Diabetes and Sarcopenia: Metabolomic Signature of Pathogenic Pathways and Targeted Therapies
Abstract
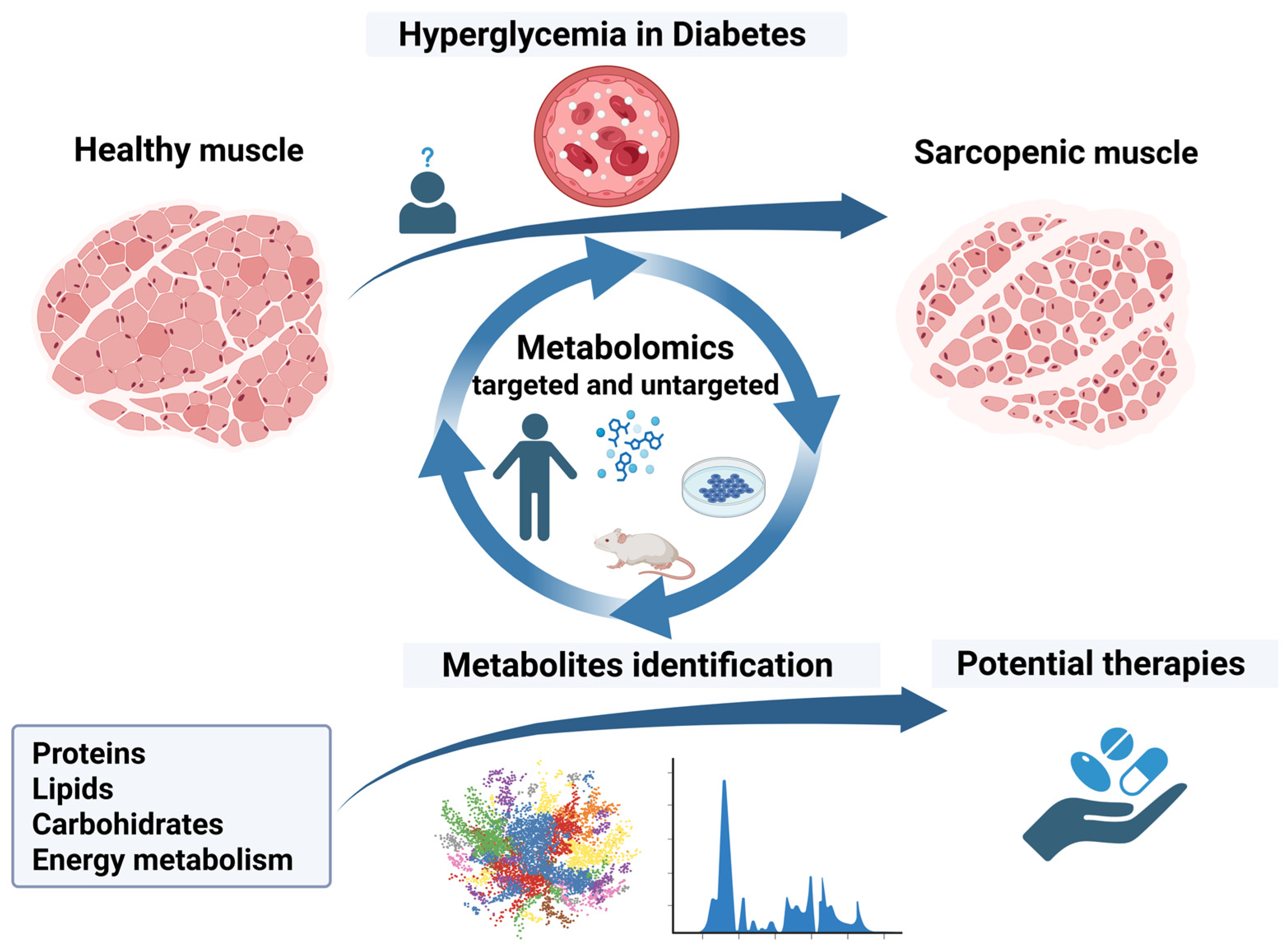
1. Introduction
2. Key Findings from Metabolomic Studies in Sarcopenia Associated with Diabetes
2.1. Amino Acids, Peptides and Proteins
2.2. Lipids
2.3. Energy Metabolism and Carbohydrates
| Metabolism | Serum Metabolites in Sarcopenia and Diabetes Mellitus | |
|---|---|---|
| Increased Levels | Decreased Levels | |
| Proteins | Targeted metabolomics: Branched-chain amino acids: valine [45] Other: alanine [41,56] arginine [34,56] L-(+)-arginine [22] citrulline [34] glutamine [22,30,33] glycine [34] proline [30] serine [27,41] D-gluconic acid [22] glutamic acid [27,56] 3-methylxanthine [22] 5′-methylthioadenosine [22] asymmetric dimethylarginine [22] N,N-dimethylarginine [22] Ubiquitin-specific protease21 [54] citric acid [41] oxalic acid [41] malonic acid [41] succinic acid [41] Untargeted metabolomics: 82 metabolites (overall) [22] 75 metabolites (overall) [31] 131 differentially expressed protein upregulated [40] 103 differentially expressed protein upregulated [42] 160 proteins (males), 46 proteins (females) [55] | Targeted metabolomics: Branched-chain amino acids: leucine [27,31,32,34,41] valine [31,32,34,41] isoleucine [31,32,34,41] Aromatic amino acids: phenylalanine [27,31,32,41] tryptophan [30,31,32,34] tyrosine [31,32] Other: alanine [27] glycine [41] glutamate [41] histidine [30] lysine [41] methionine [41] proline [34] threonine [41] isoxanthohumol [22] aspartic acid [27,41] Untargeted metabolomics: 68 differentially expressed protein downregulated [40] 52 differentially expressed protein downregulated [42] |
| Lipids | Targeted metabolomics: low-density lipoprotein triglycerides [45] Monounsaturated fatty acids: palmitoleic acid [41] oleic acid [41] Aaturated fatty acids: pentadecanoic acid [43] myristic acid [41] palmitic acid [41] lauric acid [41] stearic acid [41] Untargeted metabolomics: 146 metabolites (overall) [46] Ceramides (14:0), (16:0), (24:0) [46] sphingomyelin (24:0) [46] sphingomyelins (16:1), (24:1) [46] ceramide (24:1) [46] lysophosphatidylcholines (17:0; 18:1; 18:2) [47] phosphatidylcholine [47] sphingomyelins (16:1;18:0;18:1) [47] | Targeted metabolomics: - Untargeted metabolomics: most ceramides, sphingomyelins evaluated [46] long-chain acylcarnitines [50] |
| Carbohydrates and Energy | Targeted metabolomics: lactic acid [45] lactase dehydrogenase [31] respiratory chain complex III subunits [55] Untargeted metabolomics: fatty acid oxidation related pathways [40] intermediate metabolites of Krebs cycle [41] intermediate metabolites of glycogen metabolism [41] | Targeted metabolomics: Hexoses [32,47] alfa-ketoglutarate [50] malate [50] fumarate [50] pyruvate [31] respiratory chain complex I and V [55] free fatty acids beta-oxidation [55] tricarboxylic acid cycle [55] Untargeted metabolomics: - |
2.4. Therapeutic Approaches
| Therapy/Intervention | Effect on Diabetes Mellitus and Sarcopenia |
|---|---|
| D-pinitol | favorable [59] |
| Ceramides | unfavorable [46] |
| Aerobic exercise | favorable [42] |
| 2 weeks step reduction, followed by 2 weeks recovery | unfavorable [33] |
| Genetic USP21 ablation | favorable [54] |
| Inhibitors of sodium-glucose co-transporter 2 (luseogliflozin, canagliflozin) | favorable [70,71,72] |
| Glucagon-like peptide-1 receptor agonists (liraglutide, semaglutide) | uncertain, probably positive [73,74,75,76,77,78] |
| Conventional open surgical intervention vs. laparoscopic intervention | unfavorable [79] |
3. Conclusions and Further Needs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUROC | Area under the receiver operating characteristic curve |
| ATP | Adenosine triphosphate |
| BCAA | Branched-chain amino acids |
| CI | Confidence interval |
| DALYs | Disability-adjusted life years |
| DEP | Differentially expressed protein |
| DM | Diabetes mellitus |
| GC-MS | Gas chromatography mass spectrometry |
| GLP-1 RA | Glucagon like peptide-1 receptor agonists |
| HPLC/ESI-MS | Precolumn derivatization high-performance liquid chromatography/electrospray mass spectrometry |
| LC–ESI–MS/MS | Liquid chromatography coupled to electrospray tandem mass spectrometry |
| LC-MS | Liquid chromatography mass spectroscopy |
| LC-MS/MS | Liquid chromatography tandem mass spectroscopy |
| MSI-CE-MS | Multisegment injection capillary electrophoresis mass spectrometry |
| NAFLD | Non-alcoholic fatty liver disease |
| OR | Odds ratio |
| ROC | Receiver operating characteristic |
| SGLT2i | Inhibitor of sodium-glucose co-transporter 2 |
| SMI | Skeletal muscle mass index |
| TCA | Tricarboxylic acid cycle |
| UHPLC-ESI-MS/MS | Ultra-high-performance liquid chromatography electrospray-ionization tandem mass spectroscopy |
| UPLC/MS | Ultraperformance liquid chromatography/mass spectrometry |
| USP21 | Ubiquitin-specific protease21 |
References
- IDF Diabetes Atlas 2025|Global Diabetes Data & Insights. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 2 June 2025).
- Sun, J.; Hu, W.; Ye, S.; Deng, D.; Chen, M. The Description and Prediction of Incidence, Prevalence, Mortality, Disability-Adjusted Life Years Cases, and Corresponding Age-Standardized Rates for Global Diabetes. J. Epidemiol. Glob. Health 2023, 13, 566–576. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Beaudart, C.; Alcazar, J.; Aprahamian, I.; Batsis, J.A.; Yamada, Y.; Prado, C.M.; Reginster, J.Y.; Sanchez-Rodriguez, D.; Lim, W.S.; Sim, M.; et al. Health outcomes of sarcopenia: A consensus report by the outcome working group of the Global Leadership Initiative in Sarcopenia (GLIS). Aging Clin. Exp. Res. 2025, 37, 100. [Google Scholar] [CrossRef]
- Mesinovic, J.; Fyfe, J.J.; Talevski, J.; Wheeler, M.J.; Leung, G.K.W.; George, E.S.; Hunegnaw, M.T.; Glavas, C.; Jansons, P.; Daly, R.M.; et al. Type 2 Diabetes Mellitus and Sarcopenia as Comorbid Chronic Diseases in Older Adults: Established and Emerging Treatments and Therapies. Diabetes Metab. J. 2023, 47, 719–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, X.; Dong, M.; Wen, S.; Zhou, L.; Yuan, X. The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy. Diabetes Metab. Syndr. Obes. 2023, 16, 1541–1554. [Google Scholar] [CrossRef]
- Wen, C.Y.; Lien, A.S.Y.; Jiang, Y.D. Sarcopenia in elderly diabetes. J. Diabetes Investig. 2022, 13, 944–946. [Google Scholar] [CrossRef]
- Lisco, G.; Disoteo, O.E.; De Tullio, A.; De Geronimo, V.; Giagulli, V.A.; Monzani, F.; Jirillo, E.; Cozzi, R.; Guastamacchia, E.; De Pergola, G.; et al. Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age. Nutrients 2024, 16, 63. [Google Scholar] [CrossRef]
- Dai, S.; Shu, D.; Meng, F.; Chen, Y.; Wang, J.; Liu, X.; Xiao, X.; Guo, W.; Chen, F. Higher Risk of Sarcopenia in Older Adults with Type 2 Diabetes: NHANES 1999–2018. Obes. Facts 2023, 16, 237–248. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Demurtas, J.; Soysal, P.; Smith, L.; Sieber, C.; Strandberg, T.; Bourdel-Marchasson, I.; Sinclair, A.; Petrovic, M.; et al. Association between sarcopenia and diabetes: A systematic review and meta-analysis of observational studies. Eur. Geriatr. Med. 2019, 10, 685–696. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhang, Y.; Wu, J.; Lin, J.; Yan, S. Predictive efficacy of different diagnostic criteria for sarcopenia in osteoporosis and fractures. Sci. Rep. 2025, 15, 9473. [Google Scholar] [CrossRef]
- Al-Akl, N.S.; Khalifa, O.; Ponirakis, G.; Parray, A.; Ramadan, M.; Khan, S.; Chandran, M.; Ayadathil, R.; Elsotouhy, A.; Own, A.; et al. Untargeted Metabolomic Profiling Reveals Differentially Expressed Serum Metabolites and Pathways in Type 2 Diabetes Patients with and without Cognitive Decline: A Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 2247. [Google Scholar] [CrossRef] [PubMed]
- Bala, C.; Rusu, A.; Ciobanu, D.M.; Roman, G.; Crăciun, A.E. Metabolomics in Pathogenic Pathways and Targeted Therapies for Diabetic Neuropathy: A Comprehensive Review. Metabolites 2025, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Chen, X.; Liang, Z.; Liu, Y.; Yu, X.; Song, P.; Zhao, Y.; Zhang, H.; Zhu, S.; Shi, X.; et al. Metabolic signatures and risk of sarcopenia in suburb-dwelling older individuals by LC-MS–based untargeted metabonomics. Front. Endocrinol. 2024, 15, 1308841. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Wang, S.Y.; Chao, Y.M.; Chang, K.V.; Han, D.S.; Lin, Y.L. Novel metabolic and lipidomic biomarkers of sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 2175–2186. [Google Scholar] [CrossRef]
- Marques, J.; Shokry, E.; Uhl, O.; Baber, L.; Hofmeister, F.; Jarmusch, S.; Bidlingmaier, M.; Ferrari, U.; Koletzko, B.; Drey, M. Sarcopenia: Investigation of metabolic changes and its associated mechanisms. Skelet. Muscle 2023, 13, 2. [Google Scholar] [CrossRef]
- Alldritt, I.; Greenhaff, P.L.; Wilkinson, D.J. Metabolomics as an Important Tool for Determining the Mechanisms of Human Skeletal Muscle Deconditioning. Int. J. Mol. Sci. 2021, 22, 13575. [Google Scholar] [CrossRef]
- Mathioudaki, A.; Fanni, G.; Eriksson, J.W.; Pereira, M.J. Metabolomic Profiling of Adipose Tissue in Type 2 Diabetes: Associations with Obesity and Insulin Resistance. Metabolites 2024, 14, 411. [Google Scholar] [CrossRef]
- Hammad, S.M.; Lopes-Virella, M.F. Circulating Sphingolipids in Insulin Resistance, Diabetes and Associated Complications. Int. J. Mol. Sci. 2023, 24, 14015. [Google Scholar] [CrossRef]
- Ali, S.R.; Nkembo, A.; Tipparaju, S.M.; Ashraf, M.; Wanling, X. Sarcopenia: Recent advances for detection, progression, and metabolic alterations along with therapeutic targets. Can. J. Physiol. Pharmacol. 2024, 102, 697–708. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, S.; Cao, Z.; Yu, R.; Fan, Y.; Chen, J. The role of chronic low-grade inflammation in the development of sarcopenia: Advances in molecular mechanisms. Int. Immunopharmacol. 2025, 147, 114056. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Yang, Y.; Li, B.; Yu, F.; Zhao, W.; Fu, C.; Yu, X.; Han, Z.; Cheng, M. Metabolomics analysis reveals serum biomarkers in patients with diabetic sarcopenia. Front. Endocrinol. 2023, 14, 1119782. [Google Scholar] [CrossRef]
- Xiao, W.; Huang, T.E.; Zhou, J.; Wang, B.; Wang, X.; Zeng, W.; Wang, Q.; Lan, X.; Xiang, Y. Inhibition of MAT2A Impairs Skeletal Muscle Repair Function. Biomolecules 2024, 14, 1098. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Q.; Wang, H.; Xin, G.; Wang, T.; Zhang, K.; Yu, X.; Wen, A.; Wu, Q.; Li, X.; et al. Isoxanthohumol improves hepatic lipid metabolism via regulating the AMPK/PPARα and PI3K/AKT signaling pathways in hyperlipidemic mice. Food Sci. Nutr. 2024, 12, 8846–8857. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wei, Y.; Feng, Y.; Zhang, S.; Ma, N.; Wang, K.; Tan, P.; Zhao, Y.; Zhao, J.; Ma, X. Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 6184. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.E.; Won, C.W.; Kim, M. Metabolomic profiles to explore biomarkers of severe sarcopenia in older men: A pilot study. Exp. Gerontol. 2022, 167, 111924. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Okada, H.; Kobayashi, A.; Takahashi, F.; Okamura, T.; Hashimoto, Y.; Nakanishi, N.; Senmaru, T.; Ushigome, E.; Hamaguchi, M.; et al. Leucine and Glutamic Acid as a Biomarker of Sarcopenic Risk in Japanese People with Type 2 Diabetes. Nutrients 2023, 15, 2400. [Google Scholar] [CrossRef]
- Otvos, J.D.; Shalaurova, I.; May, H.T.; Muhlestein, J.B.; Wilkins, J.T.; McGarrah, R.W., 3rd; Kraus, W.E. Multimarkers of metabolic malnutrition and inflammation and their association with mortality risk in cardiac catheterisation patients: A prospective, longitudinal, observational, cohort study. Lancet Healthy Longev. 2023, 4, e72–e82. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, H.; Liu, J.; Chiu, C.Y.; Su, K.J.; Tian, Q.; Kakhniashvili, D.; Qiu, C.; Zhao, L.J.; Luo, Z.; et al. Pathway-based metabolomics study of sarcopenia-related traits in two US cohorts. Aging 2022, 14, 2101–2112. [Google Scholar] [CrossRef]
- Toyoshima, K.; Nakamura, M.; Adachi, Y.; Imaizumi, A.; Hakamada, T.; Abe, Y.; Kaneko, E.; Takahashi, S.; Shimokado, K. Increased plasma proline concentrations are associated with sarcopenia in the elderly. PLoS ONE 2017, 12, e0185206. [Google Scholar] [CrossRef]
- Madrid-Gambin, F.; Pérez-Sáez, M.J.; Gómez-Gómez, A.; Haro, N.; Redondo-Pachón, D.; Dávalos-Yerovi, V.; Marco, E.; Crespo, M.; Pozo, O.J.; Pascual, J.; et al. Frailty and sarcopenia metabolomic signatures in kidney transplant candidates: The FRAILMar study. Clin. Kidney J. 2025, 18, sfae366. [Google Scholar] [CrossRef]
- Yao, S.; Marron, M.M.; Farsijani, S.; Miljkovic, I.; Tseng, G.C.; Shah, R.V.; Murthy, V.L.; Newman, A.B. Metabolomic characterization of unintentional weight loss among community-dwelling older Black and White men and women. Aging Cell 2025, 24, e14410. [Google Scholar] [CrossRef] [PubMed]
- Saoi, M.; Li, A.; McGlory, C.; Stokes, T.; von Allmen, M.T.; Phillips, S.M.; Britz-McKibbin, P. Metabolic Perturbations from Step Reduction in Older Persons at Risk for Sarcopenia: Plasma Biomarkers of Abrupt Changes in Physical Activity. Metabolites 2019, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Wang, J.; Moh, A.; Ang, S.F.; Ang, K.; Shao, Y.M.; Ching, J.; Wee, H.N.; Lee, L.S.; Kovalik, J.P.; et al. Amino acid profile of skeletal muscle loss in type 2 diabetes: Results from a 7-year longitudinal study in asians. Diabetes Res. Clin. Pract. 2022, 186, 109803. [Google Scholar] [CrossRef] [PubMed]
- Ottestad, I.; Ulven, S.M.; Øyri, L.K.L.; Sandvei, K.S.; Gjevestad, G.O.; Bye, A.; Sheikh, N.A.; Biong, A.S.; Andersen, L.F.; Holven, K.B. Reduced plasma concentration of branched-chain amino acids in sarcopenic older subjects: A cross-sectional study. Br. J. Nutr. 2018, 120, 445–453. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hao, Q.; Li, Q.; Yang, L.; Yang, X.; Wang, K.; Teng, J.; Gong, Z.; Jia, Y. Associations between sarcopenia and circulating branched-chain amino acids: A cross-sectional study over 100,000 participants. BMC Geriatr. 2024, 24, 541. [Google Scholar] [CrossRef]
- Ter Borg, S.; Luiking, Y.C.; van Helvoort, A.; Boirie, Y.; Schols, J.M.G.A.; de Groot, C.P.G.M. Low Levels of Branched Chain Amino Acids, Eicosapentaenoic Acid and Micronutrients are Associated with Low Muscle Mass, Strength and Function in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 27–34. [Google Scholar] [CrossRef]
- Imai, D.; Nakanishi, N.; Shinagawa, N.; Yamamoto, S.; Ichikawa, T.; Sumi, M.; Matsui, T.; Hosomi, Y.; Hasegawa, Y.; Munekawa, C.; et al. Association of Elevated Serum Branched-chain Amino Acid Levels With Longitudinal Skeletal Muscle Loss. J. Endocr. Soc. 2024, 8, bvad178. [Google Scholar] [CrossRef]
- Li, C.W.D.; Herpich, C.; Haß, U.; Kochlik, B.; Weber, D.; Grune, T.; Norman, K. Essential amino acids and branched-chain amino acids are associated with skeletal muscle and inflammatory parameters in older age. Biogerontology 2025, 26, 66. [Google Scholar] [CrossRef]
- Wu, J.; Wang, S.; Zhuang, H.; Wang, W.; Wang, Y.; Chen, Y.; Huang, Z.; Chen, C.; Chen, X. Proteomics Analysis Provides Insights into the Role of Lipid Metabolism in T2DM-Related Sarcopenia. ACS Omega 2024, 9, 34056–34069. [Google Scholar] [CrossRef]
- Okamura, T.; Hamaguchi, M.; Kobayashi, G.; Ichikawa, T.; Hasegawa, Y.; Miyoshi, T.; Senmaru, T.; Nakanishi, N.; Sasano, R.; Fukui, M. A multi-omics approach to overeating and inactivity-induced muscle atrophy in db/db mice. J. Cachexia Sarcopenia Muscle 2024, 15, 2030–2045. [Google Scholar] [CrossRef]
- Huang, Y.C.; Sanotra, M.R.; Huang, C.C.; Hsu, Y.J.; Liao, C.C. Aerobic Exercise Modulates Proteomic Profiles in Gastrocnemius Muscle of db/db Mice, Ameliorating Sarcopenia. Life 2024, 14, 412. [Google Scholar] [CrossRef]
- Chen, F.X.; Du, N.; Hu, J.; Ning, F.; Mei, X.; Li, Q.; Peng, L. Intramuscular accumulation of pentadecanoic acid activates AKT1 to phosphorylate NCOR1 and triggers FOXM1-mediated apoptosis in the pathogenesis of sarcopenia. Am. J. Transl. Res. 2020, 12, 5064–5079. [Google Scholar] [PubMed]
- Losasso, M.R.; Parussolo, M.L.C.; Oliveira Silva, A.; Direito, R.; Quesada, K.; Penteado Detregiachi, C.R.; Bechara, M.D.; Méndez-Sánchez, N.; Abenavoli, L.; Araújo, A.C.; et al. Unraveling the Metabolic Pathways Between Metabolic-Associated Fatty Liver Disease (MAFLD) and Sarcopenia. Int. J. Mol. Sci. 2025, 26, 4673. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, Q.; Ma, S.; Chen, L.; Wu, Q.; Wu, L.; Ma, H.; Li, X.; Li, Q.; Aleteng, Q.; et al. Presence of sarcopenia identifies a special group of lean NAFLD in middle-aged and older people. Hepatol. Int. 2023, 17, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Koh, J.M.; Cho, H.J.; Kim, H.; Lee, Y.S.; Kim, S.J.; Yoon, P.W.; Kim, W.; Bae, S.J.; Kim, H.K.; et al. Sphingolipid metabolites as potential circulating biomarkers for sarcopenia in men. J. Cachexia Sarcopenia Muscle 2024, 15, 2476–2486. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; Moaddel, R.; Sun, K.; Fabbri, E.; Zhang, P.; Khadeer, M.; Salem, N.; Ferrucci, L., Jr.; Semba, R.D. Targeted Metabolomics Shows Low Plasma Lysophosphatidylcholine 18:2 Predicts Greater Decline of Gait Speed in Older Adults: The Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 62–67. [Google Scholar] [CrossRef]
- Liu, N.; Chen, Y.; An, T.; Tao, S.; Lv, B.; Dou, J.; Deng, R.; Zhen, X.; Zhang, Y.; Lu, C.; et al. Lysophosphatidylcholine trigger myocardial injury in diabetic cardiomyopathy via the TLR4/ZNF480/AP-1/NF-kB pathway. Heliyon 2024, 10, e33601. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, Y.; Yan, S.; Yan, D.; Jiang, D. Lysophosphatidylcholine (17:0) Improves HFD-Induced Hyperglycemia & Insulin Resistance: A Mechanistic Mice Model Study. Diabetes Metab. Syndr. Obes. 2022, 15, 3511–3517. [Google Scholar]
- Zhang, X.; Trevino, M.B.; Wang, M.; Gardell, S.J.; Ayala, J.E.; Han, X.; Kelly, D.P.; Goodpaster, B.H.; Vega, R.B.; Coen, P.M. Impaired Mitochondrial Energetics Characterize Poor Early Recovery of Muscle Mass Following Hind Limb Unloading in Old Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1313–1322. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Musci, R.V.; Hamilton, K.L.; Miller, B.F. Targeting mitochondrial function and proteostasis to mitigate dynapenia. Eur. J. Appl. Physiol. 2018, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Koo, J.H.; Jin, X.; Kim, W.; Park, S.Y.; Park, S.; Rhee, E.P.; Choi, C.S.; Kim, S.G. Ablation of USP21 in skeletal muscle promotes oxidative fibre phenotype, inhibiting obesity and type 2 diabetes. J. Cachexia Sarcopenia Muscle 2021, 12, 1669–1689. [Google Scholar] [CrossRef] [PubMed]
- Moriggi, M.; Belloli, S.; Barbacini, P.; Murtaj, V.; Torretta, E.; Chaabane, L.; Canu, T.; Penati, S.; Malosio, M.L.; Esposito, A.; et al. Skeletal muscle proteomic profile revealed gender-related metabolic responses in a diet-induced obesity animal model. Int. J. Mol. Sci. 2021, 22, 4680. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Rodriguez-Mañas, L.; Tosato, M.; Coelho-Júnior, H.J.; Biancolillo, A.; Laosa, O.; Gervasoni, J.; Primiano, A.; Santucci, L.; et al. Amino Acid Profiles in Older Adults with Frailty: Secondary Analysis from MetaboFrail and BIOSPHERE Studies. Metabolites 2023, 13, 542. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.J.; Choi, Y.J.; Rho, Y.H.; Kang, T.S.; Kim, Y.G.; Kang, K.S. The Glucose-Lowering Effect of Mesembryanthemum crystallinum and D-Pinitol: Studies on Insulin Secretion in INS-1 Cells and the Reduction of Blood Glucose in Diabetic Rats. Nutrients 2025, 17, 193. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M.; Chandrashekar, N. Effects of d-pinitol on diabetes mellitus: An updated review. Bull. Natl. Res. Cent. 2022, 46, 130. [Google Scholar] [CrossRef]
- Yu, X.; Li, P.; Li, B.; Yu, F.; Zhao, W.; Wang, X.; Wang, Y.; Gao, H.; Cheng, M.; Li, X. d-Pinitol Improves Diabetic Sarcopenia by Regulation of the Gut Microbiome, Metabolome, and Proteome in STZ-Induced SAMP8 Mice. J. Agric. Food Chem. 2024, 72, 14466–14478. [Google Scholar] [CrossRef]
- Turpin, S.M.; Lancaster, G.I.; Darby, I.; Febbraio, M.A.; Watt, M.J. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1341–E1350. [Google Scholar] [CrossRef]
- Tan-Chen, S.; Guitton, J.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism and Signaling in Skeletal Muscle: From Physiology to Physiopathology. Front. Endocrinol. 2020, 11, 491. [Google Scholar] [CrossRef]
- Sawicka, A.K.; Hartmane, D.; Lipinska, P.; Wojtowicz, E.; Lysiak-Szydlowska, W.; Olek, R.A. l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Nutrients 2018, 10, 255. [Google Scholar] [CrossRef]
- Elgizawy, E.I.; Amer, G.S.; Ali, E.A.; Alqalashy, F.S.; Ibrahim, M.M.; Latif, A.A.A.; Shaban, A.M. Comparing the efficacy of concomitant treatment of resistance exercise and creatine monohydrate versus multiple individual therapies in age related sarcopenia. Sci. Rep. 2024, 14, 9798. [Google Scholar] [CrossRef] [PubMed]
- Mezincescu, A.M.; Rudd, A.; Cheyne, L.; Horgan, G.; Philip, S.; Cameron, D.; van Loon, L.; Whitfield, P.; Gribbin, R.; Hu, M.K.; et al. Comparison of intramyocellular lipid metabolism in patients with diabetes and male athletes. Nat. Commun. 2024, 15, 3690. [Google Scholar] [CrossRef] [PubMed]
- Sarma, M.K.; Saucedo, A.; Sadananthan, S.A.; Darwin, C.H.; Felker, E.R.; Raman, S.; Velan, S.S.; Thomas, M. Lipid Deposition in Skeletal Muscle Tissues and Its Correlation with Intra-Abdominal Fat: A Pilot Investigation in Type 2 Diabetes Mellitus. Metabolites 2025, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Luna-Marco, C.; Iannantuoni, F.; Hermo-Argibay, A.; Devos, D.; Salazar, J.D.; Víctor, V.M.; Rovira-Llopis, S. Cardiovascular benefits of SGLT2 inhibitors and GLP-1 receptor agonists through effects on mitochondrial function and oxidative stress. Free Radic. Biol. Med. 2024, 213, 19–35. [Google Scholar] [CrossRef]
- Yalçın, N.; Aktaş, S.; Uyar, S.; Koca, N. Impact of SGLT2 Inhibitors on Cardiovascular Risk Scores, Metabolic Parameters, and Laboratory Profiles in Type 2 Diabetes. Life 2025, 15, 722. [Google Scholar] [CrossRef]
- Sobczyk, M.; Żuraw, D.; Oleksa, P.; Jasiński, K.; Porzak, M.; Dacka, M. SGLT2 inhibitors and their possible use in prevention and treatment of neurological diseases. Prospect. Pharm. Sci. 2024, 22, 16–22. [Google Scholar] [CrossRef]
- Marcus, R.L.; Addison, O.; Dibble, L.E.; Foreman, K.B.; Morrell, G.; Lastayo, P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J. Aging Res. 2012, 2012, 629637. [Google Scholar] [CrossRef]
- Bamba, R.; Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Takakuwa, H.; et al. Extracellular lipidome change by an SGLT2 inhibitor, luseogliflozin, contributes to prevent skeletal muscle atrophy in db/db mice. J. Cachexia Sarcopenia Muscle 2022, 13, 574–588. [Google Scholar] [CrossRef]
- Otsuka, H.; Yokomizo, H.; Nakamura, S.; Izumi, Y.; Takahashi, M.; Obara, S.; Nakao, M.; Ikeda, Y.; Sato, N.; Sakamoto, R.; et al. Differential effect of canagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, on slow and fast skeletal muscles from nondiabetic mice. Biochem. J. 2022, 479, 425–444. [Google Scholar] [CrossRef]
- Nakamura, S.; Miyachi, Y.; Shinjo, A.; Yokomizo, H.; Takahashi, M.; Nakatani, K.; Izumi, Y.; Otsuka, H.; Sato, N.; Sakamoto, R.; et al. Improved endurance capacity of diabetic mice during SGLT2 inhibition: Role of AICARP, an AMPK activator in the soleus. J. Cachexia Sarcopenia Muscle 2023, 14, 2866–2881. [Google Scholar] [CrossRef]
- Memel, Z.; Gold, S.L.; Pearlman, M.; Muratore, A.; Martindale, R. Impact of GLP-1 Receptor Agonist Therapy in Patients High Risk for Sarcopenia. Curr. Nutr. Rep. 2025, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Mantzoros, C.S. Effect of glucagon-like peptide-1 receptor agonists and co-agonists on body composition: Systematic review and network meta-analysis. Metabolism 2025, 164, 156113. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, S.; Kim, E.K. Glucagon-like peptide-1 analog liraglutide leads to multiple metabolic alterations in diet-induced obese mice. J. Biol. Chem. 2022, 298, 102682. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Chen, S.; Chen, X.; Niu, S.; Yue, L.; Pan, X.; Li, Z.; Chen, X. An Effective Glucagon-Like Peptide-1 Receptor Agonists, Semaglutide, Improves Sarcopenic Obesity in Obese Mice by Modulating Skeletal Muscle Metabolism. Drug Des. Devel. Ther. 2022, 16, 3723–3735. [Google Scholar] [CrossRef]
- Xiang, J.; Qin, L.; Zhong, J.; Xia, N.; Liang, Y. GLP-1RA Liraglutide and Semaglutide Improves Obesity-Induced Muscle Atrophy via SIRT1 Pathway. Diabetes Metab. Syndr. Obes. 2023, 16, 2433–2446. [Google Scholar] [CrossRef]
- Benabdelkamel, H.; Sebaa, R.; AlMalki, R.H.; Masood, A.; Alfadda, A.A.; Abdel Rahman, A.M. Untargeted metabolomics reveals the impact of Liraglutide treatment on metabolome profiling and metabolic pathways in type-2 diabetes mellitus. Saudi Pharm. J. 2024, 32, 102172. [Google Scholar] [CrossRef]
- Gau, R.Y.; Tsai, H.I.; Yu, M.C.; Chan, K.M.; Lee, W.C.; Wang, H.E.; Wang, S.F.; Cheng, M.L.; Chiu, C.C.; Chen, H.Y.; et al. Laparoscopic liver resection is associated with less significant muscle loss than the conventional open approach. World J. Surg. Oncol. 2022, 20, 385. [Google Scholar] [CrossRef]
- Gaglio, A.; Grancini, V.; Giacchetti, F.; Mirani, M.; Orsi, E.; Resi, V. Role of Medical Nutrition Therapy as Treatment of Sarcopenia in Older People with Type 2 Diabetes. Nutrients 2025, 17, 172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danciu, A.A.; Bala, C.; Inceu, G.; Vonica, C.L.; Rusu, A.; Roman, G.; Ciobanu, D.M. Diabetes and Sarcopenia: Metabolomic Signature of Pathogenic Pathways and Targeted Therapies. Int. J. Mol. Sci. 2025, 26, 7574. https://doi.org/10.3390/ijms26157574
Danciu AA, Bala C, Inceu G, Vonica CL, Rusu A, Roman G, Ciobanu DM. Diabetes and Sarcopenia: Metabolomic Signature of Pathogenic Pathways and Targeted Therapies. International Journal of Molecular Sciences. 2025; 26(15):7574. https://doi.org/10.3390/ijms26157574
Chicago/Turabian StyleDanciu, Anamaria Andreea, Cornelia Bala, Georgeta Inceu, Camelia Larisa Vonica, Adriana Rusu, Gabriela Roman, and Dana Mihaela Ciobanu. 2025. "Diabetes and Sarcopenia: Metabolomic Signature of Pathogenic Pathways and Targeted Therapies" International Journal of Molecular Sciences 26, no. 15: 7574. https://doi.org/10.3390/ijms26157574
APA StyleDanciu, A. A., Bala, C., Inceu, G., Vonica, C. L., Rusu, A., Roman, G., & Ciobanu, D. M. (2025). Diabetes and Sarcopenia: Metabolomic Signature of Pathogenic Pathways and Targeted Therapies. International Journal of Molecular Sciences, 26(15), 7574. https://doi.org/10.3390/ijms26157574








