The Procaine-Based ProcCluster® Impedes the Second Envelopment Process of Herpes Simplex Virus Type 1
Abstract
1. Introduction
2. Results
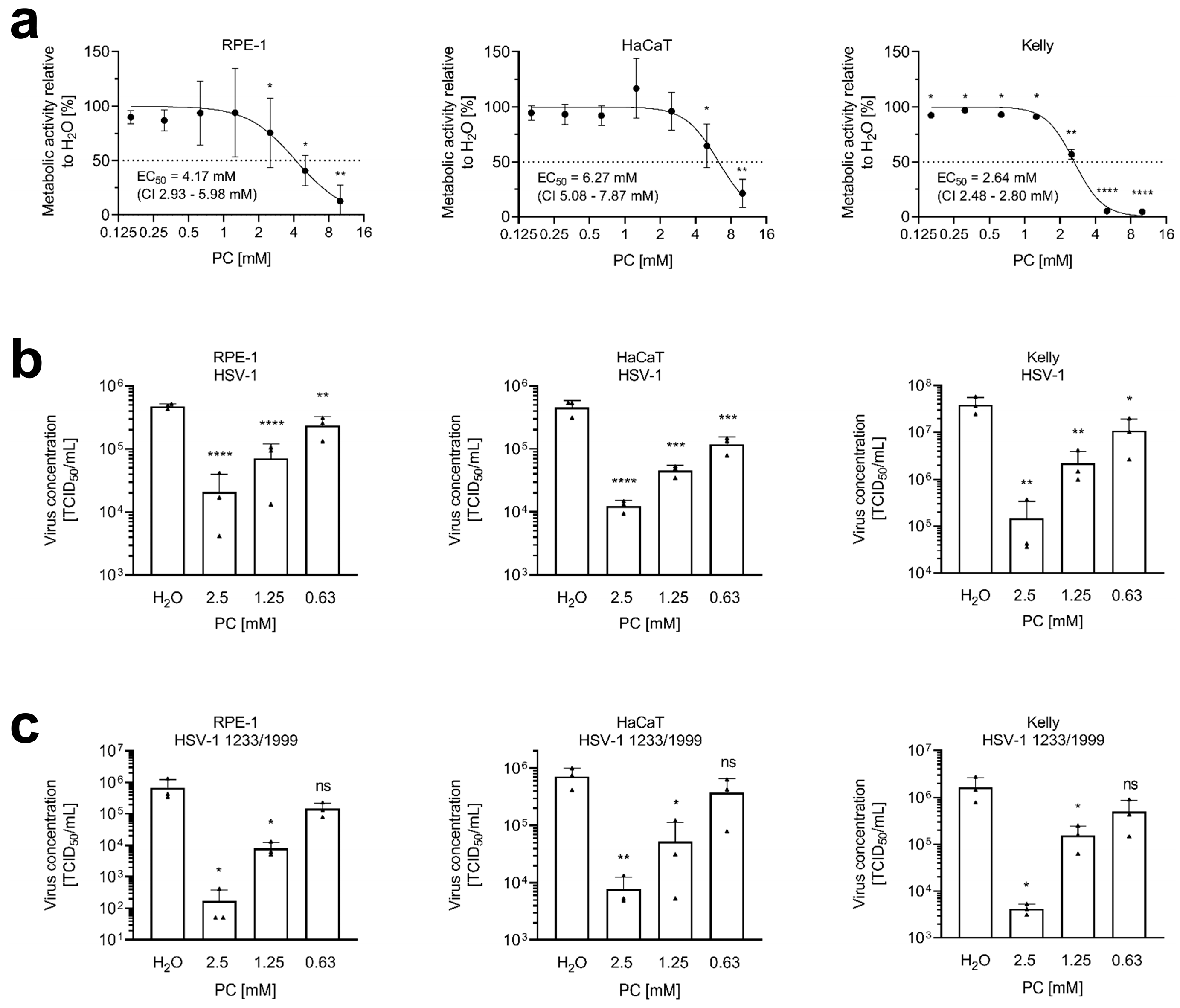
2.1. ProcCluster® Inhibits HSV-1 Replication
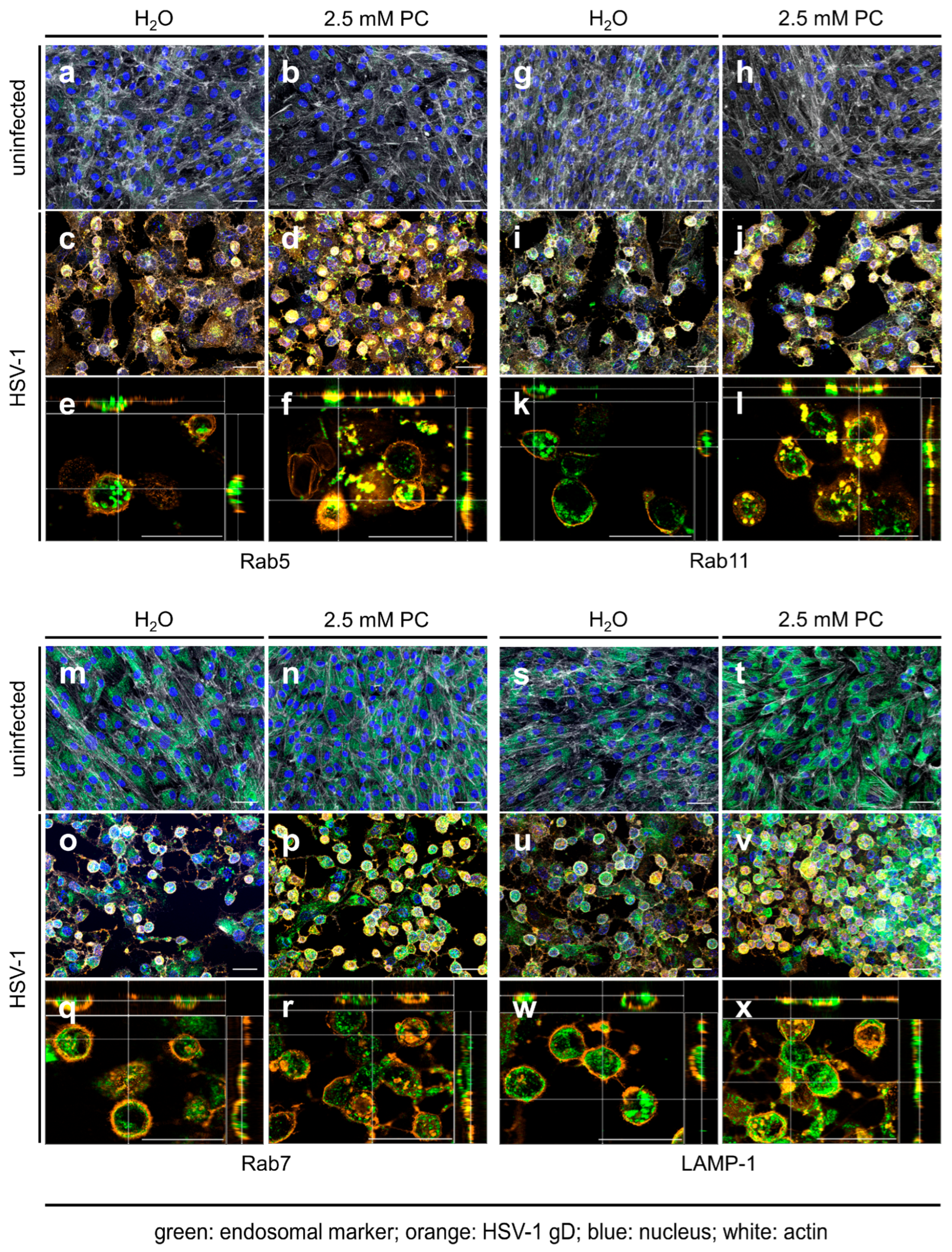
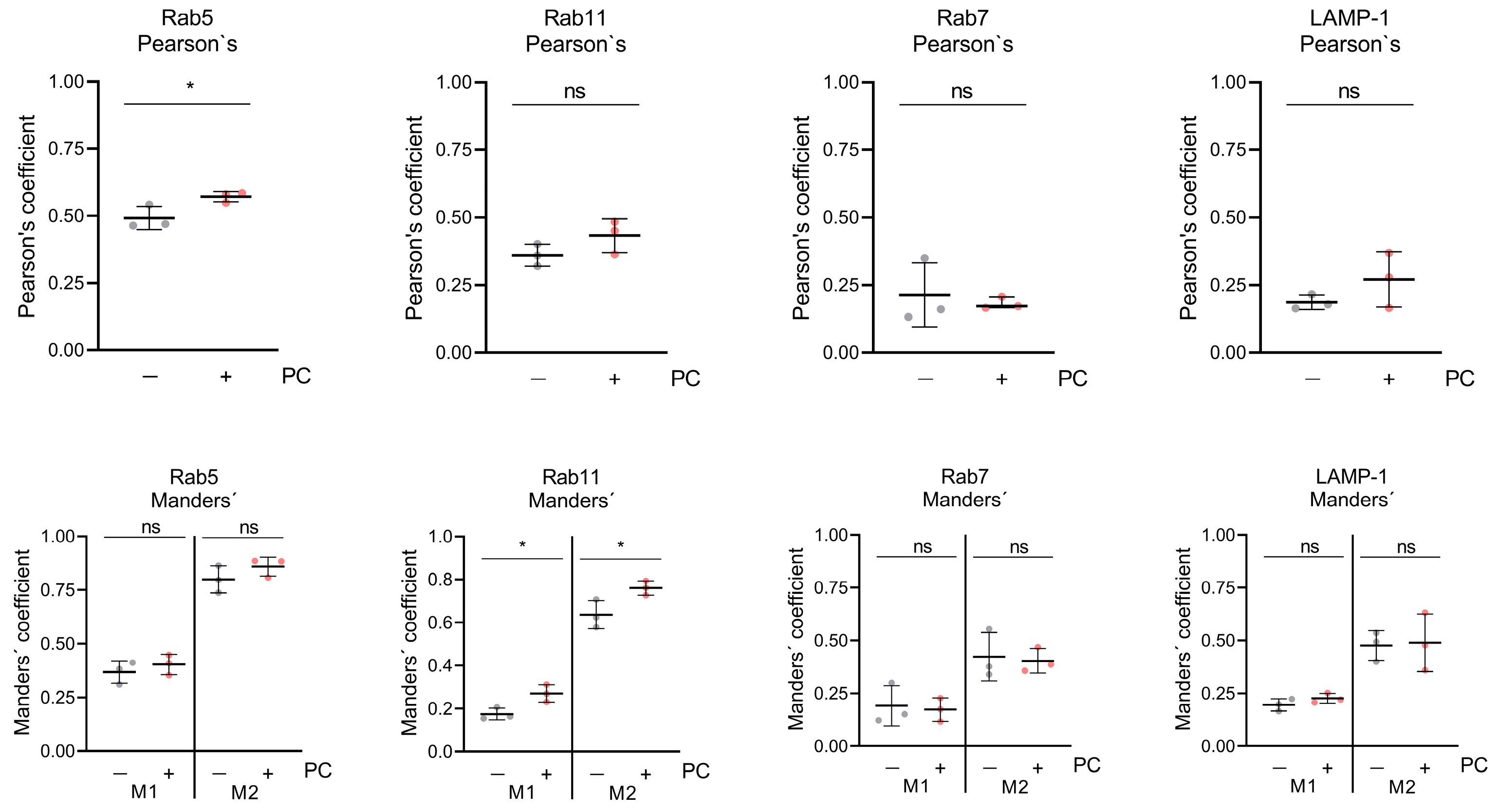
2.2. ProcCluster® Application Induces the Accumulation of the Viral Glycoprotein gD with Endosomal Host Cell Proteins
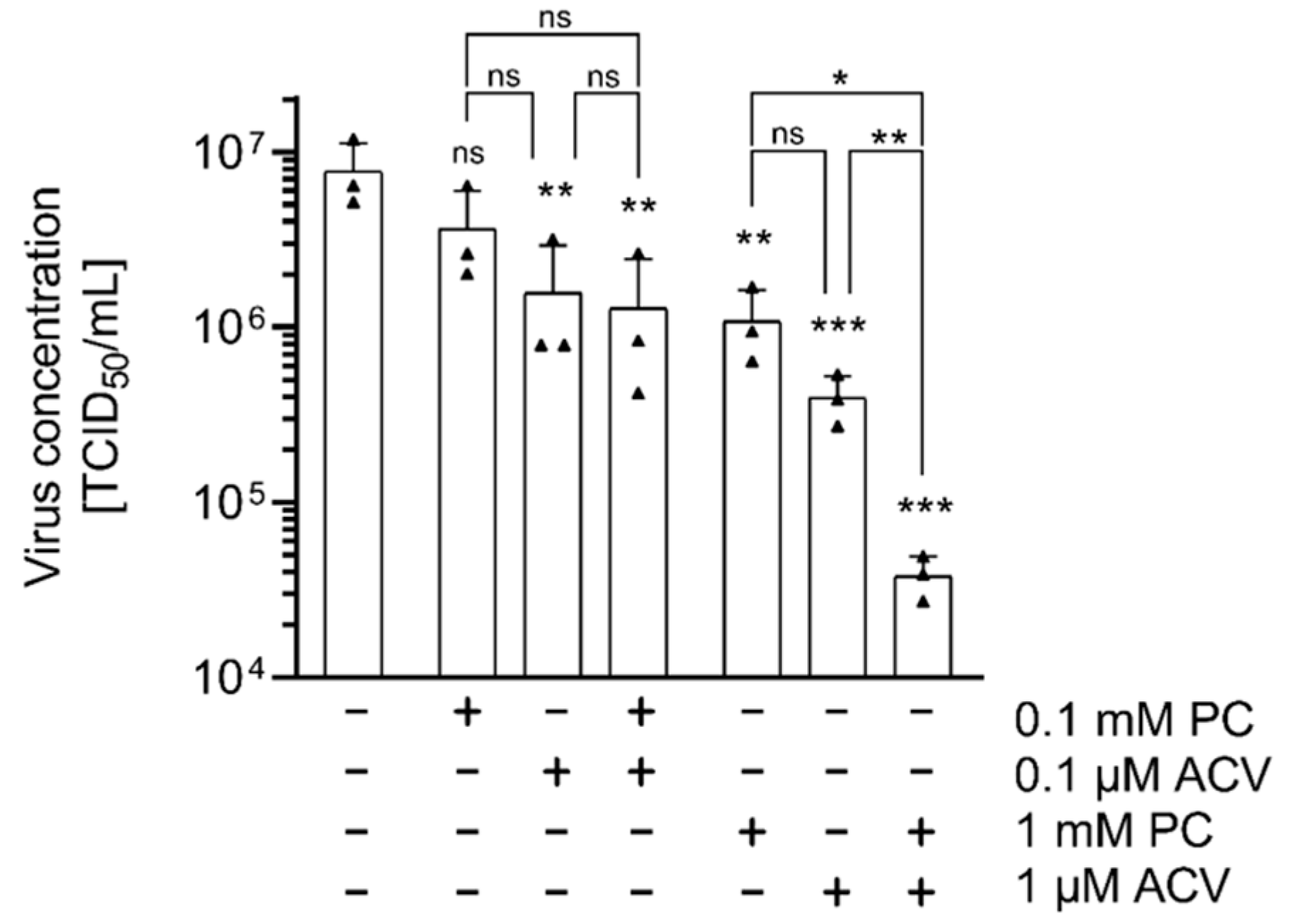
2.3. HSV-1 Replication Is Strongly Inhibited by the Combinatorial Treatment with PC and ACV
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Viruses
4.2. MTT Assay
4.3. Viral Infection and TCID50 Titration
4.4. Immunoblot and Antibodies
4.5. Antibody Staining for Immunofluorescence Microscopy
4.6. Wide-Field Fluorescence Microscopy
4.7. Co-Localization Analysis
4.8. Quantification of the gD Fluorescence Signal
4.9. Statistics
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACV | Acyclovir |
| CDV | Cidofovir |
| CI | Confidence interval |
| dsDNA | Double-stranded DNA |
| E | Early |
| EBV | Epstein–Barr virus |
| FBS | Fetal bovine serum |
| GDP | Guanosine diphosphate |
| GTP | Guanosine triphosphate |
| GTPases | Guanosine triphosphatases |
| HaCaT | Human adult keratinocytes |
| HBV | Hepatitis B virus |
| HIV | Human immunodeficiency virus |
| HSV-1 | Herpes simplex virus type 1 |
| IAV | Influenza A virus |
| IE | Immediate early |
| L | Late |
| MIP | Maximum intensity projection |
| PLA2 | Phospholipase A2 |
| PC | ProcCluster® |
| POI | Protein of interest |
| POL | Polymerase |
| Rab | Ras-related protein in brain |
| ROI | Region of interest |
| RPE-1 | Human retinal pigmented epithelial cell |
| RSV | Respiratory syncytial virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TK | Thymidine kinase |
| vRNP | Viral ribonucleoprotein |
| VPs | Virus-encoded proteins |
| WHO | World Health Organisation |
References
- Fauquet, C.M. Taxonomy, Classification and Nomenclature of Viruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 9–23. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jaggi, U.; Ghiasi, H. Knockout of signal peptide peptidase in the eye reduces HSV-1 replication and eye disease in ocularly infected mice. PLoS Pathog. 2022, 18, e1010898. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Berrington, W.R.; Jerome, K.R.; Cook, L.; Wald, A.; Corey, L.; Casper, C. Clinical correlates of herpes simplex virus viremia among hospitalized adults. Clin. Infect. Dis. 2009, 49, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- James, S.H.; Prichard, M.N. Current and future therapies for herpes simplex virus infections: Mechanism of action and drug resistance. Curr. Opin. Virol. 2014, 8, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Toma, H.S.; Murina, A.T.; Areaux, R.G., Jr.; Neumann, D.M.; Bhattacharjee, P.S.; Foster, T.P.; Kaufman, H.E.; Hill, J.M. Ocular HSV-1 latency, reactivation and recurrent disease. Semin. Ophthalmol. 2008, 23, 249–273. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, D.J.; Rixon, F.J.; Davison, A.J. Topics in herpesvirus genomics and evolution. Virus Res. 2006, 117, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C. Budding events in herpesvirus morphogenesis. Virus Res. 2004, 106, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, R.J.; Miranda-Saksena, M.; Douglas, M.W.; Cunningham, A.L. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 2008, 18, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Shukla, D. Viral entry mechanisms: Cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009, 276, 7228–7236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef] [PubMed]
- Honess, R.W.; Roizman, B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 1974, 14, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wang, M.; Cheng, A.; Jia, R.; Yang, Q.; Wu, Y.; Zhu, D.; Zhao, X.; Chen, S.; Liu, M.; et al. The Role of VP16 in the Life Cycle of Alphaherpesviruses. Front. Microbiol. 2020, 11, 1910. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C.; Klupp, B.G.; Granzow, H. Herpesvirus assembly: A tale of two membranes. Curr. Opin. Microbiol. 2006, 9, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Skepper, J.N.; Whiteley, A.; Browne, H.; Minson, A. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 2001, 75, 5697–5702. [Google Scholar] [CrossRef] [PubMed]
- Hollinshead, M.; Johns, H.L.; Sayers, C.L.; Gonzalez-Lopez, C.; Smith, G.L.; Elliott, G. Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus. EMBO J. 2012, 31, 4204–4220. [Google Scholar] [CrossRef] [PubMed]
- Johns, H.L.; Gonzalez-Lopez, C.; Sayers, C.L.; Hollinshead, M.; Elliott, G. Rab6 dependent post-Golgi trafficking of HSV1 envelope proteins to sites of virus envelopment. Traffic 2014, 15, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Albecka, A.; Laine, R.F.; Janssen, A.F.; Kaminski, C.F.; Crump, C.M. HSV-1 Glycoproteins Are Delivered to Virus Assembly Sites Through Dynamin-Dependent Endocytosis. Traffic 2016, 17, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Wisner, T.W.; Wright, C.C. Herpes simplex virus glycoproteins gB and gD function in a redundant fashion to promote secondary envelopment. J. Virol. 2011, 85, 4910–4926. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 2021, 288, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Herve, J.C.; Bourmeyster, N. Rab GTPases, master controllers of eukaryotic trafficking. Small GTPases 2018, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Waschbusch, D.; Khan, A.R. Phosphorylation of Rab GTPases in the regulation of membrane trafficking. Traffic 2020, 21, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Brishti, M.A.; Leo, M.D. Rab GTPases as Modulators of Vascular Function. Cells 2022, 11, 3061. [Google Scholar] [CrossRef] [PubMed]
- Spearman, P. Viral interactions with host cell Rab GTPases. Small GTPases 2018, 9, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A. Epstein-Barr Virus Exploits the Secretory Pathway to Release Virions. Microorganisms 2020, 8, 729. [Google Scholar] [CrossRef] [PubMed]
- Caillet, M.; Janvier, K.; Pelchen-Matthews, A.; Delcroix-Genete, D.; Camus, G.; Marsh, M.; Berlioz-Torrent, C. Rab7A is required for efficient production of infectious HIV-1. PLoS Pathog. 2011, 7, e1002347. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.J.; Bruce, E.A.; Read, E.K.; Foeglein, A.; Mahen, R.; Stuart, A.D.; Digard, P. A Rab11- and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA. J. Virol. 2011, 85, 4143–4156. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, A.J.; Kawakami, E.; Watanabe, T.; Neumann, G.; Kawaoka, Y. RAB11A is essential for transport of the influenza virus genome to the plasma membrane. J. Virol. 2011, 85, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Momose, F.; Sekimoto, T.; Ohkura, T.; Jo, S.; Kawaguchi, A.; Nagata, K.; Morikawa, Y. Apical transport of influenza A virus ribonucleoprotein requires Rab11-positive recycling endosome. PLoS ONE 2011, 6, e21123. [Google Scholar] [CrossRef] [PubMed]
- de Castro Martin, I.F.; Fournier, G.; Sachse, M.; Pizarro-Cerda, J.; Risco, C.; Naffakh, N. Influenza virus genome reaches the plasma membrane via a modified endoplasmic reticulum and Rab11-dependent vesicles. Nat. Commun. 2017, 8, 1396. [Google Scholar] [CrossRef] [PubMed]
- Utley, T.J.; Ducharme, N.A.; Varthakavi, V.; Shepherd, B.E.; Santangelo, P.J.; Lindquist, M.E.; Goldenring, J.R.; Crowe, J.E., Jr. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. Proc. Natl. Acad. Sci. USA 2008, 105, 10209–10214. [Google Scholar] [CrossRef] [PubMed]
- Zeyen, L.; Prange, R. Host Cell Rab GTPases in Hepatitis B Virus Infection. Front. Cell Dev. Biol. 2018, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Han, L.; Shi, C.; Li, Y.; Qian, S.; Feng, Z.; Yu, L. An updated review of HSV-1 infection-associated diseases and treatment, vaccine development, and vector therapy application. Virulence 2024, 15, 2425744. [Google Scholar] [CrossRef] [PubMed]
- Elion, G.B.; Furman, P.A.; Fyfe, J.A.; de Miranda, P.; Beauchamp, L.; Schaeffer, H.J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. USA 1977, 74, 5716–5720. [Google Scholar] [CrossRef] [PubMed]
- Bacon, T.H.; Levin, M.J.; Leary, J.J.; Sarisky, R.T.; Sutton, D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003, 16, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Piperi, E.; Papadopoulou, E.; Georgaki, M.; Dovrat, S.; Bar Illan, M.; Nikitakis, N.G.; Yarom, N. Management of oral herpes simplex virus infections: The problem of resistance. A narrative review. Oral Dis. 2024, 30, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.M.; Faulds, D. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs 1996, 52, 754–772. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Uta, D.; Tanbo, S.; Kawabata, A.; Kanayama, S.; Osaki, M.; Nozawa, N.; Matsumoto, T.; Andoh, T. Inhibitory effect of amenamevir on acute herpetic pain and postherpetic neuralgia in mice infected with herpes simplex virus-1. J. Dermatol. Sci. 2020, 98, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Greeley, Z.W.; Giannasca, N.J.; Porter, M.J.; Margulies, B.J. Acyclovir, cidofovir, and amenamevir have additive antiviral effects on herpes simplex virus TYPE 1. Antivir. Res. 2020, 176, 104754. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, H.H.; Andrei, G.; Snoeck, R. Combined use of pritelivir with acyclovir or foscarnet suppresses evolution of HSV-1 drug resistance. Virus Evol. 2024, 10, veae101. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, A.; Bohn-Wippert, K.; Kaspar, M.; Krumbholz, A.; Karrasch, M.; Zell, R. Database on natural polymorphisms and resistance-related non-synonymous mutations in thymidine kinase and DNA polymerase genes of herpes simplex virus types 1 and 2. J Antimicrob. Chemother. 2016, 71, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Brunnemann, A.K.; Baukmann, S.; Buhler, S.; Fickenscher, H.; Sauerbrei, A.; Zell, R.; Krumbholz, A. Antiviral susceptibility of recombinant Herpes simplex virus 1 strains with specific polymerase amino acid changes. Antivir. Res. 2021, 195, 105166. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A.; Bellera, C.L. Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics. Expert Opin. Drug Discov. 2020, 15, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Gayretli Aydin, Z.G.; Tanir, G.; Genc Sel, C.; Tasci Yildiz, Y.; Aydin Teke, T.; Kaman, A. Acyclovir Unresponsive Herpes Simplex Encephalitis in a child successfully treated with the addition of Foscarnet: Case report. Arch. Argent Pediatr. 2019, 117, e47–e51. [Google Scholar] [CrossRef] [PubMed]
- Katyal, N.; Taqui, A.M.; Tepper, D.; Beary, J.M.; Newey, C.R. Fulminant Herpes Simplex Virus Type I Encephalitis Despite Maximal Medical Therapy. Cureus 2018, 10, e2467. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, H.H.; Shewakramani, N.R.; Das, K.; Andrei, G.; Snoeck, R. Combination of ganciclovir and trifluridine prevents drug-resistance emergence in HSV-1. Antimicrob. Agents Chemother. 2024, 68, e0011024. [Google Scholar] [CrossRef] [PubMed]
- Hobden, J.A.; Kumar, M.; Kaufman, H.E.; Clement, C.; Varnell, E.D.; Bhattacharjee, P.S.; Hill, J.M. In vitro synergism of trifluorothymidine and ganciclovir against HSV-1. Investig. Ophthalmol. Vis. Sci. 2011, 52, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Priengprom, T.; Ekalaksananan, T.; Kongyingyoes, B.; Suebsasana, S.; Aromdee, C.; Pientong, C. Synergistic effects of acyclovir and 3, 19-isopropylideneandrographolide on herpes simplex virus wild types and drug-resistant strains. BMC Complement Altern Med. 2015, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.; Levanon, A. Inhibition of adsorption of West-Nile and herpes simplex viruses by procaine. Arch. Virol. 1978, 56, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, J.E. Chapter 59—Local Anesthesia. In Pharmacology and Therapeutics; Waldman, S.A., Terzic, A., Egan, L.J., Elghozi, J.-L., Jahangir, A., Kane, G.C., Kraft, W.K., Lewis, L.D., Morrow, J.D., Zingman, L.V., et al., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 863–872. [Google Scholar] [CrossRef]
- Yanagi, K.; Harada, S. Destabilization of herpes simplex virus type 1 virions by local anesthetics, alkaline pH, and calcium depletion. Arch. Virol. 1989, 108, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.D.R.; de Figueiredo, F.A.T.; Macedo, A.P.; Silva, A.C.F.; Ferreira, M.P.; de Freitas, O.; Pedrazzi, V. Local anesthetic improves individuals affected with herpes simplex type 1 labialis. J. Med. Virol. 2020, 92, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Silva-Alvarez, A.F.; de Carvalho, A.C.W.; Benassi-Zanqueta, E.; Oliveira, T.Z.; Fonseca, D.P.; Ferreira, M.P.; Vicentini, F.; Ueda-Nakamura, T.; Pedrazzi, V.; de Freitas, O. Herpes Labialis: A New Possibility for Topical Treatment with Well-Elucidated Drugs. J. Pharm. Sci. 2021, 110, 3450–3456. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.; Jungwirth, J.; Schroeder, J.; Loffler, B.; Engert, B.; Ehrhardt, C. The Local Anaesthetic Procaine Prodrugs ProcCluster® and Procaine Hydrochloride Impair SARS-CoV-2 Replication and Egress In Vitro. Int. J. Mol. Sci. 2023, 24, 14584. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.; Schroeder, J.; Jungwirth, J.; Loffler, B.; Henke, A.; Engert, B.; Ehrhardt, C. ProcCluster® and procaine hydrochloride inhibit the replication of influenza A virus in vitro. Front. Microbiol. 2024, 15, 1422651. [Google Scholar] [CrossRef] [PubMed]
- Konig, S.; Schroeder, J.; Heinekamp, T.; Brakhage, A.A.; Loffler, B.; Engert, B.; Ehrhardt, C. ProcCluster® and procaine hydrochloride inhibit the growth of Aspergillus species and exert antimicrobial properties during coinfection with influenza A viruses and A. fumigatus in vitro. Front. Cell Infect. Microbiol. 2024, 14, 1445428. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Li Puma, D.D.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Paavilainen, H.; Lehtinen, J.; Romanovskaya, A.; Nygardas, M.; Bamford, D.H.; Poranen, M.M.; Hukkanen, V. Inhibition of clinical pathogenic herpes simplex virus 1 strains with enzymatically created siRNA pools. J. Med. Virol. 2016, 88, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Sayers, C.L.; Elliott, G. Herpes Simplex Virus 1 Enters Human Keratinocytes by a Nectin-1-Dependent, Rapid Plasma Membrane Fusion Pathway That Functions at Low Temperature. J. Virol. 2016, 90, 10379–10389. [Google Scholar] [CrossRef] [PubMed]
- Llorente, P.; Mejias, V.; Sastre, I.; Recuero, M.; Aldudo, J.; Bullido, M.J. Matrix metalloproteinase 14 regulates HSV-1 infection in neuroblastoma cells. Antivir. Res. 2021, 192, 105116. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Xu, J.; Zeng, L.; Zhang, L.; Zhou, F. A review of HSV pathogenesis, vaccine development, and advanced applications. Mol. Biomed. 2024, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Zenner, H.L.; Yoshimura, S.; Barr, F.A.; Crump, C.M. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J. Virol. 2011, 85, 8012–8021. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Alvisi, G.; Shahin, F.; Husain, U.; Rabbani, M.; Yaqub, T.; Anjum, A.A.; Sheikh, A.A.; Nawaz, M.; Ali, M.A. Role of Rab GTPases in HSV-1 infection: Molecular understanding of viral maturation and egress. Microb. Pathog. 2018, 118, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, D.; Ungurianu, A.; Margina, D.; Moreno-Villanueva, M.; Burkle, A. Procaine-The Controversial Geroprotector Candidate: New Insights Regarding Its Molecular and Cellular Effects. Oxid. Med. Cell Longev. 2021, 2021, 3617042. [Google Scholar] [CrossRef] [PubMed]
- Grage, S.L.; Culetto, A.; Ulrich, A.S.; Weinschenk, S. Membrane-Mediated Activity of Local Anesthetics. Mol. Pharmacol. 2021, 100, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Cheshenko, N.; Del Rosario, B.; Woda, C.; Marcellino, D.; Satlin, L.M.; Herold, B.C. Herpes simplex virus triggers activation of calcium-signaling pathways. J. Cell Biol. 2003, 163, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xiang, Y.; Wang, Q.; Jin, F.; Chen, M.; Ma, K.; Ren, Z.; Wang, Y. Calcium-signal facilitates herpes simplex virus type 1 nuclear transport through slingshot 1 and calpain-1 activation. Virus Res. 2014, 188, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hsia, S.C.; Martin-Caraballo, M. Regulation of T-type Ca2+ channel expression by interleukin-6 in sensory-like ND7/23 cells post-herpes simplex virus (HSV-1) infection. J. Neurochem. 2019, 151, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, G.; Pritchard, S.M.; Nicola, A.V. Herpes Simplex Virus Entry by a Nonconventional Endocytic Pathway. J. Virol. 2020, 94, e01910-20. [Google Scholar] [CrossRef] [PubMed]
- Hilterbrand, A.T.; Daly, R.E.; Heldwein, E.E. Contributions of the Four Essential Entry Glycoproteins to HSV-1 Tropism and the Selection of Entry Routes. mBio 2021, 12, e00143-21. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Ren, Y.; Li, S.; Xu, J.; Wu, Y.; Cao, Z. ML-SA1 and SN-2 inhibit endocytosed viruses through regulating TRPML channel expression and activity. Antivir. Res. 2021, 195, 105193. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.S.; He, R.; Hoffmann, H.H.; Das, T.; Thinon, E.; Rice, C.M.; Peng, T.; Chandran, K.; Hang, H.C. IFITM3 directly engages and shuttles incoming virus particles to lysosomes. Nat. Chem. Biol. 2019, 15, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Welsch, S.; Muller, B.; Krausslich, H.G. More than one door—Budding of enveloped viruses through cellular membranes. FEBS Lett. 2007, 581, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Calistri, A.; Sette, P.; Salata, C.; Cancellotti, E.; Forghieri, C.; Comin, A.; Gottlinger, H.; Campadelli-Fiume, G.; Palu, G.; Parolin, C. Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies. J. Virol. 2007, 81, 11468–11478. [Google Scholar] [CrossRef] [PubMed]
- Erard, V.; Wald, A.; Corey, L.; Leisenring, W.M.; Boeckh, M. Use of long-term suppressive acyclovir after hematopoietic stem-cell transplantation: Impact on herpes simplex virus (HSV) disease and drug-resistant HSV disease. J. Infect. Dis. 2007, 196, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Zuckerman, R.A.; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13526. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Bacon, T.H.; Leary, J.J. Resistance of herpes simplex virus infections to nucleoside analogues in HIV-infected patients. Clin. Infect. Dis. 2004, 39, S248–S257. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, E.; Clementi, N.; Mancini, N.; Burioni, R.; Miduri, M.; Castelli, M.; Clementi, M. Synergy evaluation of anti-Herpes Simplex Virus type 1 and 2 compounds acting on different steps of virus life cycle. Antivir. Res. 2018, 151, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, T.; Mallick, S.; Patel, P.; Koganti, R.; Shukla, D.; Date, A.A. Pharmaceutically Acceptable Carboxylic Acid-Terminated Polymers Show Activity and Selectivity against HSV-1 and HSV-2 and Synergy with Antiviral Drugs. ACS Infect. Dis. 2020, 6, 2926–2937. [Google Scholar] [CrossRef] [PubMed]
- Graber, K.; Khan, F.; Gluck, B.; Weigel, C.; Marzo, S.; Doshi, H.; Ehrhardt, C.; Heller, R.; Graler, M.; Henke, A. The role of sphingosine-1-phosphate signaling in HSV-1-infected human umbilical vein endothelial cells. Virus Res. 2020, 276, 197835. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Linkert, M.; Rueden, C.T.; Allan, C.; Burel, J.M.; Moore, W.; Patterson, A.; Loranger, B.; Moore, J.; Neves, C.; Macdonald, D.; et al. Metadata matters: Access to image data in the real world. J. Cell Biol. 2010, 189, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Zack, G.W.; Rogers, W.E.; Latt, S.A. Automatic measurement of sister chromatid exchange frequency. J. Histochem. Cytochem. 1977, 25, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K. Mathematical Contributions to the Theory of Evolution. III. Regression, Heredity, and Panmixia. Philos. Trans. R. Soc. Lond. Ser. A 1896, 187, 253–318. [Google Scholar] [CrossRef]
- Manders, E.M.; Stap, J.; Brakenhoff, G.J.; van Driel, R.; Aten, J.A. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J. Cell Sci. 1992, 103, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Chiaruttini, N.; Burri, O. ijp-jacop-b. Available online: https://github.com/BIOP/ijp-jacop-b (accessed on 12 July 2025).
- Li, C.H.; Tam, P.K.S. An iterative algorithm for minimum cross entropy thresholding. Pattern Recognit. Lett. 1998, 19, 771–776. [Google Scholar] [CrossRef]
| Antibody | Source and Catalogue Number |
|---|---|
| mAb HSV-1 icp0 | sc-53070 (Santa Cruz Biotechnology, Heidelberg, Germany) |
| mAb HSV-1 icp8 | sc-53329 (Santa Cruz Biotechnology, Heidelberg, Germany) |
| mAb HSV-1 gD | sc-21719 (Santa Cruz Biotechnology, Heidelberg, Germany) |
| mAb α-tubulin | 2125 (Cell signaling Technology, Wetzlar, Germany) |
| pAb HSP90 | 4877 (Cell signaling Technology, Wetzlar, Germany) |
| WesternSure® HRP goat anti-mouse IgG | 926-80010 (LICORbio, Bad Homburg, Germany) |
| WesternSure® HRP goat anti-rabbit IgG | 926-80011 (LICORbio, Bad Homburg, Germany) |
| Antibody | Source and Catalogue Number |
|---|---|
| mAb HSV-1 gD (1:125) | sc-21719 (Santa Cruz Biotechnology, Heidelberg, Germany) |
| mAb Rab11 (1:125) | 5589 (Cell signaling Technology, Wetzlar, Germany) |
| mAb Rab5 (1:125) | 3547 (Cell signaling Technology, Wetzlar, Germany) |
| mAb Rab7 (1:125) | 9367 (Cell signaling Technology, Wetzlar, Germany) |
| mAb LAMP1 (1:125) | 9091 (Cell signaling Technology, Wetzlar, Germany) |
| AffiniPure Goat Anti-Mouse Cy 3 (1:500) | 115-165-003 (Dianova, Hamburg, Germany) |
| AffiniPure Goat Anti-Rabbit IgG Alexa Fluor 488 (1:500) | 111-545-144 (Dianova, Hamburg, Germany) |
| Hoechst 33342 (1:1000) | 14533 (Sigma-Aldrich®, Taufkirchen, Germany) |
| Phalloidin-iFluorTM 647 (1:1000) | ABD-23127 (Biomol, Hamburg, Germany) |
| Figure | Component | Chromophore | Light Intensity [%] | Exposure Time [ms] | Displayed Dynamic Range |
|---|---|---|---|---|---|
| a–d | Actin | iFluorTM 647 | 100 | 1000 | 200–10,000 |
| HSV-1 gD | Cy3 | 70 | 70 | 200–4000 | |
| Rab5 | Alexa Fluor 488 | 80 | 400 | 200–4000 | |
| Nucleus | Hoechst33342 | 80 | 200 | 200–7000 | |
| e,f | HSV-1 gD | Cy3 | 80 | 100 | 200–3000 |
| Rab5 | Alexa Fluor 488 | 90 | 650 | 200–2000 | |
| g–j | Actin | iFluorTM 647 | 100 | 600 | 200–8000 |
| HSV-1 gD | Cy3 | 70 | 50 | 200–4000 | |
| Rab11 | Alexa Fluor 488 | 100 | 1000 | 200–4000 | |
| Nucleus | Hoechst33342 | 80 | 100 | 200–7000 | |
| k,l | Nucleus | Hoechst33342 | 80 | 100 | 200–7000 |
| HSV-1 gD | Cy3 | 100 | 400 | 30–2000 | |
| m–p | Actin | iFluorTM 647 | 100 | 800 | 200–6000 |
| HSV-1 gD | Cy3 | 70 | 50 | 200–3000 | |
| Rab7 | Alexa Fluor 488 | 100 | 1000 | 200–3000 | |
| Nucleus | Hoechst33342 | 80 | 150 | 200–5000 | |
| q,r | HSV-1 gD | Cy3 | 100 | 400 | 200–4000 |
| Rab7 | Alexa Fluor 488 | 100 | 5000 | 200–4000 | |
| s–v | Actin | iFluorTM 647 | 100 | 500 | 200–7000 |
| HSV-1 gD | Cy3 | 80 | 90 | 200–5000 | |
| LAMP1 | Alexa Fluor 488 | 100 | 400 | 200–5000 | |
| Nucleus | Hoechst33342 | 80 | 70 | 200–7000 | |
| w,x | HSV-1 gD | Cy3 | 100 | 200 | 200–3000 |
| LAMP1 | Alexa Fluor 488 | 100 | 2500 | 200–2000 |
| Brightest Slice in Orange Channel | Brightest Slice in Green Channel | Chosen Slice for Co-Localization Analysis |
|---|---|---|
| 19 | 21 | 20 |
| 23 | 27 | 25 |
| 20 | 23 | 21 |
| 17 | 22 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungwirth, J.; Siegert, L.; Gauthier, L.; Henke, A.; Krämer, O.H.; Engert, B.; Ehrhardt, C. The Procaine-Based ProcCluster® Impedes the Second Envelopment Process of Herpes Simplex Virus Type 1. Int. J. Mol. Sci. 2025, 26, 7185. https://doi.org/10.3390/ijms26157185
Jungwirth J, Siegert L, Gauthier L, Henke A, Krämer OH, Engert B, Ehrhardt C. The Procaine-Based ProcCluster® Impedes the Second Envelopment Process of Herpes Simplex Virus Type 1. International Journal of Molecular Sciences. 2025; 26(15):7185. https://doi.org/10.3390/ijms26157185
Chicago/Turabian StyleJungwirth, Johannes, Lisa Siegert, Lena Gauthier, Andreas Henke, Oliver H. Krämer, Beatrice Engert, and Christina Ehrhardt. 2025. "The Procaine-Based ProcCluster® Impedes the Second Envelopment Process of Herpes Simplex Virus Type 1" International Journal of Molecular Sciences 26, no. 15: 7185. https://doi.org/10.3390/ijms26157185
APA StyleJungwirth, J., Siegert, L., Gauthier, L., Henke, A., Krämer, O. H., Engert, B., & Ehrhardt, C. (2025). The Procaine-Based ProcCluster® Impedes the Second Envelopment Process of Herpes Simplex Virus Type 1. International Journal of Molecular Sciences, 26(15), 7185. https://doi.org/10.3390/ijms26157185








