Cytochrome C-like Domain Within the Human BK Channel
Abstract
1. Introduction
2. Results
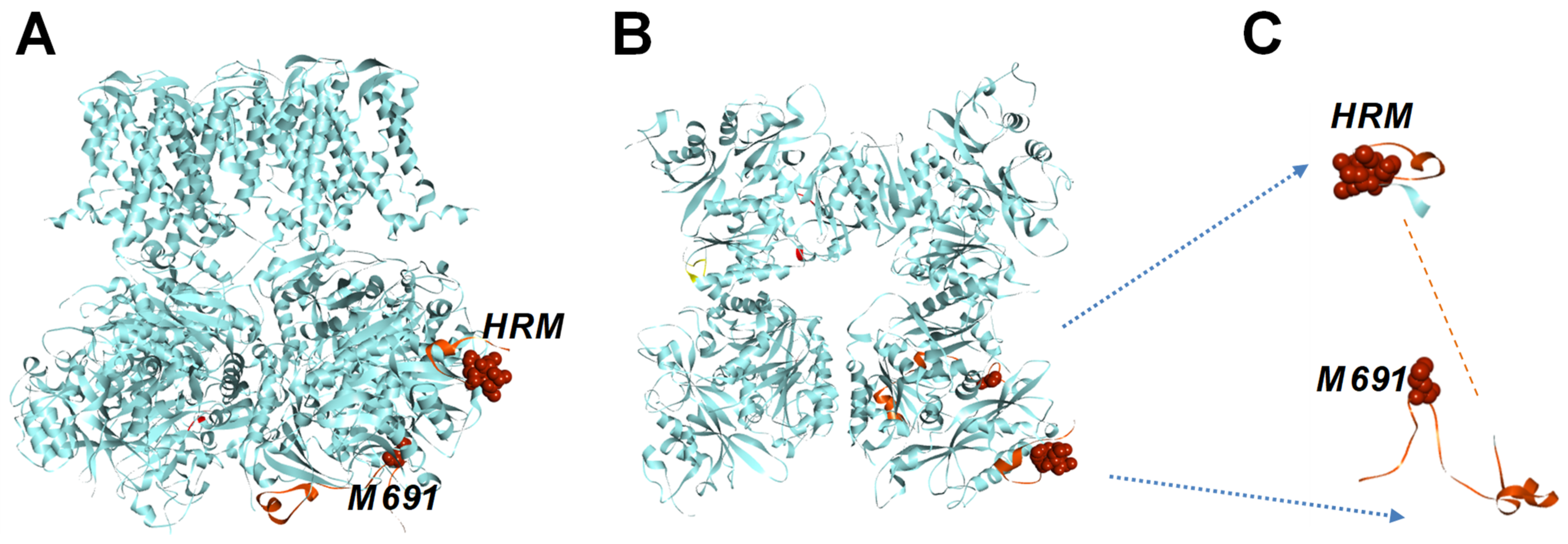
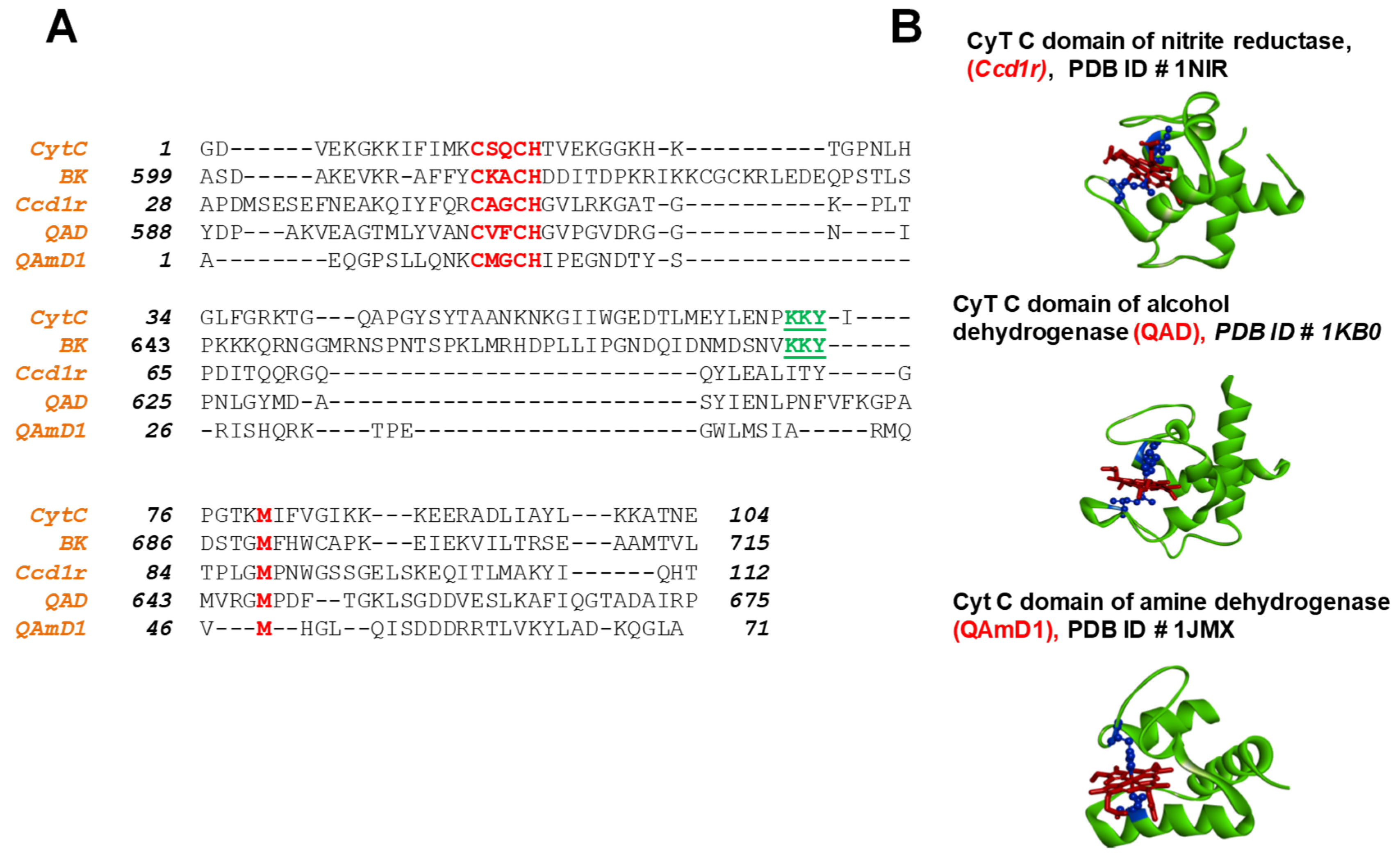
2.1. The Human BK Channel Structure Comprises a Cytochrome C-like Feature Within the Gating Ring
2.2. The Human BK Channel Confers Peroxidase Activity
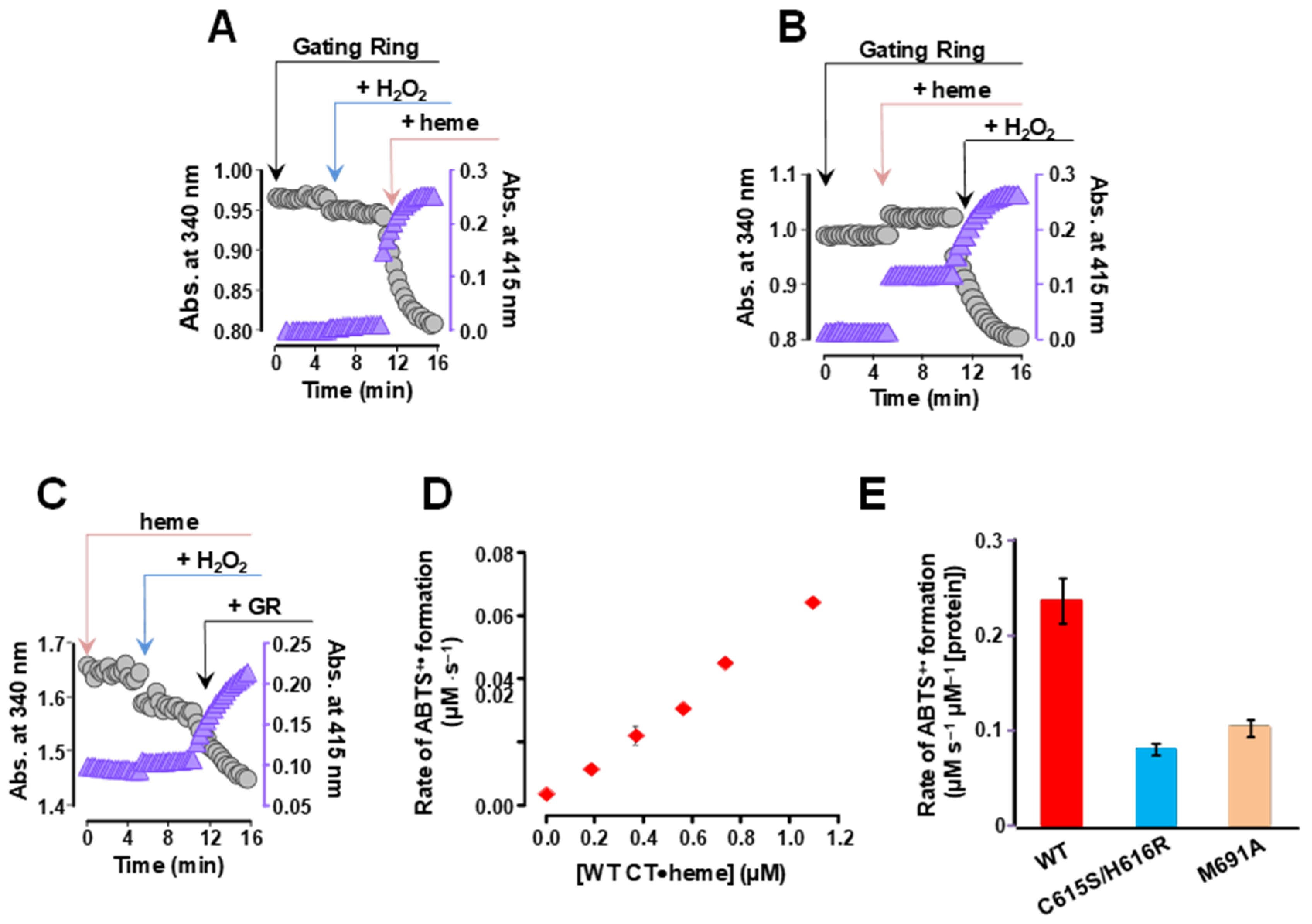
2.3. Peroxidase Activity of the Human BK Channel C-Terminal Domain
2.4. STREX Significantly Augmented CTD Peroxidase Activity
2.5. The Full Human BK Channel Exhibits Peroxidase Activity
3. Discussion
3.1. Cytochrome-C-like Structure Within BK Channel Gating Ring
3.2. The Novel Multifunctional Module Within BK Channels
4. Materials and Methods
4.1. Expression and Purification of BK-Terminal Proteins
4.2. ABTS Assay
4.3. Peroxidase Activity Assay in Cell Lysates
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoshi, T.; Pantazis, A.; Olcese, R. Transduction of voltage and Ca2+ signals by Slo1 BK channels. Physiology 2013, 28, 172–189. [Google Scholar] [CrossRef]
- Cui, J. BK Channel Gating Mechanisms: Progresses Toward a Better Understanding of Variants Linked Neurological Diseases. Front. Physiol. 2021, 12, 762175. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Gonzalez-Sanabria, N.; Alvarado-Sanchez, R.; Fernández, M.; Castillo, K.; Latorre, R. Large conductance voltage-and calcium-activated K+ (BK) channel in health and disease. Front. Pharmacol. 2024, 15, 1373507. [Google Scholar] [CrossRef] [PubMed]
- Sancho, M.; Kyle, B.D. The Large-Conductance, Calcium-Activated Potassium Channel: A Big Key Regulator of Cell Physiology. Front. Physiol. 2021, 12, 750615. [Google Scholar] [CrossRef]
- Meredith, A.L. BK Channelopathies and KCNMA1-Linked Disease Models. Annu. Rev. Physiol. 2024, 86, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Yusifov, T.; Qudretova, F.; Aliyeva, A. Role of Bioelectrical Signaling Networks in Tumor Growth. Am. J. Biomed. Life Sci. 2024, 12, 83–92. [Google Scholar] [CrossRef]
- Yusifov, T.; Savalli, N.; Pantazis, A.; Heinemann, S.H.; Hoshi, T.; Olcese, R. Carbon Monoxide May Regulate BK slo1 Channel Activity by Partially Disrupting Heme Coordination. Biophys. J. 2017, 112, 112a. [Google Scholar] [CrossRef]
- Yusifov, T.; Javaherian, A.; Gandhi, C.; Stefan, H.; Heinemann, T.; Hoshinori, T.; Olcese, R. Heme-Driven Conformational Changes in the Human Slo1 BKCa Channel Gating Ring. Biophys. J. 2010, 100, 581a. [Google Scholar] [CrossRef]
- Yusifov, T.; Javaherian, A.D.; Heinemann, S.H.; Hoshi, T.; Olcese, R. The human BK Channel Gating Ring is a PIP2 Sensor. In Proceedings of the American Society of Anesthesiologists (ASA) 2012 Annual Meeting, Washington, DC, USA, 13–17 October 2012. [Google Scholar] [CrossRef]
- Javaherian, A.D.; Yusifov, T.; Pantazis, A.; Franklin, S.; Gandhi, C.S.; Olcese, R. Metal-driven operation of the human large-conductance voltage- and Ca2+-dependent potassium channel (BK) gating ring apparatus. J. Biol. Chem. 2011, 286, 20701–20709. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, A.; Olcese, R. Biophysics of BK Channel Gating. Int. Rev. Neurobiol. 2016, 128, 1–49. [Google Scholar]
- Hou, S.; Heinemann, S.H.; Hoshi, T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology 2009, 24, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, J.; Kalenik, B.; Wrzosek, A.; Szewczyk, A. Redox Regulation of Mitochondrial Potassium Channels Activity. Antioxidants 2024, 13, 434. [Google Scholar] [CrossRef]
- Hoshi, T.; Heinemann, S.H. Modulation of BK Channels by Small Endogenous Molecules and Pharmaceutical Channel Openers. Int. Rev. Neurobiol. 2016, 541, 193–237. [Google Scholar] [CrossRef]
- Tao, X.; Mackinnon, R. Molecular structures of the human slo1 k+ channel in complex with b4. elife 2019, 8, e51409. [Google Scholar] [CrossRef]
- Hite, R.K.; Tao, X.; MacKinnon, R. Structural basis for gating the high conductance Ca2+-activated K+ channel. Nature 2017, 541, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Leonetti, M.D.; Hsiung, Y.; MacKinnon, R. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature 2012, 481, 94–98. [Google Scholar] [CrossRef]
- Tao, X.; Hite, R.K.; MacKinnon, R. Cryo-EM structure of the open high conductance Ca2+-activated K+ channel. Nature 2017, 541, 46–51. [Google Scholar] [CrossRef]
- Yuan, P.; Leonetti, M.D.; Pico, A.R.; Hsiung, Y.; MacKinnon, R. Structure of the Human BK Channel Ca2+-Activation Apparatus at 3.0 Å Resolution. Science 2010, 80, 182–186. [Google Scholar] [CrossRef]
- Walewska, A.; Szewczyk, A.; Koprowski, P. External Hemin as an Inhibitor of Mitochondrial Large-Conductance Calcium-Activated Potassium Channel Activity. Int. J. Mol. Sci. 2022, 23, 13391. [Google Scholar] [CrossRef]
- Horrigan, F.T.; Heinemann, S.H.; Hoshi, T. Heme regulates allosteric activation of the Slo1 BK channel. J. Gen. Physiol. 2005, 126, 7–21. [Google Scholar] [CrossRef]
- Tang, X.D.; Xu, R.; Reynolds, M.F.; Garcia, M.L.; Heinemann, S.H.; Hoshi, T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature 2003, 425, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Goradia, N.; Ohlenschläger, O.; Schönherr, R.; Friedrich, M.; Plass, W.; Kappl, R.; Hoshi, T.; Heinemann, S.H. Heme impairs the ball-and-chain inactivation of potassium channels. Proc. Natl. Acad. Sci. USA 2013, 110, E4036–E4044. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Kapetanaki, S.M.; Chernova, T.; Jamieson, A.G.; Dorlet, P.; Santolini, J.; Moody, P.C.E.; Mitcheson, J.S.; Davies, N.W.; Schmid, R.; et al. A heme-binding domain controls regulation of ATP-dependent potassium channels. Proc. Natl. Acad. Sci. USA 2016, 113, 3785–3790. [Google Scholar] [CrossRef]
- Wang, S.; Publicover, S.; Gu, Y. An oxygen-sensitive mechanism in regulation of epithelial sodium channel. Proc. Natl. Acad. Sci. USA 2009, 106, 2957–2962. [Google Scholar] [CrossRef]
- Sahoo, N.; Yang, K.; Coburger, I.; Bernert, A.; Swain, S.M.; Gessner, G.; Kappl, R.; Kühl, T.; Imhof, D.; Hoshi, T.; et al. Intracellular hemin is a potent inhibitor of the voltage-gated potassium channel Kv10.1. Sci. Rep. 2022, 12, 14645. [Google Scholar] [CrossRef] [PubMed]
- Coburger, I.; Yang, K.; Bernert, A.; Wiesel, E.; Sahoo, N.; Swain, S.M.; Hoshi, T.; Schönherr, R.; Heinemann, S.H. Impact of intracellular hemin on N-type inactivation of voltage-gated K+ channels. Pflug. Arch. Eur. J. Physiol. 2020, 472, 551–560. [Google Scholar] [CrossRef]
- Williams, S.E.; Wootton, P.; Mason, H.S.; Bould, J.; Iles, D.E.; Riccardi, D.; Peers, C.; Kemp, P.J. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 2004, 306, 2093–2097. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, J.H.; Li, A.; Parfenova, H.; Liu, J.; Umstot, E.S.; Dopico, A.M.; Leffler, C.W. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ. Res. 2005, 97, 805–812. [Google Scholar] [CrossRef]
- Yi, L.; Morgan, J.T.; Ragsdale, S.W. Identification of a thiol/disulfide redox switch in the human BK channel that controls its affinity for heme and CO. J. Biol. Chem. 2010, 285, 20117–20127. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Cavallaro, G.; Rosato, A. Cytochrome c: Occurrence and functions. J. Biol. Chem. 2006, 106, 15194–15200. [Google Scholar] [CrossRef]
- Kagan, V.E. Cytochrome c/cardiolipin relations in mitochondria: A kiss of death. Free Radic. Biol. Med. 2010, 46, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Jeng, W.-Y.; Shiu, J.-H.; Tsai, Y.-H.; Chuang, W.-J. Solution Structure of Reduced Recombinant Human Cytochrome c. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Baistrocchi, P.; Banci, L.; Bertini, I.; Turano, P.; Bren, K.L.; Gray, H.B. Three-dimensional solution structure of Saccharomyces cerevisiae reduced iso-1-cytochrome c. Biochemistry 1996, 35, 13788–13796. [Google Scholar] [CrossRef]
- Nurizzo, D.; Silvestrini, M.C.; Mathieu, M.; Cutruzzolà, F.; Bourgeois, D.; Fülöp, V.; Hajdu, J.; Brunori, M.; Tegoni, M.; Cambillau, C. N-terminal arm exchange is observed in the 2.15 A crystal structure of oxidized nitrite reductase from Pseudomonas aeruginosa. Structure 1997, 5, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Oubrie, A.; Rozeboom, H.J.; Kalk, K.H.; Huizinga, E.G.; Dijkstra, B.W. Crystal structure of quinohemoprotein alcohol dehydrogenase from Comamonas testosteroni: Structural basis for substrate oxidation and electron transfer. J. Biol. Chem. 2002, 277, 3727–3732. [Google Scholar] [CrossRef]
- Satoh, A.; Kim, J.K.; Miyahara, I.; Devreese, B.; Vandenberghe, I.; Hacisalihoglu, A.; Okajima, T.; Kuroda, S.; Adachi, O.; Duine, J.A.; et al. Crystal structure of quinohemoprotein amine dehydrogenase from Pseudomonas putida. Identification of a novel quinone cofactor encaged by multiple thioether cross-bridges. J. Biol. Chem. 2002, 277, 2830–2834. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Polticelli, F.; Marino, M.; Santucci, R.; Coletta, M. Cardiolipin drives cytochrome c proapoptotic and antiapoptotic actions. IUBMB Life 2011, 63, 160–165. [Google Scholar] [CrossRef]
- Belikova, N.A.; Jiang, J.; Tyurina, Y.Y.; Zhao, Q.; Epperly, M.W.; Greenberger, J.; Kagan, V.E. Cardiolipin-Specific Peroxidase Reactions of Cytochrome c in Mitochondria During Irradiation-Induced Apoptosis. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 176–186. [Google Scholar] [CrossRef]
- Ferrer, J.; Wasson, J.; Salkoff, L.; Permutt, M.A. Cloning of human pancreatic islet large conductance Ca2+-activated K+ channel (hSlo) cDNAs: Evidence for high levels of expression in pancreatic islets and identification of a flanking genetic marker. Diabetologia 1996, 39, 891–898. [Google Scholar] [CrossRef]
- Saito, M.; Nelson, C.; Salkoff, L.; Lingle, C.J. A cysteine-rich domain defined by a novel exon in a slo variant in rat adrenal chromaffin cells and PC12 cells. J. Biol. Chem. 1997, 272, 11710–11717. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; McCobb, D.P. Control of alternative splicing of potassium channels by stress hormones. Science 1998, 280, 443–446. [Google Scholar] [CrossRef]
- Tian, L.; Coghill, L.S.; McClafferty, H.; MacDonald, S.H.F.; Antoni, F.A.; Ruth, P.; Knaus, H.G.; Shipston, M.J. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 11897–11902. [Google Scholar] [CrossRef] [PubMed]
- Shipston, M.J. Ion channel regulation by protein palmitoylation. J. Biol. Chem. 2011, 286, 8709–8716. [Google Scholar] [CrossRef]
- Yeudall, S.; Upchurch, C.M.; Leitinger, N. The clinical relevance of heme detoxification by the macrophage heme oxygenase system. Front. Immunol. 2024, 15, 1379967. [Google Scholar] [CrossRef]
- Kubo, Y. A new world of heme function. Pflug. Arch. Eur. J. Physiol. 2020, 472, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Cresser-Brown, J.; Thomas, M.; Portolano, N.; Basran, J.; Freeman, S.L.; Kwon, H.; Bottrill, A.R.; Llansola-Portoles, M.J.; Pascal, A.A.; et al. Discovery of a heme-binding domain in a neuronal voltage-gated potassium channel. J. Biol. Chem. 2020, 295, 13277–13286. [Google Scholar] [CrossRef]
- Kang, S.A.; Crane, B.R. Effects of interface mutations on association modes and electron-transfer rates between proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 15465–15470. [Google Scholar] [CrossRef]
- Nugraheni, A.D.; Ren, C.; Matsumoto, Y.; Nagao, S.; Yamanaka, M.; Hirota, S. Oxidative modification of methionine80 in cytochrome c by reaction with peroxides. J. Inorg. Biochem. 2018, 182, 200–207. [Google Scholar] [CrossRef]
- Zhou, Y.; Lingle, C.J. Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J. Gen. Physiol. 2014, 144, 415–440. [Google Scholar] [CrossRef]
- Shipston, M.J.; Tian, L. Posttranscriptional and Posttranslational Regulation of BKChannels. Int. Rev. Neurobiol. 2016, 128, 91–126. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.E.; McClafferty, H.; Huibant, J.M.; Rowan, E.G.; Shipston, M.J.; Rowe, I.C. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel alpha-subunits. Proc. Natl. Acad. Sci. USA 2005, 102, 17870. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.H.; Johannesen, J.; Shah, K.; Goswami, S.K.; Patel, N.J.; Ponnalagu, D.; Kohut, A.R.; Singh, H. Inhibition of BKCa negatively alters cardiovascular function. Physiol. Rep. 2018, 6, e13748. [Google Scholar] [CrossRef]
- Szteyn, K.; Singh, H. Bkca channels as targets for cardioprotection. Antioxidants 2020, 9, 760. [Google Scholar] [CrossRef]
- Soltysinska, E.; Bentzen, B.H.; Barthmes, M.; Hattel, H.; Thrush, A.B.; Harper, M.-E.; Qvortrup, K.; Larsen, F.J.; Schiffer, T.A.; Losa-Reyna, J.; et al. KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury. PLoS ONE 2014, 9, e103402. [Google Scholar] [CrossRef]
- Yusifov, T.; Savalli, N.; Gandhi, C.S.; Ottolia, M.; Olcese, R. The RCK2 domain of the human BKCa channel is a calcium sensor. Proc. Natl. Acad. Sci. USA 2008, 105, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Yusifov, T.; Javaherian, A.D.; Pantazis, A.; Gandhi, C.S.; Olcese, R. The RCK1 domain of the human BKCa channel transduces Ca2+ binding into structural rearrangements. J. Gen. Physiol. 2010, 136, 189–202. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Computation and Analysis of Protein Circular Dichroism Spectra. Methods Enzymol. 2004, 383, 318–351. [Google Scholar]




|
WT GR [10] |
(C615S/H616R) GR in This Study |
M691A GR in This Study | |
|---|---|---|---|
| α-helix (%) | 28.6 | 27.8 | 28.0 |
| nα-helix | 21.9 | 21.5 | 21.3 |
| β-strand (%) | 21.8 | 22.1 | 21.1 |
| nβ-strand | 31.2 | 30.9 | 30.5 |
| Turn + Unordered (%) | 49.7 | 50.2 | 50.8 |
| NRMSD | 0.018 | 0.015 | 0.017 |
| Protein | KmH2O2 (mM) | KmABTS (mM) | kcat |
|---|---|---|---|
| GR | 0.512 ± 0.1 | 0.012 ± 0.001 | 0.14 ± 0.02 |
| GR/STREX | 0.15 ± 0.01 | 0.0043 ± 0.0009 | 0.29 ± 0.018 |
| CytC | 5.49 ± 0.34 | 0.1 ± 0.002 | 0.13 ± 0.0165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusifov, T.; Qudretova, F.; Aliyeva, A. Cytochrome C-like Domain Within the Human BK Channel. Int. J. Mol. Sci. 2025, 26, 7053. https://doi.org/10.3390/ijms26157053
Yusifov T, Qudretova F, Aliyeva A. Cytochrome C-like Domain Within the Human BK Channel. International Journal of Molecular Sciences. 2025; 26(15):7053. https://doi.org/10.3390/ijms26157053
Chicago/Turabian StyleYusifov, Taleh, Fidan Qudretova, and Aysel Aliyeva. 2025. "Cytochrome C-like Domain Within the Human BK Channel" International Journal of Molecular Sciences 26, no. 15: 7053. https://doi.org/10.3390/ijms26157053
APA StyleYusifov, T., Qudretova, F., & Aliyeva, A. (2025). Cytochrome C-like Domain Within the Human BK Channel. International Journal of Molecular Sciences, 26(15), 7053. https://doi.org/10.3390/ijms26157053





