTimofeeva, A. M., Aulova, K. S., Mustaev, E. A., & Nevinsky, G. A. (2025). SARS-CoV-2 Spike Protein and Molecular Mimicry: An Immunoinformatic Screen for Cross-Reactive Autoantigen Candidates. International Journal of Molecular Sciences, 26(18), 8793. https://doi.org/10.3390/ijms26188793









