A 30-Year Experience in Fragile X Syndrome Molecular Diagnosis from a Laboratory in Thailand
Abstract
1. Introduction
2. Results
2.1. Frequency of FXS
2.2. Prenatal FXS Diagnosis
2.3. Full Mutation Expansion in Maternal Transmission
3. Discussion
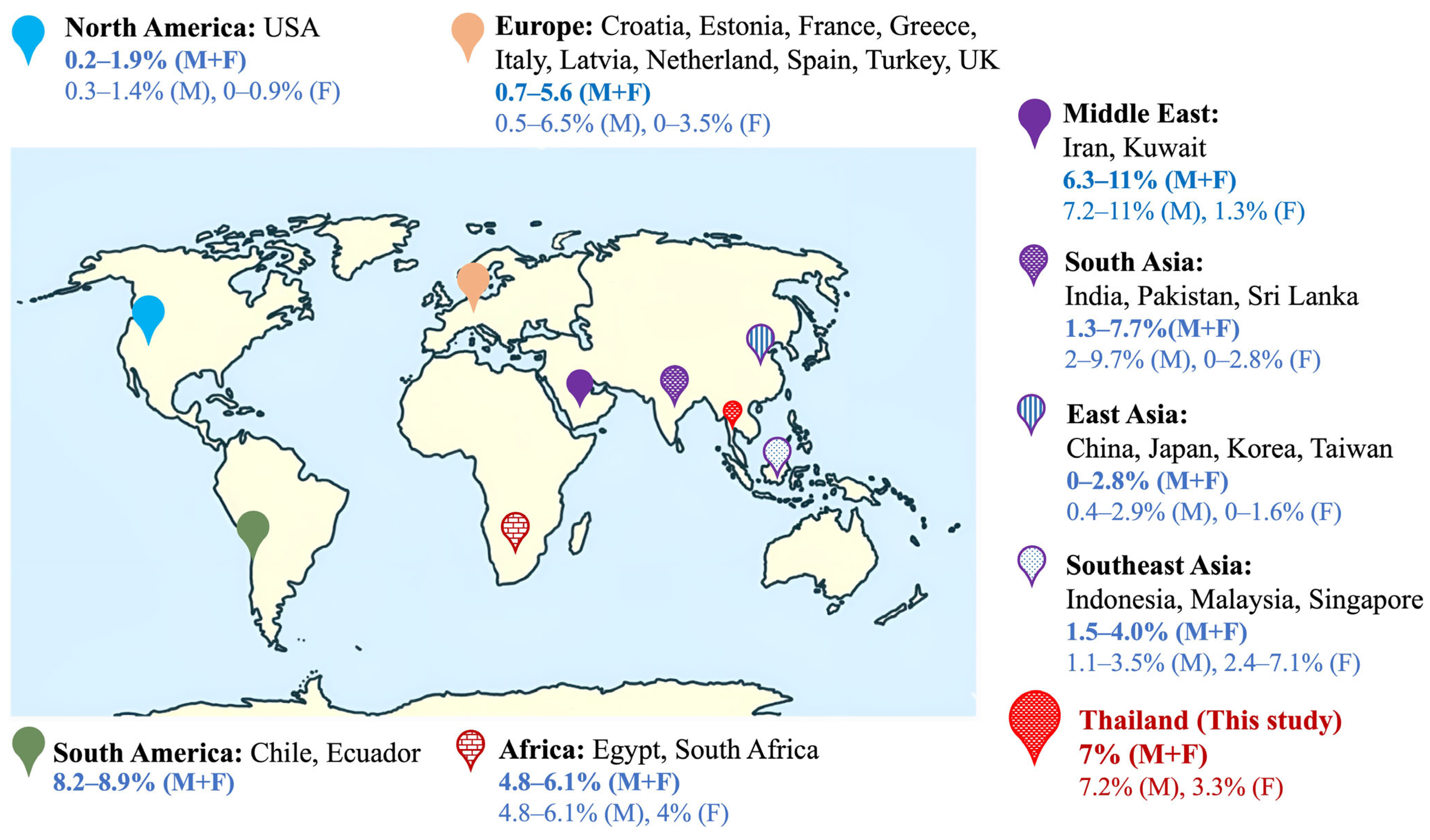
3.1. Frequency of FXS in Males
3.2. Frequency of FXS in Females
3.3. Distribution of CGG Repeats
3.4. Prenatal FXS Diagnosis
3.5. Full Mutation Expansion in Maternal Transmission and AGG Interruptions
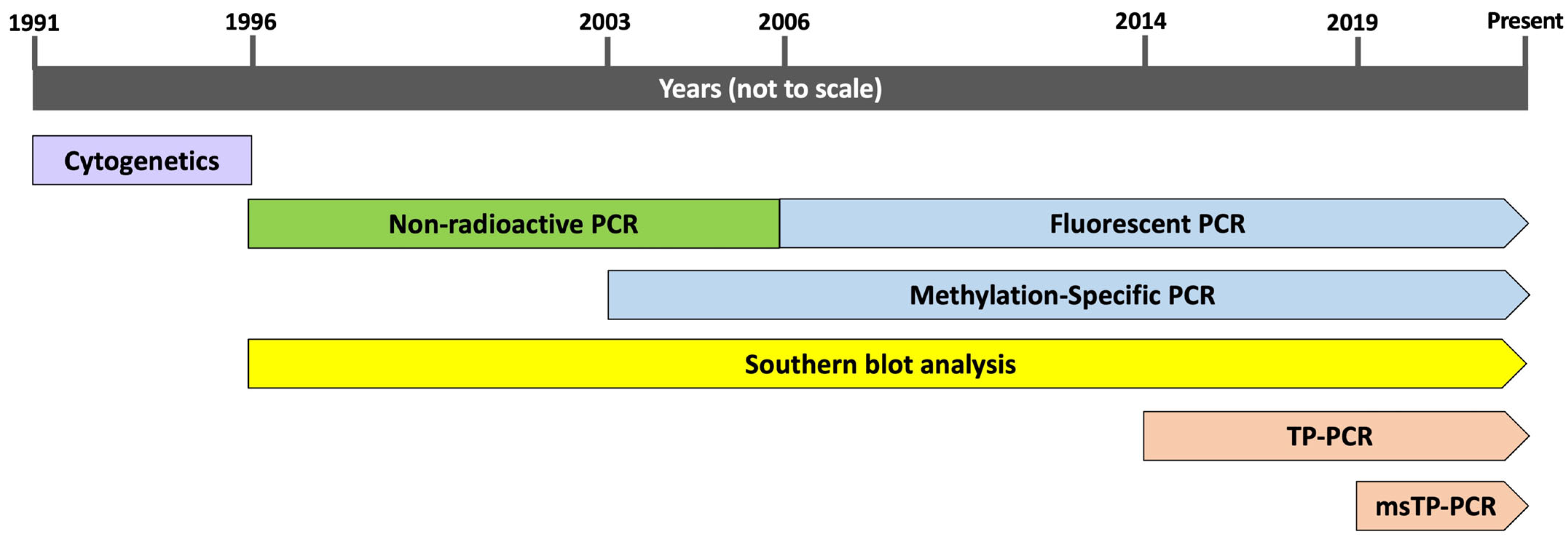
3.6. Molecular Diagnosis of FXS
4. Materials and Methods
4.1. Cohort in This Study
4.2. FXS DNA Analysis
4.2.1. Nonradioactive PCR
4.2.2. MS-PCR and msTP-PCR
4.2.3. Fluorescent PCR
4.2.4. TP-PCR
4.2.5. Southern Blot Analysis
4.3. Data Collection and Search Strategy for Literature Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism spectrum disorder |
| ADHD | Attention deficit hyperactivity disorder |
| C | Child |
| DD | Developmental delay |
| DNA | Deoxyribonucleic acid |
| F | Female |
| FXS | Fragile X syndrome |
| FMR1 | Fragile X messenger ribonucleoprotein 1 |
| FXPOI | Fragile X-associated primary ovarian insufficiency |
| FXTAS | Fragile X tremor/ataxia syndrome |
| FXAND | Fragile X-associated neuropsychiatric disorders |
| FM | Full mutation |
| ID | Intellectual disability |
| IM | Intermediate |
| LD | Learning disabilities |
| M | Male |
| MS-PCR | Methylation-specific PCR |
| msTP-PCR | Methylation-specific triplet-primed PCR |
| NA | Not available |
| P | Fetus with a prenatal diagnosis |
| PCR | Polymerase chain reaction |
| PM | Premutation |
| TP-PCR | Triplet-repeat-primed PCR |
References
- Charalsawadi, C.; Wirojanan, J.; Jaruratanasirikul, S.; Ruangdaraganon, N.; Geater, A.; Limprasert, P. Common Clinical Characteristics and Rare Medical Problems of Fragile X Syndrome in Thai Patients and Review of the Literature. Int. J. Pediatr. 2017, 2017, 9318346. [Google Scholar] [CrossRef]
- Spector, E.; Behlmann, A.; Kronquist, K.; Rose, N.C.; Lyon, E.; Reddi, H.V. ACMG Laboratory Quality Assurance Committee Laboratory Testing for Fragile X, 2021 Revision: A Technical Standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 799–812. [Google Scholar] [CrossRef]
- Hnoonual, A.; Plong-On, O.; Worachotekamjorn, J.; Charalsawadi, C.; Limprasert, P. Clinical and Molecular Characteristics of FMR1 Microdeletion in Patient with Fragile X Syndrome and Review of the Literature. Clin. Chim. Acta 2024, 553, 117728. [Google Scholar] [CrossRef]
- Jiraanont, P.; Manor, E.; Tabatadze, N.; Zafarullah, M.; Mendoza, G.; Melikishvili, G.; Tassone, F. De Novo Large Deletion Leading to Fragile X Syndrome. Front. Genet. 2022, 13, 884424. [Google Scholar] [CrossRef]
- Monaghan, K.G.; Lyon, E.; Spector, E.B. American College of Medical Genetics and Genomics ACMG Standards and Guidelines for Fragile X Testing: A Revision to the Disease-Specific Supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics. Genet. Med. 2013, 15, 575–586. [Google Scholar] [CrossRef]
- Limprasert, P.; Thanakitgosate, J.; Jaruthamsophon, K.; Sripo, T. Unique AGG Interruption in the CGG Repeats of the FMR1 Gene Exclusively Found in Asians Linked to a Specific SNP Haplotype. Genet. Res. Int. 2016, 2016, 8319287. [Google Scholar] [CrossRef]
- Yrigollen, C.M.; Martorell, L.; Durbin-Johnson, B.; Naudo, M.; Genoves, J.; Murgia, A.; Polli, R.; Zhou, L.; Barbouth, D.; Rupchock, A.; et al. AGG Interruptions and Maternal Age Affect FMR1 CGG Repeat Allele Stability during Transmission. J. Neurodev. Disord. 2014, 6, 24. [Google Scholar] [CrossRef]
- Nolin, S.L.; Glicksman, A.; Ersalesi, N.; Dobkin, C.; Brown, W.T.; Cao, R.; Blatt, E.; Sah, S.; Latham, G.J.; Hadd, A.G. Fragile X Full Mutation Expansions Are Inhibited by One or More AGG Interruptions in Premutation Carriers. Genet. Med. 2015, 17, 358–364. [Google Scholar] [CrossRef]
- Tassone, F.; Protic, D.; Allen, E.G.; Archibald, A.D.; Baud, A.; Brown, T.W.; Budimirovic, D.B.; Cohen, J.; Dufour, B.; Eiges, R.; et al. Insight and Recommendations for Fragile X-Premutation-Associated Conditions from the Fifth International Conference on FMR1 Premutation. Cells 2023, 12, 2330. [Google Scholar] [CrossRef]
- Hunter, J.; Rivero-Arias, O.; Angelov, A.; Kim, E.; Fotheringham, I.; Leal, J. Epidemiology of Fragile X Syndrome: A Systematic Review and Meta-Analysis. Am. J. Med. Genet. Part A 2014, 164A, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Owens, K.M.; Dohany, L.; Holland, C.; DaRe, J.; Mann, T.; Settler, C.; Longman, R.E. FMR1 Premutation Frequency in a Large, Ethnically Diverse Population Referred for Carrier Testing. Am. J. Med. Genet. Part A 2018, 176, 1304–1308. [Google Scholar] [CrossRef]
- Ain, Q.; Hwang, Y.H.; Yeung, D.; Panpaprai, P.; Iamurairat, W.; Chutimongkonkul, W.; Trachoo, O.; Tassone, F.; Jiraanont, P. Population-Based FMR1 Carrier Screening among Reproductive Women. J. Assist. Reprod. Genet. 2024, 41, 3237–3243. [Google Scholar] [CrossRef]
- Hnoonual, A.; Kaewfai, S.; Limwongse, C.; Limprasert, P. Prevalence and Implications of Fragile X Premutation Screening in Thailand. Sci. Rep. 2024, 14, 26257. [Google Scholar] [CrossRef]
- Zhong, N.; Ju, W.; Xu, W.; Ye, L.; Shen, Y.; Wu, G.; Chen, S.H.; Jin, R.; Hu, X.F.; Yang, A.; et al. Frequency of the Fragile X Syndrome in Chinese Mentally Retarded Populations Is Similar to That in Caucasians. Am. J. Med. Genet. 1999, 84, 191–194. [Google Scholar] [CrossRef]
- Biancalana, V.; Beldjord, C.; Taillandier, A.; Szpiro-Tapia, S.; Cusin, V.; Gerson, F.; Philippe, C.; Mandel, J.-L. Five Years of Molecular Diagnosis of Fragile X Syndrome (1997–2001): A Collaborative Study Reporting 95% of the Activity in France. Am. J. Med. Genet. Part A 2004, 129A, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Pouya, A.R.; Abedini, S.S.; Mansoorian, N.; Behjati, F.; Nikzat, N.; Mohseni, M.; Nieh, S.E.; Abbasi Moheb, L.; Darvish, H.; Monajemi, G.B.; et al. Fragile X Syndrome Screening of Families with Consanguineous and Non-Consanguineous Parents in the Iranian Population. Eur. J. Med. Genet. 2009, 52, 170–173. [Google Scholar] [CrossRef]
- de Vries, B.B.; van den Ouweland, A.M.; Mohkamsing, S.; Duivenvoorden, H.J.; Mol, E.; Gelsema, K.; van Rijn, M.; Halley, D.J.; Sandkuijl, L.A.; Oostra, B.A.; et al. Screening and Diagnosis for the Fragile X Syndrome among the Mentally Retarded: An Epidemiological and Psychological Survey. Collaborative Fragile X Study Group. Am. J. Hum. Genet. 1997, 61, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Essop, F.B.; Krause, A. Diagnostic, Carrier and Prenatal Genetic Testing for Fragile X Syndrome and Other FMR-1-Related Disorders in Johannesburg, South Africa: A 20-Year Review. S. Afr. Med. J. 2013, 103, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, B.; Wijesundera, S.; Chong, S.S.; Perera, H.N. Prevalence of Fragile X Syndrome among Children Receiving Special Education and Carrier States in First Degree Relatives. Ceylon Med. J. 2017, 62, 92–96. [Google Scholar] [CrossRef]
- Strom, C.M.; Crossley, B.; Redman, J.B.; Buller, A.; Quan, F.; Peng, M.; McGinnis, M.; Fenwick, R.G.; Sun, W. Molecular Testing for Fragile X Syndrome: Lessons Learned from 119,232 Tests Performed in a Clinical Laboratory. Genet. Med. 2007, 9, 46–51. [Google Scholar] [CrossRef]
- Santa María, L.; Aliaga, S.; Faundes, V.; Morales, P.; Pugin, Á.; Curotto, B.; Soto, P.; Peña, M.I.; Salas, I.; Alliende, M.A. FMR1 Gene Mutations in Patients with Fragile X Syndrome and Obligate Carriers: 30 Years of Experience in Chile. Genet. Res. 2016, 98, e11. [Google Scholar] [CrossRef]
- Hantash, F.M.; Goos, D.G.; Tsao, D.; Quan, F.; Buller-Burckle, A.; Peng, M.; Jarvis, M.; Sun, W.; Strom, C.M. Qualitative Assessment of FMR1 (CGG)n Triplet Repeat Status in Normal, Intermediate, Premutation, Full Mutation, and Mosaic Carriers in Both Sexes: Implications for Fragile X Syndrome Carrier and Newborn Screening. Genet. Med. 2010, 12, 162–173. [Google Scholar] [CrossRef]
- Ali, E.Z.; Yakob, Y.; Md Desa, N.; Ishak, T.; Zakaria, Z.; Ngu, L.K.; Keng, W.T. Molecular Analysis of Fragile X Syndrome (FXS) among Malaysian Patients with Developmental Disability. Malays. J. Pathol. 2017, 39, 99–106. [Google Scholar] [PubMed]
- Limprasert, P.; Ruangdaraganon, N.; Sura, T.; Vasiknanonte, P.; Jinorose, U. Molecular Screening for Fragile X Syndrome in Thailand. Southeast Asian J. Trop. Med. Public Health 1999, 30 (Suppl. S2), 114–118. [Google Scholar]
- Hnoonual, A.; Jankittunpaiboon, C.; Limprasert, P. Screening for FMR1 CGG Repeat Expansion in Thai Patients with Autism Spectrum Disorder. BioMed Res. Int. 2021, 2021, 4359308. [Google Scholar] [CrossRef]
- Fazna, A.; Hagerman, R.J. Prevalence of Fragile X Syndrome in South Asia, and Importance of Diagnosis. Med. Rev. 2025, 5, 164–173. [Google Scholar] [CrossRef]
- Bastaki, L.A.; Hegazy, F.; Al-Heneidi, M.M.; Turki, N.; Azab, A.S.; Naguib, K.K. Fragile X Syndrome: A Clinico-Genetic Study of Mentally Retarded Patients in Kuwait. East. Mediterr. Health J. 2004, 10, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.P.; Poon, P.M.; Chen, Q.L.; Lai, K.Y.; Yin, C.H.; Zhao, Z.; Zhong, N.; Lau, C.H.; Lam, S.T.; Wong, C.K.; et al. Trinucleotide CGG Repeat in the FMR1 Gene in Chinese Mentally Retarded Patients. Am. J. Med. Genet. 1999, 84, 179–183. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Xie, H.; Zhou, W.; Wu, Y.; Wang, J.; Qin, J.; Guo, J.; Gu, Q.; Zhang, X.; et al. Fragile X Syndrome Screening in Chinese Children with Unknown Intellectual Developmental Disorder. BMC Pediatr. 2015, 15, 77. [Google Scholar] [CrossRef]
- Hofstee, Y.; Arinami, T.; Hamaguchi, H. Comparison between the Cytogenetic Test for Fragile X and the Molecular Analysis of the FMR-1 Gene in Japanese Mentally Retarded Individuals. Am. J. Med. Genet. 1994, 51, 466–470. [Google Scholar] [CrossRef]
- Nanba, E.; Kohno, Y.; Matsuda, A.; Yano, M.; Sato, C.; Hashimoto, K.; Koeda, T.; Yoshino, K.; Kimura, M.; Maeoka, Y.; et al. Non-Radioactive DNA Diagnosis for the Fragile X Syndrome in Mentally Retarded Japanese Males. Brain Dev. 1995, 17, 317–321. [Google Scholar] [CrossRef]
- Otsuka, S.; Sakamoto, Y.; Siomi, H.; Itakura, M.; Yamamoto, K.; Matumoto, H.; Sasaki, T.; Kato, N.; Nanba, E. Fragile X Carrier Screening and FMR1 Allele Distribution in the Japanese Population. Brain Dev. 2010, 32, 110–114. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, K.S.; Hyun, M.C.; Song, K.E.; Kim, J.K. Molecular Screening for Fragile X Syndrome in Mentally Handicapped Children in Korea. J. Korean Med. Sci. 2001, 16, 271–275. [Google Scholar] [CrossRef]
- Tzeng, C.C.; Lin, S.J.; Chen, Y.J.; Kuo, P.L.; Jong, Y.J.; Tsai, L.P.; Chen, R.M. An Effective Strategy of Using Molecular Testing to Screen Mentally Retarded Individuals for Fragile X Syndrome. Diagn. Mol. Pathol. 2001, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.-H.; Chen, W.-C.; Tzeng, C.-C.; Fang, J.-S.; Chu, S.-Y. Molecular Screening of Fragile X Syndrome in Children with Mental Retardation in Hualien. Tzu Chi Med. J. 2008, 20, 309–313. [Google Scholar] [CrossRef]
- Winarni, T.I.; Utari, A.; Mundhofir, F.E.P.; Tong, T.; Durbin-Johnson, B.; Faradz, S.M.H.; Tassone, F. Identification of Expanded Alleles of the FMR1 Gene among High-Risk Population in Indonesia by Using Blood Spot Screening. Genet. Test. Mol. Biomark. 2012, 16, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Faradz, S.M.; Buckley, M.; Tang, L.P.; Leigh, D.; Holden, J.J. Molecular Screening for Fragile X Syndrome among Indonesian Children with Developmental Disability. Am. J. Med. Genet. 1999, 83, 350–351. [Google Scholar] [CrossRef]
- Fatima, T.; Zaidi, S.A.H.; Sarfraz, N.; Perween, S.; Khurshid, F.; Imtiaz, F. Frequency of FMR1 Gene Mutation and CGG Repeat Polymorphism in Intellectually Disabled Children in Pakistan. Am. J. Med. Genet. Part A 2014, 164A, 1151–1161. [Google Scholar] [CrossRef]
- Tan, B.S.; Law, H.Y.; Zhao, Y.; Yoon, C.S.; Ng, I.S. DNA Testing for Fragile X Syndrome in 255 Males from Special Schools in Singapore. Ann. Acad. Med. Singap. 2000, 29, 207–212. [Google Scholar]
- Tzeng, C.C.; Tzeng, P.Y.; Sun, H.S.; Chen, R.M.; Lin, S.J. Implication of Screening for FMR1 and FMR2 Gene Mutation in Individuals with Nonspecific Mental Retardation in Taiwan. Diagn. Mol. Pathol. 2000, 9, 75–80. [Google Scholar] [CrossRef]
- Crawford, D.C.; Meadows, K.L.; Newman, J.L.; Taft, L.F.; Scott, E.; Leslie, M.; Shubek, L.; Holmgreen, P.; Yeargin-Allsopp, M.; Boyle, C.; et al. Prevalence of the Fragile X Syndrome in African-Americans. Am. J. Med. Genet. 2002, 110, 226–233. [Google Scholar] [CrossRef]
- Reddy, K.S. Cytogenetic Abnormalities and Fragile-X Syndrome in Autism Spectrum Disorder. BMC Med. Genet. 2005, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Choudhary, N.S.; Tassone, F.; Durbin-Johnson, B.; Hansen, R.; Hertz-Picciotto, I.; Pessah, I. Identification of Expanded Alleles of the FMR1 Gene in the CHildhood Autism Risks from Genes and Environment (CHARGE) Study. J. Autism Dev. Disord. 2013, 43, 530–539. [Google Scholar] [CrossRef][Green Version]
- Pandey, U.B.; Phadke, S.; Mittal, B. Molecular Screening of FRAXA and FRAXE in Indian Patients with Unexplained Mental Retardation. Genet. Test. 2002, 6, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Gupta, M.; Thelma, B.K. Expansion Mutation Frequency and CGG/GCC Repeat Polymorphism in FMR1 and FMR2 Genes in an Indian Population. Genet. Epidemiol. 2001, 20, 129–144. [Google Scholar] [CrossRef]
- Kanwal, M.; Alyas, S.; Afzal, M.; Mansoor, A.; Abbasi, R.; Tassone, F.; Malik, S.; Mazhar, K. Molecular Diagnosis of Fragile X Syndrome in Subjects with Intellectual Disability of Unknown Origin: Implications of Its Prevalence in Regional Pakistan. PLoS ONE 2015, 10, e0122213. [Google Scholar] [CrossRef]
- Goldman, A.; Jenkins, T.; Krause, A. Molecular Evidence That Fragile X Syndrome Occurs in the South African Black Population. J. Med. Genet. 1998, 35, 878–879. [Google Scholar] [CrossRef] [PubMed]
- Meguid, N.; Abdel-Raouf, E.; Daedir, A.; Awady, M. Prevalence of Fragile X Syndrome among School-Age Egyptian Males. World J. Pediatr. 2007, 3, 271–275. [Google Scholar]
- Hećimović, S.; Tarnik, I.P.; Barić, I.; Cakarun, Z.; Pavelić, K. Screening for Fragile X Syndrome: Results from a School for Mentally Retarded Children. Acta Paediatr. 2002, 91, 535–539. [Google Scholar] [CrossRef]
- Puusepp, H.; Kahre, T.; Sibul, H.; Soo, V.; Lind, I.; Raukas, E.; Ounap, K. Prevalence of the Fragile X Syndrome among Estonian Mentally Retarded and the Entire Children’s Population. J. Child Neurol. 2008, 23, 1400–1405. [Google Scholar] [CrossRef]
- Gérard, B.; Le Heuzey, M.F.; Brunie, G.; Lewine, P.; Saiag, M.C.; Cacheux, V.; Da Silva, F.; Dugas, M.; Mouren-Simeoni, M.C.; Elion, J.; et al. Systematic Screening for Fragile X Syndrome in a Cohort of 574 Mentally Retarded Children. Ann. Genet. 1997, 40, 139–144. [Google Scholar]
- Sofocleous, C.; Kitsiou, S.; Fryssira, H.; Kolialexi, A.; Kalaitzidaki, M.; Roma, E.; Tsangaris, G.T.; Chistofidou, C.; Metaxotou, C.; Kanavakis, E.; et al. 10 Years’ Experience in Fragile X Testing among Mentally Retarded Individuals in Greece: A Molecular and Epidemiological Approach. In Vivo 2008, 22, 451–455. [Google Scholar] [PubMed]
- Esposito, G.; Ruggiero, R.; Savarese, G.; Savarese, M.; Tremolaterra, M.R.; Salvatore, F.; Carsana, A. A 15-Year Case-Mix Experience for Fragile X Syndrome Molecular Diagnosis and Comparison between Conventional and Alternative Techniques Leading to a Novel Diagnostic Procedure. Clin. Chim. Acta 2013, 417, 85–89. [Google Scholar] [CrossRef]
- Daneberga, Z.; Krūmiņa, Z.; Lāce, B.; Bauze, D.; Lugovska, R. The Fragile X Syndrome: 13 Years of Experience. Proc. Latv. Acad. Sci. Sect. B 2011, 65, 67–72. [Google Scholar] [CrossRef]
- van den Ouweland, A.M.; de Vries, B.B.; Bakker, P.L.; Deelen, W.H.; de Graaff, E.; van Hemel, J.O.; Oostra, B.A.; Niermeijer, M.F.; Halley, D.J. DNA Diagnosis of the Fragile X Syndrome in a Series of 236 Mentally Retarded Subjects and Evidence for a Reversal of Mutation in the FMR-1 Gene. Am. J. Med. Genet. 1994, 51, 482–485. [Google Scholar] [CrossRef]
- Milà, M.; Sànchez, A.; Badenas, C.; Brun, C.; Jiménez, D.; Villa, M.P.; Castellví-Bel, S.; Estivill, X. Screening for FMR1 and FMR2 Mutations in 222 Individuals from Spanish Special Schools: Identification of a Case of FRAXE-Associated Mental Retardation. Hum. Genet. 1997, 100, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.M.; Martínez, F.; Cadroy, A.; Gandía, J.; Casquero, M.; Beneyto, M.; Badía, L.; Prieto, F. Screening for FMR1 Mutations among the Mentally Retarded: Prevalence of the Fragile X Syndrome in Spain. Clin. Gen. 1999, 56, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Tunçbilek, E.; Alikasifoğlu, M.; Boduroğlu, K.; Aktas, D.; Anar, B. Frequency of Fragile X Syndrome among Turkish Patients with Mental Retardation of Unknown Etiology. Am. J. Med. Genet. 1999, 84, 202–203. [Google Scholar] [CrossRef]
- Slaney, S.F.; Wilkie, A.O.; Hirst, M.C.; Charlton, R.; McKinley, M.; Pointon, J.; Christodoulou, Z.; Huson, S.M.; Davies, K.E. DNA Testing for Fragile X Syndrome in Schools for Learning Difficulties. Arch. Dis. Child. 1995, 72, 33–37. [Google Scholar] [CrossRef][Green Version]
- Youings, S.A.; Murray, A.; Dennis, N.; Ennis, S.; Lewis, C.; McKechnie, N.; Pound, M.; Sharrock, A.; Jacobs, P. FRAXA and FRAXE: The Results of a Five Year Survey. J. Med. Genet. 2000, 37, 415–421. [Google Scholar] [CrossRef]
- Chiu, H.-H.; Tseng, Y.-T.; Hsiao, H.-P.; Hsiao, H.-H. The AGG Interruption Pattern within the CGG Repeat of the FMR1 Gene among Taiwanese Population. J. Genet. 2008, 87, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Faradz, S.M.; Pattiiha, M.Z.; Leigh, D.A.; Jenkins, M.; Leggo, J.; Buckley, M.F.; Holden, J.J. Genetic Diversity at the FMR1 Locus in the Indonesian Population. Ann. Hum. Genet. 2000, 64, 329–339. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, K.; Law, H.-Y.; Ng, I.S.L.; Lee, C.G.L.; Chong, S.S. FMR1 CGG Repeat Patterns and Flanking Haplotypes in Three Asian Populations and Their Relationship with Repeat Instability. Ann. Hum. Genet. 2006, 70, 784–796. [Google Scholar] [CrossRef]
- Saha, S.; Karmakar, P.; Chatterjee, C.; Banerjee, D.; Das, S.; Dasgupta, U.B. Fragile X Syndrome in Calcutta, India. Ann. Clin. Biochem. 2001, 38, 264–271. [Google Scholar] [CrossRef]
- Jara, L.; Aspillaga, M.; Avendaño, I.; Obreque, V.; Blanco, R.; Valenzuela, C.Y. Distribution of (CGG)n and FMR-1 Associated Microsatellite Alleles in a Normal Chilean Population. Am. J. Med. Genet. 1998, 75, 277–282. [Google Scholar] [CrossRef]
- Dokić, H.; Barisić, I.; Culić, V.; Lozić, B.; Hećimović, S. Haplotype and AGG Interspersion Analysis of FMR1 Alleles in a Croatian Population: No Founder Effect Detected in Patients with Fragile X Syndrome. Hum. Biol. 2008, 80, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.P.; Mingroni-Netto, R.C.; Vianna-Morgante, A.M. Structure and Stability upon Maternal Transmission of Common and Intermediate FMR1 (Fragile X Mental Retardation 1) Alleles in a Sample of the Brazilian Population. Genet. Mol. Biol. 2005, 28, 10–15. [Google Scholar] [CrossRef]
- Pastore, L.M.; Manichaikul, A.; Wang, X.Q.; Finkelstein, J.S. FMR1 CGG Repeats: Reference Levels and Race-Ethnic Variation in Women with Normal Fertility (Study of Women’s Health Across the Nation). Reprod. Sci. 2016, 23, 1225–1233. [Google Scholar] [CrossRef]
- Xunclà, M.; Badenas, C.; Domínguez, M.; Rodríguez-Revenga, L.; Madrigal, I.; Jiménez, L.; Soler, A.; Borrell, A.; Sánchez, A.; Milà, M. Fragile X Syndrome Prenatal Diagnosis: Parental Attitudes and Reproductive Responses. Reprod. Biomed. Online 2010, 21, 560–565. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Huang, W.; Luo, S.; Lin, Y.; Duan, R. Attitude of Medical School Students in China towards Genetic Testing and Counseling Issues in FXS. J. Genet. Couns. 2013, 22, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Fanos, J.H.; Spangner, K.A.; Musci, T.J. Attitudes toward Prenatal Screening and Testing for Fragile X. Genet. Med. 2006, 8, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Nolin, S.L.; Sah, S.; Glicksman, A.; Sherman, S.L.; Allen, E.; Berry-Kravis, E.; Tassone, F.; Yrigollen, C.; Cronister, A.; Jodah, M.; et al. Fragile X AGG Analysis Provides New Risk Predictions for 45-69 Repeat Alleles. Am. J. Med. Genet. Part A 2013, 161A, 771–778. [Google Scholar] [CrossRef]
- Yrigollen, C.M.; Durbin-Johnson, B.; Gane, L.; Nelson, D.L.; Hagerman, R.; Hagerman, P.J.; Tassone, F. AGG Interruptions within the Maternal FMR1 Gene Reduce the Risk of Offspring with Fragile X Syndrome. Genet. Med. 2012, 14, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Owens, K.M.; Terhaar, C.; Zdrodowski, J.; Johnson, L.R.; Eveleigh, D. Refining Reproductive Risk for FMR1 Premutation Carriers in the General Obstetric Population. Am. J. Med. Genet. Part A 2022, 188, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Nolin, S.L.; Glicksman, A.; Tortora, N.; Allen, E.; Macpherson, J.; Mila, M.; Vianna-Morgante, A.M.; Sherman, S.L.; Dobkin, C.; Latham, G.J.; et al. Expansions and Contractions of the FMR1 CGG Repeat in 5,508 Transmissions of Normal, Intermediate, and Premutation Alleles. Am. J. Med. Genet. Part A 2019, 179, 1148–1156. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Committee Opinion No. 691: Carrier Screening for Genetic Conditions. Obstet. Gynecol. 2017, 129, e41–e55. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Jang, D.-H. Combining Chromosomal Microarray and Clinical Exome Sequencing for Genetic Diagnosis of Intellectual Disability. Sci. Rep. 2023, 13, 22807. [Google Scholar] [CrossRef]
- Manickam, K.; McClain, M.R.; Demmer, L.A.; Biswas, S.; Kearney, H.M.; Malinowski, J.; Massingham, L.J.; Miller, D.; Yu, T.W.; Hisama, F.M.; et al. Exome and Genome Sequencing for Pediatric Patients with Congenital Anomalies or Intellectual Disability: An Evidence-Based Clinical Guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 2029–2037. [Google Scholar] [CrossRef]
- Lyon, E.; Laver, T.; Yu, P.; Jama, M.; Young, K.; Zoccoli, M.; Marlowe, N. A Simple, High-Throughput Assay for Fragile X Expanded Alleles Using Triple Repeat Primed PCR and Capillary Electrophoresis. J. Mol. Diagn. 2010, 12, 505–511. [Google Scholar] [CrossRef]
- Rajan-Babu, I.-S.; Lian, M.; Chong, S.S. Triplet-Primed PCR Assays for Accurate Screening of FMR1 CGG Repeat Expansion and Genotype Verification. Curr. Protoc. 2022, 2, e427. [Google Scholar] [CrossRef]
- Rajan-Babu, I.-S.; Phang, G.-P.; Law, H.-Y.; Lee, C.G.; Chong, S.S. High-Throughput Methylation-Specific Triplet-Primed PCR and Melting Curve Analysis for Selective and Reliable Identification of Actionable FMR1 Genotypes. J. Mol. Diagn. 2022, 24, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Mao, A.; Shan, S.; Li, Y.; Meng, W.; Zhan, J.; Nie, W.; Jin, H. Evaluating the Clinical Utility of a Long-Read Sequencing-Based Approach in Genetic Testing of Fragile-X Syndrome. Clin. Chim. Acta 2023, 551, 117614. [Google Scholar] [CrossRef] [PubMed]
- Jinorose, U.; Vasiknanonte, P.; Limprasert, P.; Brown, W.T.; Panich, V. The Frequency of Fragile X Syndrome among Selected Patients at Songklanagarind Hospital during 1991–1996, Studied by Cytogenetic and Molecular Methods. Southeast Asian J. Trop. Med. Public Health 1997, 28 (Suppl. S3), 69–74. [Google Scholar]
- Weinhäusel, A.; Haas, O.A. Evaluation of the Fragile X (FRAXA) Syndrome with Methylation-Sensitive PCR. Hum. Genet. 2001, 108, 450–458. [Google Scholar] [CrossRef]
- Charalsawadi, C.; Sripo, T.; Limprasert, P. Multiplex Methylation Specific PCR Analysis of Fragile X Syndrome: Experience in Songklanagarind Hospital. J. Med. Assoc. Thai. 2005, 88, 1057–1061. [Google Scholar]
- Rajan-Babu, I.-S.; Teo, C.R.L.; Lian, M.; Lee, C.G.; Law, H.-Y.; Chong, S.S. Single-Tube Methylation-Specific Duplex-PCR Assay for Rapid and Accurate Diagnosis of Fragile X Mental Retardation 1-Related Disorders. Expert Rev. Mol. Diagn. 2015, 15, 431–441. [Google Scholar] [CrossRef]
- Brown, W.T.; Houck, G.E.; Jeziorowska, A.; Levinson, F.N.; Ding, X.; Dobkin, C.; Zhong, N.; Henderson, J.; Brooks, S.S.; Jenkins, E.C. Rapid Fragile X Carrier Screening and Prenatal Diagnosis Using a Nonradioactive PCR Test. JAMA 1993, 270, 1569–1575. [Google Scholar] [CrossRef]
- Tassone, F.; Pan, R.; Amiri, K.; Taylor, A.K.; Hagerman, P.J. A Rapid Polymerase Chain Reaction-Based Screening Method for Identification of All Expanded Alleles of the Fragile X (FMR1) Gene in Newborn and High-Risk Populations. J. Mol. Diagn. 2008, 10, 43–49. [Google Scholar] [CrossRef]
- Pozo-Palacios, J.; Llamos-Paneque, A.; Rivas, C.; Onofre, E.; López-Cáceres, A.; Villareal, J. Experiences of the Molecular Diagnosis of Fragile X Syndrome in Ecuador. Front. Psychiatry 2021, 12, 716311. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.J.; Holden, J.J.A.; Zhang, C.; Curlis, Y.; Slater, H.R.; Burgess, T.; Kirkby, K.C.; Carmichael, A.; Heading, K.D.; Loesch, D.Z. FMR1 Alleles in Tasmania: A Screening Study of the Special Educational Needs Population. Clin. Genet. 2005, 67, 38–46. [Google Scholar] [CrossRef]
| Cohort Characteristic | Setting/Region of Thailand | Period of Sample Collection | Source | Total Cases a | No. of Cases with FM (%) | No. of Cases with IM (%) | No. of Cases with NL (%) |
|---|---|---|---|---|---|---|---|
| Males with ID | Pediatric clinic/Southern Thailand | 1991–1999 | Limprasert et al., 1999 [24] and unpublished data | Male: 132 | Male: 9 (6.8%) | Male: 0 | Male: 123 (93.2%) |
| Males with ID | Pediatric clinic/Central Thailand | 1997–1999 | Limprasert et al., 1999 [24] and unpublished data | Male: 161 | Male: 12 (7.5%) | Male: 0 | Male: 149 (92.5%) |
| Males with ID | Child psychiatric clinic/Northern Thailand | 2007–2008 | Present study | Male: 101 | Male: 8 (7.9%) | Male: 0 | Male: 93 (92.1%) |
| Children in routine FXS testing | Songklanagarind Hospital/All regions of Thailand | 2000–2021 | Present study | Male: 996 Female: 90 | Male: 71 (7.1%) Female: 3 (3.3%) | Male: 1 (0.1%) Female: 0 (0%) | Male: 921 (92.5%) b Female: 87 (96.7%) |
| Total | Male: 1390 Female: 90 Male + Female: 1480 | Male: 100 (7.2%) Female: 3 (3.3%) Male + Female: 103 (7.0%) | Male: 1 (0.07%) Female: 0 (0%) Male + Female: 1 (0.07%) | Male: 1286 (92.5%) Female: 87 (96.7%) Male + Female: 1373 (92.8%) | |||
| Family ID of Prenatal Testing | Maternal Alleles | Paternal Allele | FMR1 CGG Repeats of the Child (C) Alleles and Fetus (P) Alleles |
|---|---|---|---|
| RM10 family | 29,PM (80) | 29 | C1 (M): FM |
| C2 (M): mosaic PM (130) and FM | |||
| P1 (M): 29 | |||
| F4 family | NA | PM (~75) | P1 (M): 29 |
| P2 (F): 28, PM (~96–130) | |||
| F10-2 family | 29,PM (75,100) | 30 | C1 (M): FM |
| P1 (F): 29,30 | |||
| F10-6 family | 29,PM (85) | 36 | C1 (M): FM |
| P1 (M): 29 | |||
| F12-2 family | 37,PM (~140)/FM | 29 | C1 (M): mosaic PM (~101)/313 bp deletion [3] |
| C2 (M): FM | |||
| P1 (M): 37 | |||
| F12-6 family | 29,PM (113,167) | 29 | P1 (F): 29,FM |
| P2: mosaic FM as female pattern (47,XXY) | |||
| P3 (F): 29,FM | |||
| F20 family | 30,FM | NA | C1 (F): 29,FM |
| P1 (M): FM | |||
| F31 family | 32,PM (108) | 30 | C1 (M): FM |
| P1 (M): FM | |||
| F33 family | 29,PM (104) | 29 | C1 (M): FM |
| P1 (F): 29,29 | |||
| P2 (M-twinA): 29 | |||
| P3 (M-twinB): 29 | |||
| F34 family | 30,PM (82) | 29 | C1 (M): FM |
| P1 (M): 30 | |||
| F2 (M): FM | |||
| F35 family | 29,PM (100) | 29 | C1 (M): FM |
| C2 (F): 29, ~200 | |||
| P1 (F): 29,29 | |||
| P2 (F): 29,29 | |||
| F37 family | 23,PM (86) | 32 | C1 (M): FM |
| P1 (F): 23,32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hnoonual, A.; Plong-On, O.; Tangviriyapaiboon, D.; Charalsawadi, C.; Limprasert, P. A 30-Year Experience in Fragile X Syndrome Molecular Diagnosis from a Laboratory in Thailand. Int. J. Mol. Sci. 2025, 26, 7418. https://doi.org/10.3390/ijms26157418
Hnoonual A, Plong-On O, Tangviriyapaiboon D, Charalsawadi C, Limprasert P. A 30-Year Experience in Fragile X Syndrome Molecular Diagnosis from a Laboratory in Thailand. International Journal of Molecular Sciences. 2025; 26(15):7418. https://doi.org/10.3390/ijms26157418
Chicago/Turabian StyleHnoonual, Areerat, Oradawan Plong-On, Duangkamol Tangviriyapaiboon, Chariyawan Charalsawadi, and Pornprot Limprasert. 2025. "A 30-Year Experience in Fragile X Syndrome Molecular Diagnosis from a Laboratory in Thailand" International Journal of Molecular Sciences 26, no. 15: 7418. https://doi.org/10.3390/ijms26157418
APA StyleHnoonual, A., Plong-On, O., Tangviriyapaiboon, D., Charalsawadi, C., & Limprasert, P. (2025). A 30-Year Experience in Fragile X Syndrome Molecular Diagnosis from a Laboratory in Thailand. International Journal of Molecular Sciences, 26(15), 7418. https://doi.org/10.3390/ijms26157418







