Vitamin D, Gut Microbiota, and Cancer Immunotherapy—A Potentially Effective Crosstalk
Abstract
1. Introduction
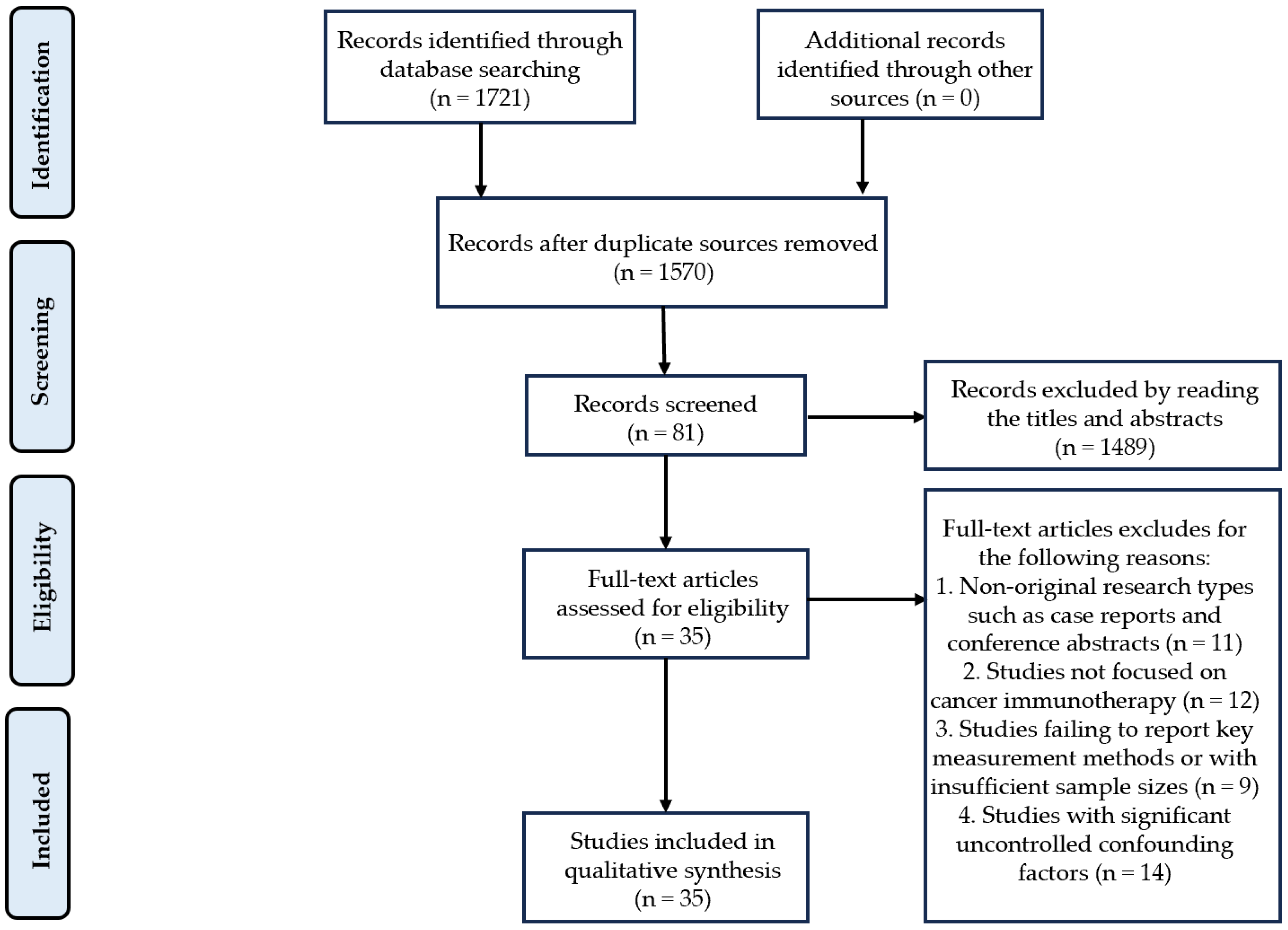
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
- (a)
- Study types include randomized controlled trials, cohort studies, case–control studies, cross-sectional studies, and systematic reviews. Animal or cellular experimental studies with clearly defined mechanistic investigations are also eligible;
- (b)
- For human studies, participants must be cancer patients receiving immunotherapy. Animal studies must examine the effects of VD or gut microbiota on immunotherapy outcomes;
- (c)
- Studies must explicitly report VD status or gut microbiota composition and their interactions, with particular focus on the bidirectional regulatory relationship between VD and gut microbiota;
- (d)
- Primary outcomes include immunotherapy response rates and survival data. Secondary outcomes encompass immune-related adverse events and changes in microbiota diversity.
2.3. Exclusion Criteria
- (a)
- Non-original research types such as case reports and conference abstracts will be excluded;
- (b)
- Studies not focused on cancer immunotherapy will be excluded;
- (c)
- Studies failing to report key measurement methods or with insufficient sample sizes will be excluded based on data quality considerations;
- (d)
- Studies with significant uncontrolled confounding factors will be excluded;
- (e)
- Duplicate publications or studies irrelevant to the research topic will not be considered.
2.4. Quality Assessment
- (a)
- Selection of Study Groups:
- Representativeness of the exposed cohort (★): Rated from “fully representative of population” to “not described” (4 tiers).
- Selection of non-exposed group (★): Highest score if drawn from the same community; lower if from different sources/not described.
- Ascertainment of exposure (★): Priority given to secure records or structured interviews; self-reports or no description score lower.
- Absence of outcome at baseline (★): Must confirm no pre-existing outcome (“yes” scores).
- (b)
- Comparability:
- Control for the most important confounder (★), with additional control for other factors (★).
- (c)
- Outcome Assessment:
- Evaluation method (★): Blind independent assessment or record linkage scores highest; self-reports or no description score lower.
- Follow-up duration (★): Must be sufficient and predefined.
- Follow-up adequacy (★): Complete follow-up or low-bias attrition (e.g., <20% loss) scores highest.
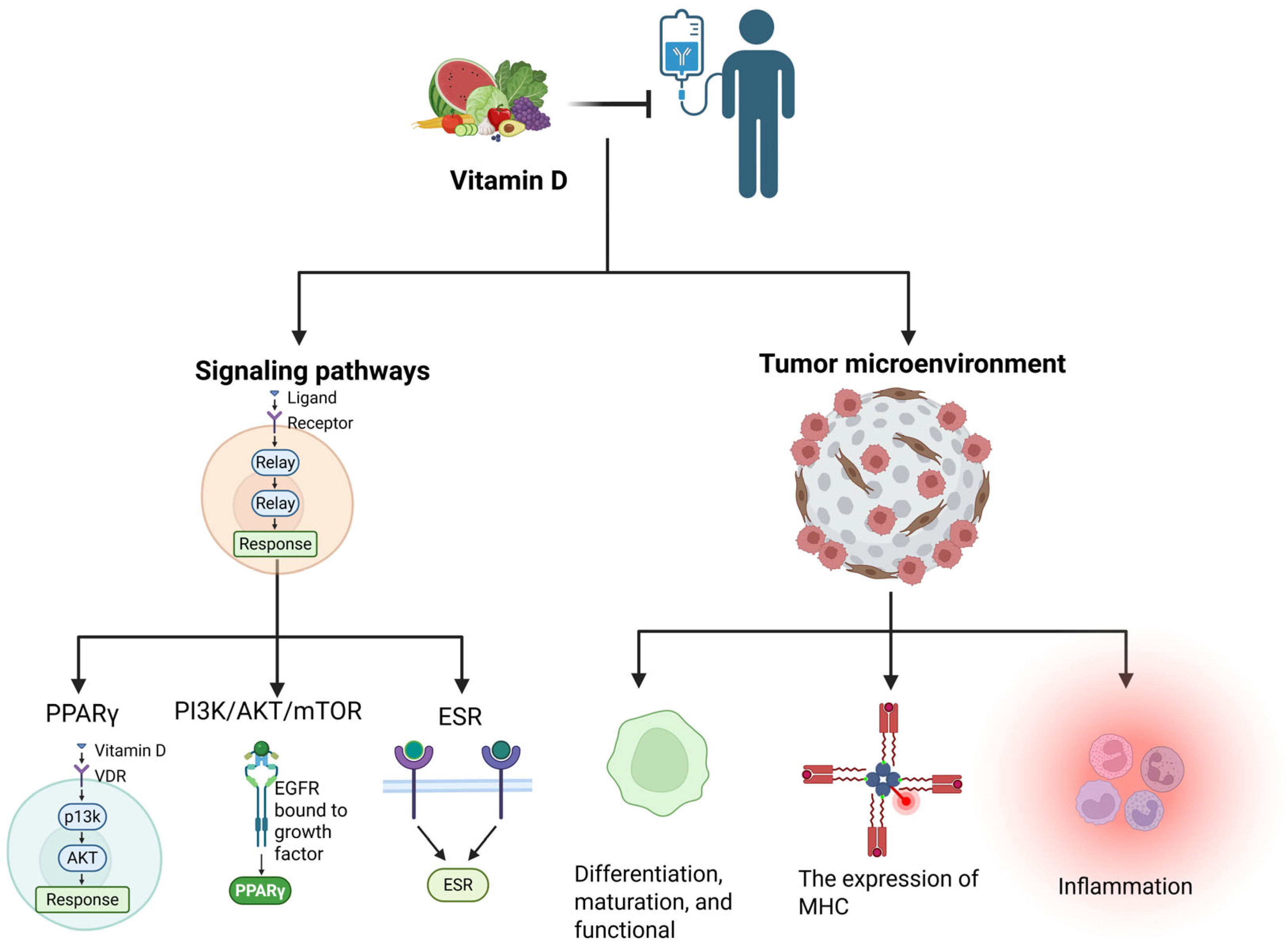
3. VD Metabolism and Function
4. Effects of VD-Based Cancer Immunotherapy
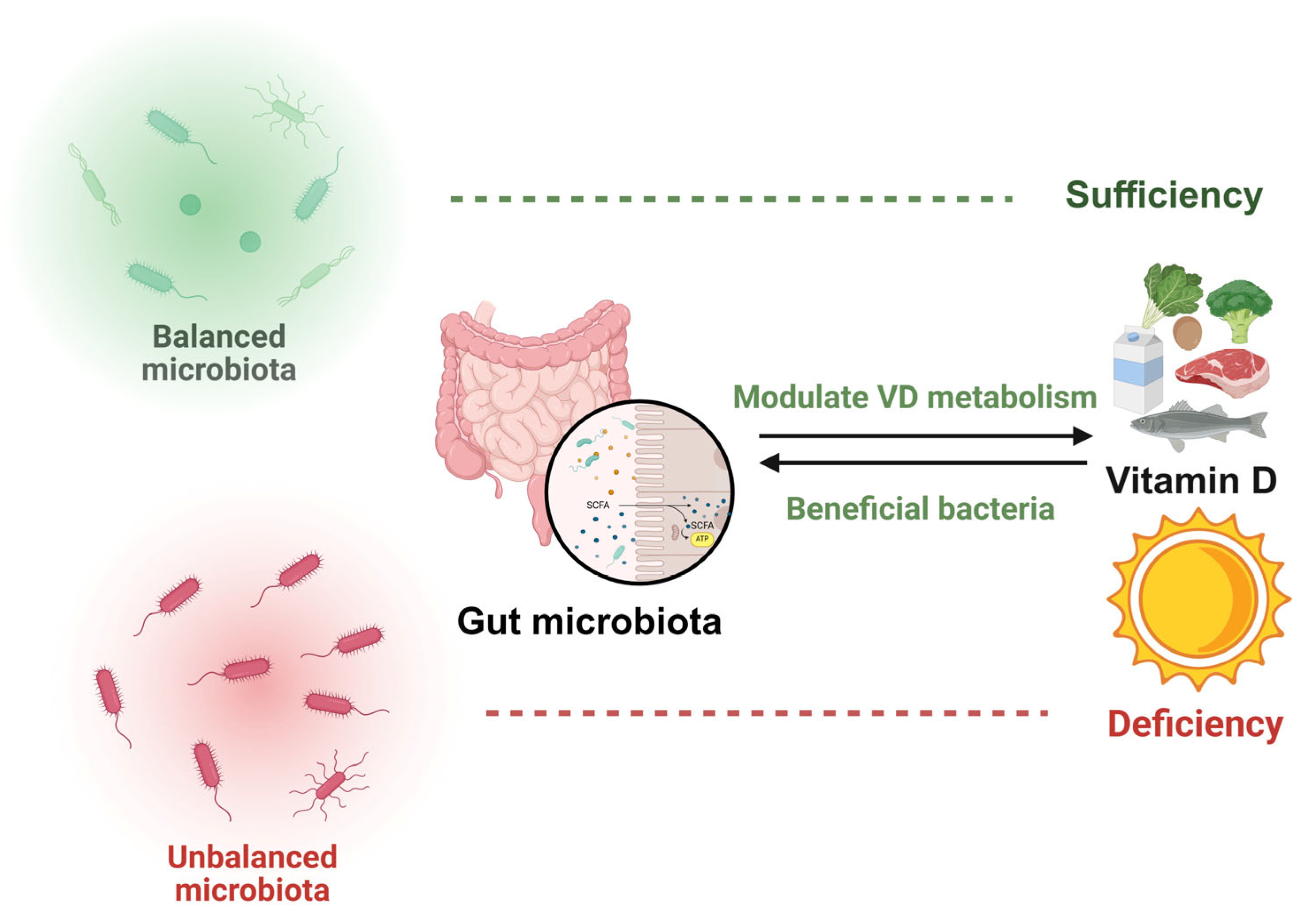
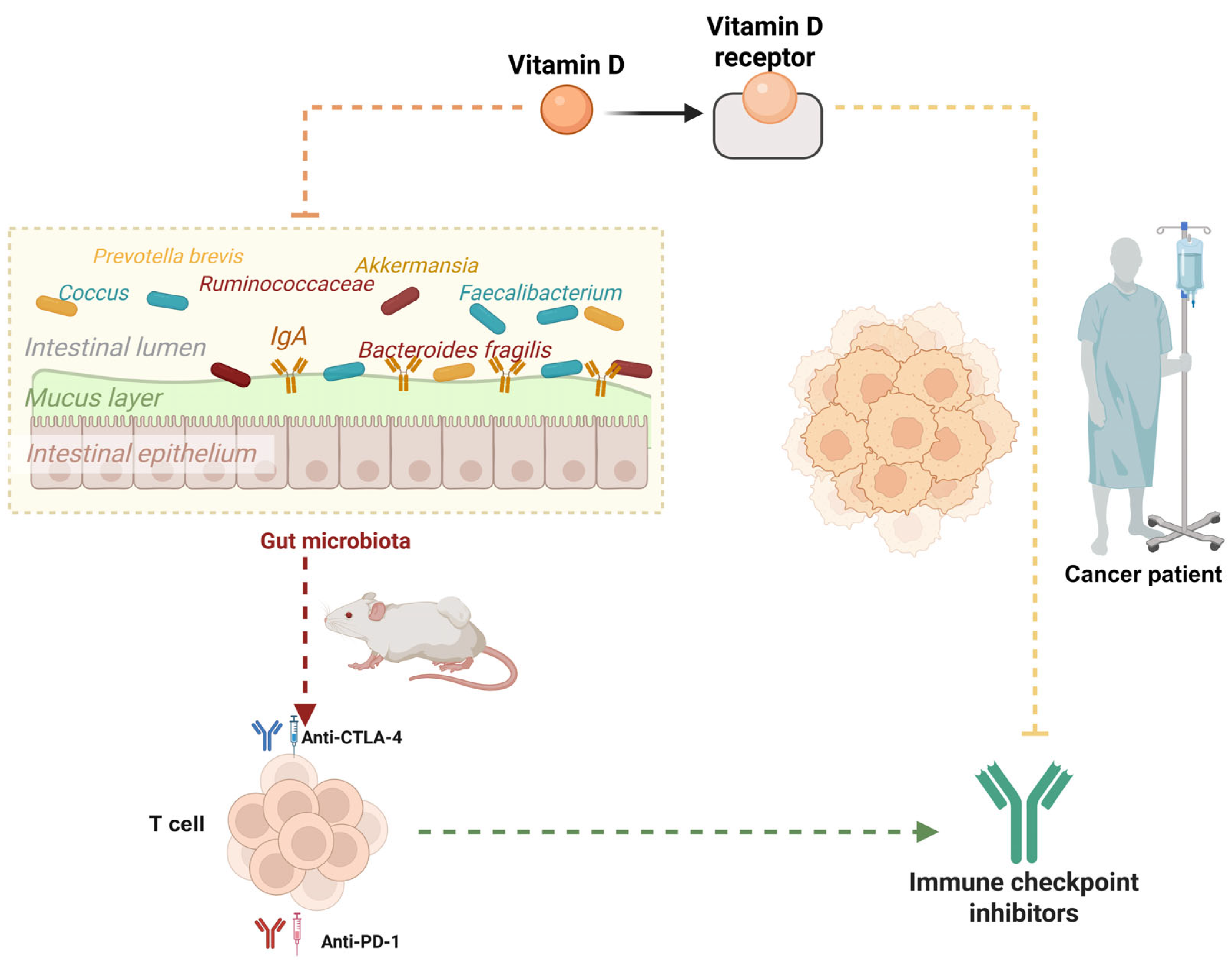
5. VD Interacts with Gut Microbiota
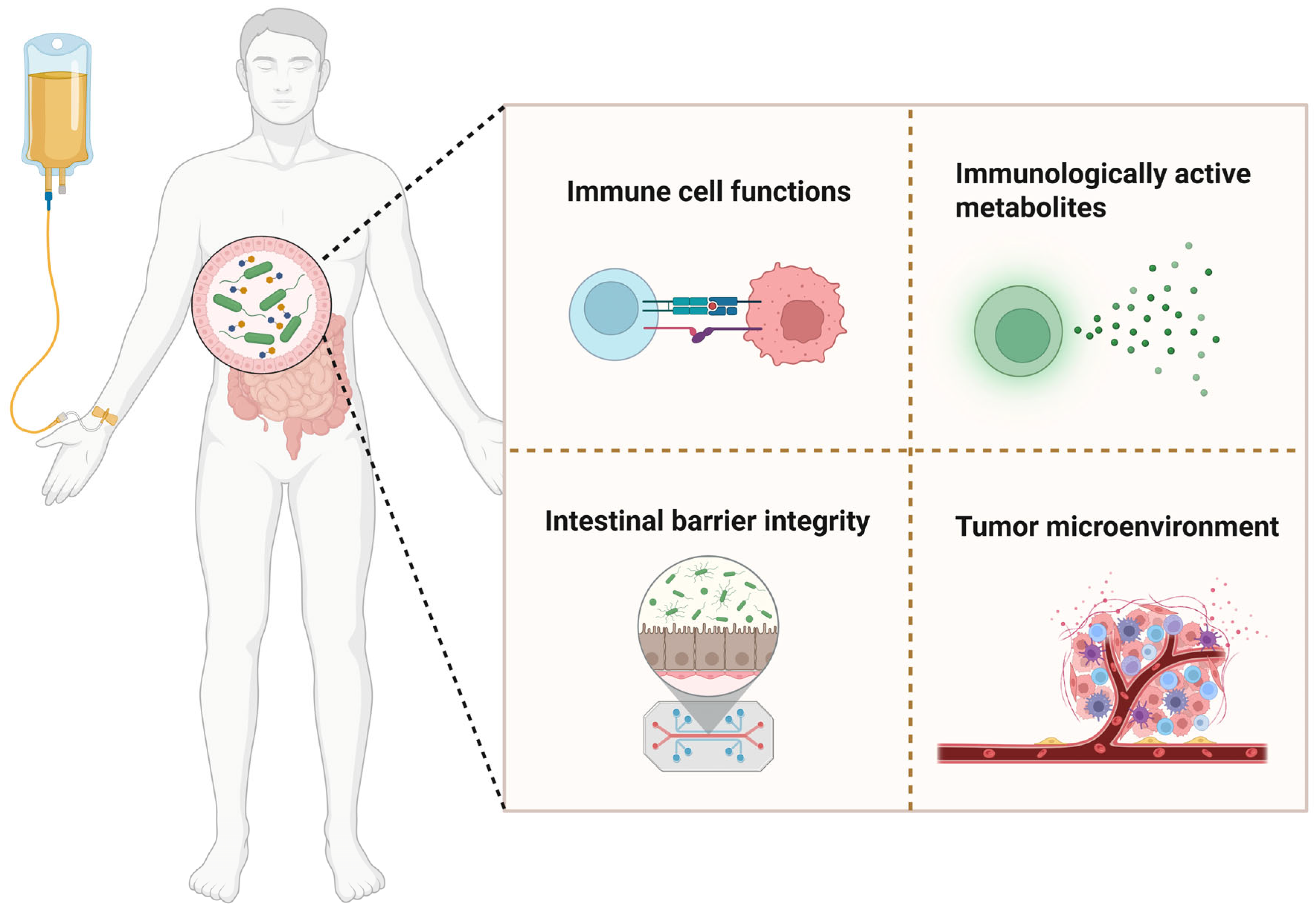
6. Gut Microbiota as a Determinant of Immunotherapy Efficacy
7. Gut Microbiota and VD Synergy in Modulating Cancer Immunotherapy
8. Publication Bias and Evidence Gaps
9. Discussion and Conclusions
10. Future Perspectives
- (a)
- Mechanistic Research: Further exploration is needed to understand how VD influences immune cell function via gut microbiota, particularly its bidirectional regulation of the Th17/Treg balance [9,86]. Studies should also investigate VD’s crosstalk with other critical signaling pathways (e.g., PPARγ and PI3K/AKT/mTOR) and its dynamic impact on PD-L1 expression [6,35].
- (b)
- Personalized Treatment Strategies: Multi-omics data (e.g., metagenomics, metabolomics, and immunomics) should be integrated to develop predictive models identifying patients who may benefit from VD supplementation [13,15]. Precision intervention strategies, such as combining probiotics, prebiotics, or FMT, should be explored to optimize immunotherapy outcomes [16,33].
- (c)
- Clinical Translation: Large-scale RCTs are required to determine the optimal VD dosage, timing, and target populations while avoiding the immunosuppressive risks of excessive supplementation [34,85]. The combined use of VD with other immunomodulators should be investigated to develop more effective combination therapies [6].
- (d)
- Technological Advancements: Rapid and cost-effective VD and gut microbiota detection methods should be developed to facilitate routine clinical monitoring [47]. Organoid or humanized mouse models could help simulate VD–microbiota–immune system interactions, accelerating mechanistic research.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Szeto, G.L.; Finley, S.D. Integrative Approaches to Cancer Immunotherapy. Trends Cancer 2019, 5, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Dougan, M.; Tyan, K.; Giobbie-Hurder, A.; Blum, S.M.; Ishizuka, J.; Qazi, T.; Elias, R.; Vora, K.B.; Ruan, A.B.; et al. Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis. Cancer 2020, 126, 3758–3767. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Yoshikawa, S.; Morikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Asai, T.; Matsuda, S. Potential tactics with vitamin D and certain phytochemicals for enhancing the effectiveness of immune-checkpoint blockade therapies. Explor. Target. Antitumor Ther. 2023, 4, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Giampazolias, E.; Pereira da Costa, M.; Lam, K.C.; Lim, K.H.J.; Cardoso, A.; Piot, C.; Chakravarty, P.; Blasche, S.; Patel, S.; Biram, A.; et al. Vitamin D regulates microbiome-dependent cancer immunity. Science 2024, 384, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Kanstrup, C.; Teilum, D.; Rejnmark, L.; Bigaard, J.V.; Eiken, P.; Kroman, N.; Tjønneland, A.; Mejdahl, M.K. 25-Hydroxyvitamin D at time of breast cancer diagnosis and breast cancer survival. Breast Cancer Res. Treat. 2020, 179, 699–708. [Google Scholar] [CrossRef]
- Filip-Psurska, B.; Zachary, H.; Strzykalska, A.; Wietrzyk, J. Vitamin D, Th17 Lymphocytes, and Breast Cancer. Cancers 2022, 14, 3649. [Google Scholar] [CrossRef] [PubMed]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, X.; Hu, M.; Sun, R.; Li, J.; Wang, H.; Pan, X.; Ma, Y.; Ning, L.; Tong, T.; et al. A specific enterotype derived from gut microbiome of older individuals enables favorable responses to immune checkpoint blockade therapy. Cell Host Microbe 2024, 32, 489–505.e5. [Google Scholar] [CrossRef] [PubMed]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H. Gut-vitamin D interplay: Key to mitigating immunosenescence and promoting healthy ageing. Immun. Ageing 2025, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Barbáchano, A.; Fernández-Barral, A.; Ferrer-Mayorga, G.; Costales-Carrera, A.; Larriba, M.J.; Muñoz, A. The endocrine vitamin D system in the gut. Mol. Cell Endocrinol. 2017, 453, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Lehoczki, A.; Szappanos, Á.; Zábó, V.; Kaposvári, C.; Horváth, A.; Farkas, Á.; Fazekas-Pongor, V.; Major, D.; Lipécz, Á.; et al. Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications. Nutrients 2025, 17, 1351. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D exposure and Risk of Breast Cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Layne, T.M.; Albanes, D. Vitamin D and Cancer Risk and Mortality: State of the Science, Gaps, and Challenges. Epidemiol. Rev. 2017, 39, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Tsounis, E.P.; Mouzaki, A.; Triantos, C. Exploring the Role of Vitamin D and the Vitamin D Receptor in the Composition of the Gut Microbiota. Front. Biosci. (Landmark Ed.) 2023, 28, 116. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: A systematic review of in vivo studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discovery. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Vanhooren, J.; Derpoorter, C.; Depreter, B.; Deneweth, L.; Philippé, J.; De Moerloose, B.; Lammens, T. TARP as antigen in cancer immunotherapy. Cancer Immunol. Immunother. 2021, 70, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Paleari, L. Personalized Assessment for Cancer Prevention, Detection, and Treatment. Int. J. Mol. Sci. 2024, 25, 8140. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.; Jeon, Y.W.; Suh, Y.J. Association Between Alterations in the Serum 25-Hydroxyvitamin D Status During Follow-Up and Breast Cancer Patient Prognosis. Asian Pac. J. Cancer Prev. 2015, 16, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, L.; Almquist, M.; Borgquist, S.; Malm, J.; Manjer, J. Serum vitamin D (25OHD3) levels and the risk of different subtypes of breast cancer: A nested case–control study. Breast 2016, 28, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Ruiz-Limón, P.; Pilo, J.; Lisbona-Montañez, J.M.; Tinahones, F.J.; Moreno Indias, I.; Macías-González, M. Linking serum vitamin D levels with gut microbiota after 1-year lifestyle intervention with Mediterranean diet in patients with obesity and metabolic syndrome: A nested cross-sectional and prospective study. Gut Microbes 2023, 15, 2249150. [Google Scholar] [CrossRef] [PubMed]
- Sardar, P.; Beresford-Jones, B.S.; Xia, W.; Shabana, O.; Suyama, S.; Ramos, R.J.F.; Soderholm, A.T.; Tourlomousis, P.; Kuo, P.; Evans, A.C.; et al. Gut microbiota-derived hexa-acylated lipopolysaccharides enhance cancer immunotherapy responses. Nat. Microbiol. 2025, 10, 795–807. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kanno, K.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect of Vitamin D Supplements on Relapse or Death in a p53-Immunoreactive Subgroup With Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2328886. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barral, A.; Peña, C.; Pisano, D.G.; Cantero, R.; Rojo, F.; Muñoz, A.; Larriba, M.J. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut 2017, 66, 1449–1462. [Google Scholar] [CrossRef]
- Fan, L.; Xia, Y.; Wang, Y.; Han, D.; Liu, Y.; Li, J.; Fu, J.; Wang, L.; Gan, Z.; Liu, B.; et al. Gut microbiota bridges dietary nutrients and host immunity. Sci. China Life Sci. 2023, 66, 2466–2514. [Google Scholar] [CrossRef] [PubMed]
- Galus, Ł.; Michalak, M.; Lorenz, M.; Stoińska-Swiniarek, R.; Tusień Małecka, D.; Galus, A.; Kolenda, T.; Leporowska, E.; Mackiewicz, J. Vitamin D supplementation increases objective response rate and prolongs progression-free time in patients with advanced melanoma undergoing anti-PD-1 therapy. Cancer 2023, 129, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lum, D.; Haiyum, M.; Fairbairn, K.A. Vitamin D Status of Elite Athletes in Singapore and Its Associations With Muscle Function and Bone Health. J. Sci. Sport Exerc. 2021, 3, 385–393. [Google Scholar] [CrossRef]
- Jamshidi, S.; Masoumi, S.J.; Abiri, B.; Vafa, M. The effects of synbiotic and/or vitamin D supplementation on gut-muscle axis in overweight and obese women: A study protocol for a double-blind, randomized, placebo-controlled trial. Trials 2022, 23, 631. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 5997. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Role of Vitamin D in the Hygiene Hypothesis: The Interplay between Vitamin D, Vitamin D Receptors, Gut Microbiota, and Immune Response. Front. Immunol. 2016, 7, 627. [Google Scholar] [CrossRef] [PubMed]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wei, X.; Ge, X.; Chen, Y.; Li, Y.C. Microbiota-Dependent Induction of Colonic Cyp27b1 Is Associated With Colonic Inflammation: Implications of Locally Produced 1,25-Dihydroxyvitamin D3 in Inflammatory Regulation in the Colon. Endocrinology 2017, 158, 4064–4075. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef]
- Xiang, H.; Zhou, C.; Gan, X.; Huang, Y.; He, P.; Ye, Z.; Liu, M.; Yang, S.; Zhang, Y.; Zhang, Y.; et al. Relationship of Serum 25-Hydroxyvitamin D Concentrations, Diabetes, Vitamin D Receptor Gene Polymorphisms and Incident Venous Thromboembolism. Diabetes Metab. Res. Rev. 2025, 41, e70014. [Google Scholar] [CrossRef]
- Carlberg, C.; Raczyk, M.; Zawrotna, N. Vitamin D: A master example of nutrigenomics. Redox Biol. 2023, 62, 102695. [Google Scholar] [CrossRef] [PubMed]
- Delrue, C.; Speeckaert, M.M. Vitamin D and Vitamin D-Binding Protein in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4642. [Google Scholar] [CrossRef] [PubMed]
- Thouvenot, E.; Laplaud, D.; Lebrun-Frenay, C.; Derache, N.; Le Page, E.; Maillart, E.; Froment-Tilikete, C.; Castelnovo, G.; Casez, O.; Coustans, M.; et al. High-Dose Vitamin Din Clinically Isolated Syndrome Typical of Multiple Sclerosis: The D-Lay MSRandomized Clinical Trial. JAMA 2025, 333, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.; Bodea, J.; Garcia, A.; Beebe, K.; Campbell, C.; Schwalbach, C.; Salzberg, D.; Miller, H.; Adams, R.; Mirea, L.; et al. Vitamin D Supplementation: Association With Serum Cytokines in Pediatric Hematopoietic Stem Cell Transplantation. Front. Pediatr. 2022, 13, 913586. [Google Scholar] [CrossRef] [PubMed]
- Ros-Soto, J.; Anthias, C.; Madrigal, A.; Snowden, J.A. Vitamin D: Is it important in haematopoietic stem cell transplantation? A review. Bone Marrow Transplant. 2019, 54, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Bodea, J.; Beebe, K.; Campbell, C.; Salzberg, D.; Schwalbach, C.; Miller, H.; Adams, R.; Mirea, L.; Castillo, P.; Horn, B.; et al. Impact of Adequate Day 30 Post-Pediatric Hematopoietic Stem Cell Transplantation Vitamin D Level on Clinical Outcome: An Observational Cohort Study. Transplant. Cell Ther. 2022, 28, 514.e1–514.e5. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, P.; Suchitra, M.M.; Bitla, A.R.; Sachan, A. Attenuation of Oxidative Stress, Interleukin-6, High-Sensitivity C-Reactive Protein, Plasminogen Activator Inhibitor-1, and Fibrinogen with Oral Vitamin D Supplementation in Patients with T2DM having Vitamin D Deficiency. J. Lab. Physicians 2021, 14, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Miao, Y.; Gan, L.; Zhao, B.; Fang, F.; Wang, R.; Chen, X.; Huang, J. Associations of Serum 25-Hydroxyvitamin D Concentrations with Risks of Mortality and Cardiovascular Disease among Individuals with Psoriasis. J. Am. Acad. Dermatol. 2025, 1380, S0190–S9622. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Xie, R.; Gwenzi, T.; Wang, Y.; Brenner, H.; Schöttker, B. Real-world evidence for an association of vitamin D supplementation with atherosclerotic cardiovascular disease in the UK Biobank. Clin. Nutr. 2025, 49, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, A.; Pallarés-Carratalá, V.; Turégano-Yedro, M.; Torres, F.; Sapena, V.; Martin-Gorgojo, A.; Martin-Moreno, J.M. Vitamin D Supplementation and Its Impact on Mortality and Cardiovascular Outcomes: Systematic Review and Meta-Analysis of 80 Randomized Clinical Trials. Nutrients 2023, 15, 1810. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Lyu, Z.; Wu, Y. Supplementing with Vitamin D during Pregnancy Reduces Inflammation and Prevents Autism-Related Behaviors in Offspring Caused by Maternal Immune Activation. Biol. Pharm. Bull. 2025, 48, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Addressing cancer’s grand challenges. Nat. Rev. Drug Discov. 2020, 19, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Elkin, E.B.; Bach, P.B. Cancer’s next frontier: Addressing high and increasing costs. JAMA 2010, 303, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ding, J.; Li, S.; Li, Y. Autophagy in cancer immunotherapy: Perspective on immune evasion and cell death interactions. Cancer Lett. 2024, 590, 216856. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Woo, H.D.; Lyu, J.; Song, B.M.; Lim, J.Y.; Park, H.Y. Serum 25-hydroxyvitamin D levels and risk of overall and site-specific cancers in Korean adults: Results from two prospective cohort studies. Nutr. J. 2025, 24, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.; Wang, B.; Jia, X.; Yu, J.; Zhang, Y.; Sang, D.; Zhang, Y. Exercise Interventions for the Prevention and Treatment of Anthracycline-Induced Cardiotoxicity in Women with Breast Cancer: A Systematic Review. J. Sci. Sport Exerc. 2024, 7, 14–27. [Google Scholar] [CrossRef]
- Ordóñez-Mena, J.M.; Schöttker, B.; Fedirko, V.; Jenab, M.; Olsen, A.; Halkjær, J.; Kampman, E.; de Groot, L.; Jansen, E.; Bueno-de-Mesquita, H.B.; et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: An analysis of cohorts participating in the CHANCES consortium. Eur. J. Epidemiol. 2016, 31, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Cullup, H.; Dickinson, A.M.; Norden, J.; Jackson, G.H.; Taylor, P.R.; Cavet, J. Vitamin D receptor gene polymorphism associates with graft-versus-host disease and survival in HLA-matched sibling allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002, 30, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.D.; Sun, G.; Li, J.; Xu, J.; Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019, 452, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Semeraro, M.D.; Herrmann, M.; Absenger, G.; Gerger, A.; Renner, W. Immune Aging and Immunotherapy in Cancer. Int. J. Mol. Sci. 2021, 22, 7016. [Google Scholar] [CrossRef] [PubMed]
- Pouliliou, S.; Nikolaidis, C.; Drosatos, G. Current trends in cancer immunotherapy: A literature-mining analysis. Cancer Immunol. Immunother. 2020, 69, 2425–2439. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Weiner, G.J.; Pardoll, D.M. Cancer Immunotherapy Comes of Age. J. Clin. Oncol. 2011, 29, 4828–4836. [Google Scholar] [CrossRef] [PubMed]
- Rui, R.; Zhou, L.; He, S. Cancer immunotherapies: Advances and bottlenecks. Front. Immunol. 2023, 14, 1212476. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 2, 1248. [Google Scholar] [CrossRef] [PubMed]
- Artusa, P.; White, J.H. Vitamin D and its analogs in immune system regulation. Pharmacol. Rev. 2025, 77, 100032. [Google Scholar] [CrossRef] [PubMed]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Karthik, N.; Taneja, R. Crosstalk Between Inflammatory Signaling and Methylation in Cancer. Front. Cell Dev. Biol. 2021, 9, 756458. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, A.; van Etten, E.; Overbergh, L.; Stoffels, K.; Bouillon, R.; Mathieu, C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res. Clin. Pract. 2007, 77, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Stucci, L.S.; D’Oronzo, S.; Tucci, M.; Macerollo, A.; Ribero, S.; Spagnolo, F.; Marra, E.; Picasso, V.; Orgiano, L.; Marconcini, R.; et al. Italian Melanoma Intergroup (IMI). Vitamin D in melanoma: Controversies and potential role in combination with immune check-point inhibitors. Cancer Treat. Rev. 2018, 69, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Cortellini, A.; Leonetti, A.; Parisi, A.; Tiseo, M.; Bordi, P.; Michiara, M.; Bui, S.; Cosenza, A.; Ferri, L.; et al. Systematic vitamin D supplementation is associated with improved outcomes and reduced thyroid adverse events in patients with cancer treated with immune checkpoint inhibitors: Results from the prospective PROVIDENCE study. Cancer Immunol. Immunother. 2023, 72, 3707–3716. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Daniel, K.C.; Penna, G. Vitamin D receptor agonists, cancer and the immune system: An intricate relationship. Curr. Top. Med. Chem. 2006, 6, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lan, Y.; Wang, W.; Zhang, J.; Shao, R.; Yin, Z.; Gudmundsson, G.H.; Bergman, P.; Mai, K.; Ai, Q.; et al. Vitamin D influences gut microbiota and acetate production in zebrafish (Danio rerio) to promote intestinal immunity against invading pathogens. Gut Microbes 2023, 15, 2187575. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota gut- brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.F.; Pang, Q.; Yao, L.P.; Zhang, Y.; Peng, C.; Huang, W.; Han, B. Gut microbiota: A magical multifunctional target regulated by medicine food homology species. J. Adv. Res. 2023, 52, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Dong, H.; Sun, J. Lack of Vitamin D Receptor Causes Dysbiosis and Changes the Functions of the Murine Intestinal Microbiome. Clin. Ther. 2015, 7, 996–1009.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, G.; Pei, Z.; Yu, X.; Wang, Y.; Xu, F.; Zhao, J.; Lu, S.; Lu, W. Bifidobacterium longum increases serum vitamin D metabolite levels and modulates intestinal flora to alleviate osteoporosis in mice. mSphere 2025, 10, e0103924. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, Q.; Zhang, L.; Pei, Y.; Xu, X.; Liu, X.; Lu, G.; Pan, J.; Wang, Y. Causal relationship between gut microbiota and serum vitamin D: Evidence from genetic correlation and Mendelian randomization study. Eur. J. Clin. Nutr. 2022, 76, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.M. Vitamin D-dependent microbiota-enhancing tumor immunotherapy. Cell Mol. Immunol. 2024, 21, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.; McCoy, K.D. Microbes and vitamin D aid immunotherapy. Science 2024, 384, 384–385. [Google Scholar] [CrossRef] [PubMed]

| Author/Year | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ | ⑩ | ⑪ | Total Score | Literature Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fekete M. et al., 2025 [19] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 6 | Medium |
| Estébanez N. et al., 2018 [20] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | High |
| Mondul A.M. et al., 2017 [21] | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Medium |
| Aggeletopoulou I. et al., 2023 [22] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 8 | High |
| Waterhouse M. et al., 2018 [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 10 | High |
| Author/Year | Selection | Comparability | Outcome | Total Score | Literature Quality |
|---|---|---|---|---|---|
| Grover S. et al., 2020 [4] | ★★★★ | ★★ | ★★★ | Nine | High |
| Kanstrup C. et al., 2020 [8] | ★★★★ | ★★ | ★★★ | Nine | High |
| Chaput N. et al., 2017 [12] | ★★★ | ★ | ★★★ | Seven | High |
| Zhu X. et al., 2024 [13] | ★★★★ | ★★ | ★★★ | Nine | High |
| Gopalakrishnan V. et al., 2018 [15] | ★★★★ | ★★ | ★★★ | Nine | High |
| Matson V. et al., 2018 [16] | ★★★★ | ★★ | ★★★ | Nine | High |
| Song S. et al., 2025 [24] | ★★★★ | ★★ | ★★★ | Nine | High |
| Lim S.T. et al., 2015 [25] | ★★★ | ★★ | ★ | Six | Medium |
| Shirazi L. et al., 2016 [26] | ★★★ | ★★ | ★★★ | Eight | High |
| Ordóñez-Mena J.M. et al., 2016 [27] | ★★★★ | ★★ | ★★★ | Nine | High |
| Middleton P.G. et al., 2002 [28] | ★★★★ | ★★ | ★★★ | Nine | High |
| Galus Ł. et al., 2023 [29] | ★★★★ | ★★ | ★★ | Eight | High |
| Boughanem H. et al., 2023 [30] | ★★★ | ★★ | ★★★ | Eight | High |
| Sardar P. et al., 2025 [31] | ★★★★ | ★★ | ★★ | Eight | High |
| Zitvogel L. et al., 2018 [32] | ★★★ | ★★ | ★★ | Seven | High |
| Routy B. et al., 2018 [33] | ★★★★ | ★★ | ★★ | Eight | High |
| Kanno K. et al., 2023 [34] | ★★★★ | ★★ | ★★★ | Nine | High |
| Ferrer-Mayorga G. et al., 2017 [35] | ★★★★ | ★★ | ★★ | Eight | High |
| Author/Year | Random Sequence Generation | Randomization Concealment | Blinding | Withdrawals and Dropouts | Total Score | Literature Quality |
|---|---|---|---|---|---|---|
| Jamshidi S. et al., 2022 [36] | +2 | +2 | +2 | +1 | 7 | High |
| Author/Year | Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Blinding | Random Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Bias |
|---|---|---|---|---|---|---|---|---|---|
| Giampazolias E. et al., 2024 [7] | High | Low | Unclear | Unclear | Unclear | Low | Low | Low | Moderate |
| Vétizou M. et al., 2015 [11] | Unclear | Low | Unclear | Low | Unclear | Low | Low | Low | Low |
| Gopalakrishnan V. et al., 2018 [15] | Unclear | Low | Unclear | Low | Unclear | Low | Low | Low | Low |
| Matson V. et al., 2018 [16] | Unclear | Low | Unclear | Low | Unclear | Low | Low | Low | Low |
| Liao X. et al., 2023 [37] | Unclear | Unclear | Unclear | Low | Unclear | Low | Low | Low | Unclear |
| Assa A. et al., 2014 [38] | Unclear | Low | Unclear | Low | Unclear | Low | Low | Low | Unclear |
| Du J. et al., 2022 [39] | Unclear | Unclear | Unclear | Low | Unclear | Low | Low | Low | Low |
| Jin D. et al., 2015 [40] | Unclear | Unclear | Unclear | Low | Unclear | Low | Low | Low | Low |
| Wang H. et al., 2025 [41] | Unclear | Low | Unclear | Low | Unclear | Low | Low | Low | Unclear |
| Ma C. et al., 2018 [42] | Unclear | Low | Unclear | Low | Unclear | Low | Low | Low | Low |
| Song X. et al., 2020 [43] | Unclear | Low | Unclear | Low | Unclear | Low | Low | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Guo, Y.; Li, Y.; Jiang, Q.; Yuan, C.; Zhao, L.; Mao, S. Vitamin D, Gut Microbiota, and Cancer Immunotherapy—A Potentially Effective Crosstalk. Int. J. Mol. Sci. 2025, 26, 7052. https://doi.org/10.3390/ijms26157052
Yan Y, Guo Y, Li Y, Jiang Q, Yuan C, Zhao L, Mao S. Vitamin D, Gut Microbiota, and Cancer Immunotherapy—A Potentially Effective Crosstalk. International Journal of Molecular Sciences. 2025; 26(15):7052. https://doi.org/10.3390/ijms26157052
Chicago/Turabian StyleYan, Yizhen, Yi Guo, Yiting Li, Qingrui Jiang, Chenhang Yuan, Li Zhao, and Shanshan Mao. 2025. "Vitamin D, Gut Microbiota, and Cancer Immunotherapy—A Potentially Effective Crosstalk" International Journal of Molecular Sciences 26, no. 15: 7052. https://doi.org/10.3390/ijms26157052
APA StyleYan, Y., Guo, Y., Li, Y., Jiang, Q., Yuan, C., Zhao, L., & Mao, S. (2025). Vitamin D, Gut Microbiota, and Cancer Immunotherapy—A Potentially Effective Crosstalk. International Journal of Molecular Sciences, 26(15), 7052. https://doi.org/10.3390/ijms26157052






