The Efficacy of Pembrolizumab Immunotherapy in the Treatment of Endometrial Cancer: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Review Design and Search Strategy
- -
- Population: The target population is patients over 18 years of age with advanced, metastatic, or recurrent endometrial cancer, regardless of histological type, excluding sarcomas and angiosarcomas.
- -
- Intervention: Interventions include the use of pembrolizumab therapy, either in monotherapy or together with chemotherapy or lenvatinib.
- -
- Comparison: The efficacy and safety of pembrolizumab therapy for the treatment of endometrial cancer were compared with those of standard cancer treatments. This comparison will allow for an assessment of the relative risks and benefits of the said therapy.
- -
- Results: The results analyzed include the efficacy and safety of pembrolizumab therapy for the treatment of endometrial cancer. Efficacy is measured by PFS and OS. In addition, the frequency of side effects events associated with the use of pembrolizumab will be assessed.
2.2. Data Collection
2.3. Assessment of Risk of Bias in Included Studies
3. Results
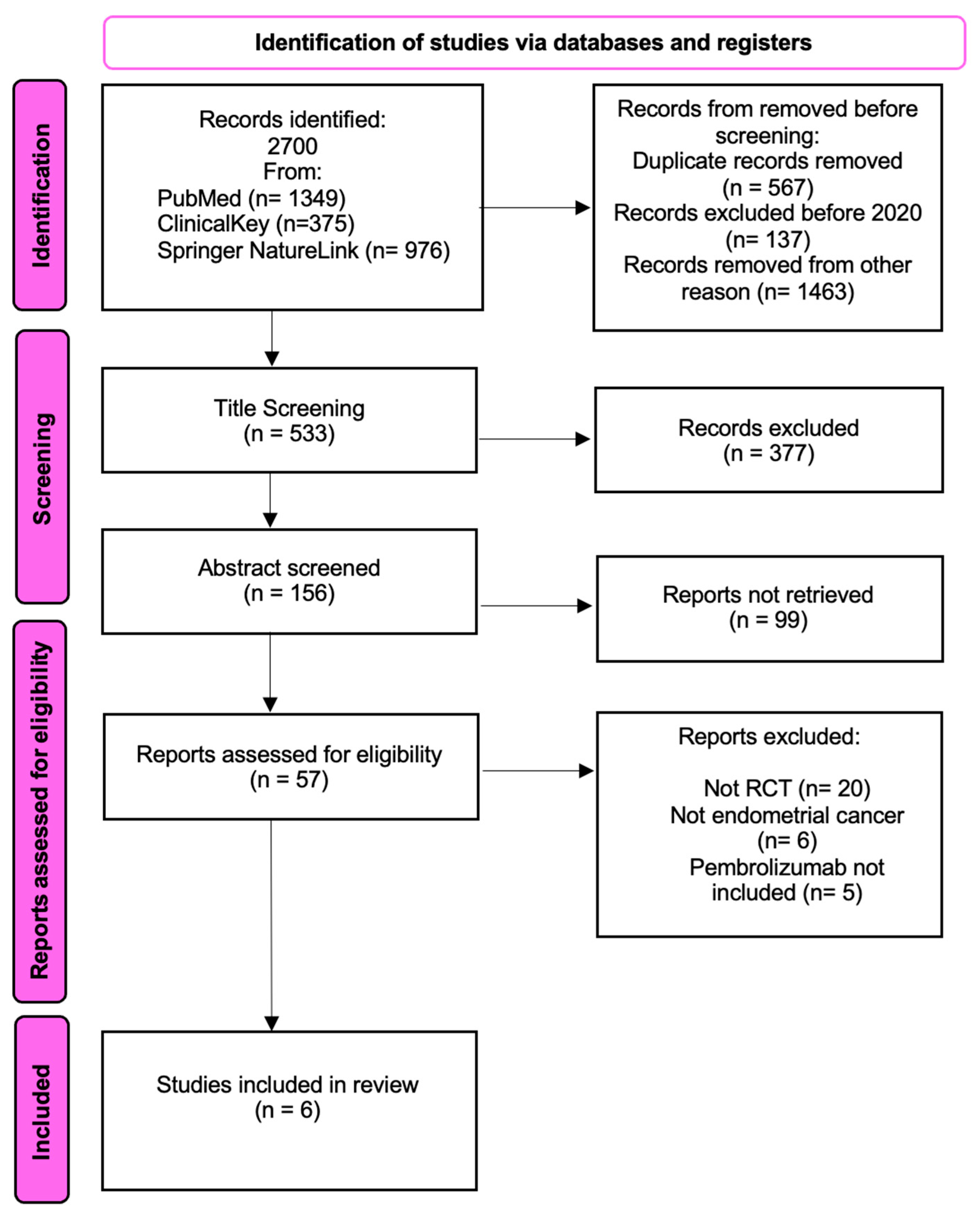
3.1. Selection and Identification of Studies
3.2. Molecular Classification of Endometrial Cancer
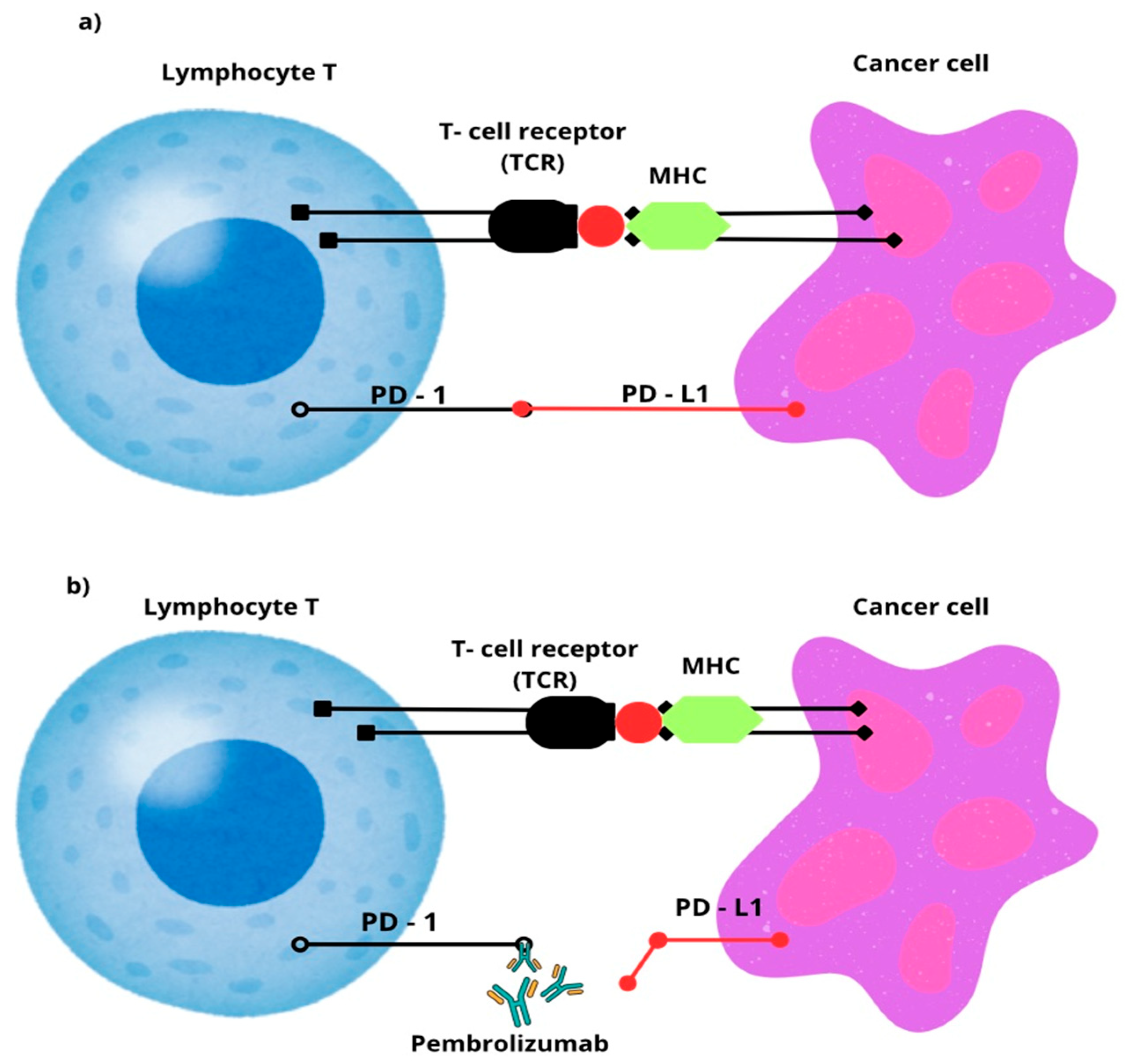
3.3. Pembrolizumab
3.4. Research
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASMR | Age-Standardized Mortality Rate |
| CBR | Clinical Benefit Rate |
| CTNNB1 | β-Catenin Gene |
| DFS | Disease-Free Survival |
| dMMR | Deficient Mismatch Repair |
| ECOG | Eastern Cooperative Oncology Group |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast Growth Factor Receptor |
| FIGO | International Federation of Gynecology and Obstetrics |
| HNPCC | Hereditary Non-Polyposis Colorectal Cancer |
| HR | Hazard Ratio |
| IDO1 | Inhibitor of Indoleamine 2,3-Dioxygenase |
| IgG G4 | Immunoglobulin G4 |
| irAEs | Immune-related adverse events |
| L1CAM | L1 Cell Adhesion Molecule |
| LAG-3 | Lymphocyte-Activation Gene 3 |
| MDM2 | Mouse Double Minute 2 Homolog |
| MDMX | Murine Double Minute X |
| MHC | Major Tissue Compatibility Complex |
| MMR | Mismatch Repair |
| MLH-1 | MutL homolog 1 |
| MLH-3 | MutL homolog 3 |
| MSH-2 | MutS homolog 2 |
| MSH-6 | MutS homolog 6 |
| MSI-H | Microsatellite Instability-High |
| MSS | Microsatellite Stable |
| NCCN | National Comprehensive Cancer Network |
| NGS | Next-Generation Sequencing |
| NSMP | No Specific Molecular Profile |
| ORR | Overall Response Rate |
| OS | Overall Survival |
| OSF | Open Science Framework |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PD-L2 | Programmed Death-Ligand 2 |
| PFS | Progression-Free Survival |
| pMMR | Proficient Mismatch Repair |
| PMS-1 | Post-Meiotic Segregation Increase 1 |
| PMS-2 | Post-Meiotic Segregation Increase 2 |
| POLEmut | POLE Mutation |
| Pol ε | Polymerase Epsilon |
| p53abn | p53 abnormal |
| RCTs | Randomized Controlled Trials |
| TCR | T-cell Receptor |
| TMB | Tumor Mutational Burden |
| TMB-H | Tumor Mutational Burden-High |
| uLMS | Uterine Leiomyosarcoma |
References
- Králíčková, M.; Vetvicka, V.; Laganà, A.S. Endometrial cancer—Is our knowledge changing? Transl. Cancer Res. 2020, 9, 7734–7745. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Gu, B.; Shang, X.; Yan, M.; Li, X.; Wang, W.; Wang, Q. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol. Oncol. 2021, 161, 573–580. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mahdy, H.; Vadakekut, E.S.; Crotzer, D. Endometrial Cancer. 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK525981/ (accessed on 29 May 2025).
- Baker-Rand, H.; Kitson, S.J. Recent Advances in Endometrial Cancer Prevention, Early Diagnosis and Treatment. Cancers 2024, 16, 1028. [Google Scholar] [CrossRef]
- Niu, S.; Molberg, K.; Castrillon, D.H.; Lucas, E.; Chen, H. Biomarkers in the Diagnosis of Endometrial Precancers. Cancers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Raffone, A.; Troisi, J.; Boccia, D.; Travaglino, A.; Capuano, G.; Insabato, L. Metabolomics in endometrial cancer diagnosis: A systematic review. Acta Obstet. Gynecol. Scand. 2020, 99, 1135–1146. [Google Scholar] [CrossRef]
- Barretina-Ginesta, M.P.; Quindós, M.; Alarcón, J.D.; Esteban, C.; Gaba, L.; Gómez, C.; Fidalgo, J.A.P.; Romero, A.; Santaballa, A.; Rubio-Pérez, M.J. SEOM-GEICO clinical guidelines on endometrial cancer (2021). Clin. Transl. Oncol. 2022, 24, 625–634. [Google Scholar] [CrossRef]
- El-Ghazzi, N.; Durando, X.; Giro, A.; Herrmann, T. Targeted Treatment of Advanced Endometrial Cancer: Focus on Pembrolizumab. Onco Targets Ther. 2023, 16, 359–369. [Google Scholar] [CrossRef]
- Tronconi, F.; Nero, C.; Giudice, E.; Salutari, V.; Musacchio, L.; Ricci, C. Advanced and recurrent endometrial cancer: State of the art and future perspectives. Crit. Rev. Oncol. Hematol. 2022, 180, 103851. [Google Scholar] [CrossRef]
- Karpel, H.; Slomovitz, B.; Coleman, R.L.; Pothuri, B. Biomarker-driven therapy in endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.; Chelariu-Raicu, A.; Slomovitz, B.M. Immunotherapy in endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.; Aimagambetova, G.; Kunz, J.; Bapayeva, G.; Aitbayeva, B.; Terzic, S. Molecular Basis of Endometriosis and Endometrial Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9274. [Google Scholar] [CrossRef]
- Li, Y.T.; Liu, C.H.; Wang, P.H. Integrating molecular pathology to endometrial cancer. Taiwan J. Obstet. Gynecol. 2023, 62, 792–794. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S. FIGO staging of endometrial cancer: 2023. J. Gynecol. Oncol. 2023, 34, e85. [Google Scholar] [CrossRef]
- Jamieson, A.; Bosse, T.; McAlpine, J.N. The emerging role of molecular pathology in directing the systemic treatment of endometrial cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211035959. [Google Scholar] [CrossRef]
- Vermij, L.; Jobsen, J.J.; León-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef]
- Bateman, A.C. DNA mismatch repair proteins: Scientific update and practical guide. J. Clin. Pathol. 2021, 74, 264–268. [Google Scholar] [CrossRef]
- Riedinger, C.J.; Esnakula, A.; Haight, P.J.; Suarez, A.A.; Chen, W.; Gillespie, J. Characterization of mismatch-repair/microsatellite instability-discordant endometrial cancers. Cancer 2024, 130, 385–399. [Google Scholar] [CrossRef]
- Puliga, E.; Corso, S.; Pietrantonio, F.; Giordano, S. Microsatellite instability in Gastric Cancer: Between lights and shadows. Cancer Treat. Rev. 2021, 95, 102175. [Google Scholar] [CrossRef]
- Peltomäki, P.; Nyström, M.; Mecklin, J.P.; Seppälä, T.T. Lynch Syndrome Genetics and Clinical Implications. Gastroenterology 2023, 164, 783–799. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, L.; Zang, Y.; Liu, W.; Liu, S.; Teng, F. Endometrial cancer in Lynch syndrome. Int. J. Cancer 2022, 150, 7–17. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Vermij, L.; Léon-Castillo, A.; Singh, N.; Powell, M.E.; Edmondson, R.J.; Genestie, C. p53 immunohistochemistry in endometrial cancer: Clinical and molecular correlates in the PORTEC-3 trial. Mod. Pathol. 2022, 35, 1475–1483. [Google Scholar] [CrossRef]
- Tang, M.; Yin, S.; Zeng, H.; Huang, A.; Huang, Y.; Hu, Z. The P286R mutation of DNA polymerase ε activates cancer-cell-intrinsic immunity and suppresses endometrial tumorigenesis via the cGAS-STING pathway. Cell Death Dis. 2024, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Galant, N.; Krawczyk, P.; Monist, M.; Obara, A.; Gajek, Ł.; Grenda, A. Molecular Classification of Endometrial Cancer and Its Impact on Therapy Selection. Int. J. Mol. Sci. 2024, 25, 5893. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Ji, Z.; Wang, J.; Meng, J.; Bi, R.; Ren, Y. Characterization of hotspot exonuclease domain mutations in the DNA polymerase ϵ gene in endometrial cancer. Front. Oncol. 2022, 12, 1018034. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Khan, F.I. Investigation of Molecular Interactions Mechanism of Pembrolizumab and PD-1. Int. J. Mol. Sci. 2023, 24, 10684. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef]
- Ciesielska-Figlon, K.; Lisowska, K.A. The Role of the CD28 Family Receptors in T-Cell Immunomodulation. Int. J. Mol. Sci. 2024, 25, 1274. [Google Scholar] [CrossRef]
- Javed, S.A.; Najmi, A.; Ahsan, W.; Zoghebi, K. Targeting PD-1/PD-L-1 immune checkpoint inhibition for cancer immunotherapy: Success and challenges. Front. Immunol. 2024, 15, 1383456. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Wang, H. Soluble B7-CD28 Family Inhibitory Immune Checkpoint Proteins and Anti-Cancer Immunotherapy. Front. Immunol. 2021, 12, 651634. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. 2020, 9, 8086–8121. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Prabhu, V.S.; Corman, S.; Odak, S.; Rusibamayila, N.; Macahilig, C. Treatment patterns and real-world clinical outcomes in patients with advanced endometrial cancer who are microsatellite instability (MSI)-high or are mismatch repair deficient (dMMR) in the United States. Gynecol. Oncol. 2023, 169, 154–163. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Guidelines Detail. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1473 (accessed on 29 May 2025).
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- Van Gorp, T.; Cibula, D.; Lv, W.; Backes, F.; Ortaç, F.; Hasegawa, K. ENGOT-en11/GOG-3053/KEYNOTE-B21: A randomised, double-blind, phase III study of pembrolizumab or placebo plus adjuvant chemotherapy with or without radiotherapy in patients with newly diagnosed, high-risk endometrial cancer. Ann. Oncol. 2024, 35, 968–980. [Google Scholar] [CrossRef]
- Slomovitz, B.M.; Cibula, D.; Lv, W.; Ortaç, F.; Hietanen, S.; Backes, F. Pembrolizumab or Placebo Plus Adjuvant Chemotherapy With or Without Radiotherapy for Newly Diagnosed, High-Risk Endometrial Cancer: Results in Mismatch Repair-Deficient Tumors. J. Clin. Oncol. 2025, 43, 251–259. [Google Scholar] [CrossRef]
- Makker, V.; Colombo, N.; Casado Herráez, A.; Santin, A.D.; Colomba, E.; Miller, D.S. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef]
- Marth, C.; Moore, R.G.; Bidziński, M.; Pignata, S.; Ayhan, A.; Rubio, M.J. First-Line Lenvatinib Plus Pembrolizumab Versus Chemotherapy for Advanced Endometrial Cancer: A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2025, 43, 1083–1100. [Google Scholar] [CrossRef]
- Yonemori, K.; Yunokawa, M.; Ushijima, K.; Sakata, J.; Shikama, A.; Minobe, S. Lenvatinib plus pembrolizumab in Japanese patients with endometrial cancer: Results from Study 309/KEYNOTE-775. Cancer Sci. 2022, 113, 3489–3497. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H., Jr.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients with Microsatellite Instability-High Advanced Endometrial Cancer: Results from the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Colombo, N.; Herráez, A.C.; Monk, B.J.; Mackay, H.; Santin, A.D. Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775. J. Clin. Oncol. 2023, 41, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Simultaneous Review Decisions for Pembrolizumab Plus Lenvatinib (Australia, Canada, and the US). Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/simultaneous-review-decisions-pembrolizumab-plus-lenvatinib-australia-canada-and-us (accessed on 2 July 2025).
- Food and Drug Administration. FDA Approves Pembrolizumab with Chemotherapy for Primary Advanced or Recurrent Endometrial Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-chemotherapy-primary-advanced-or-recurrent-endometrial-carcinoma (accessed on 28 May 2025).
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J. Clinical activity and safety of the anti-PD-1 monoclonal antibody dostarlimab for patients with recurrent or advanced dMMR endometrial cancer. Future Oncol. 2021, 17, 3781–3785. [Google Scholar] [CrossRef]
- Oaknin, A.; Pothuri, B.; Gilbert, L.; Sabatier, R.; Brown, J.; Ghamande, S.; Mathews, C.; O’Malley, D.M.; Kristeleit, R.; Boni, V.; et al. Safety, Efficacy, and Biomarker Analyses of Dostarlimab in Patients with Endometrial Cancer: Interim Results of the Phase I GARNET Study. Clin. Cancer Res. 2023, 29, 4564–4574. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Colombo, N.; Biagioli, E.; Harano, K.; Galli, F.; Hudson, E.; Antill, Y.; Choi, C.H.; Rabaglio, M.; Marmé, F.; Marth, C.; et al. Atezolizumab and chemotherapy for advanced or recurrent endometrial cancer (AtTEnd): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024, 25, 1135–1146. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Dostarlimab-Gxly With Chemotherapy for Endometrial Cancer; FDA: Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-dostarlimab-gxly-chemotherapy-endometrial-cancer (accessed on 22 August 2025).
- Lheureux, S.; Matei, D.E.; Konstantinopoulos, P.A.; Wang, B.X.; Gadalla, R.; Block, M.S.; Jewell, A.; Gaillard, S.L.; McHale, M.; McCourt, C.; et al. Translational randomized phase II trial of cabozantinib in combination with nivolumab in advanced, recurrent, or metastatic endometrial cancer. J. Immunother. Cancer 2022, 10, e004233. [Google Scholar] [CrossRef]
- Incyte Corporation. A Study of INCMGA00012 in Participants with Select Solid Tumors (POD1UM-204). 2020. Available online: https://clinicaltrials.gov/study/NCT04463771 (accessed on 29 May 2025).
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Nagashima, T.; Imai, Y.; Akitsu, K.; Yamanaka, Z.; Nishi, H. A case of recurrent endometrial cancer with long-term complete remission following pembrolizumab-induced severe immune-related adverse event colitis. J. Obstet. Gynaecol. Res. 2022, 48, 2630–2634. [Google Scholar] [CrossRef] [PubMed]
- Khetan, V.; Blake, E.A.; Ciccone, M.A.; Matsuo, K. Rhabdomyolysis following single administration of pembrolizumab: Is severe immune-reaction a marker for durable treatment response? Gynecol. Oncol. Rep. 2021, 35, 100700. [Google Scholar] [CrossRef] [PubMed]
| Indication | Use of Pembrolizumab |
|---|---|
| Cancers with dMMR/MSI-H | Monotherapy as the preferred option |
| Cancers with TMB-H | Monotherapy (as the second line) |
| Cancers with pMMR | Combination with lenvatinib |
| Uterine LMS with TMB-H | Second-line treatment |
| Author and Type of Intervention | Side Effects | Intervention: Frequency [%] | Intervention: Severity ≤ 3 Grade [%] | Control Group: Frequency [%] | Control Group: Severity ≥ 3 Grade [%] |
|---|---|---|---|---|---|
| Slomovitz BM. et al. Pembrolizumab + chemotherapy | Anemia | 54.3% | 0% | 50% | 9.3% |
| Diarrhea | 47.9% | 2.9% | 36.4% | 3.6% | |
| Neutropenia | 23.6% | 17.1% | 23.6% | 14.3% | |
| Hypothyroidism | 22.1% | 0.7% | 3.6% | 0% | |
| Hyperthyroidism | 11.4% | 0% | 3.6% | 0% | |
| Thyroiditis | 3.6% | 0% | 0.7% | 0% | |
| Arthritis | <1% | 0% | 0% | 0% | |
| Marth C. et al. Pembrolizumab + lenvatinib | Hypertension | 63% | 43% | 3% | 1% |
| Hypothyroidism | 59% | 1% | <1% | 0% | |
| Diarrhea | 42% | 8% | 16% | <1% | |
| Proteinuria | 31% | 7% | 1% | <1% | |
| Neutropenia | 4% | 1% | 30% | 23% | |
| Van Gorp T. et al. Pembrolizumab + adjuvant chemotherapy | Anemia | 52% | 11% | 52% | 9% |
| Diarrhea | 40% | 2% | 38% | 3% | |
| Decreased neutrophil count | 35% | 23% | 32% | 22% | |
| Hypothyroidism | 23% | <1% | 4% | 0% | |
| Hyperthyroidism | 12% | 0% | 3% | 0% | |
| Thyroiditis | 2% | 0% | <1% | 0% | |
| Myositis | <1% | 0% | <1% | 0% | |
| Arthritis | <1% | 0% | 0% | 0% | |
| Eskander RN. et al. Pembrolizumab added to chemotherapy | Fatigue | 65.7% | <1% | 59% | <1% |
| Sensory peripheral neuropathy | 58.2% | <1% | 58.7% | <1% | |
| Anemia | 55.8% | 15.3% | 53.2% | <1% | |
| Thrombocytopenia | 31.4% | <1% | 23.7% | <1% | |
| Hypothyroidism | 13.2% | 0% | <1% | 0% | |
| Hyperthyroidism | 6.7% | 0% | 2.8% | 0% | |
| Myositis | <1% | 0% | 0% | 0% | |
| Makker V. et al. Pembrolizumab + lenvatinib | Hypertension | 64% | 37.9% | 5.2% | 2.3% |
| Hypothyroidism | 57.4% | 1.2% | 0.8% | 0% | |
| Diarrhea | 54.2% | 7.6% | 20.1% | 2.1% | |
| Proteinuria | 28.8% | 5.4% | 2.8% | 0.3% | |
| Anemia | 26.1% | 6.2% | 48.7% | 14.7% | |
| Yonemori K. et al. Pembrolizumab + lenvatinib | Hypertension | 78.8% | 30.8% | 0% | 0% |
| Hypothyroidism | 75% | 0% | 0% | 0% | |
| Proteinuria | 63.5% | 17.3% | 7.8% | 2% | |
| Platelet count decreased | 48.1% | 11.5% | 13.7% | 2% | |
| Anemia | 42.3% | 13.5% | 47.1% | 19.6% |
| Author and Year | Number of Patients | Intervention | Type of Examination | Results |
|---|---|---|---|---|
| Slomovitz BM. et al., 2025 | 281 | Pembrolizumab + chemotherapy vs. placebo + chemotherapy | RCT, double-blinded | DFS 2 years: 92.4% vs. 80.2%; fewer relapses: 8 vs. 25 OS: the results were only 3.6% mature, so it was not mentioned; PFS: not reported |
| Marth C. et al., 2025 | 842 | Pembrolizumab + lenvatinib vs. chemotherapy | RCT, double-blinded | DFS 2 years: 92.4% vs. 80.2%; fewer relapses: 8 vs. 25 OS: the results were only 3.6% mature, so it was not mentioned; PFS: not reported |
| Van Gorp T. et al., 2024 | 1095 | Pembrolizumab + adjuvant chemotherapy vs. placebo + chemotherapy | RCT, double-blinded | DFS 2 years: 92.4% vs. 80.2%; fewer relapses: 8 vs. 25 OS: the results were only 3.6% mature, so it was not mentioned; PFS: not reported |
| Eskander RN. et al., 2023 | 816 | Pembrolizumab added to chemotherapy (carboplatin + paclitaxel) vs. placebo with chemotherapy | RCT, double-blinded | DFS 2 years: not reported OS: not mentioned PFS: dMMR: 12 months 74% vs. 38% (HR = 0.3); pMMR: 13.1 vs. 8.7 months (HR = 0.54) |
| Makker V. et al., 2022 | 827 | Pembrolizumab + lenvatinib vs. chemotherapy | RCT, double-blinded | DFS 2 years: not reported OS: pMMR: 17.4 vs. 12 months, overall: 18.3 vs. 11.4 months, (HR = 0.62) PFS: pMMR: 6.6 vs. 3.8 months, (HR = 0.6) overall 7.2 vs. 3.8 months; |
| Yonemori K. et al., 2022 | 104 | Pembrolizumab + lenvatinib vs. chemotherapy | RCT, double-blinded | DFS 2 years: not reported OS: pMMR 16.7 vs. 12.2 (HR = 0.74) dMMR not reached vs. 8 months (HR = 0.11) PFS: pMMR 5.6 vs. 5.6 months (HR = 1.04) dMMR not reached vs. 3.7 months (HR = 0.17) overall: 7.2 vs. 5.4 months, (HR = 0.81) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picheta, N.; Piekarz, J.; Kułak, K.; Tarkowski, R. The Efficacy of Pembrolizumab Immunotherapy in the Treatment of Endometrial Cancer: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 8789. https://doi.org/10.3390/ijms26188789
Picheta N, Piekarz J, Kułak K, Tarkowski R. The Efficacy of Pembrolizumab Immunotherapy in the Treatment of Endometrial Cancer: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(18):8789. https://doi.org/10.3390/ijms26188789
Chicago/Turabian StylePicheta, Natalia, Julia Piekarz, Krzysztof Kułak, and Rafał Tarkowski. 2025. "The Efficacy of Pembrolizumab Immunotherapy in the Treatment of Endometrial Cancer: A Systematic Review" International Journal of Molecular Sciences 26, no. 18: 8789. https://doi.org/10.3390/ijms26188789
APA StylePicheta, N., Piekarz, J., Kułak, K., & Tarkowski, R. (2025). The Efficacy of Pembrolizumab Immunotherapy in the Treatment of Endometrial Cancer: A Systematic Review. International Journal of Molecular Sciences, 26(18), 8789. https://doi.org/10.3390/ijms26188789






