Research Advances in Multiple Embryos and Apomixis in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Multiple Embryos in Rice
2.1. Origins and Classification of Multiple Embryos
2.2. Frequency and Influencing Factors of Multiple Embryos
2.2.1. Frequency of Multiple Embryos
2.2.2. Influencing Factors of Multiple Embryos
2.3. Genetic Mechanisms of Multiple Embryos
2.4. Genes of Multiple Embryos in Rice
3. Overview of Apomixis in Rice
3.1. Classification of Apomixis in Rice
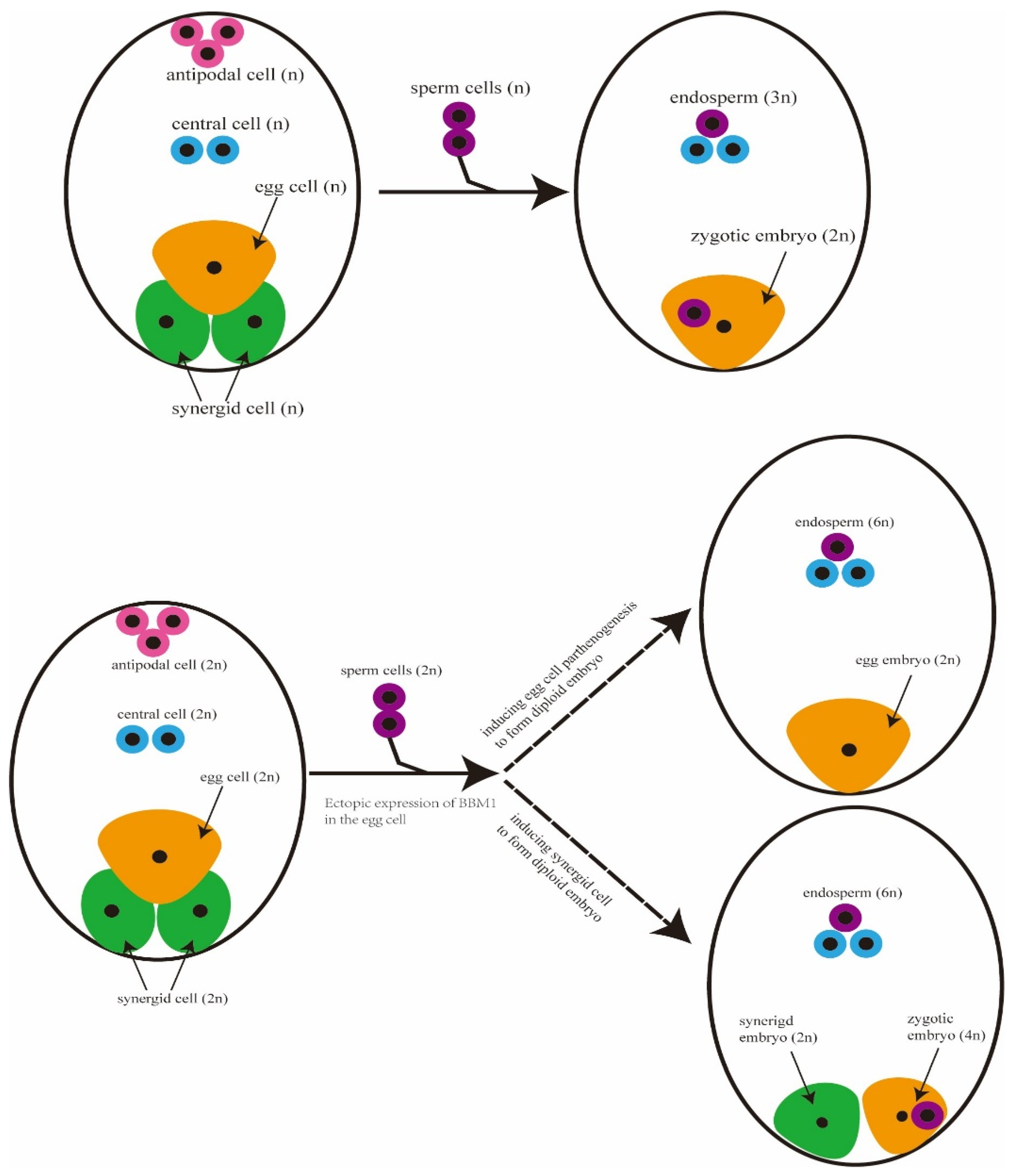
3.2. The Process of Apomixis in Rice
3.3. Advances of Synthetic Apomixis in Rice
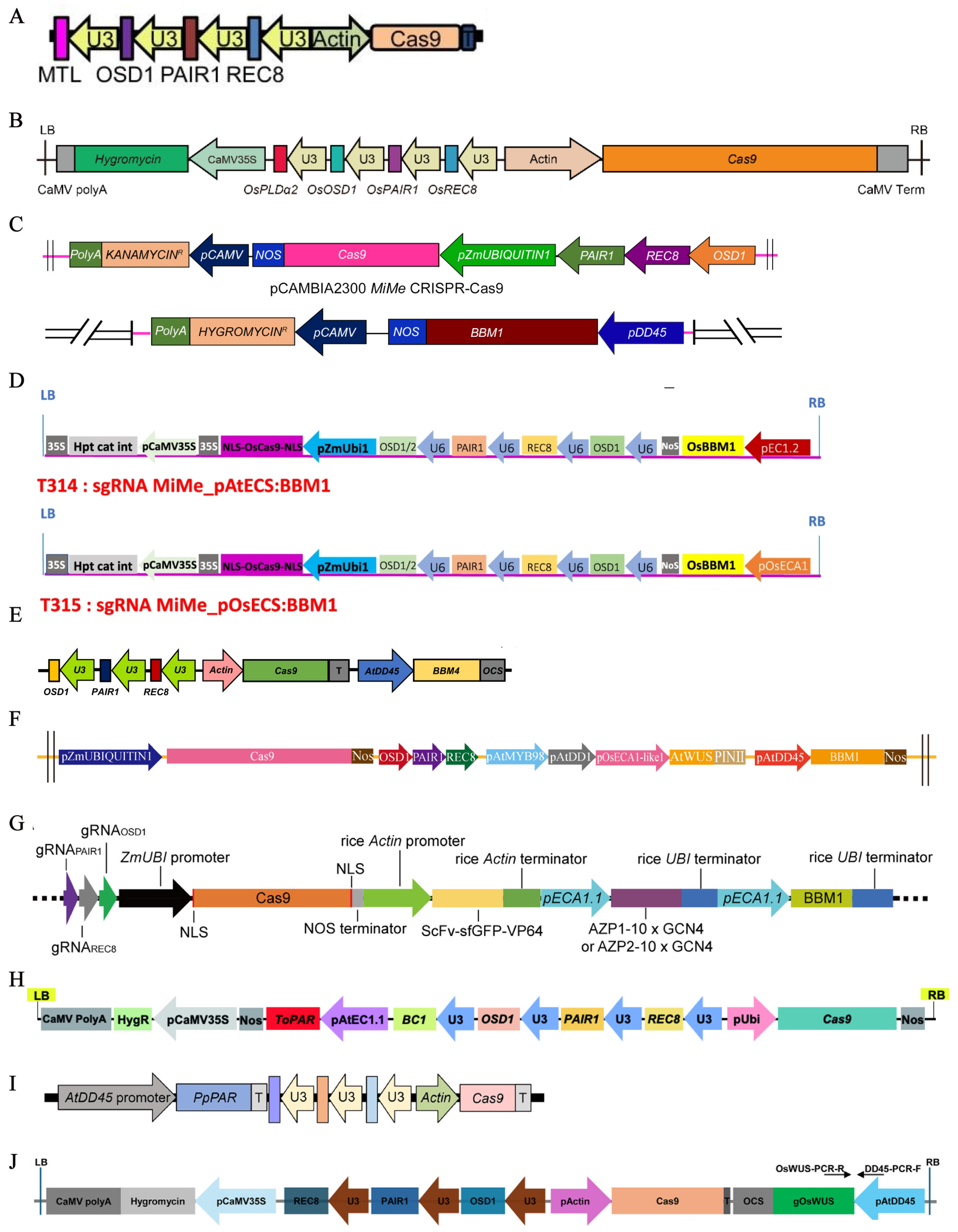
3.3.1. Generation of MiMe Mutants
3.3.2. Establishment of a Synthetic Apomixis System
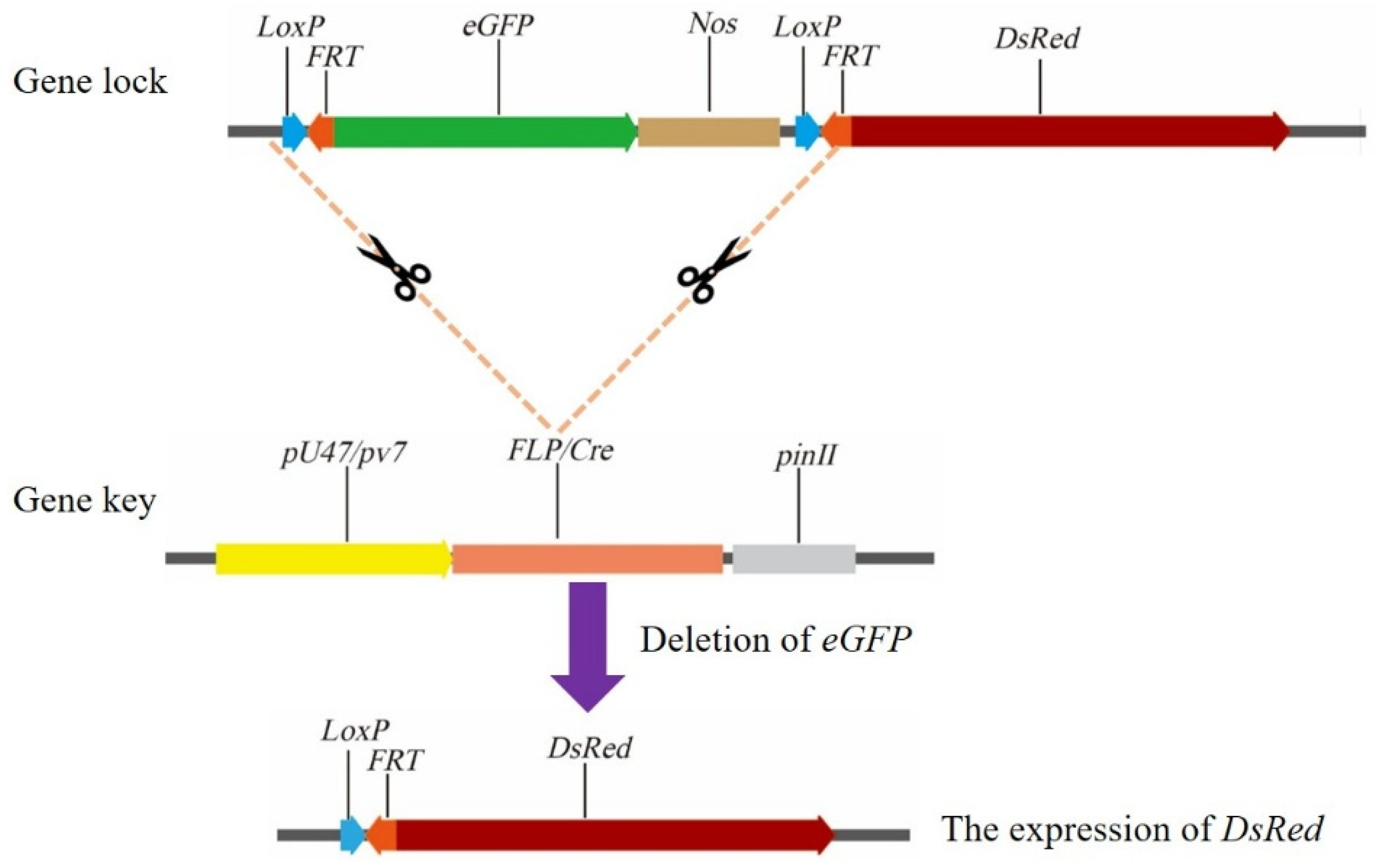
3.3.3. Clonal Seed Sorting System
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAIR1 | HOMOLOGOUS PAIRING ABERRATION IN RICE MEIOSIS 1 |
| REC8 | RECOMBINATION8 |
| OSD1 | OMISSION OF SECOND DIVISION |
| MiMe | mitosis instead of meiosis |
| WOC9A | WUSCHEL-LIKE HOMEODOMAIN 9 |
| DD45 | DOWNREGULATED IN DETERMINANT INFERTILE1 45 |
| BBM | BABY BOOM |
| SPO11-1 | SPORULATION11-1 |
| MTL | MATRILINEAL |
| Fix | Fixation of hybrids |
| PLD | Phospholipase D |
| AOP | Apomictic Offspring Producer |
| AP2/ERF | APETALA 2/ETHYLENE RESPONSE FACTOR |
| PAR | PARTHENOGENESIS |
| EAR | ethylene-responsive element-binding factor-associated amphiphilic repression |
| WUS | WUSCHEL |
| WIH1 | WINDHOSE1 |
| WIH2 | WINDHOSE2 |
| FIS | fertilization-independent seed |
| FIE | FERTILIZATION-INDEPENDENT ENDOSPERM |
| FLP | Flipase recombinase |
References
- Batygina, T.B.; Vinogradova, G.Y. Phenomenon of polyembryony. Genetic heterogeneity of seeds. Russ. J. Dev. Biol. 2007, 38, 126–151. [Google Scholar] [CrossRef]
- Zhang, S.Q. Genetic Analysis of Citrus Apomixis and Its Related Gene Discovery. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2017. [Google Scholar]
- Xu, Y.T. Molecular Basis of Apomixis and Grafting Chimerism in Citrus. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar]
- Mo, R.; Zhen, C.M.; Zhu, W.; Han, P.-Y. The polyembryony and morphogenesis of multi-seedlings in coffee. Chin. Bull. Bot. 2004, 21, 189–194. [Google Scholar]
- Fei, X.T. Analysis of Apomixis Characteristics and Functional Verification of Key Genes in Zanthoxylum bungeanum. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2021. [Google Scholar]
- Mendes-Rodrigues, C.; Oliveira, P.E. Polyembryony in melastomataceae from brazilian cerrado: Multiple embryos in a small world. Plant Biol. 2012, 14, 845–853. [Google Scholar] [CrossRef]
- Puri, A.; Basha, P.O.; Kumar, M.; Rajpurohit, D.; Randhawa, G.S.; Kianian, S.F.; Rishi, A.; Dhaliwal, H.S. The polyem-bryo gene (OsPE) in rice. Funct. Integr. Genom. 2010, 10, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.J.; Jin, B.; Teng, N.J. Studies on the early development of zygotic and synergid embryo and endosperm in polyembryonic rice ApIII. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 404–410. [Google Scholar] [CrossRef]
- Guo, M.Q. Discovery and research progress on twin embryo seedlings in rice. Hybrid Rice 1990, 5, 26–27. [Google Scholar]
- Xue, L.; Zhang, Y.; Wei, F.; Shi, G.; Tian, B.; Yuan, Y.; Jiang, W.; Zhao, M.; Hu, L.; Xie, Z.; et al. Recent progress on plant apomixis for genetic improvement. Int. J. Mol. Sci. 2024, 25, 11378. [Google Scholar] [CrossRef]
- Hand, M.L.; Koltunow, A.M. The genetic control of apomixis: Asexual seed formation. Genetics 2014, 197, 441–450. [Google Scholar] [CrossRef]
- Rodkiewicz, B.; Fyk, B.; Szczuka, E. Ultrastructure of meiotic restitution nuclei in ovules of Taraxacum palustre (Asteraceae). Acta Biol. Cracov. Bot. 1996, 3, 51–58. [Google Scholar]
- Noyes, R.D. Apomixis in the Asteraceae: Diamonds in the rough. Funct. Plant Sci. Biotechnol. 2007, 1, 207–222. [Google Scholar]
- Hofmann, N.R. Apomixis and gene expression in Boechera. Plant Cell 2010, 22, 539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conner, J.A.; Mookkan, M.; Huo, H.; Chae, K.; Ozias-Akins, P. A parthenogenesis gene of apomict origin elicits embryo formation from unfertilized eggs in a sexual plant. Proc. Natl. Acad. Sci. USA 2015, 112, 11205–11210. [Google Scholar] [CrossRef] [PubMed]
- Spillane, C.; Curtis, M.D.; Grossniklaus, U. Apomixis technology development-virgin births in farmers’ fields. Nat. Biotechnol. 2004, 22, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.P. The strategy for hybrid rice development. Hybrid Rice 2018, 33, 1–2. [Google Scholar]
- Ogawa, D.; Johnson, S.D.; Henderson, S.T.; Koltunow, A.M. Genetic separation of autonomous endosperm formation (AutE) from the two other components of apomixis in Hieracium. Plant Reprod. 2013, 26, 113–123. [Google Scholar] [CrossRef]
- Suzuki, D. Recent progress in plant reproduction research: The story of the male gametophyte through to successful fertilization. Plant Cell Physiol. 2009, 50, 1857–1864. [Google Scholar] [CrossRef]
- Liu, Y.S.; Sun, J.S.; Wang, F.X.; Zhou, K.D. Embryo sac characteristics and abnormal reproductive behavior of the polyembryonic rice (Oryza sativa) line SB-1. Chin Bull Bot. 1994, 11, 71. [Google Scholar]
- Jiang, Q.G. Study on twin-seedlings of rice W3338-986 by embryogenesis and heredity research. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2007. [Google Scholar]
- Paul, P.; Awasthi, A.; Kumar, S.; Verma, S.K.; Prasad, R.; Dhaliwal, H.S. Development of multiple embryos in polyembryonic insertional mutant OsPE of rice. Plant Cell Rep. 2012, 31, 1779–1787. [Google Scholar] [CrossRef]
- Mu, X.J.; Zhu, Z.Q.; Cai, X.; Sun, D.L.; Lin, J.X. Embryogenesis of polyembryonic rice ApIII: Structural and histochemical studies of egg apparatus around fertilization. J. Integr. Plant Biol. 2002, 22, 1387–1395. [Google Scholar]
- Wu, X.J.; Zhou, K.D. Embryogeny for poly-embryo strain 9003 in rice (Oryza sativa L.). J.-Sichuan Univ. Nat. Sci. Ed. 2003, 40, 966–969. [Google Scholar]
- Liu, Y.S.; Sun, J.S.; Wang, F.X.; Zhou, K.D. Cytoembryological studies on polyembryonic line SB-1 of Oryza sativa apogamy of synergids. J. Integr. Plant Biol. 1994, 36, 828–832. [Google Scholar]
- Li, Y.Q.; Deng, H.D.; Yuan, L.P. New progress in apomixis research in rice. Hybrid Rice 1990, 5, 29–32. [Google Scholar]
- Guan, H.X.; Cai, D.T.; Yao, J.L.; Ma, P.F.; Zhu, H.; Xie, G.S. Studies of twin-seedling in rice (Oryza sativa L.) by synthetic methods of embryology and genetics. Acta Genet. Sin. 1998, 25, 237–244. [Google Scholar]
- León-Martínez, G.; Vielle-Calzada, J.P. Apomixis in flowering plants: Developmental and evolutionary considerations. Curr. Top. Dev. Biol. 2019, 131, 565–604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.D.; Wang, X.D.; Luo, M.; Gao, K.M.; Zhou, S.L.; Yan, Z.B.; Li, P.; Chen, F. Progress of research on apomixis of rice. Southwest China J. Agric. Sci. 1991, 4, 109–110. [Google Scholar]
- Li, Y.Q.; Yuan, L.P. Studies on genetics of twin seedlings in rice. Acta Agron Sin. 1990, 16, 176–182. [Google Scholar]
- Sun, M.X.; Yao, J.L.; Li, H.P.; Cai, D.T.; Wang, Z.A. Embryological research on the polyembryony types of polyembryonic rice. J. Huazhong Agric. Univ. 1992, 11, 21–25. [Google Scholar]
- Xiao, W. Embryonic and Genetic Study on Twin-Seedling in Rice. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2006. [Google Scholar]
- Dai, X.M.; Huang, Q.C.; Hu, X.M.; Qin, G.Y. Development and character observation of autotetraploid polyembryonic mutant rice induced by ion beam implantation. Acta Agric. Nucleatae Sin. 2007, 21, 1–4. [Google Scholar]
- Hu, X.M. The Frequencies of Autotetraploid Rice with Polyembryonic Seedling and Its Reproductive Characeristics. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2007. [Google Scholar]
- Huang, Y.Q. Studies on Biological Characteristics of the Twin-Embryo Seedling Rice. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2010. [Google Scholar]
- Xia, Y.M.; Wang, Y.; Hu, Y.Y.; Zhan, Y.J.; Dan, J.H.; Tang, N.; Tian, J.Y.; Cao, M.L. Double-seedlings and embryo-free seeds generated by genetic engineering. Front. Plant Sci. 2022, 13, 999031. [Google Scholar] [CrossRef]
- Dan, J.H.; Xia, Y.M.; Wang, Y.; Zhan, Y.J.; Tian, J.Y.; Tang, N.; Deng, H.F.; Cao, M.L. One-line hybrid rice with high-efficiency synthetic apomixis and near-normal fertility. Plant Cell Rep. 2024, 43, 79. [Google Scholar] [CrossRef]
- Ren, H.; Shankle, K.; Cho, M.J.; Tjahjadi, M.; Khanday, I.; Sundaresan, V. Synergistic induction of fertilization-independent embryogenesis in rice egg cells by paternal-genome-expressed transcription factors. Nat. Plants 2024, 10, 1892–1899. [Google Scholar] [CrossRef]
- Luo, W.X.; Zhou, K.D. Studies on genetic of rice twins. J. Sichuan Agri. Univ. 1992, 10, 453–459. [Google Scholar]
- Xiong, X.; He, Y.; Liu, H.H.; Liu, Z.E.; Tian, Z.H. Identify and analyze the splice variants of polyembryo candidate gene OsPE in rice. Acta Agric. Boreali-Sin. 2023, 38, 55–60. [Google Scholar]
- Li, J.Q.; Li, M.Q.; Wang, Y.T.; Liu, X.L.; Zhang, X.H.; Li, X.A. Research progress in hormonal regulation of AP2/ERF transcription factors in fruits and vegetables in response to stress. J. Food Sci. 2024, 45, 315–323. [Google Scholar] [CrossRef]
- Shirasawa, K.; Hand, M.L.; Henderson, S.T.; Okada, T.; Johnson, S.D.; Taylor, J.M.; Spriggs, A.; Siddons, H.; Hirakawa, H.; Isobe, S.; et al. A reference genetic linkage map of apomictic Hieracium species based on expressed markers derived from developing ovule transcripts. Ann. Bot. 2015, 115, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Hojsgaard, D.; Hörandl, E. The rise of apomixis in natural plant populations. Front. Plant. Sci. 2019, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Carman, J.G. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory tetraspory and polyembryony. Biol. J. Linn. Soc. 1997, 61, 51–94. [Google Scholar] [CrossRef]
- Susmita, C.; Kumar, S.P.J.; Chintagunta, A.D.; Agarwal, D.K. Apomixis: A foresight from genetic mechanisms to molecular perspectives. Bot. Rev. 2022, 88, 220–256. [Google Scholar] [CrossRef]
- Bicknell, R.A.; Catanach, A.S. The Molecular biology of Apomixis. Mol. Biol. Biotechnol. Flower. 2006, 2, 354–390. [Google Scholar] [CrossRef]
- Peel, M.D.; Carman, J.G.; Leblanc, O. Megasporocyte callose in apomictic buffelgrass, Kentucky bluegrass, Pennisetum Squamulatum Fresen, Tripsacum L. and weeping lovegrass. Crop Sci. 1997, 37, 724–732. [Google Scholar] [CrossRef]
- Koltunow, A.M.; Grossniklaus, U. Apomixis: A developmental perspective. Annu. Rev. Plant Biol. 2003, 54, 547–574. [Google Scholar] [CrossRef]
- Dusi, D.M.A.; Alves, E.R.; Cabral, G.B.; Mello, L.V.; Rigden, D.J.; Silveira, E.D.; Alves-Ferreira, M.; Guimarães, L.A.; Gomes, A.C.M.M.; Rodrigues, J.C.M.; et al. An exonuclease V homologue is expressed predominantly during early megasporogenesis in apomictic Brachiaria brizantha. Planta 2023, 258, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wang, K.J. Research advances on plant synthetic apomixis. Sci. Bull. 2020, 65, 2999–3007. [Google Scholar] [CrossRef]
- Chen, Q.F.; Huang, Q.C. Progress of apomixis in Chinese rice. Chin. Agric. Sci. Bull. 2005, 8, 120–124. [Google Scholar]
- Conner, J.A.; Podio, M.; Ozias-Akins, P. Haploid embryo production in rice and maize induced by PsASGR-BBML transgenes. Plant Reprod. 2017, 30, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, R.A.; Koltunow, A.M. Understanding apomixis: Recent advances and remaining conundrums. Plant Cell 2004, 16, S228–S245. [Google Scholar] [CrossRef]
- D’erfueth, I.; Jolivet, S.; Froger, N.; Catrice, O.; Novatchkova, M.; Mercier, R. Turning meiosis into mitosis. PLoS Biol. 2009, 7, e1000124. [Google Scholar] [CrossRef]
- Mieulet, D.; Jolivet, S.; Rivard, M.; Cromer, L.; Vernet, A.; Mayonove, P.; Pereira, L.; Droc, G.; Courtois, B.; Guiderdoni, E.; et al. Turning rice meiosis into mitosis. Cell Res. 2016, 26, 1242–1254. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Shen, Y.; Hua, Y.F.; Wang, J.J.; Lin, J.R.; Wu, M.G.; Sun, T.T.; Cheng, Z.K.; Mercier, R.; et al. Clonal seeds from hybrid rice by simultaneous genome engineering of meiosis and fertilization genes. Nat. Biotechnol. 2019, 37, 283–286. [Google Scholar] [CrossRef]
- Hu, F.Y.; Liu, C.L.; Jin, X.C.; Sun, T.T.; Hong, L.; Rao, Y.C.; Qian, Q.; Wang, K.J. OsPLDα2-dependent synthetic apomixis enables normal seed setting in hybrid rice via genome editing. Sci. Bull. 2025, 70, S2095–S9273. [Google Scholar] [CrossRef]
- Khanday, I.; Skinner, D.; Yang, B.; Mercier, R.; Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 2019, 565, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, K.; Ozias-Akins, P.; Schranz, M.E. Identifying and engineering genes for parthenogenesis in plants. Front. Plant Sci. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, C.L.; Chen, X.; Lu, H.W.; Wang, J.; Yang, S.L.; Wang, K.J. Synthetic apomixis with normal hybrid rice seed production. Mol. Plant 2023, 16, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.M.A.; Gilles, L.M.; Pyott, D.E.; Martinant, J.P.; Rogowsky, P.M.; Widiez, T. Puzzling out plant reproduction by haploid induction for innovations in plant breeding. Nat. Plants 2020, 6, 610–619. [Google Scholar] [CrossRef]
- Ji, J.H.; Tang, D.; Shen, Y.; Xue, Z.H.; Wang, H.J.; Shi, W.Q.; Zhang, C.; Du, G.J.; Li, Y.F.; Cheng, Z.K. P31comet, a member of the synaptonemal complex, participates in meiotic DSB formation in rice. Proc. Natl. Acad. Sci. USA 2016, 113, 10577–10582. [Google Scholar] [CrossRef]
- Vernet, A.; Meynard, D.; Guiderdoni, E. Clonal reproduction by seed of acultivated hybrid plant: A new perspective for small-scale rice growers. Comptes Rendus. Biol. 2024, 346, 107–116. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Y.; Liu, C.X.; Wang, Y.L.; Liang, D.W.; Liu, J.T.; Sahoo, G.; Kelliher, T. OsMATL mutation induces haploid seed formation in indica rice. Nat. Plants 2018, 4, 530–533. [Google Scholar] [CrossRef]
- Liu, C.L.; He, Z.X.; Zhang, Y.; Hu, F.Y.; Li, M.Q.; Liu, Q.; Huang, Y.; Wang, J.; Zhang, W.L.; Wang, C.; et al. Synthetic apomixis enables stable transgenerational transmission of heterotic phenotypes in hybrid rice. Plant Commun. 2023, 4, 100470. [Google Scholar] [CrossRef]
- Xie, E.; Li, Y.F.; Tang, D.; Lv, Y.L.; Shen, Y.; Cheng, Z.K. A strategy for generating rice apomixis by gene editing. J. Integr. Plant Biol. 2019, 61, 911–916. [Google Scholar] [CrossRef]
- Ali, U.; Lu, S.; Fadlalla, T.; Iqbal, S.; Yue, H.; Yang, B.; Hong, Y.Y.; Wang, X.M.; Guo, L. The functions of phospholipases and their hydrolysis products in plant growth, development and stress responses. Prog. Lipid Res. 2022, 86, 101158. [Google Scholar] [CrossRef]
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.M.; Hattori, J.; Liu, C.M.; van Lammeren, A.A.M.; Miki, B.L.A.; et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 2002, 14, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- El Ouakfaoui, S.; Schnell, J.; Abdeen, A.; Colville, A.; Labbé, H.; Han, S.Y.; Baum, B.; Laberge, S.; Miki, B. Control of somatic embryogenesis and embryo development by AP2 transcription factors. Plant Mol. Biol. 2010, 74, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Schmidi, A. Controlling apomixis: Shared features and distinct characteristics of gene regulation. Genes 2020, 11, 329. [Google Scholar] [CrossRef]
- Vernet, A.; Meynard, D.; Lian, Q.C.; Mieulet, D.; Gibert, O.; Bissah, M.; Rivallan, R.; Autran, D.; Leblanc, O.; Meunier, A.C.; et al. High-frequency synthetic apomixis in hybrid rice. Nat. Commun. 2022, 13, 7963. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.N.; Johnson, C.S.; Chesnut, J.; Jones, D.S.; Khanday, I.; Woodhouse, M.; Li, C.X.; Conrad, L.J.; Russell, S.D.; Sundaresan, V. The zygotic transition is initiated in unicellular plant zygotes with asymmetric activation of parental genomes. Dev. Cell 2017, 43, 349–358. [Google Scholar] [CrossRef]
- Song, M.L.; Li, F.; Chen, Z.; Hou, H.N.; Wang, Y.; Liu, H.X.; Liu, D.; Li, J.Z.; Peng, T.; Zhao, Y.F.; et al. Engineering high-frequency apomixis with normal seed production in hybrid rice. Iscience 2024, 27, 111479. [Google Scholar] [CrossRef]
- Van Dijk, P.J.; Op den Camp, R.; Schauer, S.E. Genetic dissection of apomixis in dandelions identifies a dominant parthenogenesis locus and highlights the complexity of autonomous endosperm formation. Genes 2020, 11, 961. [Google Scholar] [CrossRef]
- Vijverberg, K.; Milanovic-Ivanovic, S.; Bakx-Schotman, T.; van Dijk, P.J. Genetic fine-mapping of DIPLOSPOROUS in Taraxacum (dandelion; Asteraceae) indicates a duplicated DIP-gene. BMC Plant Biol. 2010, 10, 154. [Google Scholar] [CrossRef]
- Underwood, C.J.; Vijverberg, K.; Rigola, D.; Okamoto, S.; Oplaat, C.; Op den Camp, R.H.M.; Radoeva, T.; Schauer, S.E.; Fierens, J.; Jansen, K.; et al. A PARTHENOGENESIS allele from apomictic dandelion can induce egg cell division without fertilization in lettuce. Nat. Genet. 2022, 54, 84–93. [Google Scholar] [CrossRef]
- Song, M.Q.; Wang, W.M.; Ji, C.; Li, S.N.; Liu, W.; Hu, X.Y.; Feng, A.H.; Ruan, S.; Du, S.Y.; Wang, H.; et al. Simultaneous production of high-frequency synthetic apomixis with high fertility and improved agronomic traits in hybrid rice. Mol. Plant 2024, 17, 4–7. [Google Scholar] [CrossRef]
- Xiong, J.; Ji, Y.J.; Yang, S.L.; Huang, Y.; Qiu, X.J.; Qian, Q.; Underwood, C.J.; Wang, K.J. Extending Mendel’s legacy: The application of hawkweed PpPAR for inducing synthetic apomixis in hybrid rice. Plant Commun. 2025. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, X.B.; Rao, Y.C.; Xie, Y.Y.; Sun, T.T.; Chen, W.Q.; Wei, X.; Xiong, J.; Yu, H.; Li, J.Y.; et al. OsWUS-driven synthetic apomixis in hybrid rice. Plant Commun. 2025, 6, 1011136. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.F.X.; Schoof, H.; Haecker, A.; Lenhard, M.; Jürgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Nardmann, J.; Werr, W. The shoot stem cell niche in angiosperms: Expression patterns of WUS orthologues in rice and maize imply major modifications in the course of mono-and dicot evolution. Mol. Biol. Evol. 2006, 23, 2492–2504. [Google Scholar] [CrossRef] [PubMed]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.X.; Jürgens, G.; Laux, T. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef]
- Lieber, D.; Lora, J.; Schrempp, S.; Lenhard, M.; Laux, T. Arabidopsis WIH1 and WIH2 genes act in the transition from somatic to reproductive cell fate. Curr. Biol. 2011, 21, 1009–1017. [Google Scholar] [CrossRef]
- Zuo, J.R.; Niu, Q.W.; Frugis, G.; Chua, N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef]
- Cao, M.L.; Xia, Y.M.; Zhan, Y.J.; Tang, N.; Bu, X.L.; Yu, M.L.; Yuan, L.P. A Seed Sorting Method for Fixed Plant Heterosis. Cambodian patent. Patent WO2020211747 A1. (accessed on 28 April 2022).
- Dan, J.H.; Deng, H.F.; Xia, Y.M.; Zhan, Y.J.; Tang, N.; Wang, Y.; Cao, M.L. Application of the FLP/LoxP-FRT recombination system to switch the eGFP expression in a model prokaryote. Open Life Sci. 2022, 17, 172–179. [Google Scholar] [CrossRef]
- Zhan, Y.J.; Xia, Y.M.; Wang, Y.; Liu, S.Q.; Zhang, X.L.; Xiong, S.; Lv, Q.M.; Cao, M.L. Efficient clonal seeds sorting for apomictic hybrid rice using a pollen-specific gene switch system. Plant Biotechnol. J. 2025, 23, 2266–2275. [Google Scholar] [CrossRef]
- Peha, H.; Ren, H.; Skinner, D.; Sundaresan, V. Twin embryo formation by induced parthenogenesis. Plant Reprod. 2025, 38, 3. [Google Scholar] [CrossRef]
- Xiong, H.X.; Wang, W.; Sun, M.X. Endosperm development is an autonomously programmed process independent of embryogenesis. Plant Cell 2021, 33, 1151–1160. [Google Scholar] [CrossRef]
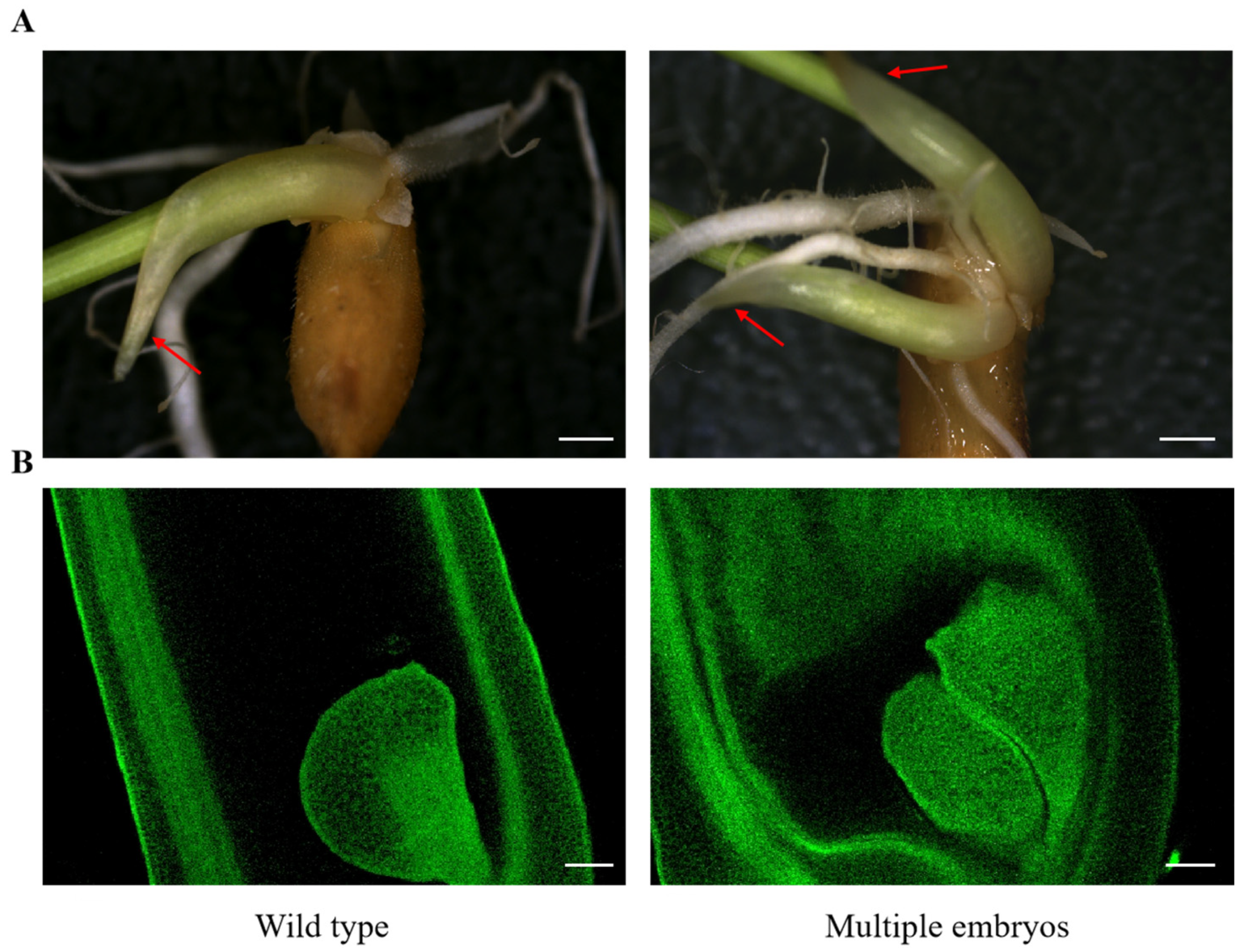

| Rice Cultivar | Multiple Embryos/% | Ref. |
|---|---|---|
| (A) Frequency of multiple embryos in different rice varieties (naturally selected diploids) | ||
| API | 16.1 | [30] |
| APII | 23.4 | [30] |
| APIII | 32.4, 55.0 | [27,30] |
| APIV | 5.0, 30.0 | [27,30] |
| W3338 | 36.4 | [32] |
| W255 | 11.0 | [32] |
| W338-986 | 43.5 | [21] |
| (B) Frequency of multiple embryos in different rice varieties (artificially induced polyploids) | ||
| IR36-Shuang | 12.7 | [33] |
| ASDR05-01 | 9.8 | [34] |
| ASDR05-02 | 3.4 | [34] |
| D07-04-01 | 1.3 | [35] |
| (C) Frequency of multiple embryos in gene-edited rice mutants | ||
| OsPE | 21.0 | [7] |
| Os02g51090:AtWUS | 0.5 | [36] |
| MiMe + pAtDD45:BBM1 | 61.0 | [37] |
| MiMe + pAtMYB98_pAtDD1_pOsECA1-like1:WUS | 44.7 | [37] |
| pEC1.2:OsWOC9A + OsBBM1 | 44.6 | [38] |
| Method | Clonal Seed Induction Rate/% | Seed-Setting Rate/% | Year | Ref. |
|---|---|---|---|---|
| MiMe + MTL | 4.7–9.5 | 3.7–5.2 | 2019 | [56] |
| MiMe + BBM1 | 11.1–29.2 | No data | 2019 | [58] |
| MiMe + BBM1 (single step) | 80.0–100.0 | 27.0–35.5 | 2022 | [71] |
| MiMe + BBM4 | 1.3–2.4 | 80.9–82.0 | 2023 | [60] |
| MiMe + ToPAR | 42.9–67.7 | 72.7–75.6 | 2024 | [77] |
| MiMe + BBM1 + AtWUS | 5.4–98.2 | 15.8–83.7 | 2024 | [37] |
| MiMe-ECA1-AZP2 + BBM1 | 1.7–100.0 | 63.8–87.7 | 2024 | [73] |
| MiMe + OsWUS | 0.5–21.7 | 72.0–85.2 | 2025 | [79] |
| MiMe + OsPLDα2 | 0.8–1.2 | 82.1–85.0 | 2025 | [57] |
| MiMe + PpPAR | 21.2–84.6 | 52.1–59.6 | 2025 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dan, J.; Long, W.; Qiu, M.; Zhang, L.; Wu, C.; Jiang, X.; Fang, S.; Zhu, S.; Deng, H. Research Advances in Multiple Embryos and Apomixis in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2025, 26, 7257. https://doi.org/10.3390/ijms26157257
Dan J, Long W, Qiu M, Zhang L, Wu C, Jiang X, Fang S, Zhu S, Deng H. Research Advances in Multiple Embryos and Apomixis in Rice (Oryza sativa L.). International Journal of Molecular Sciences. 2025; 26(15):7257. https://doi.org/10.3390/ijms26157257
Chicago/Turabian StyleDan, Junhao, Wuhua Long, Mudan Qiu, Longhui Zhang, Chaoxin Wu, Xue Jiang, Shengyan Fang, Susong Zhu, and Huafeng Deng. 2025. "Research Advances in Multiple Embryos and Apomixis in Rice (Oryza sativa L.)" International Journal of Molecular Sciences 26, no. 15: 7257. https://doi.org/10.3390/ijms26157257
APA StyleDan, J., Long, W., Qiu, M., Zhang, L., Wu, C., Jiang, X., Fang, S., Zhu, S., & Deng, H. (2025). Research Advances in Multiple Embryos and Apomixis in Rice (Oryza sativa L.). International Journal of Molecular Sciences, 26(15), 7257. https://doi.org/10.3390/ijms26157257