Clinical Significance of Nuclear Yin-Yang Overexpression Evaluated by Immunohistochemistry in Tissue Microarrays and Digital Pathology Analysis: A Useful Prognostic Tool for Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Clinical Profile and Pathological Features of the Study Population
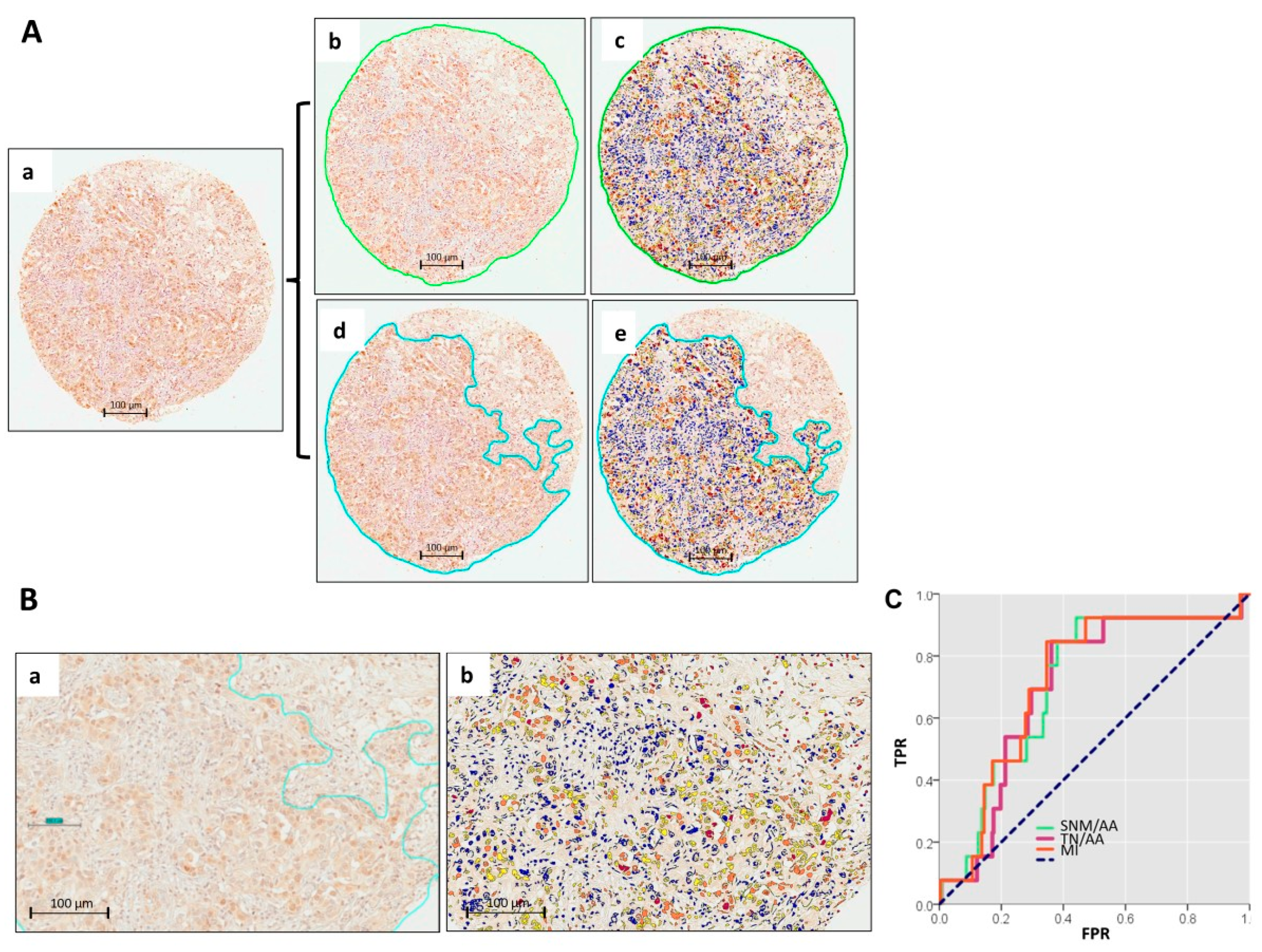
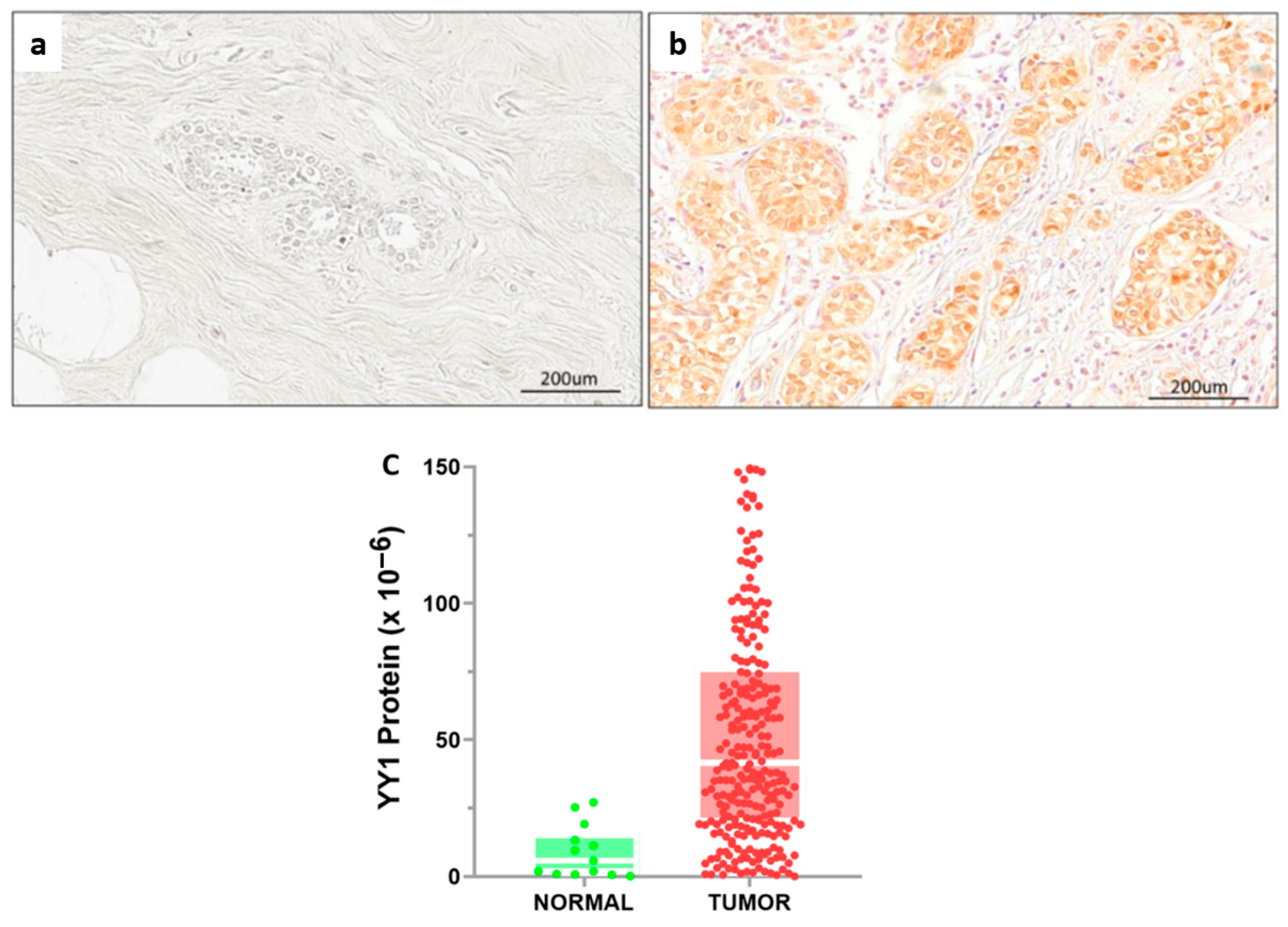
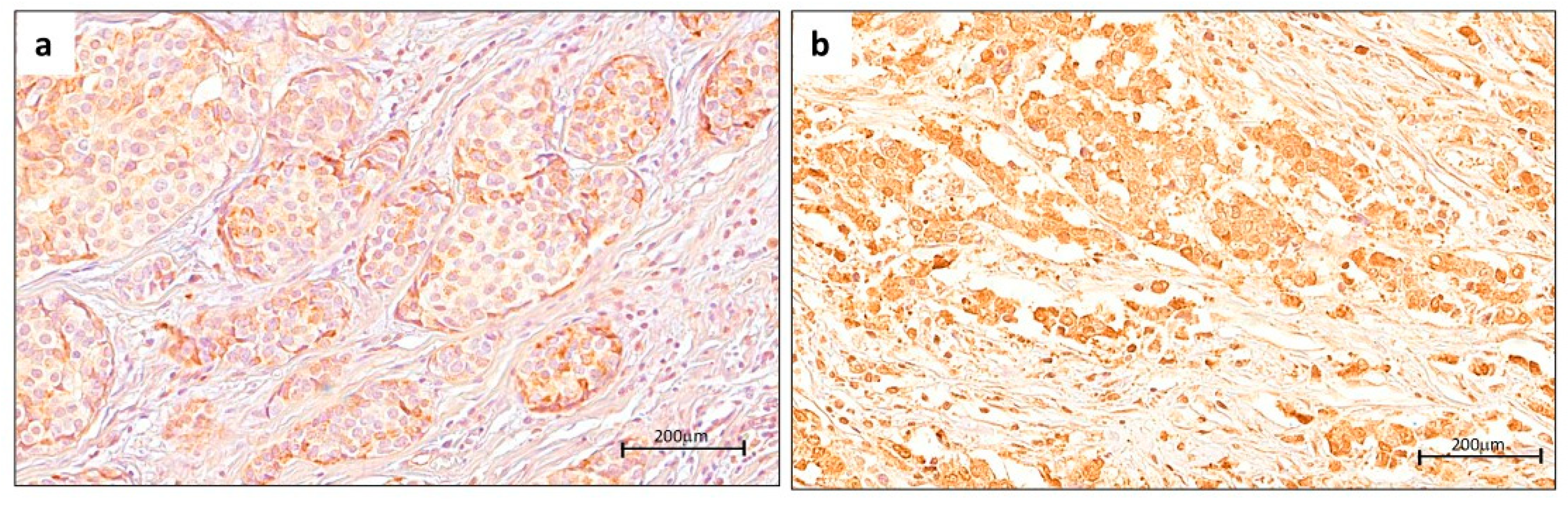
2.2. Digital Pathology Approach for Quantification of YY1 and Cutoff Point
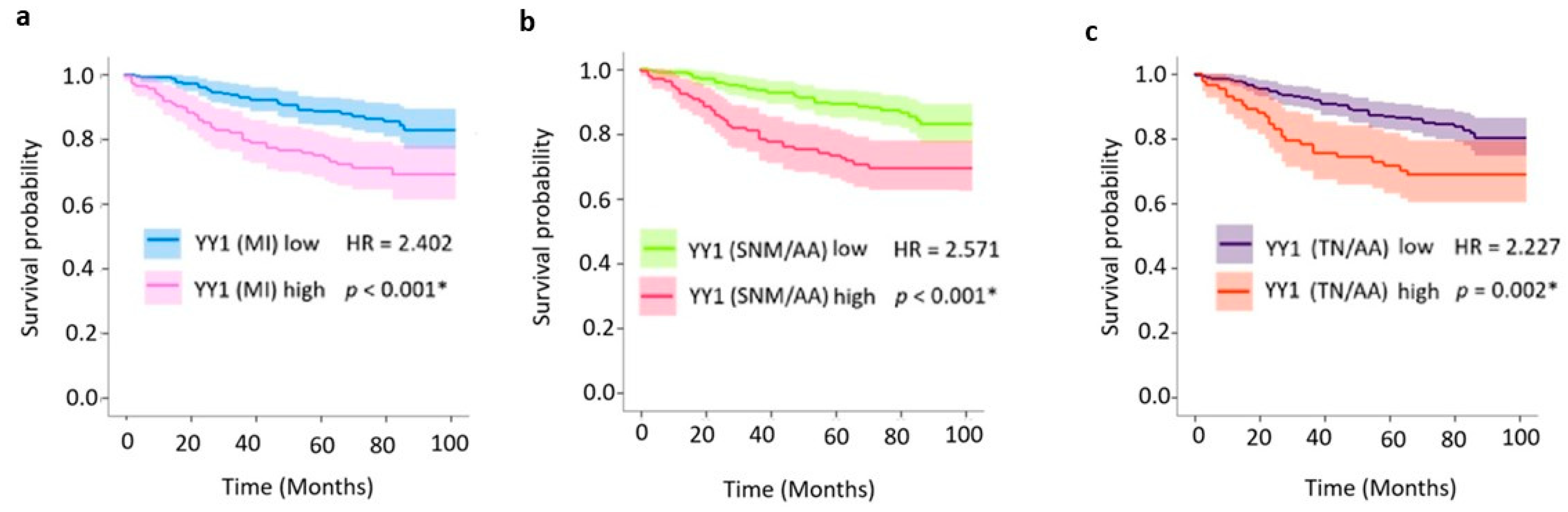
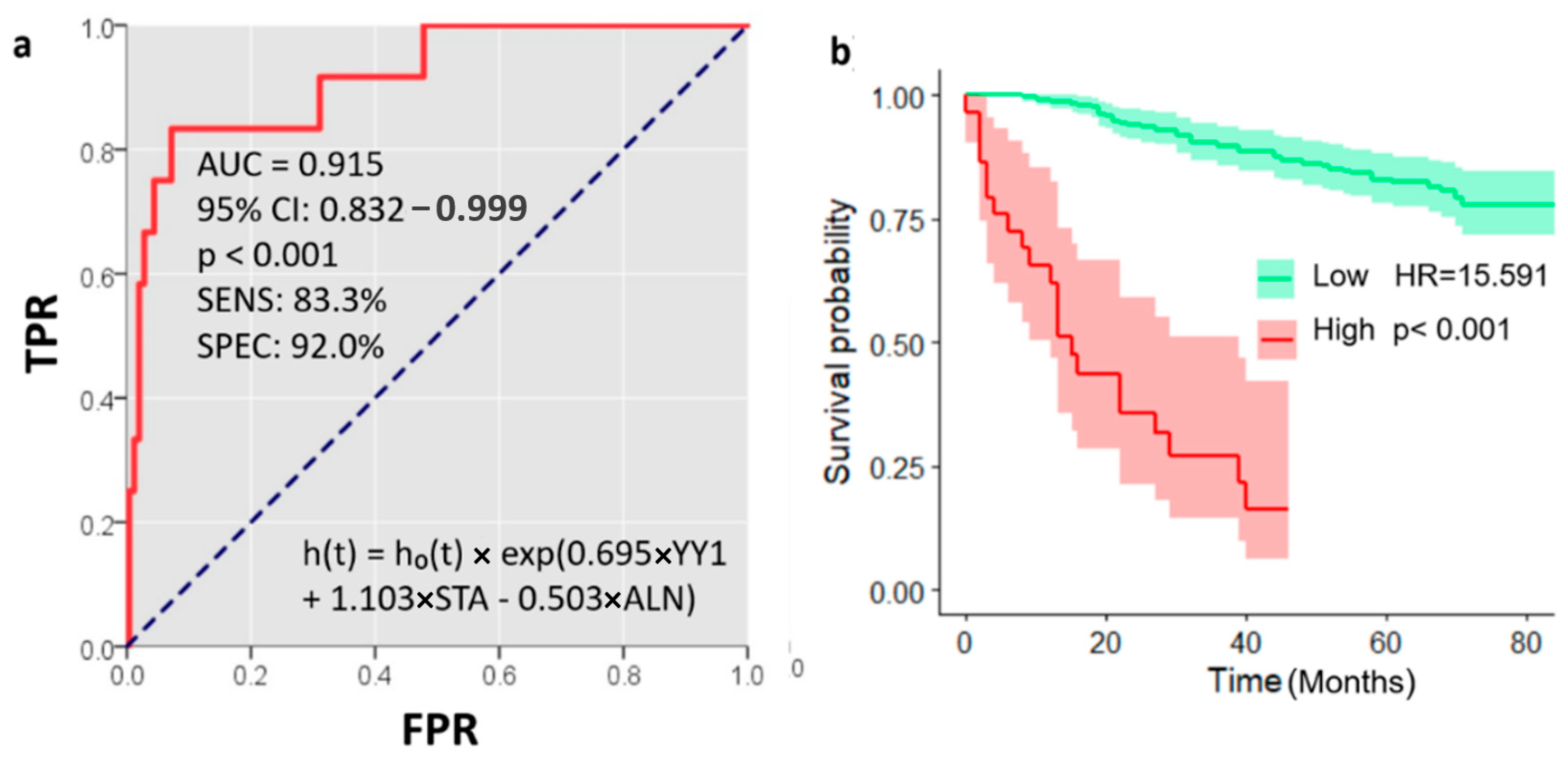
2.3. Prognostic Value of Nuclear YY1 Expression: Univariate and Multivariate Survival Analyses
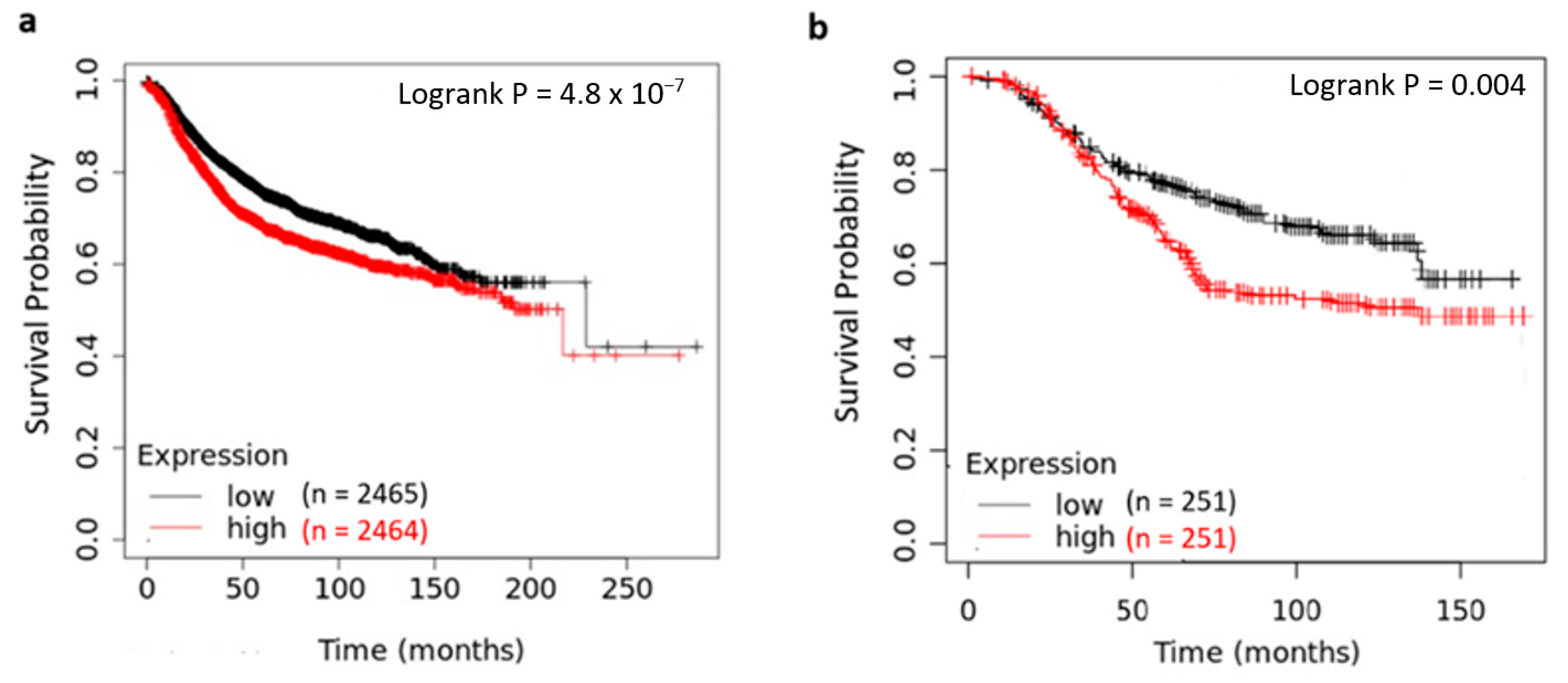
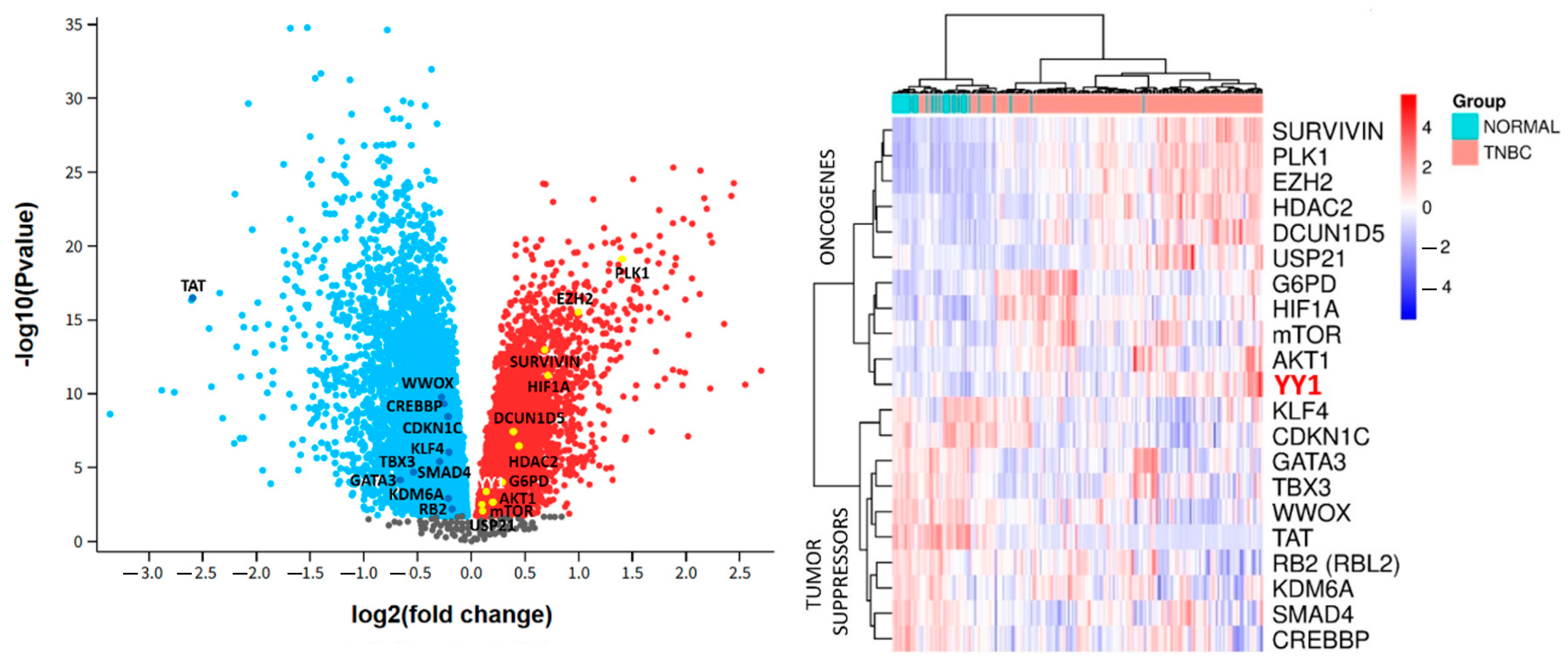
2.4. Pan-Cancer and Genomic Analyses of YY1 Expression and Prognostic Impact on Breast Cancer
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Tissue Microarray
4.3. Immunohistochemical Analysis
4.4. Digital Pathology Analysis and Automated Image Quantification
4.5. Public Datasets
4.6. Data Availability Statement
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Dogra, A.K.; Prakash, A.; Gupta, S.; Gupta, M. Prognostic Significance and Molecular Classification of Triple Negative Breast Cancer: A Systematic Review. Eur. J. Breast Health 2025, 21, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Krasnoselskyi, M.; Starikov, V.; Kartashov, S.; Zhulkevych, I.; Vlasenko, V.; Oleshko, K.; Bilodid, O.; Sadchikova, M.; Vinnyk, Y. Triple-negative breast cancer: Current treatment strategies and factors of negative prognosis. J. Med. Life 2022, 15, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Punie, K.; Kurian, A.W.; Ntalla, I.; Sjekloca, N.; Estrin, A.; Dabrowski, E.C.; Lai, C.; Hurvitz, S. Unmet need for previously untreated metastatic triple-negative breast cancer: A real-world study of patients diagnosed from 2011 to 2022 in the United States. Oncologist 2025, 30, oyaf034. [Google Scholar] [CrossRef] [PubMed]
- Karagiannakos, A.; Adamaki, M.; Tsintarakis, A.; Vojtesek, B.; Fåhraeus, R.; Zoumpourlis, V.; Karakostis, K. Targeting Oncogenic Pathways in the Era of Personalized Oncology: A Systemic Analysis Reveals Highly Mutated Signaling Pathways in Cancer Patients and Potential Therapeutic Targets. Cancers 2022, 14, 664. [Google Scholar] [CrossRef]
- Mamonova, T.; Friedman, P.A. Noncanonical Sequences Involving NHERF1 Interaction with NPT2A Govern Hormone-Regulated Phosphate Transport: Binding Outside the Box. Int. J. Mol. Sci. 2021, 22, 1087. [Google Scholar] [CrossRef]
- Uddin, M.N.; Wang, J.; Bhuiyan, M.H.R.; Rashid, M.M.; Mamun, M.Z.U.A.; Syed, A.; Roney, M. Exploring the anti-cancer potential of daidzin in breast cancer: Integrated bioinformatics and computational insights on oncogene inhibition. Comput. Biol. Chem. 2025, 119, 108590. [Google Scholar] [CrossRef]
- Gordon, S.; Akopyan, G.; Garban, H.; Bonavida, B. Transcription factor YY1: Structure, function, and therapeutic implications in cancer biology. Oncogene 2006, 25, 1125–1142. [Google Scholar] [CrossRef]
- Furlong, E.E.M.; Rein, T.; Martin, F. YY1 and NF1 Both Activate the Human p53 Promoter by Alternatively Binding to a Composite Element, and YY1 and E1A Cooperate To Amplify p53 Promoter Activity. Mol. Cell. Biol. 1996, 16, 5933–5945. [Google Scholar] [CrossRef]
- Nicholson, S.; Whitehouse, H.; Naidoo, K.; Byers, R. Yin Yang 1 in Human Cancer. Crit. Rev. Oncog. 2011, 16, 245–260. [Google Scholar] [CrossRef]
- Zapata-Tarres, M.; Juarez-Villegas, L.E.; Maldonado-Valenzuela, A.; Baay-Guzman, G.J.; Lopez-Perez, T.V.; Cabrera-Muñoz, L.; Sadowinski-Pine, S.; Huerta-Yepez, S. Expression of YY1 in Wilms tumors with favorable histology is a risk factor for adverse outcomes. Future Oncol. 2019, 15, 1231–1241. [Google Scholar] [CrossRef]
- Antonio-Andrés, G.; Rangel-Santiago, J.; Tirado-Rodríguez, B.; Martinez-Ruiz, G.U.; Klunder-Klunder, M.; Vega, M.I.; Lopez-Martinez, B.; Jiménez-Hernández, E.; Torres Nava, J.; Medina-Sanson, A.; et al. Role of Yin Yang-1 (YY1) in the transcription regulation of the multi-drug resistance (MDR1) gene. Leuk. Lymphoma 2018, 59, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- de Nigris, F.; Zanella, L.; Cacciatore, F.; De Chiara, A.; Fazioli, F.; Chiappetta, G.; Apice, G.; Infante, T.; Monaco, M.; Rossiello, R.; et al. YY1 overexpression is associated with poor prognosis and metastasis-free survival in patients suffering osteosarcoma. BMC Cancer 2011, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, Q.; Wang, A.; Jiao, J. YY1 regulates melanoma tumorigenesis through a miR-9 ~ RYBP axis. J. Exp. Clin. Cancer Res. 2015, 34, 66. [Google Scholar] [CrossRef] [PubMed]
- Baritaki, S.; Chatzinikola, A.M.; Vakis, A.F.; Soulitzis, N.; Karabetsos, D.A.; Neonakis, I.; Bonavida, B.; Spandidos, D.A. YY1 Over-Expression in Human Brain Gliomas and Meningiomas Correlates with TGF-β1, IGF-1 and FGF-2 mRNA Levels. Cancer Investig. 2009, 27, 184–192. [Google Scholar] [CrossRef]
- Berchuck, A.; Iversen, E.S.; Lancaster, J.M.; Pittman, J.; Luo, J.; Lee, P.; Murphy, S.; Dressman, H.K.; Febbo, P.G.; West, M.; et al. Patterns of Gene Expression That Characterize Long-term Survival in Advanced Stage Serous Ovarian Cancers. Clin. Cancer Res. 2005, 11, 3686–3696. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Wu, C.W.; Dong, Y.; Cai, M.; Mok, M.T.S.; Wang, H.; Chen, J.; Ng, S.S.M.; Chen, M.; et al. microRNA-7 is a novel inhibitor of YY1 contributing to colorectal tumorigenesis. Oncogene 2013, 32, 5078–5088. [Google Scholar] [CrossRef]
- Allouche, A.; Nolens, G.; Tancredi, A.; Delacroix, L.; Mardaga, J.; Fridman, V.; Winkler, R.; Boniver, J.; Delvenne, P.; Begon, D.Y. The combined immunodetection of AP-2α and YY1 transcription factors is associated with ERBB2 gene overexpression in primary breast tumors. Breast Cancer Res. 2008, 10, R9. [Google Scholar] [CrossRef]
- Thomassen, M.; Tan, Q.; Kruse, T.A. Gene expression meta-analysis identifies metastatic pathways and transcription factors in breast cancer. BMC Cancer 2008, 8, 394. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Huang, W.; Kute, T.E.; Miller, L.D.; Zhang, Q.; Hatcher, H.; Wang, J.; Stovall, D.B.; Russell, G.B.; Cao, P.D.; et al. Yin Yang 1 Plays an Essential Role in Breast Cancer and Negatively Regulates p27. Am. J. Pathol. 2012, 180, 2120–2133. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, J.; Long, Q.; Yang, Y.; Li, Y.; Li, G.; Pu, P.; Tong, S.; He, Y.; Li, Q.; et al. SETD7 promotes metastasis of triple-negative breast cancer by YY1 lysine methylation. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166780. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Li, X.; Zhang, J.; Yu, H. YY1 as a promoter regulating the circ_0001946/miR-671-5p/EGFR axis to promote chemotherapy resistance in breast cancer cells. Am. J. Transl. Res. 2022, 14, 2550–2566. [Google Scholar]
- Zhai, Z.; Ren, Y.; Shu, C.; Chen, D.; Liu, X.; Liang, Y.; Li, A.; Zhou, J. JAC1 targets YY1 mediated JWA/p38 MAPK signaling to inhibit proliferation and induce apoptosis in TNBC. Cell Death Discov. 2022, 8, 169. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, H.; Pan, Z.; Li, H.; Ding, J.; Chen, Q. YY1-inudced activation of lncRNA DUXAP8 promotes proliferation and suppresses apoptosis of triple negative breast cancer cells through upregulating SAPCD2. Cancer Biol. Ther. 2021, 22, 216–224. [Google Scholar] [CrossRef]
- Huang, R.; Li, X.; Yu, Y.; Ma, L.; Liu, S.; Zong, X.; Zheng, Q. SETD7 is a prognosis predicting factor of breast cancer and regulates redox homeostasis. Oncotarget 2017, 8, 94080–94090. [Google Scholar] [CrossRef]
- Hosea, R.; Hillary, S.; Wu, S.; Kasim, V. Targeting Transcription Factor YY1 for Cancer Treatment: Current Strategies and Future Directions. Cancers 2023, 15, 3506. [Google Scholar] [CrossRef]
- Qiao, K.; Ning, S.; Wan, L.; Wu, H.; Wang, Q.; Zhang, X.; Xu, S.; Pang, D. LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR-515-5p/MARK4/Hippo signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 418. [Google Scholar] [CrossRef]
- Li, W.; Hu, S.; Han, Z.; Jiang, X. YY1-Induced Transcriptional Activation of FAM111B Contributes to the Malignancy of Breast Cancer. Clin. Breast Cancer 2022, 22, e417–e425. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Chen, X.; Jin, X.; Jiang, M.; Xiao, H.; Chen, L.; Chen, M.; Zhang, W.; Chen, H.; et al. YY1 mediated DCUN1D5 transcriptional activation promotes triple-negative breast cancer progression by targeting FN1/PI3K/AKT pathway. Biol. Direct 2024, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.D.; Son, S.H.; Kim, C.G.; Park, H.; Chung, M.S. Prognostic Implication of YY1 and CP2c Expression in Patients with Primary Breast Cancer. Cancers 2023, 15, 3495. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Park, H.; Kim, C.G.; Chung, M.S. Abstract P4-05-13: Prognostic implication of Yin Yang 1 (YY1) overexpression in patients with primary breast cancer. Cancer Res. 2022, 82, P4-05-13. [Google Scholar] [CrossRef]
- Montecillo-Aguado, M.; Soca-Chafre, G.; Antonio-Andres, G.; Morales-Martinez, M.; Tirado-Rodriguez, B.; Rocha-Lopez, A.G.; Hernandez-Cueto, D.; Sánchez-Ceja, S.G.; Alcala-Mota-Velazco, B.; Gomez-Garcia, A.; et al. Upregulated Nuclear Expression of Soluble Epoxide Hydrolase Predicts Poor Outcome in Breast Cancer Patients: Importance of the Digital Pathology Approach. Int. J. Mol. Sci. 2024, 25, 8024. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Goding Sauer, A.; Newman, L.A.; Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA. Cancer J. Clin. 2017, 67, 439–448. [Google Scholar] [CrossRef]
- Watkins, E.J. Overview of breast cancer. JAAPA 2019, 32, 13–17. [Google Scholar] [CrossRef]
- Yap, Y.-S. Outcomes in breast cancer—Does ethnicity matter? ESMO Open 2023, 8, 101564. [Google Scholar] [CrossRef]
- Balkhi, M.Y.; Wittmann, G.; Xiong, F.; Junghans, R.P. YY1 Upregulates Checkpoint Receptors and Downregulates Type I Cytokines in Exhausted, Chronically Stimulated Human T Cells. iScience 2018, 2, 105–122. [Google Scholar] [CrossRef]
- Patten, D.K.; Corleone, G.; Győrffy, B.; Perone, Y.; Slaven, N.; Barozzi, I.; Erdős, E.; Saiakhova, A.; Goddard, K.; Vingiani, A.; et al. Enhancer mapping uncovers phenotypic heterogeneity and evolution in patients with luminal breast cancer. Nat. Med. 2018, 24, 1469–1480. [Google Scholar] [CrossRef]
- Powe, D.G.; Akhtar, G.; Habashy, H.O.; Abdel-Fatah, T.; Rakha, E.A.; Green, A.R.; Ellis, I.O. Investigating AP-2 and YY1 protein expression as a cause of high HER2 gene transcription in breast cancers with discordant HER2 gene amplification. Breast Cancer Res. 2009, 11, R90. [Google Scholar] [CrossRef]
- Shen, X.; Zhong, J.; Yu, P.; Zhao, Q.; Huang, T. YY1-regulated LINC00152 promotes triple negative breast cancer progression by affecting on stability of PTEN protein. Biochem. Biophys. Res. Commun. 2019, 509, 448–454. [Google Scholar] [CrossRef]
- Teichgraeber, D.C.; Guirguis, M.S.; Whitman, G.J. Breast Cancer Staging: Updates in the AJCC Cancer Staging Manual, 8th Edition, and Current Challenges for Radiologists, From the AJR Special Series on Cancer Staging. Am. J. Roentgenol. 2021, 217, 278–290. [Google Scholar] [CrossRef]
- Wu, S.-G.; Li, F.-Y.; Chen, Y.; Sun, J.-Y.; Lin, H.-X.; Lin, Q.; He, Z.-Y. Therapeutic role of axillary lymph node dissection in patients with stage IV breast cancer: A population-based analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Sávolt, Á.; Cserni, G.; Lázár, G.; Maráz, R.; Kelemen, P.; Kovács, E.; Győrffy, B.; Udvarhelyi, N.; Vörös, A.; Ormándi, K.; et al. Sentinel lymph node biopsy following previous axillary surgery in recurrent breast cancer. Eur. J. Surg. Oncol. 2019, 45, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xiao, H.; Yang, Q.; Hu, R.; Jiang, L.; Bi, R.; Jiang, X.; Wang, L.; Mei, J.; Ding, F.; et al. The USP21/YY1/SNHG16 axis contributes to tumor proliferation, migration, and invasion of non-small-cell lung cancer. Exp. Mol. Med. 2020, 52, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wan, M.; Shi, J.; Horita, D.A.; Miller, L.D.; Kute, T.E.; Kridel, S.J.; Kulik, G.; Sui, G. Yin Yang 1 promotes mTORC2-mediated AKT phosphorylation. J. Mol. Cell Biol. 2016, 8, 232–243. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Li, Y.; Xie, Y.; Huang, C.; Zhao, H.; Miyagishi, M.; Kasim, V. Transcription Factor YY1 Promotes Cell Proliferation by Directly Activating the Pentose Phosphate Pathway. Cancer Res. 2018, 78, 4549–4562. [Google Scholar] [CrossRef]
- Petkova, V.; Romanowski, M.J.; Sulijoadikusumo, I.; Rohne, D.; Kang, P.; Shenk, T.; Usheva, A. Interaction between YY1 and the Retinoblastoma Protein. J. Biol. Chem. 2001, 276, 7932–7936. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, L.; Zhu, Y.; Xu, R.; Deng, Z.; Liu, X.; Ding, Y.; Wang, C.; Shi, Y.; Bei, L.; et al. KDM6A promotes imatinib resistance through YY1-mediated transcriptional upregulation of TRKA independently of its demethylase activity in chronic myelogenous leukemia. Theranostics 2021, 11, 2691–2705. [Google Scholar] [CrossRef]
- Kurisaki, K.; Kurisaki, A.; Valcourt, U.; Terentiev, A.A.; Pardali, K.; ten Dijke, P.; Heldin, C.-H.; Ericsson, J.; Moustakas, A. Nuclear Factor YY1 Inhibits Transforming Growth Factor β- and Bone Morphogenetic Protein-Induced Cell Differentiation. Mol. Cell. Biol. 2003, 23, 4494–4510. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Devi, H.S.; Daddam, J.R.; Sarwar, R.; Badrealam, F.K. The HDAC2/YY1/c-Myc signaling axis regulates lung cancer cell migration and proliferation. Environ. Toxicol. 2023, 38, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chen, X.; Wu, J.; Chen, S.; Gan, Y.; Liu, C.; Ha, X.; Wu, Y.; Zhou, X.; Wu, Y.; et al. YY1-induced USP43 drives ferroptosis suppression by FASN stabilization and subsequent activation of SLC7A11 in ovarian cancer. Cell Death Dis. 2025, 16, 589. [Google Scholar] [CrossRef] [PubMed]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Antonio-Andres, G.; Morales-Martinez, M.; Tong, Z.; Yang, J.; Hammock, B.D.; Hernandez-Pando, R.; Huerta-Yepez, S. Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins. Int. J. Mol. Sci. 2022, 23, 6179. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Yepez, S.; Tirado-Rodriguez, A.; Montecillo-Aguado, M.R.; Yang, J.; Hammock, B.D.; Hankinson, O. Aryl Hydrocarbon Receptor-Dependent inductions of omega-3 and omega-6 polyunsaturated fatty acid metabolism act inversely on tumor progression. Sci. Rep. 2020, 10, 7843. [Google Scholar] [CrossRef]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Park, S.-J.; Yoon, B.-H.; Kim, S.-K.; Kim, S.-Y. GENT2: An updated gene expression database for normal and tumor tissues. BMC Med. Genomics 2019, 12, 101. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]

| Characteristics | n (%) | Total (n = 276) | p-Value |
|---|---|---|---|
| Age | 276 | <0.001 * | |
| <50 yr | 111 (40.2%) | ||
| ≥50 yr | 165 (59.8%) | ||
| Histologic Grade | 273 | <0.001 * | |
| I | 59 (21.6%) | ||
| II | 146 (53.5%) | ||
| III | 68 (24.9%) | ||
| Nuclear Grade | 273 | <0.001 * | |
| 1 | 25 (9.2%) | ||
| 2 | 171 (62.6%) | ||
| 3 | 77 (28.2%) | ||
| Border Type | 276 | <0.001 * | |
| Non-Infiltrative | 206 (74.6%) | ||
| Infiltrative | 70 (25.4%) | ||
| Tumor size | 276 | 0.952 | |
| ≤3 cm | 139 (50.4%) | ||
| >3 cm | 137 (49.6%) | ||
| ALND | 276 | <0.001 * | |
| No | 99 (35.9%) | ||
| Yes | 177 (64.1%) | ||
| ER Status | 276 | <0.001 * | |
| Negative | 86 (31.2%) | ||
| Positive | 190 (68.8%) | ||
| PR Status | 276 | 0.003 * | |
| Negative | 113 (40.9%) | ||
| Positive | 163 (59.1%) | ||
| HER2 Status | 273 | <0.001 * | |
| Negative | 227 (83.2%) | ||
| Positive | 46 (16.8%) | ||
| Molecular Subtype | 268 | <0.001 * | |
| LUM A + B | 190 (70.9%) | ||
| HER2 | 26 (9.7%) | ||
| TNBC | 52 (19.4%) | ||
| AJCC Prognostic Stage | 272 | <0.001 * | |
| I | 103 (37.9%) | ||
| II | 101 (37.1%) | ||
| III | 62 (22.8%) | ||
| IV | 6 (2.2%) | ||
| Recurrence | 276 | <0.001 * | |
| No | 215 (77.9%) | ||
| Yes | 61 (22.1%) |
| Variable n (%) | N | Low YY1 (n = 165) | High YY1 (n = 111) | Total (n = 276) | p-Value |
|---|---|---|---|---|---|
| Age | 276 | 1.000 C | |||
| <50 yr | 66 (40.0%) | 45 (40.5%) | 111 (40.2%) | ||
| ≥50 yr | 99 (60.0%) | 66 (59.5%) | 165 (59.8%) | ||
| Histologic Grade | 273 | 0.415 C | |||
| I | 37 (22.7%) | 22 (20.0%) | 59 (21.6%) | ||
| II | 90 (55.2%) | 56 (50.9%) | 146 (53.5%) | ||
| III | 36 (22.1%) | 32 (29.1%) | 68 (24.9%) | ||
| Nuclear Grade | 273 | 0.547 C | |||
| 1 | 15 (9.2%) | 10 (9.1%) | 25 (9.2%) | ||
| 2 | 106 (65.0%) | 65 (59.1%) | 171 (62.6%) | ||
| 3 | 42 (25.8%) | 35 (31.8%) | 77 (28.2%) | ||
| Border Type | 276 | 0.400 C | |||
| Non- | 120 (72.7%) | 86 (77.5%) | 206 (74.6%) | ||
| Infiltrative | 45 (27.3%) | 25 (22.5%) | 70 (25.4%) | ||
| Tumor size | 276 | 0.037 C* | |||
| ≤3 cm | 92 (55.8%) | 47 (42.3%) | 139 (50.4%) | ||
| >3 cm | 73 (44.2%) | 64 (57.7%) | 137 (49.6%) | ||
| ALND | 276 | 0.074 C | |||
| No | 52 (31.5%) | 47 (42.3%) | 99 (35.9%) | ||
| Yes | 113 (68.5%) | 64 (57.7%) | 177 (64.1%) | ||
| ER Status | 276 | 0.185 C | |||
| Negative | 46 (27.9%) | 40 (36.0%) | 86 (31.2%) | ||
| Positive | 119 (72.1%) | 71 (64.0%) | 190 (68.8%) | ||
| PR Status | 276 | 0.035 C* | |||
| Negative | 59 (35.8%) | 54 (48.6%) | 113 (40.9%) | ||
| Positive | 106 (64.2%) | 57 (51.4%) | 163 (59.1%) | ||
| HER2 Status | 273 | 0.414 C | |||
| Negative | 132 (81.5%) | 95 (85.6%) | 227 (83.2%) | ||
| Positive | 30 (18.5%) | 16 (14.4%) | 46 (16.8%) | ||
| Molecular Subtype | 268 | 0.026 F* | |||
| LUM A + B | 119 (75.3%) | 71 (64.5%) | 190 (70.9%) | ||
| HER2 | 17 (10.8%) | 9 (8.2%) | 26 (9.7%) | ||
| TNBC | 22 (13.9%) | 30 (27.3%) | 52 (19.4%) | ||
| AJCC Prog Stage | 272 | 0.003 F* | |||
| I | 74 (45.7%) | 29 (26.4%) | 103 (37.9%) | ||
| II | 58 (35.8%) | 43 (39.1%) | 101 (37.1%) | ||
| III | 27 (16.7%) | 35 (31.8%) | 62 (22.8%) | ||
| IV | 3 (1.9%) | 3 (2.7%) | 6 (2.2%) | ||
| Recurrence | 276 | 0.554 C | |||
| No | 131 (79.4%) | 84 (75.7%) | 215 (77.9%) | ||
| Yes | 34 (20.6%) | 27 (24.3%) | 61 (22.1%) |
| Univariate Analysis | ||||
|---|---|---|---|---|
| Variable | Category | HR | 95% CI | p Value |
| Age | <50 | 1 | ||
| ≥50 | 1.040 | 0.36–1.700 | 0.875 | |
| Molecular Subtype | LUMINAL A + B | 1 | ||
| HER2 | 2.317 | 1.155–4.647 | 0.018 * | |
| TNBC | 2.018 | 1.153–3.531 | 0.014 * | |
| ER Status | Negative | 1 | ||
| Positive | 0.504 | 0.311–0.819 | 0.006 * | |
| PR Status | Negative | 1 | ||
| Positive | 0.529 | 0.327–0.855 | 0.009 * | |
| HER2 Status | Negative | 1 | ||
| Positive | 1.004 | 0.525–1.917 | 0.991 | |
| Tumor size | <3 cm | 1 | ||
| ≥3 cm | 1.852 | 1.132–3.028 | 0.014 * | |
| Recurrence | No | 1 | ||
| Yes | 3.963 | 2.445–6.424 | <0.001 * | |
| Nuclear Grade | 1 + 2 | 1 | ||
| 3 | 1.748 | 1.060–2.882 | 0.029 * | |
| ALND | No | 1 | ||
| Yes | 0.459 | 0.284–0.742 | 0.001 * | |
| AJCC Prognostic Stage | I | 1 | ||
| II | 2.230 | 1.115–4.460 | 0.023 * | |
| III | 5.882 | 2.973–11.637 | <0.001 * | |
| IV | 5.240 | 1.169–23.494 | 0.030 * | |
| Histologic Grade | I + II | 1 | ||
| III | 1.289 | 0.755–2.201 | 0.351 | |
| YY1 Protein Expression | Low | 1 | ||
| High | 2.402 | 1.479–3.899 | <0.001 * | |
| Multivariate Analysis | ||||
|---|---|---|---|---|
| Variable | Category | HR | 95% CI | p Value |
| Molecular Subtype | LUMINAL A + B | 1 | ||
| HER2 | 0.080 | 0.007–0.881 | 0.039 * | |
| TNBC | 0.087 | 0.009–0.865 | 0.037 * | |
| ER Status | Negative | 1 | ||
| Positive | 0.050 | 0.006–0.418 | 0.006 * | |
| PR Status | Negative | 1 | ||
| Positive | 1.569 | 0.664–3.709 | 0.305 | |
| Tumor size | <3 cm | 1 | ||
| ≥3 cm | 1.117 | 0.605–2.063 | 0.724 | |
| Recurrence | No | 1 | ||
| Yes | 3.655 | 2.136–6.255 | <0.001 * | |
| Nuclear Grade | 1 + 2 | 1 | ||
| 3 | 0.790 | 0.452–1.381 | 0.408 | |
| ALND | No | |||
| Yes | 0.545 | 0.321–0.928 | 0.025 | |
| AJCC Prognostic Stage | I | 1 | ||
| II | 1.829 | 0.849–3.941 | 0.123 | |
| III | 3.575 | 1.416–9.021 | 0.007 * | |
| IV | 1.602 | 0.288–8.907 | 0.591 | |
| YY1 Protein Expression | Low | 1 | ||
| High | 1.927 | 1.144–3.247 | 0.014 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montecillo-Aguado, M.; Soca-Chafre, G.; Antonio-Andres, G.; Tirado-Rodriguez, B.; Hernández-Cueto, D.; Rivera-Pazos, C.M.; Duran-Padilla, M.A.; Sánchez-Ceja, S.G.; Alcala-Mota-Velazco, B.; Gomez-Garcia, A.; et al. Clinical Significance of Nuclear Yin-Yang Overexpression Evaluated by Immunohistochemistry in Tissue Microarrays and Digital Pathology Analysis: A Useful Prognostic Tool for Breast Cancer. Int. J. Mol. Sci. 2025, 26, 8777. https://doi.org/10.3390/ijms26188777
Montecillo-Aguado M, Soca-Chafre G, Antonio-Andres G, Tirado-Rodriguez B, Hernández-Cueto D, Rivera-Pazos CM, Duran-Padilla MA, Sánchez-Ceja SG, Alcala-Mota-Velazco B, Gomez-Garcia A, et al. Clinical Significance of Nuclear Yin-Yang Overexpression Evaluated by Immunohistochemistry in Tissue Microarrays and Digital Pathology Analysis: A Useful Prognostic Tool for Breast Cancer. International Journal of Molecular Sciences. 2025; 26(18):8777. https://doi.org/10.3390/ijms26188777
Chicago/Turabian StyleMontecillo-Aguado, Mayra, Giovanny Soca-Chafre, Gabriela Antonio-Andres, Belen Tirado-Rodriguez, Daniel Hernández-Cueto, Clara M. Rivera-Pazos, Marco A. Duran-Padilla, Sandra G. Sánchez-Ceja, Berenice Alcala-Mota-Velazco, Anel Gomez-Garcia, and et al. 2025. "Clinical Significance of Nuclear Yin-Yang Overexpression Evaluated by Immunohistochemistry in Tissue Microarrays and Digital Pathology Analysis: A Useful Prognostic Tool for Breast Cancer" International Journal of Molecular Sciences 26, no. 18: 8777. https://doi.org/10.3390/ijms26188777
APA StyleMontecillo-Aguado, M., Soca-Chafre, G., Antonio-Andres, G., Tirado-Rodriguez, B., Hernández-Cueto, D., Rivera-Pazos, C. M., Duran-Padilla, M. A., Sánchez-Ceja, S. G., Alcala-Mota-Velazco, B., Gomez-Garcia, A., Gutierrez-Castellanos, S., & Huerta-Yepez, S. (2025). Clinical Significance of Nuclear Yin-Yang Overexpression Evaluated by Immunohistochemistry in Tissue Microarrays and Digital Pathology Analysis: A Useful Prognostic Tool for Breast Cancer. International Journal of Molecular Sciences, 26(18), 8777. https://doi.org/10.3390/ijms26188777






