The Characterization of the Neuroimmune Response in Primary Pterygia
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Subjects
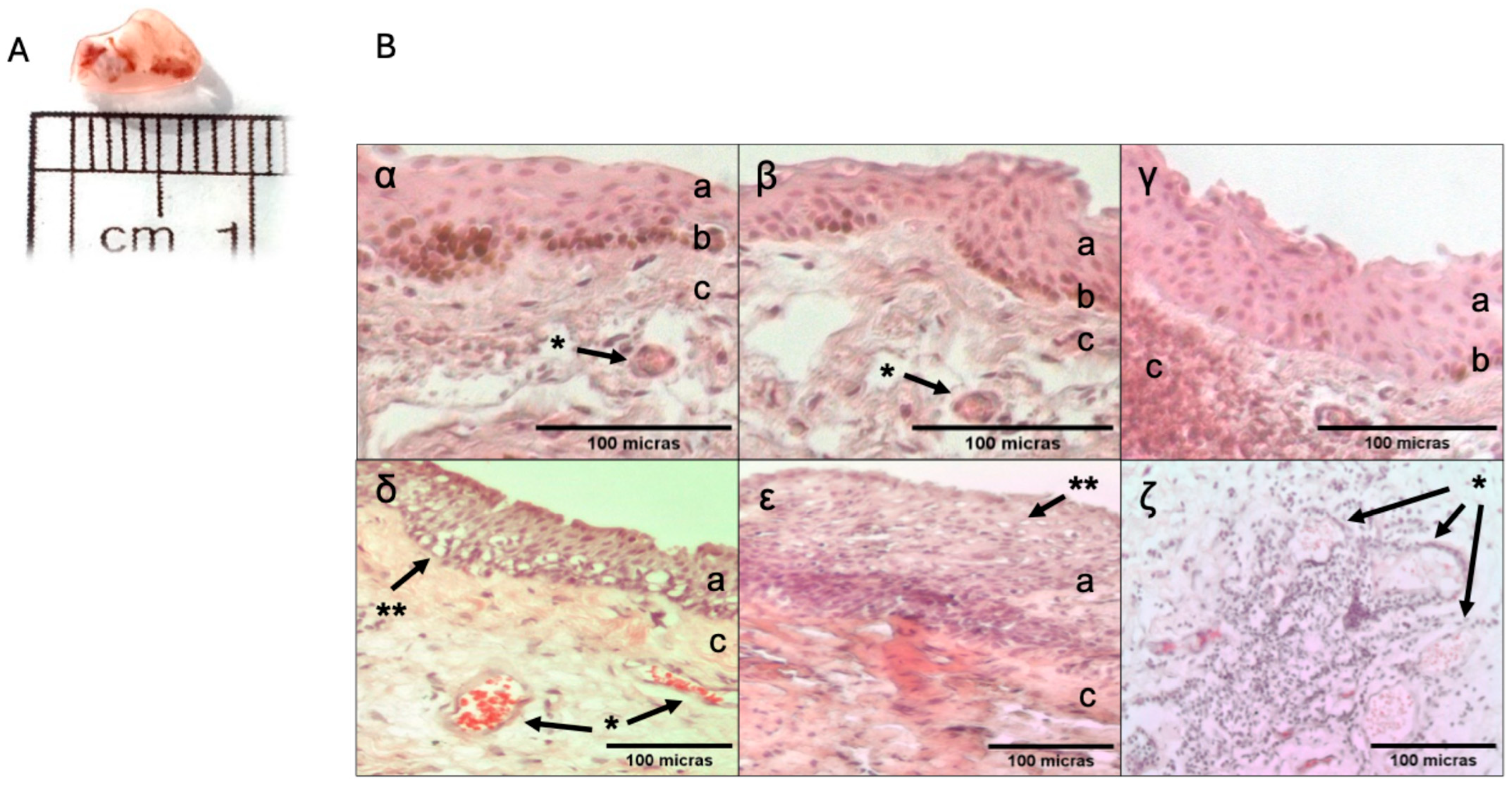
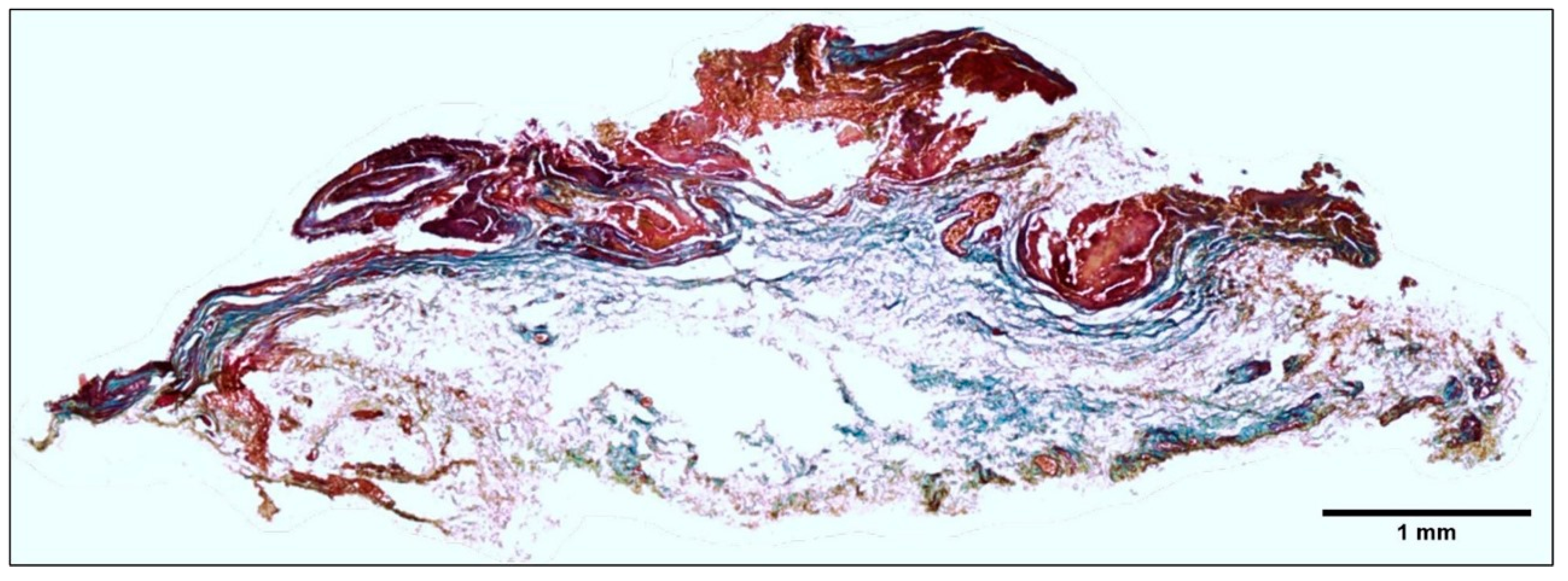
2.2. Histopathology Description of the Tissue
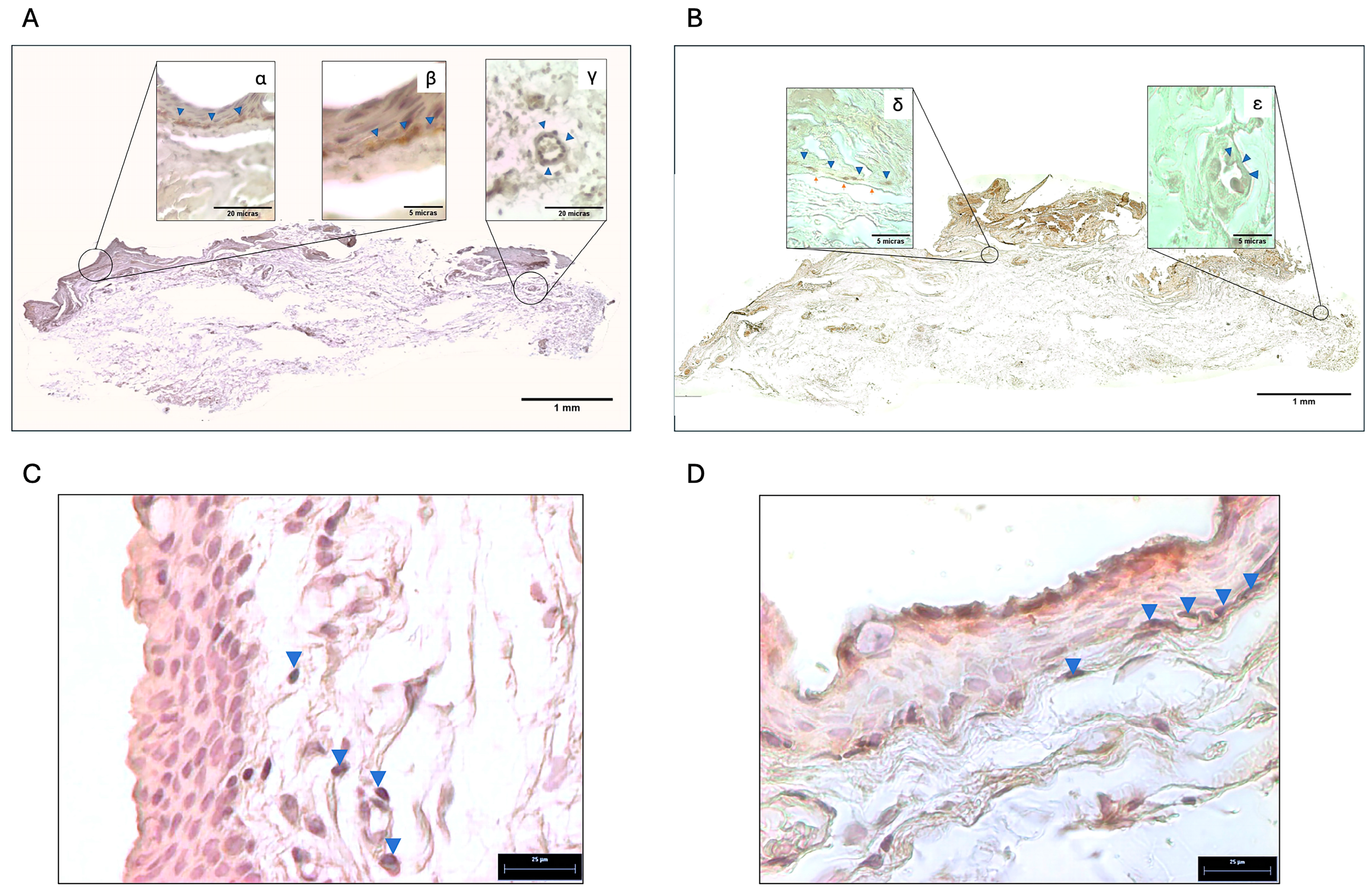
2.3. Localization of Tyrosine Hydroxylase and Adrenergic Receptors in the Pterygium
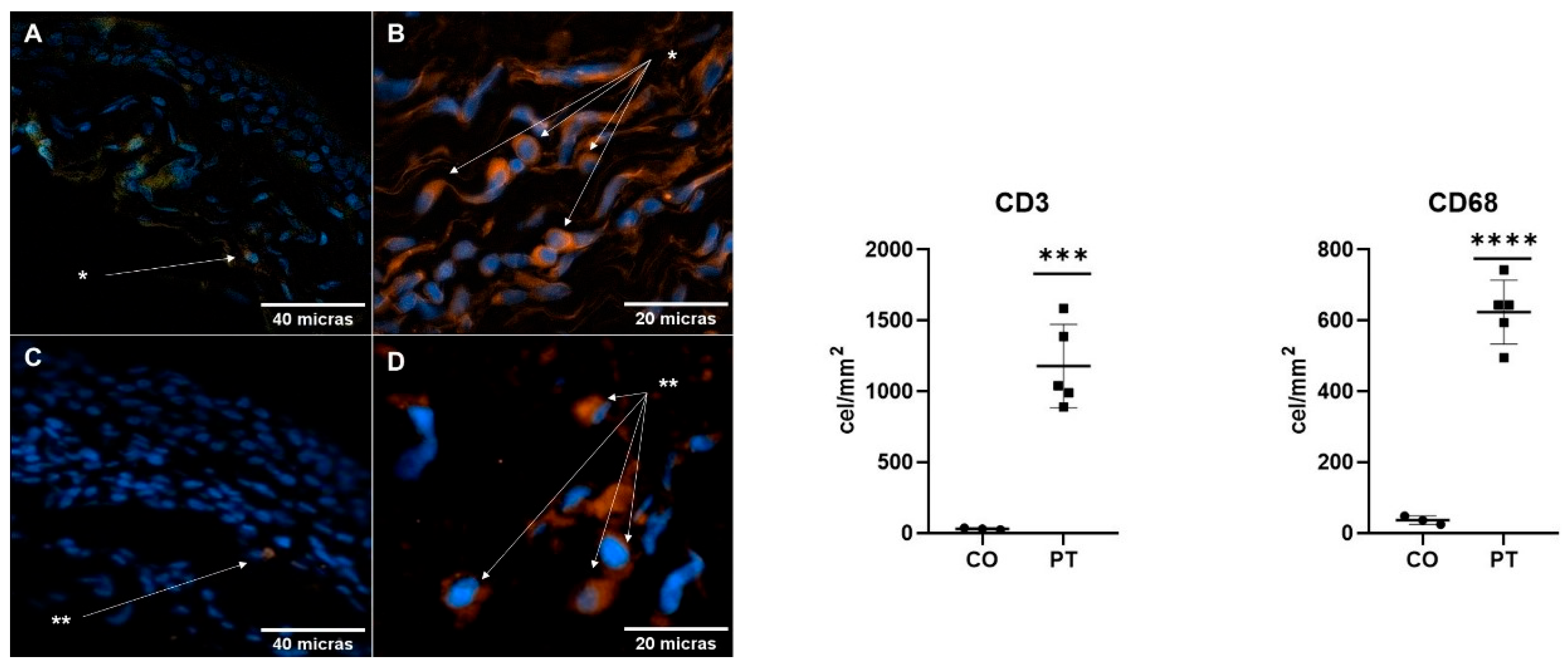
2.4. Immune Response in the Pterygium and Conjunctiva
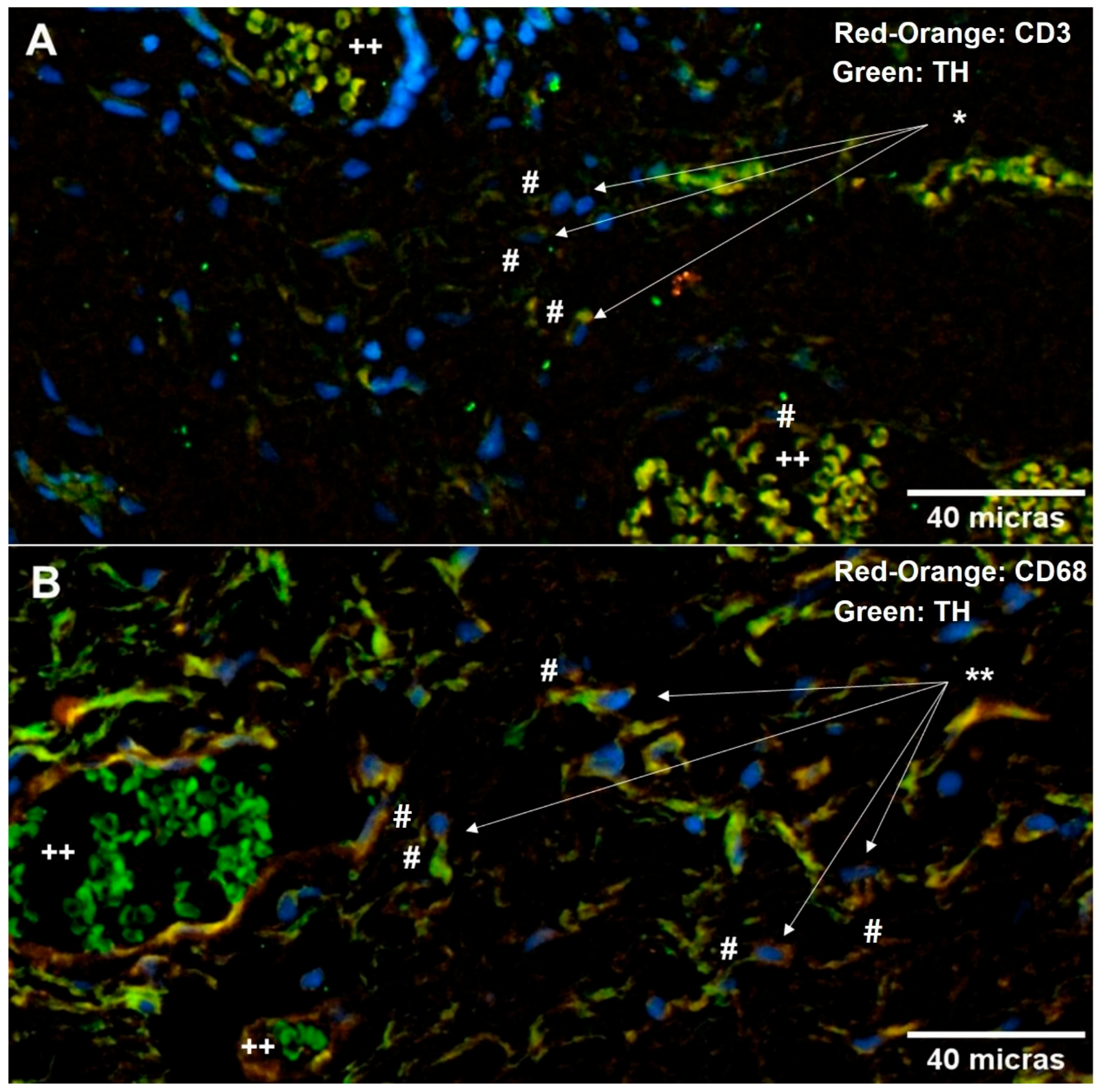
2.5. Neuroimmune Relationship in the Pterygium
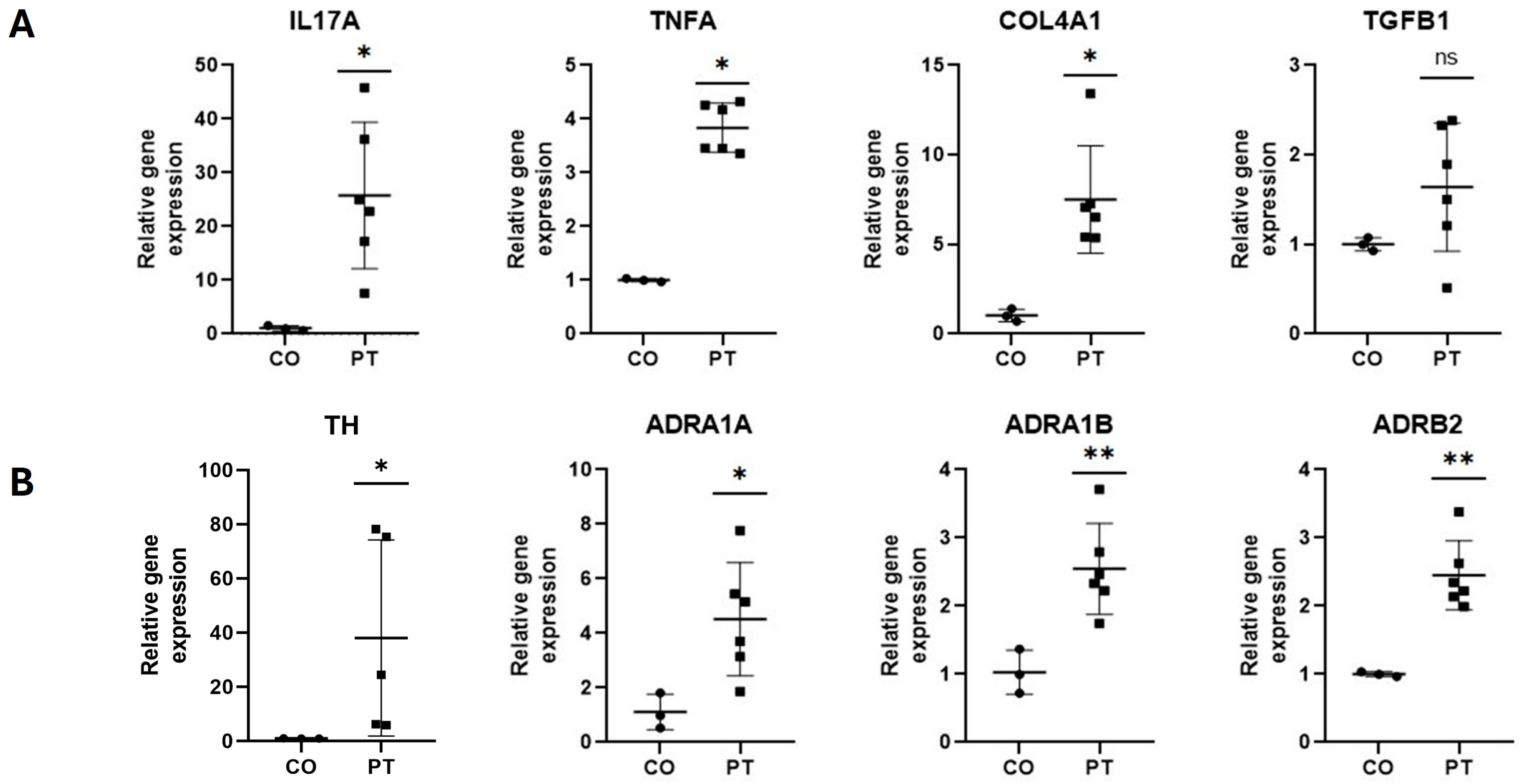
2.6. Inflammatory, Fibrogenic, and Adrenergic Markers’ Gene Expression
3. Discussion
Limitations
4. Material and Methods
4.1. Patients
4.2. Sample Selection
4.3. Histopathological Analysis
4.3.1. Histological Sections
4.3.2. Morphological Analysis
4.3.3. Immunohistochemistry
4.3.4. Immunofluorescence
4.3.5. Immunofluorescence Double Labeling
4.3.6. Image Analysis
4.4. Gene Expression Analysis
4.5. Statistical Analysis
4.6. Use of Artificial Intelligence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chui, J.; Coroneo, M.T.; Tat, L.T.; Crouch, R.; Wakefield, D.; Di Girolamo, N. Ophthalmic pterygium: A stem cell disorder with premalignant features. Am. J. Pathol. 2011, 178, 817–827. [Google Scholar] [CrossRef]
- Domdey, M.; Kluth, M.A.; Maßlo, C.; Ganss, C.; Frank, M.H.; Frank, N.Y.; Coroneo, M.; Cursiefen, C.; Notara, M. Consecutive dosing of UVB irradiation induces loss of ABCB5 expression and activation of EMT and fibrosis proteins in limbal epithelial cells similar to pterygium epithelium. Stem. Cell Res. 2022, 64, 102936. [Google Scholar] [CrossRef] [PubMed]
- Malozhen, S.A.; Trufanov, S.V.; Krakhmaleva, D.A. Pterygium: Etiology, pathogenesis, treatment. Vestn. Oftalmol. 2017, 133, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Dushku, N.; John, M.K.; Schultz, G.S.; Reid, T.W. Pterygia pathogenesis: Corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch. Ophthalmol. 2001, 119, 695–706. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The Sympathetic Nerve—An Integrative Interface between Two supersystems: The brain and the Immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix. Biol. 2018, 68–69, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis Cell Signaling Technology. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Rassler, B. Role of α- and β-adrenergic mechanisms in the pathogenesis of pulmonary injuries characterized by edema, inflammation and fibrosis. Cardiovasc. Hematol. Disord. Drug Targets. 2013, 13, 197–207. [Google Scholar] [CrossRef]
- Monroe, T.B.; Anderson, E.J. A Catecholaldehyde metabolite of norepinephrine induces myofibroblast activation and toxicity via the re-ceptor for advanced glycation endproducts: Mitigating role of l-carnosine. Chem. Res. Toxicol. 2021, 34, 2194–2201. [Google Scholar] [CrossRef]
- Uehara, A.; Motegi, S.; Yamada, K.; Uchiyama, A.; Perera, B.; Toki, S.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Ishikawa, O. Mechanistic insight into the norepinephrine-induced fibrosis in systemic sclerosis. Sci. Rep. 2016, 6, 34012. [Google Scholar] [CrossRef]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef]
- Ratnakar, K.S.; Goswamy, V.; Agarwal, L.P. Mast cells and pterygium. Acta Ophthalmol. 1976, 54, 363–368. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Chui, J.; Coroneo, M.T.; Wakefield, D. Pathogenesis of pterygia: Role of cytokines, growth factors, and matrix metalloproteinases. Prog. Retin. Eye Res. 2004, 23, 195–228. [Google Scholar] [CrossRef]
- Oben, J.A.; Diehl, A.M. Sympathetic nervous system regulation of liver repair. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2004, 280, 874–883. [Google Scholar] [CrossRef]
- Neuhuber, W.L.; Tiegs, G. Innervation of immune cells: Evidence for neuroimmunomodulation in the liver. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2004, 280, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- He, J.; Bazan, N.G.; Bazan, H.E.P. Mapping the entire human corneal nerve architecture. Exp. Eye Res. 2010, 91, 513–523. [Google Scholar] [CrossRef]
- Tortora, G.; Derrickson, B. Principios de Anatomía y Fisiología. 15°; Editorial Médica Panamericana: Madrid, Spain, 2018; pp. 403–445. [Google Scholar]
- Fernández, A.; Moreno, J.; Prósper, F.; García, M.; Echeveste, J.; Fernández, A. Regeneración de la superficie ocular: Stem cells/células madre y técnicas reconstructivas. Sist. Sanit. Navar. 2008, 31, 53–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299. [Google Scholar]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Nance, D.M.; Sanders, V.M. Autonomic innervation and regulation of the immune system. Brain Behav. Immun. 2007, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542, Erratum in Exp. Eye Res. 2003, 77, 253. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic Control of the Eye. Compr. Physiol. 2015, 5, 439. [Google Scholar] [CrossRef]
- Al-Aqaba, M.A.; Anis, F.S.; Mohammed, I.; Dua, H.S. Nerve terminals at the human corneoscleral limbus. Br. J. Ophthalmol. 2018, 102, 556–561. [Google Scholar] [CrossRef]
- Lawrenson, J.G.; Ruskell, G.L. The structure of corpuscular nerve endings in the limbal conjunctiva of the human eye. J. Anat. 1991, 177, 75–84. [Google Scholar]
- Lin, X.-H.; Liu, H.-H.; Hsu, S.-J.; Zhang, R.; Chen, J.; Chen, J.; Gao, D.-M.; Cui, J.-F.; Ren, Z.-G.; Chen, R.-X. Norepinephrine-stimulated HSCs secrete sFRP1 to promote HCC progression following chronic stress via augmentation of a Wnt16B/β-catenin positive feedback loop. J. Exp. Clin. Cancer Res. 2020, 39, 1–17. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb-prot 4986. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zuo, S.; Hou, Y.; Shang, W.; Liu, N.; Yin, Z. Inhibition of α1-adrenoceptor reduces TGF-β1-induced.epithelial-to-mesenchymal transition and attenuates UUO-induced renal fibrosis in mice. FASEB J. 2020, 34, 14892–14904. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Sequence (5′-3′) | Target Gene |
|---|---|---|
| Forward | 5′-ACC AAT CCC AAA AGG TCC TC-3′ | IL-17 |
| Reverse | 5′-GGG GAC AGA GTT CAT GTG GT-3′ | |
| Forward | 5′-CTC TTC TGC CTG CTG CAC TTT G-3′ | TNF-α |
| Reverse | 5′-ATG GGC TAC AGG CTT GTC ACT C-3′ | |
| Forward | 5′-CCC AGC ATC TGC AAA GCT C-3′ | TGFB |
| Reverse | 5′-GTC AAT GTA CAG CTG CCG CA-3′ | |
| Forward | 5′-CTG GTC CAA GAG GAT TTC CA-3′ | COL4A1 |
| Reverse | 5′-TTT TGG TCC CAG AAG GAC AC-3′ | |
| Forward | 5′-GTG TTC CAG TGC ACC CAG TA-3′ | TH |
| Reverse | 5′-AGC GTG GAC AGC TTC TCA AT-3′ | |
| Forward | 5′-TGG CCG ACC TCC TGC TCA CCT C-3′ | ADRA1A |
| Reverse | 5′-GGC CCC GGC TCT CCC TCT TG-3′ | |
| Forward | 5′-CCC CCG ACG CCG TGT TCA AGG TG-3′ | ADRA1B |
| Reverse | 5′-CTG AGG CGC GGG CAG GCT CAG GA-3′ | |
| Forward | 5′-GAT TTC AGG ATT GCC TTC CA-3′ | ADRB2 |
| Reverse | 5′-AAA TAA TGT ACG GGG GAG ATG CAT GA-3′ | |
| Forward | 5′-GAG TCA ACG GAT TTG GTC GT-3′ | GAPDH |
| Reverse | 5′-GAC AAG CTT CCC GTT CTC AG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba-Gallardo, L.F.; Ocón-Garcia, S.G.; Avila-Blanco, M.E.; Diaz-Rubio, J.L.; Ventura-Juárez, J.; Casillas-Casillas, E.; Muñoz-Ortega, M.H. The Characterization of the Neuroimmune Response in Primary Pterygia. Int. J. Mol. Sci. 2025, 26, 7417. https://doi.org/10.3390/ijms26157417
Barba-Gallardo LF, Ocón-Garcia SG, Avila-Blanco ME, Diaz-Rubio JL, Ventura-Juárez J, Casillas-Casillas E, Muñoz-Ortega MH. The Characterization of the Neuroimmune Response in Primary Pterygia. International Journal of Molecular Sciences. 2025; 26(15):7417. https://doi.org/10.3390/ijms26157417
Chicago/Turabian StyleBarba-Gallardo, Luis Fernando, Sofía Guadalupe Ocón-Garcia, Manuel Enrique Avila-Blanco, José Luis Diaz-Rubio, Javier Ventura-Juárez, Elizabeth Casillas-Casillas, and Martín Humberto Muñoz-Ortega. 2025. "The Characterization of the Neuroimmune Response in Primary Pterygia" International Journal of Molecular Sciences 26, no. 15: 7417. https://doi.org/10.3390/ijms26157417
APA StyleBarba-Gallardo, L. F., Ocón-Garcia, S. G., Avila-Blanco, M. E., Diaz-Rubio, J. L., Ventura-Juárez, J., Casillas-Casillas, E., & Muñoz-Ortega, M. H. (2025). The Characterization of the Neuroimmune Response in Primary Pterygia. International Journal of Molecular Sciences, 26(15), 7417. https://doi.org/10.3390/ijms26157417







