Investigating Seed Germination, Seedling Growth, and Enzymatic Activity in Onion (Allium cepa) Under the Influence of Plasma-Treated Water
Abstract
1. Introduction
2. Results
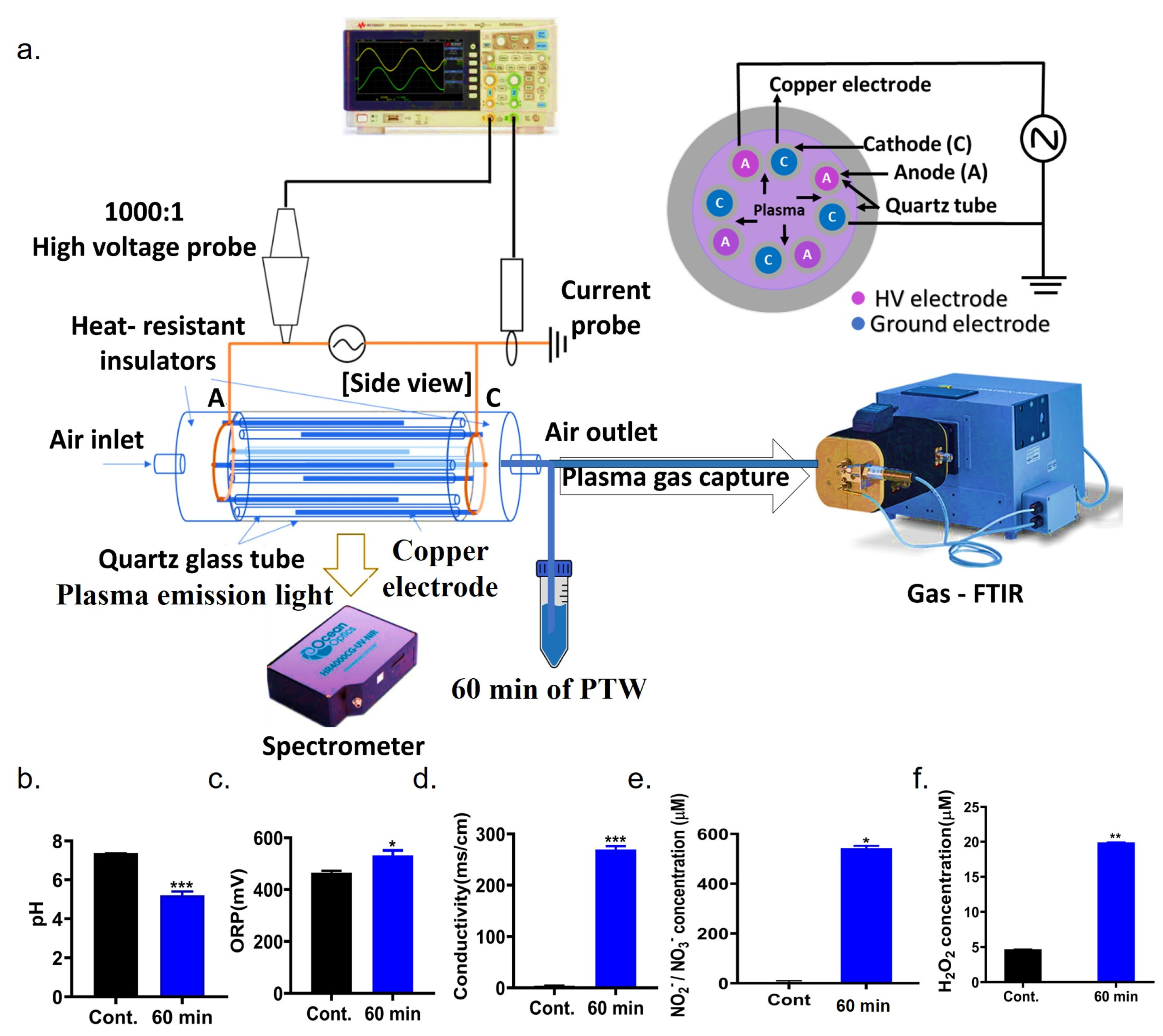
2.1. Characterization of Multi-Electrode Cylindrical Dielectric Barrier Discharge (c-DBD) Plasma System
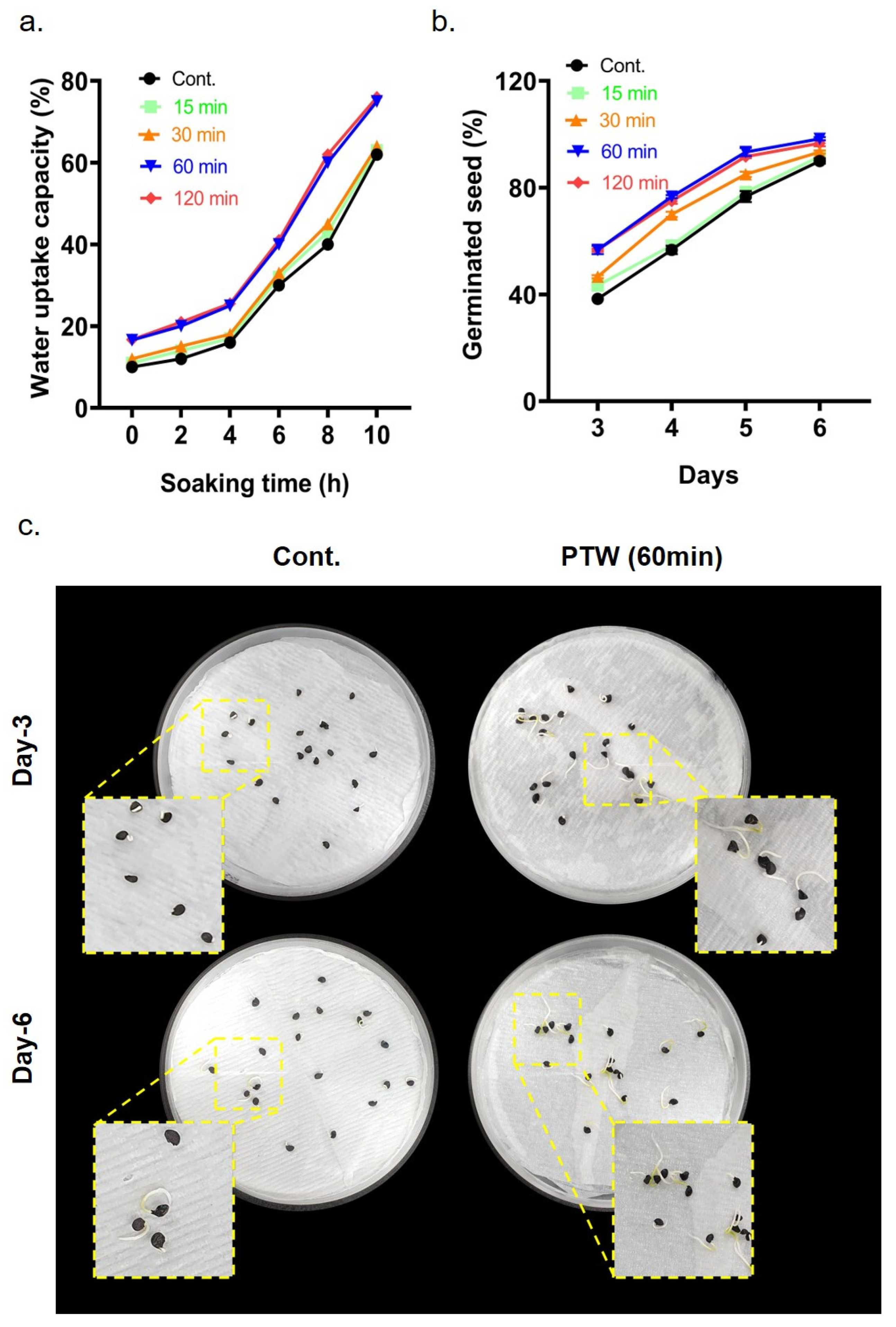
2.2. Water Uptake
2.3. Determination of Seed Germination
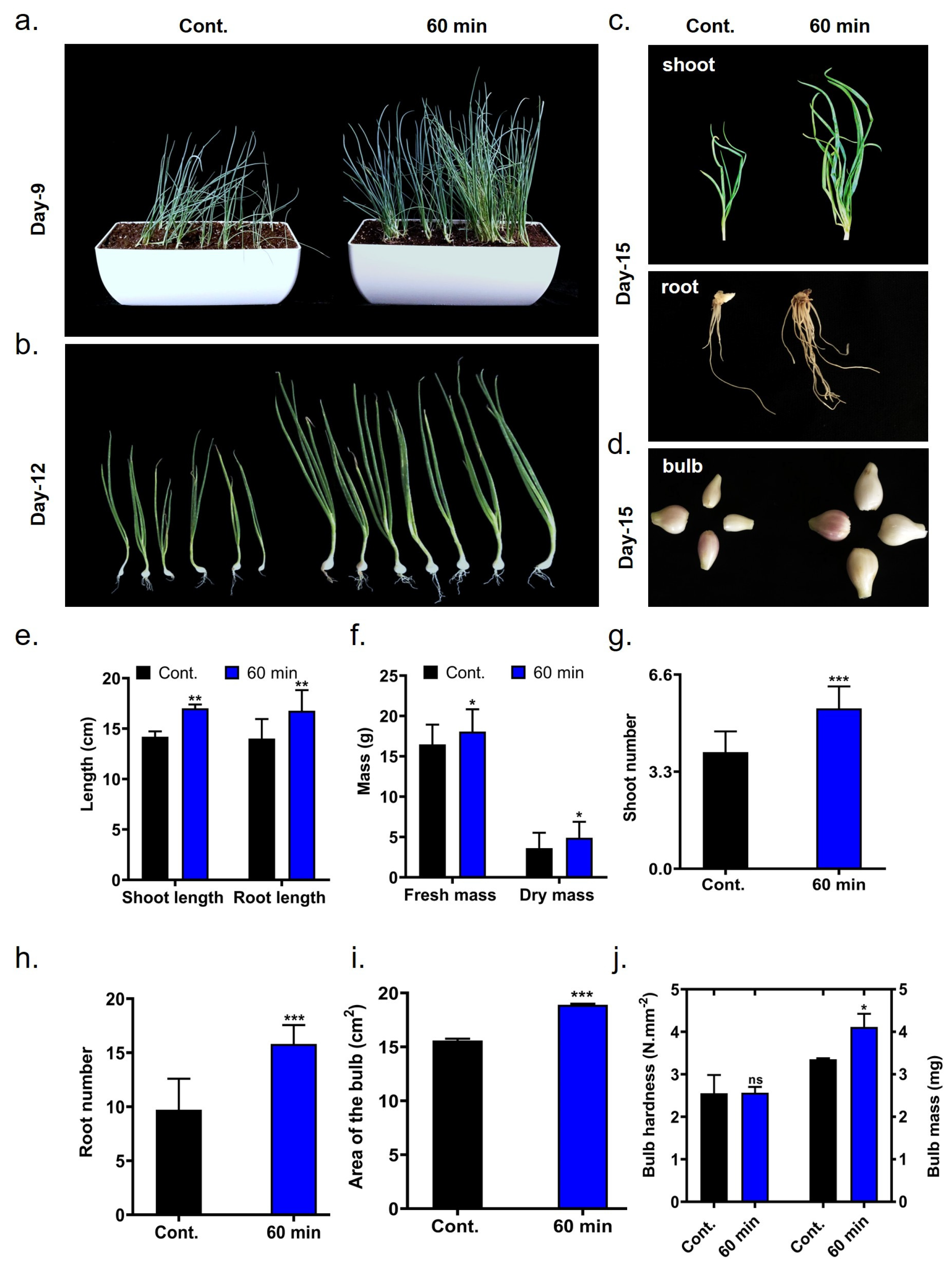
2.4. Growth Related Variables
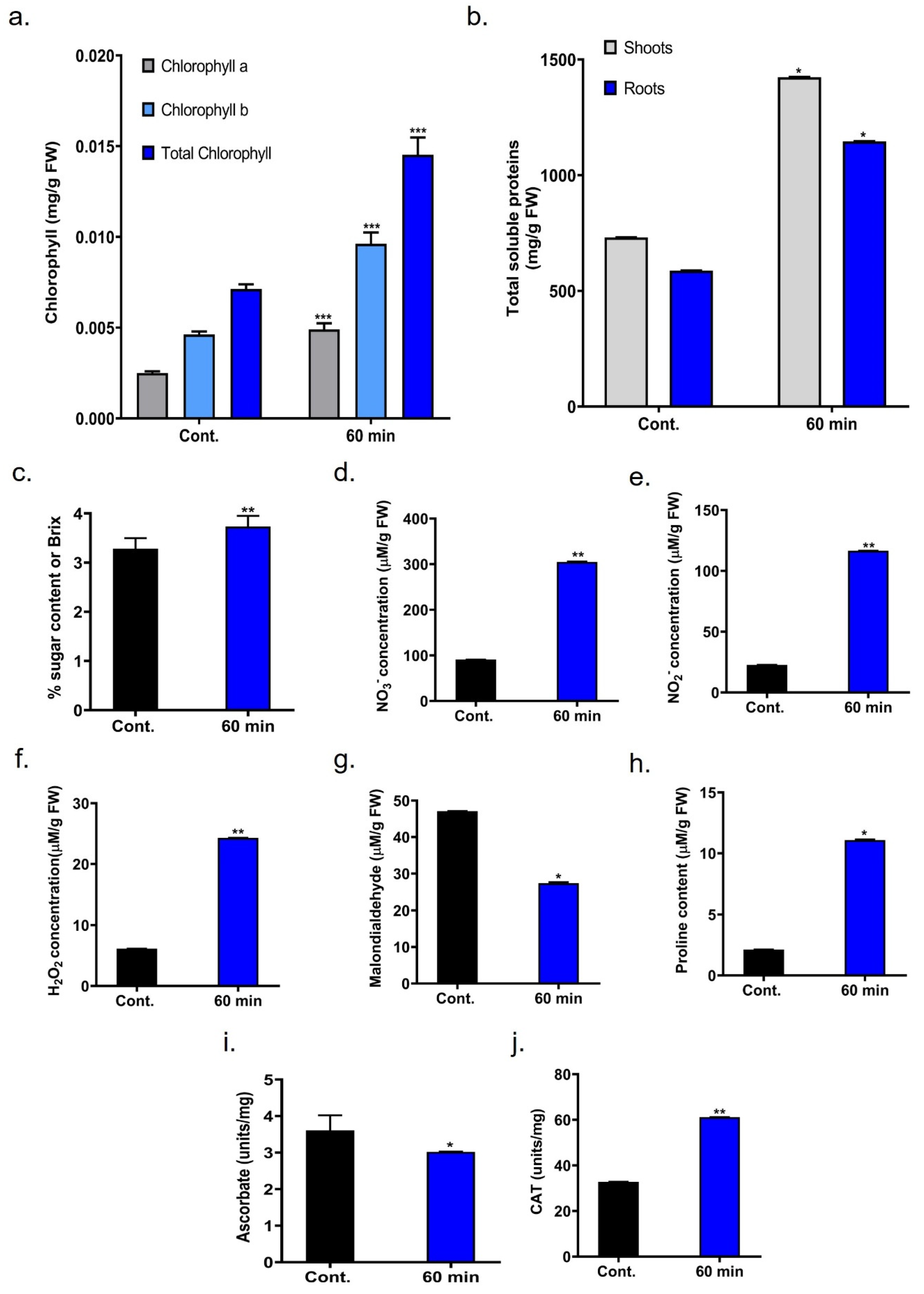
2.5. Chlorophyll Content
2.6. Total Soluble Protein and Sugar Content in Onion
2.7. c-DBD Plasma Induced Reactive Species
2.8. Malondialdehyde (MDA Content
2.9. Determination of Proline Content
2.10. Asessment of Ascorbate Content
2.11. Evalution of Catalase Activity
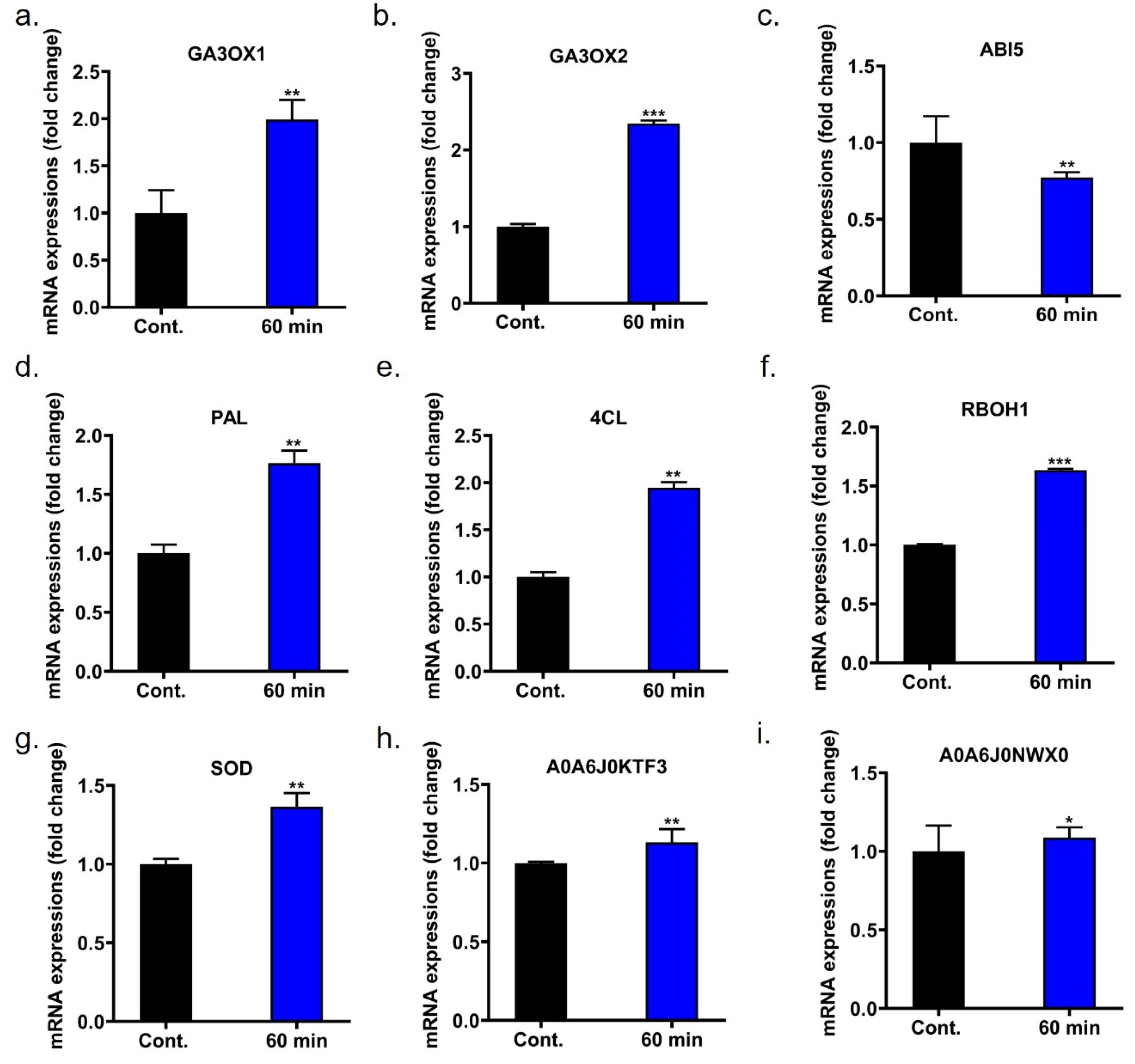
2.12. Gene Expression Alters by c-DBD Plasma Treatment
2.13. Properties of PTW
3. Discussion
4. Materials and Methods
4.1. Properties of c-DBD Plasma System
4.2. Physicochemical Characteristics of PTW
4.3. Assessment of Water Uptake
4.4. Assessment of Seed Germination Efficiency and Plant Growth
4.5. Determination of Phenotypical Traits
4.6. Determination of Chlorophyll Content
4.7. Determination of Total Soluble Protein
4.8. Determination of Total Soluble Sugar
4.9. Determination of Reactive Species
4.10. Malondialdehyde Content
4.11. Proline Content
4.12. Ascorbate Content
4.13. Catalase Activity
4.14. qPCR Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gyarmati, G. Cross-Country Analysis of Onion Trade in EU and Balkan Countries. J. Hyg. Eng. Des. 2023, 42, 190–196. [Google Scholar]
- Wakchaure, V.; Pawase, R. Analysis of Recent Trends and Developments in IoT-Based Onion Storage Monitoring Systems: A Systematic Review. Int. J. Smart Sens. Intell. Syst. 2025, 18, 1–14. [Google Scholar] [CrossRef]
- Safari, S.; Engah, W.R.W.; Sa’at, N.H.M.; Ghazali, N.S.; Basir, M.H.; Ahmad, S. Forecasting Malaysian Onion Imports Using A Moving Average Model and Strategies to Overcome Reliance on A Fully Imported Supply. Rev. Food Agric. 2024, 5, 97–101. [Google Scholar] [CrossRef]
- Colina-Coca, C.; de Ancos, B.; Sanchez-Moreno, C. Nutritional Composition of Processed Onion: S-Alk (En) Yl-L-Cysteine Sulfoxides, Organic Acids, Sugars, Minerals, and Vitamin C. Food Bioprocess Technol. 2014, 7, 289–298. [Google Scholar] [CrossRef]
- Lawande, K.E. Onion. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 417–429. [Google Scholar]
- Fayos, O.; Mallor, C.; Garces-Claver, A. Evolution of Knowledge on the Pungency of Onion (Allium cepa L.) and Pepper (Capsicum spp.): From Their Origins to Current Nutraceutical Potential. A Literature Review. Tech. Econ. Agric. Inf. 2018, 114, 99–118. [Google Scholar]
- Elouattassi, Y.; Ferioun, M.; El Ghachtouli, N.; Derraz, K.; Rachidi, F. Enhancing Onion Growth and Yield through Agroecological Practices: Organic Fertilization and Intercropping. Ecol. Front. 2024, 44, 547–557. [Google Scholar] [CrossRef]
- Imran, M.; Kang, H.; Lee, S.-G.; Kim, E.-H.; Park, H.-M.; Oh, S.-W. Current Trends and Future Prospects in Onion Production, Supply, and Demand in South Korea: A Comprehensive Review. Sustain. 2025, 17, 837. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; De Vos, O.J.; Korthals, G.W.; Van Bruggen, A.H.C. Effects of Organic versus Conventional Management on Chemical and Biological Parameters in Agricultural Soils. Appl. soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene Expression in Tomato Seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef]
- Amsalu, K.; Acharya, T.; Apurva, J.; Lamichhane, P.; Kifle, R.; Kaushik, N.; Lim, J.S.; Kim, C.T.; Kaushik, N.K.; Choi, E.H. Abatement and Biotoxicity Assessment of Chlorpyrifos Residue from Green Coffee Beans: Effect of Non-Thermal Plasma Generated Ozone and Nitric Oxide Species. Chem. Eng. J. 2024, 497, 154364. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of Plasma-Seed Treatments as a Potential Seed Processing Technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Singh, K.L.; Chaudhuri, A.; Kar, R.K. Superoxide and Its Metabolism during Germination and Axis Growth of Vigna radiata (L.) Wilczek Seeds. Plant Signal. Behav. 2014, 9, 29278. [Google Scholar] [CrossRef]
- Mazandarani, A.; Goudarzi, S.; Ghafoorifard, H.; Eskandari, A. Evaluation of DBD Plasma Effects on Barley Seed Germination and Seedling Growth. IEEE Trans. Plasma Sci. 2020, 48, 3115–3121. [Google Scholar] [CrossRef]
- Lofthus, A.; Krupenie, P.H. The Spectrum of Molecular Nitrogen. J. Phys. Chem. Ref. Data 1977, 6, 113–307. [Google Scholar] [CrossRef]
- Lee, T.; Keidar, M. Adaptive Plasma and Machine Learning. In Springer Series on Atomic, Optical, and Plasma Physics; Springer: Berlin/Heidelberg, Germany, 2020; Volume 115, pp. 223–250. [Google Scholar]
- Choi, E.H.; Kaushik, N.K.; Hong, Y.J.; Lim, J.S.; Choi, J.S.; Han, I. Plasma Bioscience for Medicine, Agriculture and Hygiene Applications. J. Korean Phys. Soc. 2022, 80, 817–851. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of Plasma-Activated Water Treatment on Seed Germination and Growth of Mung Bean Sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma, a Novel Promising Anti-Cancer Treatment Modality. Oncotarget 2016, 8, 15977. [Google Scholar] [CrossRef]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen Peroxide Signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Ono, R.; Hayashi, N. Variation of Antioxidative Activity and Growth Enhancement of Brassicaceae Induced by Low-Pressure Oxygen Plasma. Jpn. J. Appl. Phys. 2015, 54, 06GD03. [Google Scholar] [CrossRef]
- Sera, B.; Spatenka, P.; Michal, S.; Vrchotova, N.; Hruskova, I. Influence of Plasma Treatment on Wheat and Oat Germination and Early Growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Abarghuei, F.M.; Etemadi, M.; Ramezanian, A.; Esehaghbeygi, A.; Alizargar, J. An Application of Cold Atmospheric Plasma to Enhance Physiological and Biochemical Traits of Basil. Plants 2021, 10, 2088. [Google Scholar] [CrossRef]
- Khanam, S.; Amsalu, K.; Kifle, R.; Han, I.; Choi, E.H. Exploring the Combined Effects of Plasma-Treated Water and Seaweed Biostimulants on Tomato (Solanum lycopersicum) Growth and Bioactive Performance. Food Chem. 2025, 487, 144747. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Z.; Wu, W. POD, CAT and SOD Enzyme Activity of Corn Kernels as Affected by Low Plasma Pretreatment. Int. J. Food Prop. 2023, 26, 38–48. [Google Scholar] [CrossRef]
- Yoshimura, K.; Yabuta, Y.; Ishikawa, T.; Shigeoka, S. Expression of Spinach Ascorbate Peroxidase Isoenzymes in Response to Oxidative Stresses. Plant Physiol. 2000, 123, 223–234. [Google Scholar] [CrossRef]
- Hosseini, S.I.; Mohsenimehr, S.; Hadian, J.; Ghorbanpour, M.; Shokri, B. Physico-Chemical Induced Modification of Seed Germination and Early Development in Artichoke (Cynara scolymus L.) Using Low Energy Plasma Technology. Phys. Plasmas 2018, 25, 013525. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic Acid in Plant: Still a Mystery Unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Matakiadis, T.; Alboresi, A.; Jikumaru, Y.; Tatematsu, K.; Pichon, O.; Renou, J.-P.; Kamiya, Y.; Nambara, E.; Truong, H.-N. The Arabidopsis Abscisic Acid Catabolic Gene CYP707A2 Plays a Key Role in Nitrate Control of Seed Dormancy. Plant Physiol. 2009, 149, 949–960. [Google Scholar] [CrossRef]
- Boccaccini, A.; Lorrai, R.; Ruta, V.; Frey, A.; Mercey-Boutet, S.; Marion-Poll, A.; Tarkowska, D.; Strnad, M.; Costantino, P.; Vittorioso, P. The DAG1 Transcription Factor Negatively Regulates the Seed-to-Seedling Transition in Arabidopsis Acting on ABA and GA Levels. BMC Plant Biol. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid Metabolism in Ripening Fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Postharvest UV-C Irradiation Increased the Flavonoids and Anthocyanins Accumulation, Phenylpropanoid Pathway Gene Expression, and Antioxidant Activity in Sweet Cherries (Prunus avium L.). Postharvest Biol. Technol. 2021, 175, 111490. [Google Scholar] [CrossRef]
- Ngadze, E.; Icishahayo, D.; Coutinho, T.A.; der Waals, J.E. Role of Polyphenol Oxidase, Peroxidase, Phenylalanine Ammonia Lyase, Chlorogenic Acid, and Total Soluble Phenols in Resistance of Potatoes to Soft Rot. Plant Dis. 2012, 96, 186–192. [Google Scholar] [CrossRef]
- Turpaev, K. Reactive Oxygen Species and Regulation of Gene Expression. Biochem. 2002, 67, 281–292. [Google Scholar] [CrossRef]
- Xu, J.; Kang, Z.; Zhu, K.; Zhao, D.; Yuan, Y.; Yang, S.; Zhen, W.; Hu, X. RBOH1-Dependent H2O2 Mediates Spermine-Induced Antioxidant Enzyme System to Enhance Tomato Seedling Tolerance to Salinity—Alkalinity Stress. Plant Physiol. Biochem. 2021, 164, 237–246. [Google Scholar] [CrossRef]
- Koussounadis, A.; Langdon, S.P.; Um, I.H.; Harrison, D.J.; Smith, V.A. Relationship between Differentially Expressed MRNA and MRNA-Protein Correlations in a Xenograft Model System. Sci. Rep. 2015, 5, 10775. [Google Scholar] [CrossRef]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold Plasma Effects on Enzyme Activity in a Model Food System. Innov. Food Sci. Emerg. Technol. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. food Anal. Chem. 2001, 1, 3–4. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Khanam, S.; Hong, Y.J.; Kim, Y.; Choi, E.H.; Han, I. Enhancing Ferroptosis in Lung Adenocarcinoma Cells via the Synergistic Action of Nonthermal Biocompatible Plasma and a Bioactive Phenolic Compound. Biomolecules 2025, 15, 691. [Google Scholar] [CrossRef]

| Gene | Forward | Reverse |
|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) endogenous gene | AACATTATCCCCAGCAGCAC | TAGGAACTCGGAATGCCATC |
| Gibberellin 3-beta-dioxygenase2 (GA3OX1) | GTCGGAGTCCTTCCAACTGAA | GGCCGTTGGATAATATGTGG |
| Gibberellin 3-beta-dioxygenase2 (GA3OX2) | TTTGCCAAGCAAATGTGGTA | TCTGGACCAGCCCATTCTAC |
| Abscisic Acid-Insensitive 5 (ABI5) | ACCACCGCATGATCAAGAAC | GGTGGTTTAGTTCGGCTTCA |
| Phenylalanine lyase (PAL) | CCTCAACATCACTCCATGCC | CTCAAAGAAGGACGGGACGC |
| 4-Coumarate:CoA ligase (4CL) | TCGTAGACAGGGTGAAGGAGC | CTTCACCAGCAGCCTCATCT |
| Respiratory Burst Oxidase Homolog 1 (RBOH1) | CGAAGCATTTCTCGCAAG | TTGAGTCCACGAAGAGCCTT |
| Superoxide dismutase [Cu-Zn] 1 (SOD) | GACCACATTACAATCCTGCTG | CAATGATGGACTGTGGACCAG |
| Arginine-tRNAligase (A0A6J0KTF3) | ATGCAATGGGTCTCTGGTCA | GCCCATGTGTCAATTCCGTT |
| Lectin-B-like (A0A6J0NWX0) | ATGTTCCCGTCCTGCAGT | TGGTGGGATTAGGAGGAGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanam, S.; Hong, Y.J.; Choi, E.H.; Han, I. Investigating Seed Germination, Seedling Growth, and Enzymatic Activity in Onion (Allium cepa) Under the Influence of Plasma-Treated Water. Int. J. Mol. Sci. 2025, 26, 7256. https://doi.org/10.3390/ijms26157256
Khanam S, Hong YJ, Choi EH, Han I. Investigating Seed Germination, Seedling Growth, and Enzymatic Activity in Onion (Allium cepa) Under the Influence of Plasma-Treated Water. International Journal of Molecular Sciences. 2025; 26(15):7256. https://doi.org/10.3390/ijms26157256
Chicago/Turabian StyleKhanam, Sabnaj, Young June Hong, Eun Ha Choi, and Ihn Han. 2025. "Investigating Seed Germination, Seedling Growth, and Enzymatic Activity in Onion (Allium cepa) Under the Influence of Plasma-Treated Water" International Journal of Molecular Sciences 26, no. 15: 7256. https://doi.org/10.3390/ijms26157256
APA StyleKhanam, S., Hong, Y. J., Choi, E. H., & Han, I. (2025). Investigating Seed Germination, Seedling Growth, and Enzymatic Activity in Onion (Allium cepa) Under the Influence of Plasma-Treated Water. International Journal of Molecular Sciences, 26(15), 7256. https://doi.org/10.3390/ijms26157256








