Anti-Ku Antibodies: Clinical Associations, Organ Damage, and Prognostic Implications in Connective Tissue Diseases
Abstract
1. Introduction
2. Results
2.1. A Total of 47 and 33 Patients Were Identified as Anti-Ku-Positive and Anti-Ku-Borderline Patients, Respectively
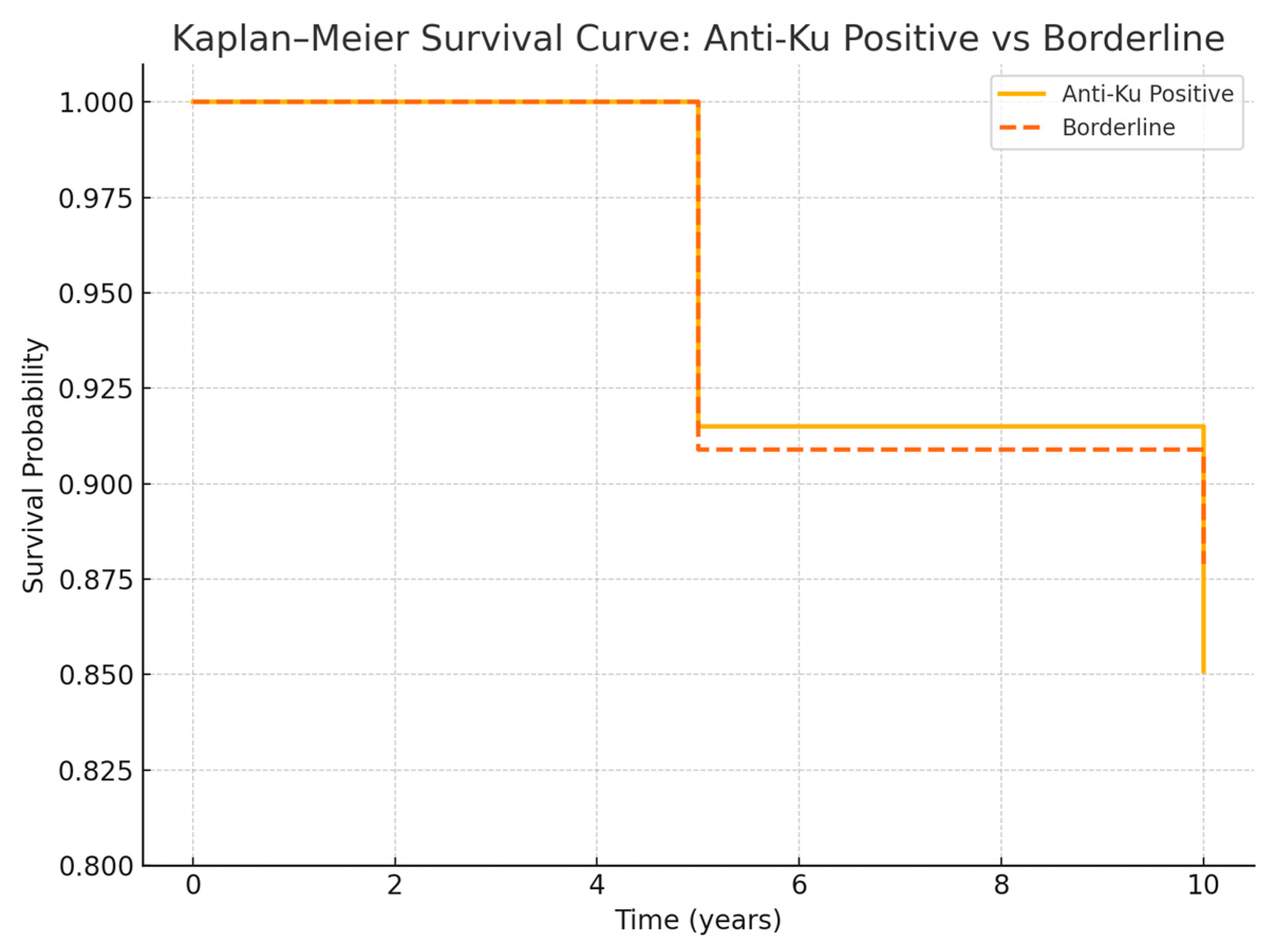
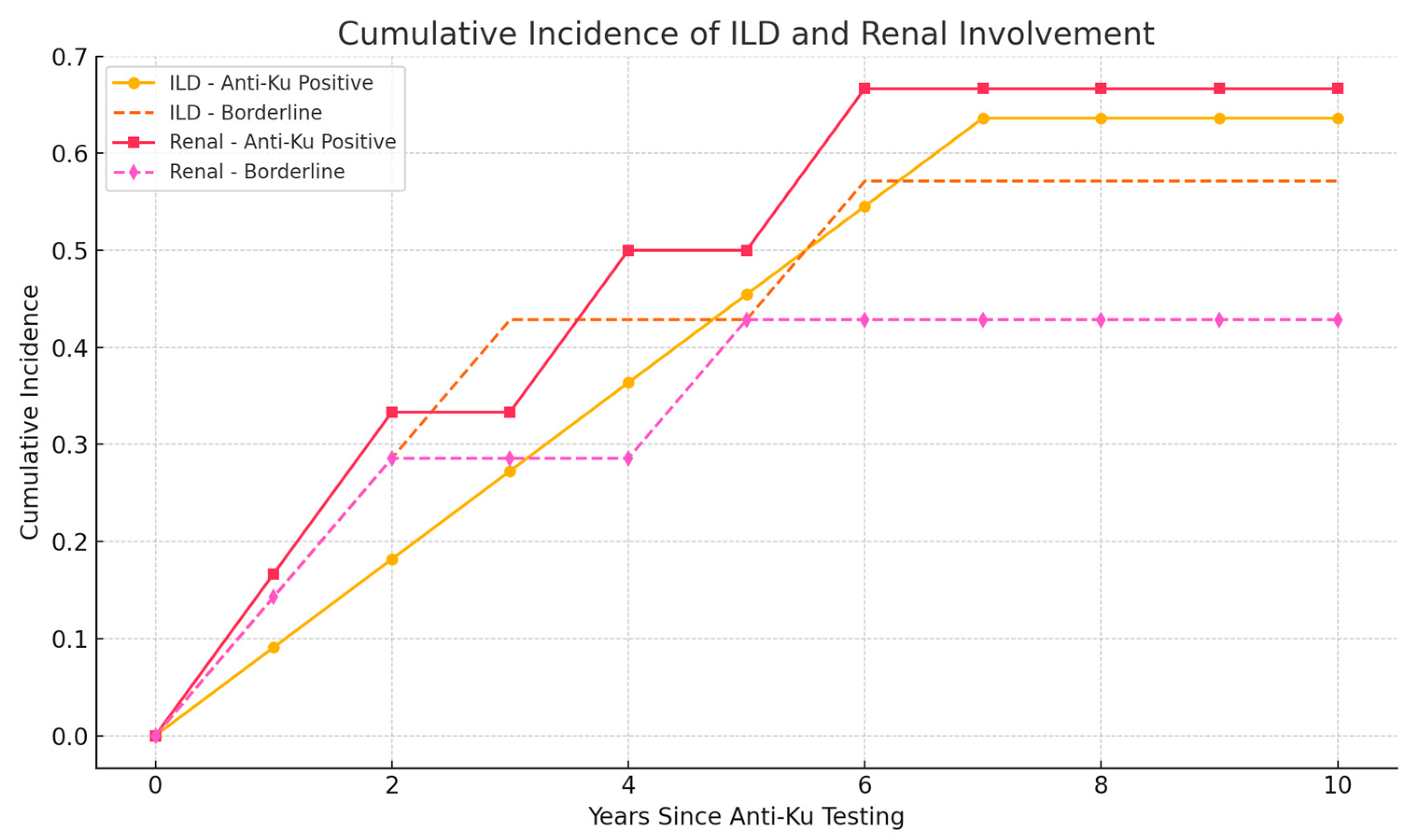
2.2. Survival Analysis
2.3. Therapeutic Burden
2.4. Risk Stratification and Prognostic Modeling
2.4.1. Predictors of Pulmonary Involvement
2.4.2. Predictors of Renal Involvement
2.4.3. Mortality Prediction
2.4.4. Risk Stratification System
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Detection of Anti-Ku Antibodies and Other Serological Data
4.3. Definitions
4.4. Statistical Analysis
4.5. Ethics Approval
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Clinical and Biological Parameters | Value |
|---|---|
| Arthralgia | 43/46 (93.5) |
| Arthritis | 19/46 (41.3) |
| Raynaud | 10/24 (41.7) |
| Muscle weakness | 15/45 (33.3) |
| Dysphagia | 2/43 (4.7) |
| Serositis | 6/42 (14.3) |
| Lupus rash | 8/41 (19.5) |
| Photosensitivity | 7/30 (23.3) |
| Thrombosis | 4/45 (8.9) |
| Neuropathy | 12/22 (54.5) |
| Sicca syndrome | 21/31 (67.7) |
| Myocarditis | 5/44 (11.4) |
| Vasculitis | 4/40 (10.0) |
| Lymphoma | 2/47 (4.3) |
| Cryoglobulinemia | 1/13 (7.7) |
| Elevated CK | 12/44 (27.3) |
| Cytopenia | 13/47 (27.7) |
| Low C3 | 7/31 (22.6) |
| Low C4 | 5/31 (16.1) |
| Elevated dsDNA | 9/45 (20.0) |
| SSA | 20/47 (42.6) |
| Ro60 | 13/27 (48.1) |
| Ro52 | 15/29 (51.7) |
| SSB | 4/47 (8.5) |
| Sm | 4/47 (8.5) |
| RNP | 6/47 (12.8) |
| Mi2 | 5/47 (10.6) |
| PMScl75 | 3/13 (23.1) |
| PMScl100 | 1/12 (8.3) |
| PL12 | 4/14 (28.6) |
| SRP | 1/6 (16.7) |
| RF | 7/37 (18.9) |
| ACPA | 2/28 (7.1) |
| Antiphospholipid biology | 11/24 (45.8) |
| ANCA | 4/33 (12.1) |
References
- Mimori, T.; Akizuki, M.; Yamagata, H.; Inada, S.; Yoshida, S.; Homma, M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J. Clin. Investig. 1981, 68, 611–620. [Google Scholar] [CrossRef]
- Mimori, T.; Hardin, J.A.; Steitz, J.A. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J. Biol. Chem. 1986, 261, 2274–2278. [Google Scholar] [CrossRef]
- Mimori, T.; Hardin, J.A. Mechanism of interaction between Ku protein and DNA. J. Biol. Chem. 1986, 261, 10375–10379. [Google Scholar] [CrossRef]
- Gottlieb, T.M.; Jackson, S.P. The DNA-dependent protein kinase: Requirement for DNA ends and association with Ku antigen. Cell 1993, 72, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; Visser, M.D.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef]
- Yaneva, M.; Arnett, F.C. Antibodies against Ku protein in sera from patients with autoimmune diseases. Clin. Exp. Immunol. 1989, 76, 366–372. [Google Scholar]
- Franceschini, F.; Cavazzana, I.; Generali, D.; Quinzanini, M.; Viardi, L.; Ghirardello, A.; Radstake, T.R.; van den Hoogen, F.H.; Doria, A. Anti-Ku antibodies in connective tissue diseases: Clinical and serological evaluation of 14 patients. J. Rheumatol. 2002, 29, 1393–1397. [Google Scholar]
- Beyne-Rauzy, O.; Couret, B.; Fortenfant, F.; Adoue, D. Anti-Ku antibodies. Study of prevalence and of clinical meaning. Rev. Med. Interne 2004, 25, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Lakota, K.; Thallinger, G.G.; Sodin-Semrl, S.; Rozman, B.; Ambrozic, A.; Tomsic, M.; Selmi, C.; Meroni, P.L.; Shoenfeld, Y. International cohort study of 73 anti-Ku-positive patients: Association of p70/p80 anti-Ku antibodies with joint/bone features and differentiation of disease populations by using principal-components analysis. Arthritis Res. Ther. 2012, 14, R2. [Google Scholar] [CrossRef]
- Fredi, M.; Cavazzana, I.; Quinzanini, M.; Taraborelli, M.; Cartella, S.; Tincani, A.; Franceschini, F. Rare autoantibodies to cellular antigens in systemic lupus erythematosus. Lupus 2014, 23, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Aguila, L.A.; Lopes, M.R.U.; Pretti, F.Z.; Sampaio-Barros, P.D.; Carlos de Souza, F.H.; Borba, E.F.; Shinjo, S.K. Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin. Rheumatol. 2014, 33, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana, I.; Ceribelli, A.; Quinzanini, M.; Scarsi, M.; Airò, P.; Cattaneo, R.; Franceschini, F. Prevalence and clinical associations of anti-Ku antibodies in systemic autoimmune diseases. Lupus 2008, 17, 727–732. [Google Scholar] [CrossRef]
- Spielmann, L.; Nespola, B.; Séverac, F.; Andres, E.; Kessler, R.; Guffroy, A.; Martin, T.; Korganow, A.S. Anti-Ku syndrome with elevated CK and anti-Ku syndrome with anti-dsDNA are two distinct entities with different outcomes. Ann. Rheum. Dis. 2019, 78, 1101–1106. [Google Scholar] [CrossRef]
- Ogawa-Momohara, M.; Muro, Y.; Akiyama, M. Overlap of systemic lupus erythematosus and myositis is rare in anti-Ku antibody-positive patients. Ann. Rheum. Dis. 2019, 78, e87. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef]
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990, 335, 1078–1080. [Google Scholar]
- Wang, J.; Satoh, M.; Kabir, F.; Shaw, M.; Domingo, M.A.; Mansoor, R.; Kenney, M.; Earthman, C.; Reeves, W.H. Increased prevalence of autoantibodies to ku antigen in African American versus white patients with systemic lupus erythematosus. Arthritis Rheum. 2001, 44, 2367–2370. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DE Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hoa, S.; Hudson, M.; Troyanov, Y.; Proudman, S.; Walker, J.; Stevens, W.; Sahhar, J.; Tatibouet, S.; Baron, M.; Fernandez-Codina, A.; et al. Single-specificity anti-Ku antibodies in an international cohort of 2140 systemic sclerosis subjects: Clinical associations. Medicine 2016, 95, e4713. [Google Scholar] [CrossRef] [PubMed]
- Casal-Dominguez, M.; Pinal-Fernandez, I.; Derfoul, A.; Graf, R.; Michelle, H.; Albayda, J.; Paik, J.J.; Christopher-Stine, L.; Lloyd, T.E.; Mammen, A.L.; et al. The phenotype of myositis patients with anti-Ku autoantibodies. Semin. Arthritis Rheum. 2021, 51, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Petitgrand, L.; Ahmad, K.; Gamondès, D.; Diesler, R.; Fabien, N.; Gallay, L.; Fort, R.; Traclet, J.; Lestelle, F.; Chapurlat, R.; et al. Interstitial Lung Disease Associated with Anti-Ku Antibodies: A Case Series of 19 Patients. J. Clin. Med. 2025, 14, 247. [Google Scholar] [CrossRef]
- Holzer, M.T.; Uruha, A.; Roos, A.; Preusse, C.; Naddaf, E.; Suzuki, S.; Schneider, U.; Radke, J.; Schmidt, K.; Seipelt, E.; et al. Anti-Ku + myositis: An acquired inflammatory protein-aggregate myopathy. Acta Neuropathol. 2024, 148, 6. [Google Scholar] [CrossRef]
- Nigro, A.; Fusco, N.; Mariniello, R.M.; Bocchino, M. Pulmonary fibrosis as the sole manifestation of anti-Ku antibody positivity in the absence of myositis: A case report. Respir. Med. Case Rep. 2025, 53, 102165. [Google Scholar] [CrossRef]
- Kanda, S.; Shimbo, A.; Nakamura, Y.; Matsuno, R.; Kaneko, S.; Irabu, H.; Kaneko, K.; Shimizu, M. Anti-Ku antibody-positive systemic sclerosis and idiopathic inflammatory myopathies overlap syndrome in children: A report of two cases and a review of the literature. Clin. Rheumatol. 2023, 42, 3411–3417. [Google Scholar] [CrossRef]
- Sjöwall, C.; Bentow, C.; Aure, M.A.; Mahler, M. Two-Parametric Immunological Score Development for Assessing Renal Involvement and Disease Activity in Systemic Lupus Erythematosus. J. Immunol. Res. 2018, 2018, 1294680. [Google Scholar] [CrossRef]
- Giannini, M.; Ellezam, B.; Leclair, V.; Lefebvre, F.; Troyanov, Y.; Hudson, M.; Senécal, J.L.; Geny, B.; Landon-Cardinal, O.; Meyer, A. Scleromyositis: A distinct novel entity within the systemic sclerosis and autoimmune myositis spectrum. Implications for care and pathogenesis. Front. Immunol. 2023, 13, 974078. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Parmar, G.; Kelly, R.D.; Balasuriya, N.; Schild-Poulter, C. The Ku complex: Recent advances and emerging roles outside of non-homologous end-joining. Cell. Mol. Life Sci. 2021, 78, 4589–4613. [Google Scholar] [CrossRef] [PubMed]
- Ouazahrou, K.; El Bakkouri, J.; Souali, M.; Jeddane, L.; Mokhantar, K.; Errami, A.; El Kabli, H.; Bousfiha, A.A.; Echchilali, K. Anti-Ku antibodies: Clinical significance and associated autoimmune connective tissue diseases in Moroccan patients. Rheumatol. Adv. Pract. 2023, 7, rkad036. [Google Scholar]
- Golovina, O.A.; Torgashina, A.V.; Gorodetskiy, V.R.; Sockol, E.V.; Sagina, E.G. Association of autoantibodies with organ damage in connective tissue diseases: Focus on anti-Ku antibodies. Clin. Rheumatol. 2024, 43, 2145–2152. [Google Scholar] [CrossRef]
- Rigolet, A.; Musset, L.; Dubourg, O.; Maisonobe, T.; Grenier, P.; Charuel, J.L.; Guillevin, L.; Benveniste, O. Inflammatory myopathies with anti-Ku antibodies: A prognosis dependent on associated lung disease. Medicine 2012, 91, 95–102. [Google Scholar] [CrossRef]
- Antiochos, B.; Casciola-Rosen, L. Interferon and autoantigens: Intersection in autoimmunity. Front. Med. 2023, 10, 1165225. [Google Scholar] [CrossRef]
- Mahler, M.; Swart, A.; Wu, J.; Szmyrka-Kaczmarek, M.; Senécal, J.L.; Troyanov, Y.; Hanly, J.G.; Fritzler, M.J. Clinical and serological associations of autoantibodies to the Ku70/Ku80 heterodimer determined by a novel chemiluminescent immunoassay. Lupus 2016, 25, 889–896. [Google Scholar] [CrossRef]
- Belizna, C.; Henrion, D.; Beucher, A.; Lavigne, C.; Ghaali, A.; Lévesque, H. Anti-Ku antibodies: Clinical, genetic and diagnostic insights. Autoimmun. Rev. 2010, 9, 691–694. [Google Scholar] [CrossRef]
- Wielosz, E.; Majdan, M.; Jeleniewicz, R.; Mazurek, M. Autoimmune diseases with the presence of anti-ku antibodies—Analysis of three cases. Wiadomości Lek. 2016, 69, 24–26. [Google Scholar]
- Kuzumi, A.; Norimatsu, Y.; Matsuda, K.M.; Ono, C.; Okumura, T.; Kogo, E.; Goshima, N.; Fukasawa, T.; Fushida, N.; Horii, M.; et al. Comprehensive autoantibody profiling in systemic autoimmunity by a highly-sensitive multiplex protein array. Front. Immunol. 2023, 14, 1255540. [Google Scholar] [CrossRef]
- Almaabdi, K.; Ahmad, Z.; Johnson, S.R. Advanced Autoantibody Testing in Systemic Sclerosis. Diagnostics 2023, 13, 851. [Google Scholar] [CrossRef]
- Connolly, C.M.; Paik, J.J. Myopathy in systemic sclerosis. Curr. Opin. Rheumatol. 2023, 35, 341–348. [Google Scholar] [CrossRef]
- Sag, E.; Demir, S.; Bilginer, Y.; Ozen, S. Further expanding the phenotype of anti-Ku antibody associated disease in children and adolescents. Neuromuscul. Disord. 2024, 34, 19–24. [Google Scholar]
- Yang, H.; Li, W.; Tian, X.; Zeng, X.; Zhang, F. Immune-mediated necrotizing myopathies and interstitial lung disease are predominant characteristics in anti-Ku positive patients with idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2022, 81, e48. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Clinical Features | Value |
|---|---|
| Age at diagnosis, years (N = 44) | 44.8 (16.6–79.5) |
| Female sex | 35 (74.5) |
| Ethnicity | |
| Caucasian | 17 (36.2) |
| Mediterranean | 18 (38.3) |
| African | 12 (25.5) |
| Initial diagnosis at onset | |
| SLE | 11 (23.4) |
| SS | 9 (19.1) |
| UCTD | 8 (17.1) |
| RA | 6 (12.8) |
| IIM | 4 (8.5) |
| AS | 2 (4.3) |
| PsA | 1 (2.1) |
| Anti-synthetase syndrome | 1 (2.1) |
| Behcet | 1 (2.1) |
| Primary sclerosing cholangitis | 1 (2.1) |
| No diagnosis | 3 (6.4) |
| Diagnosis at last follow-up | |
| No diagnosis | 3 (6.4) |
| 1 CTD | 34 (72.3) |
| >1 CTD | 10 (21.3) |
| SLE | 2 (4.3) |
| SS | 3 (6.4) |
| RA | 3 (6.4) |
| IIM | 7 (8.5) |
| Duration of follow-up, years | 5.4 (0–43.4) |
| Outcome | |
| Alive and stable | 39 (83.0) |
| Dead | 4 (8.5) |
| Chronic dialysis | 2 (4.3) |
| Graft | 1 (2.1) |
| Palliative care | 1 (2.1) |
| ILD | 11 (23.4) |
| Renal involvement | 6 (12.8) |
| Demographic Features and Diagnoses | Value |
|---|---|
| Age at diagnosis, years (N = 28) | 48.0 (12.0–89.6) |
| Female sex | 22 (66.7) |
| Ethnicity | |
| Caucasian | 16 (48.5) |
| Mediterranean | 11 (33.3) |
| African | 4 (12.1) |
| Asian | 2 (6.1) |
| Initial diagnosis at onset | |
| SLE | 8 (24.3) |
| SS | 4 (12.1) |
| SSc | 2 (6.1) |
| UCTD | 2 (6.1) |
| RA | 1 (3.0) |
| IIM | 1 (3.0) |
| PsA | 1 (3.0) |
| Cryoglobulinemia | 1 (3.0) |
| Isolated ILD | 3 (9.1) |
| IgA nephropathy | 1 (3.0) |
| Paget’s disease | 1 (3.0) |
| Others | 3 (9.1) |
| (cystic bronchiectasis, severe eczema, coxsackie myocarditis) | |
| No diagnosis | 5 (15.2) |
| Diagnosis at last follow-up | |
| No diagnosis | 5 (15.2) |
| No CTD but other diagnosis | 8 (24.2) |
| 1 CTD | 17 (51.5) |
| >1 CTD | 3 (9.1) |
| SLE | 1 (3.0) |
| SS | 1 (3.0) |
| SSc | 1 (3.0) |
| Other autoimmune diseases | |
| Autoimmune thyroiditis | 1 (3.0) |
| Crohn | 2 (6.1) |
| Ulcerative colitis | 1 (3.0) |
| Biermer’s disease | 1 (3.0) |
| Familial autoimmune diseases | |
| Crohn | 1 (3.0) |
| Duration of follow-up, years | 6.2 (0–21.6) |
| Outcome | |
| Alive and stable | 28 (84.9) |
| Dead | 3 (9.1) |
| Chronic dialysis | 1 (3.0) |
| Graft | 1 (3.0) |
| Clinical and Biological Features | Value |
|---|---|
| Arthralgia | 26/33 (78.8) |
| Arthritis | 8/25 (32.0) |
| Raynaud | 8/16 (50.0) |
| Muscle weakness | 2/33 (6.1) |
| Dysphagia | 4/33 (12.1) |
| ILD | 7/22 (31.8) |
| Renal involvement | 7/32 (21.9) |
| Serositis | 1/21 (4.8) |
| Lupus rash | 11/33 (33.3) |
| Photosensitivity | 6/22 (27.3) |
| Thrombosis | 7/33 (21.2) |
| Neuropathy | 2/6 (33.3) |
| Sicca syndrome | 8/11 (72.7) |
| Myocarditis | 1/19 (5.3) |
| Vasculitis | 4/33 (12.1) |
| Lymphoma | 0/33 (0) |
| Cryoglobulinemia | 2/8 (25.0) |
| Elevated CK | 1/31 (3.2) |
| Cytopenia | 8/33 (24.2) |
| Low C3 | 9/21 (42.9) |
| Low C4 | 3/22 (13.6) |
| Elevated dsDNA | 7/31 (22.6) |
| SSA | 9/33 (27.3) |
| Ro60 | 7/17 (41.2) |
| Ro52 | 9/17 (52.9) |
| SSB | 6/33 (18.2) |
| Sm | 2/33 (6.1) |
| RNP | 4/33 (12.1) |
| Mi2 | 3/33 (9.1) |
| PMScl75 | 2/23 (8.7) |
| PMScl100 | 0/23 (0) |
| PL12 | 0/8 (0) |
| SRP | 0/8 (0) |
| CENP-B | 1/1 (100) |
| RF | 2/21 (9.5) |
| ACPA | 0/21 (0) |
| Antiphospholipid biology | 4/15 (26.7) |
| ANCA | 7/24 (29.2) |
| Variable | Anti-Ku Positive | Anti-Ku Borderline | p-Value |
|---|---|---|---|
| Age at diagnosis (mean ± SD) | 44.8 ± 0.0 | 48.0 ± 0.0 | <0.001 |
| Female sex (%) | 74.5% | 66.7% | 0.61 |
| SLE diagnosis (%) | 23.4% | 24.3% | 1.00 |
| SS diagnosis (%) | 19.1% | 12.1% | 0.60 |
| UCTD diagnosis (%) | 17.1% | 6.1% | 0.26 |
| Smoking history (%) | 42.6% | 30.3% | 0.38 |
| Anti-SSA positivity (%) | 42.6% | 27.3% | 0.24 |
| Disease Diagnosis | Total Patients N (%) | Ku-Positive N (%) | Ku-Borderline N (%) | p-Value |
|---|---|---|---|---|
| Systemic Lupus Erythematosus (SLE) | 19 (23.8) | 11 (23.4) | 8 (24.2) | 0.93 |
| Sjögren’s Syndrome (SS) | 13 (16.3) | 9 (19.1) | 4 (12.1) | 0.39 |
| Undifferentiated CTD (UCTD) | 10 (12.5) | 8 (17.0) | 2 (6.1) | 0.12 |
| Rheumatoid Arthritis (RA) | 7 (8.8) | 6 (12.8) | 1 (3.0) | 0.11 |
| DM/PM | 5 (6.3) | 4 (8.5) | 1 (3.0) | 0.29 |
| Systemic Sclerosis (SSc) | 3 (3.8) | 0 (0) | 3 (9.1) | 0.03 * |
| Ankylosing Spondylitis (AS) | 2 (2.5) | 2 (4.3) | 0 (0) | 0.21 |
| Psoriatic Arthritis (PsA) | 2 (2.5) | 1 (2.1) | 1 (3.0) | 0.79 |
| Other CTD | 3 (3.8) | 3 (6.4) | 0 (0) | 0.11 |
| Multiple CTD Overlap | 13 (16.3) | 10 (21.3) | 3 (9.1) | 0.14 |
| No CTD Diagnosis | 8 (10.0) | 0 (0) | 8 (24.2) | <0.001 * |
| Alternative Diagnosis § | 8 (10.0) | 0 (0) | 8 (24.2) | <0.001 * |
| Total | 80 (100) | 47 (58.8) | 33 (41.3) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, C.; Smet, J.; Nagant, C.; Soyfoo, M. Anti-Ku Antibodies: Clinical Associations, Organ Damage, and Prognostic Implications in Connective Tissue Diseases. Int. J. Mol. Sci. 2025, 26, 7433. https://doi.org/10.3390/ijms26157433
La C, Smet J, Nagant C, Soyfoo M. Anti-Ku Antibodies: Clinical Associations, Organ Damage, and Prognostic Implications in Connective Tissue Diseases. International Journal of Molecular Sciences. 2025; 26(15):7433. https://doi.org/10.3390/ijms26157433
Chicago/Turabian StyleLa, Céline, Julie Smet, Carole Nagant, and Muhammad Soyfoo. 2025. "Anti-Ku Antibodies: Clinical Associations, Organ Damage, and Prognostic Implications in Connective Tissue Diseases" International Journal of Molecular Sciences 26, no. 15: 7433. https://doi.org/10.3390/ijms26157433
APA StyleLa, C., Smet, J., Nagant, C., & Soyfoo, M. (2025). Anti-Ku Antibodies: Clinical Associations, Organ Damage, and Prognostic Implications in Connective Tissue Diseases. International Journal of Molecular Sciences, 26(15), 7433. https://doi.org/10.3390/ijms26157433





