Hydroxytyrosol Reprograms the Tumor Microenvironment in 3D Melanoma Models by Suppressing ERBB Family and Kinase Pathways
Abstract
1. Introduction
2. Results
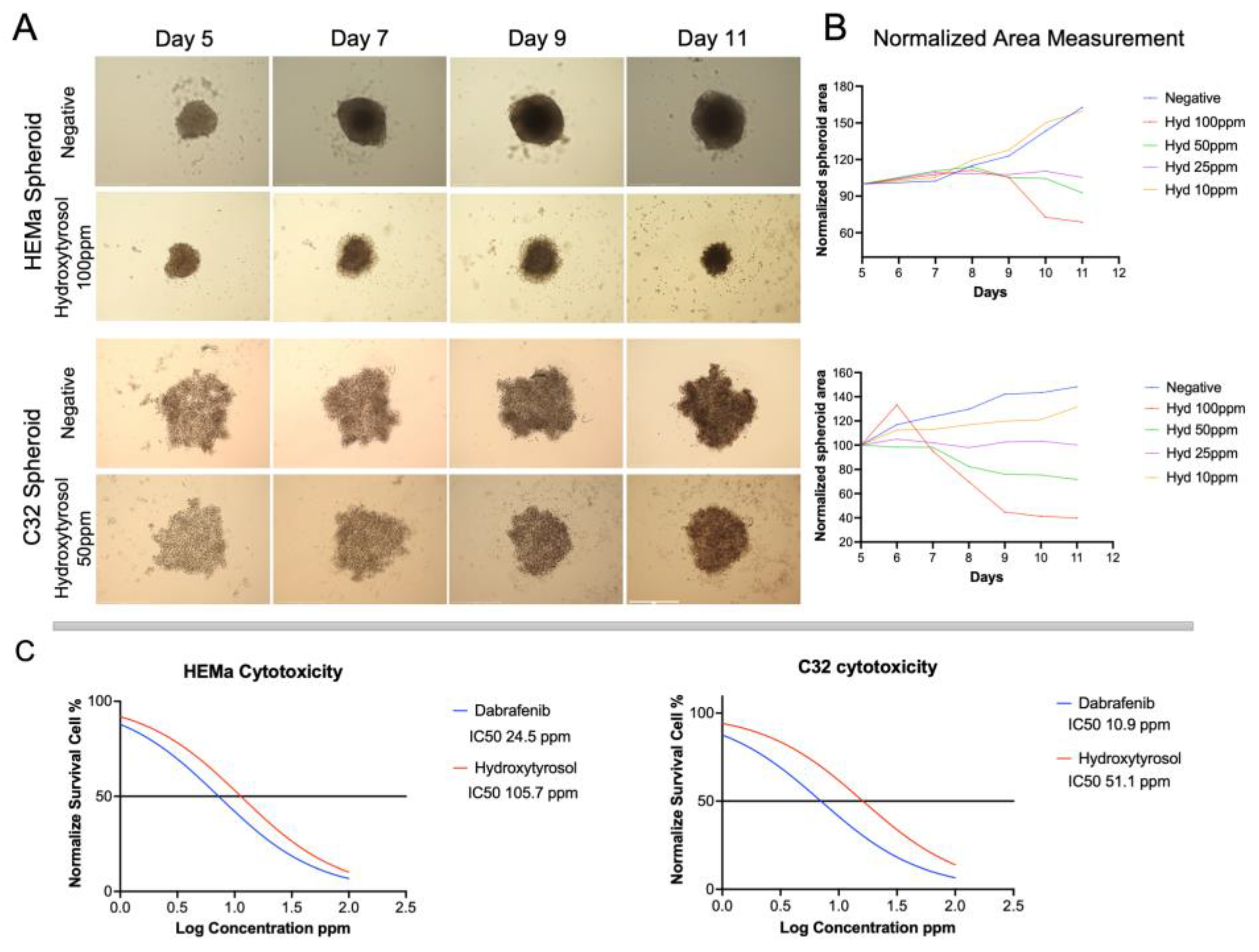
2.1. Hydroxytyrosol Reduces Spheroid Growth from Tumoral and Non-Tumoral Cells
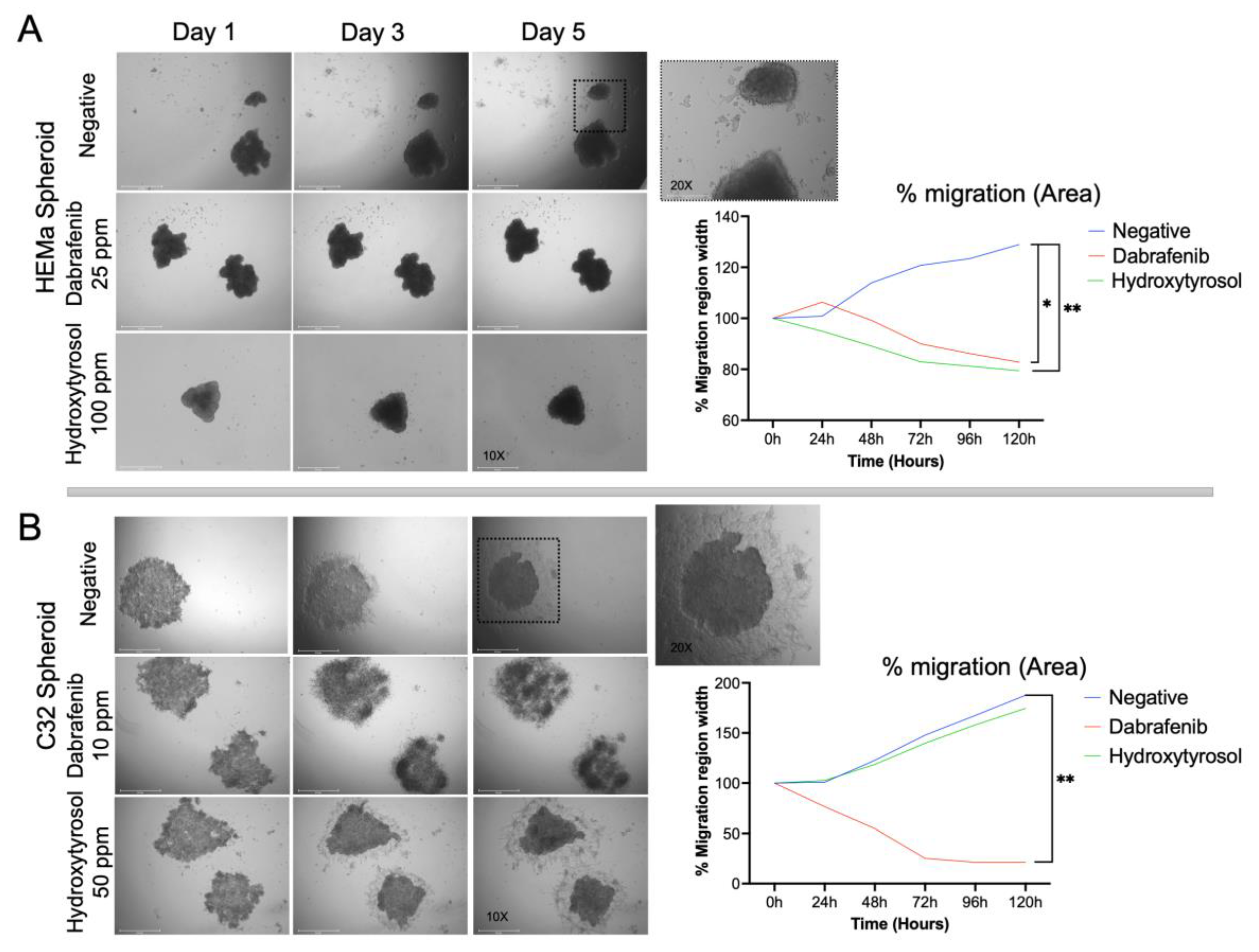
2.2. Hydroxytyrosol Reduces Spheroid Migration on Matrigel
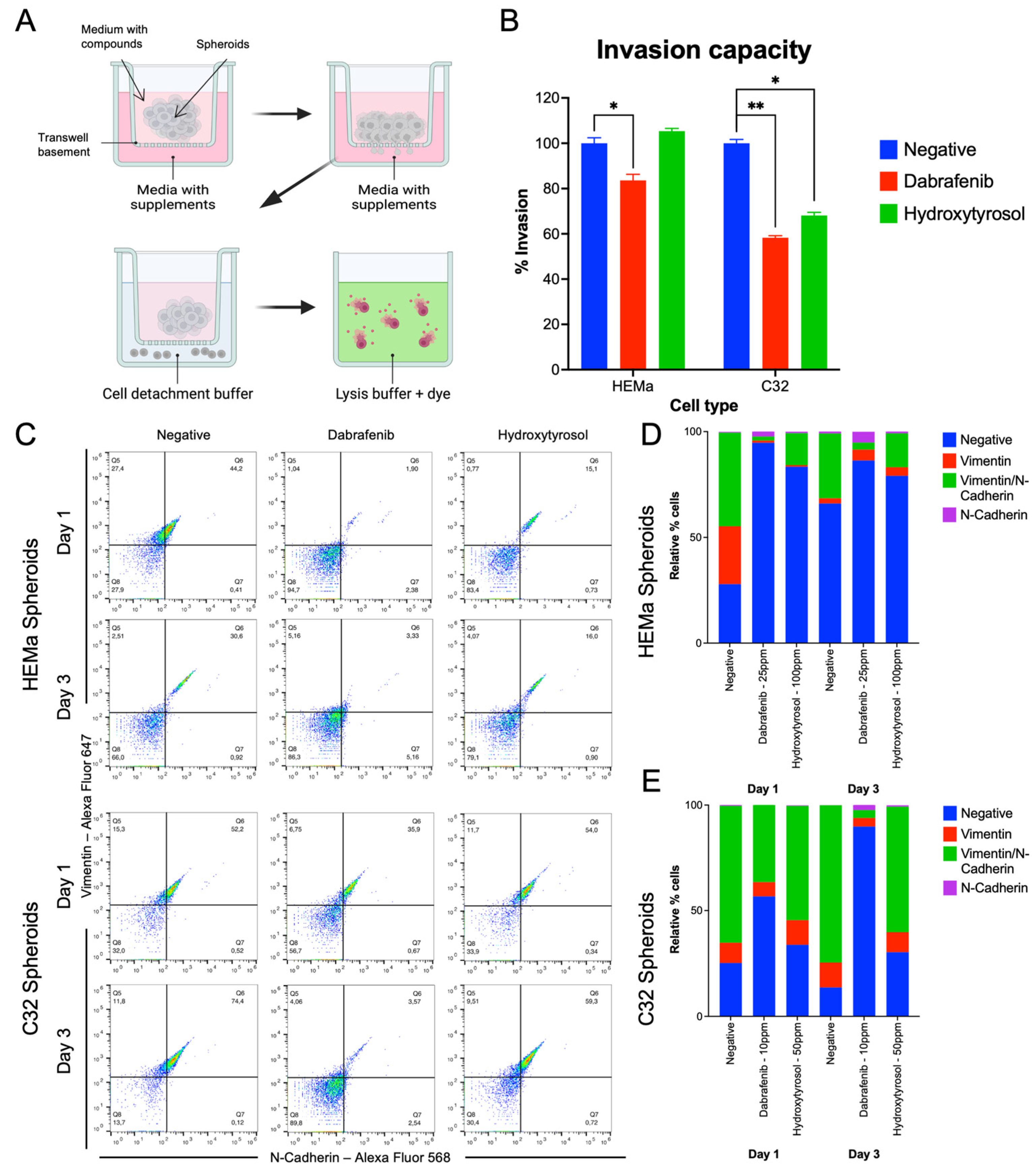
2.3. Hydroxytyrosol Inhibits Invasive Capacity in Both Tumor and Non-Tumor Spheroids
2.4. Hydroxytyrosol Downregulates Mesenchymal EMT Markers
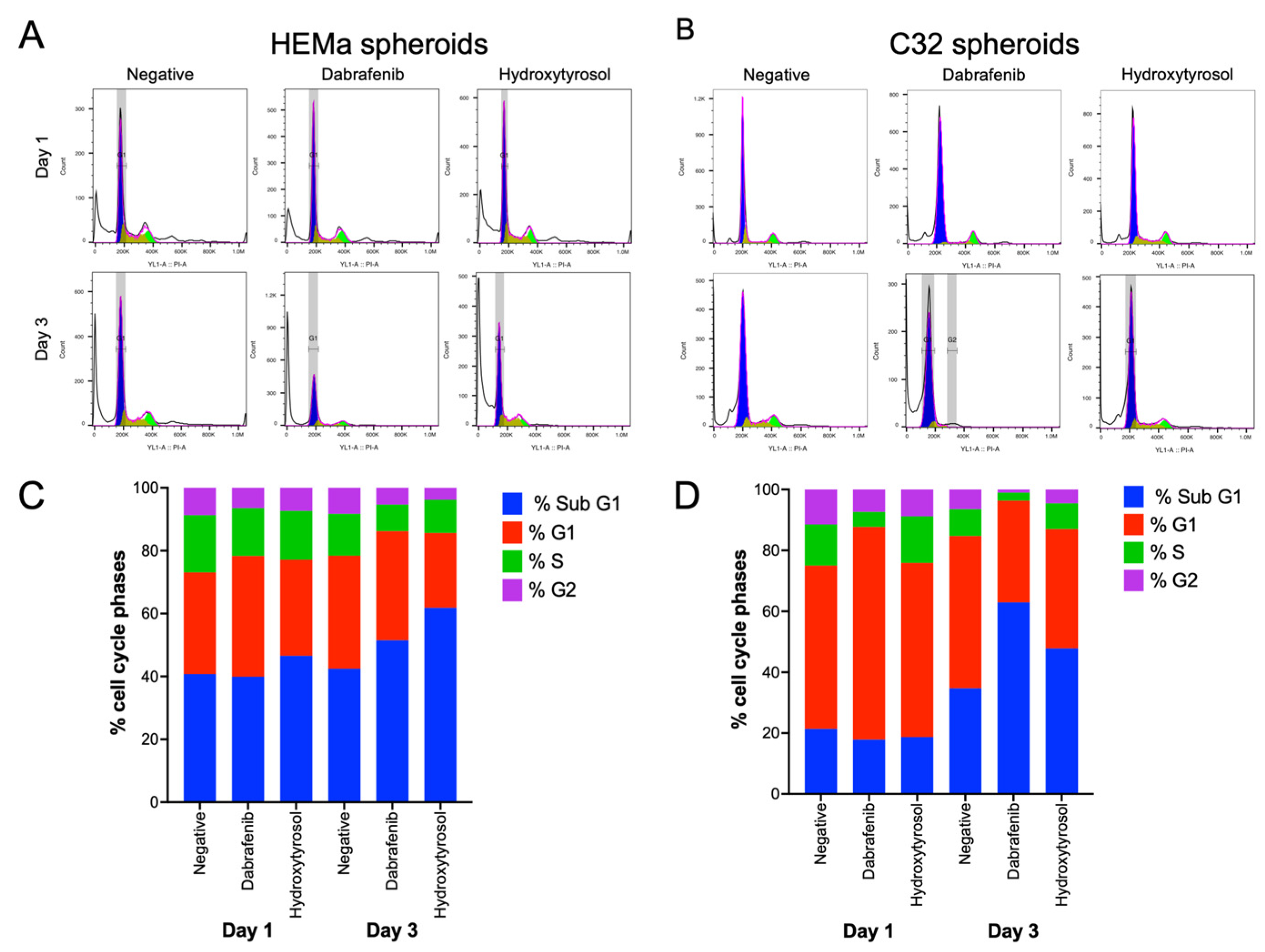
2.5. Hydroxytyrosol Modulates Cell Cycle Distribution in Melanoma Spheroids
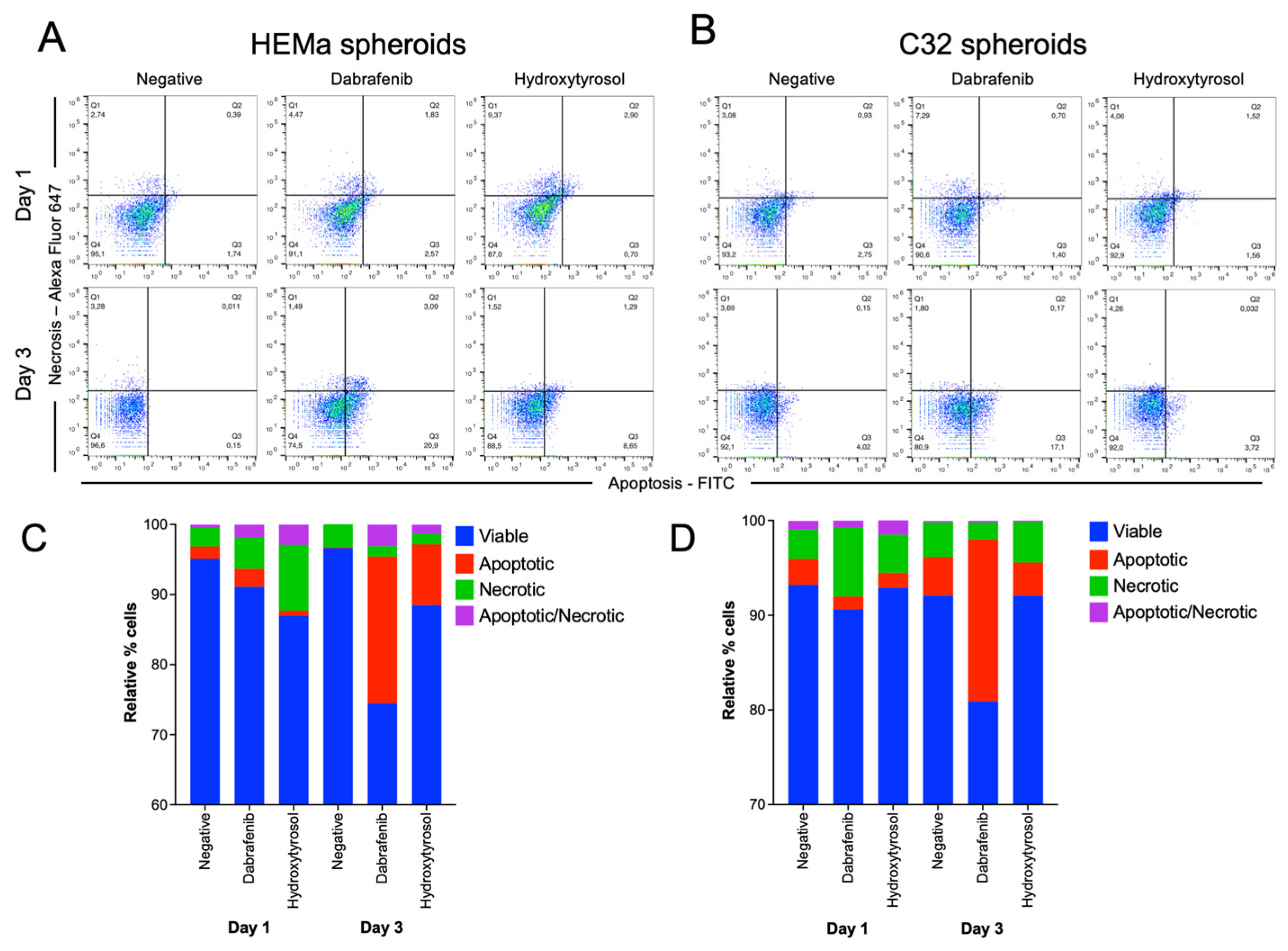
2.6. Hydroxytyrosol Induces Selective Cell Death in Melanoma and Non-Tumor Spheroids
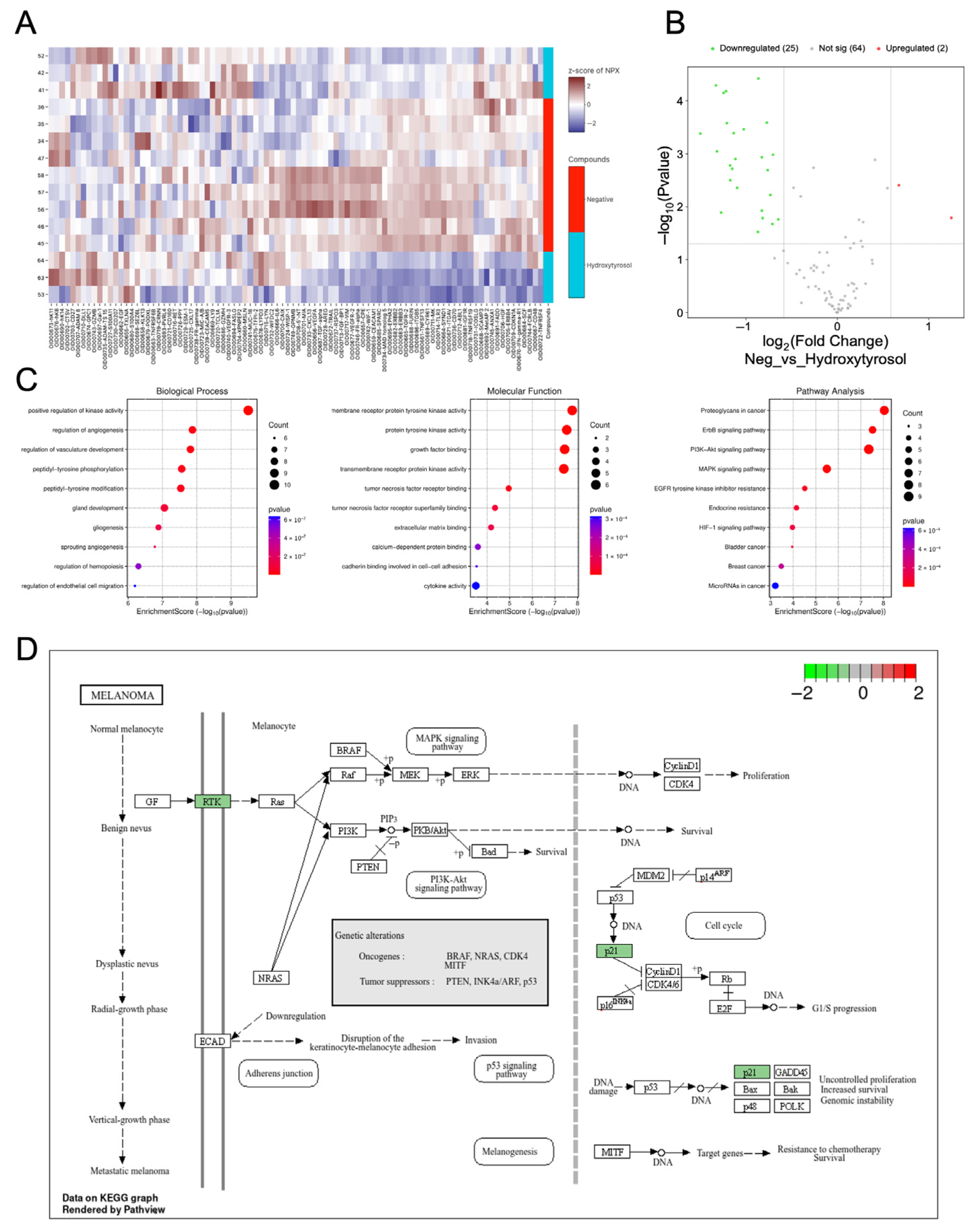
2.7. Hydroxytyrosol Downregulates Pro-Tumorigenic Protein Networks in Hema Spheroids
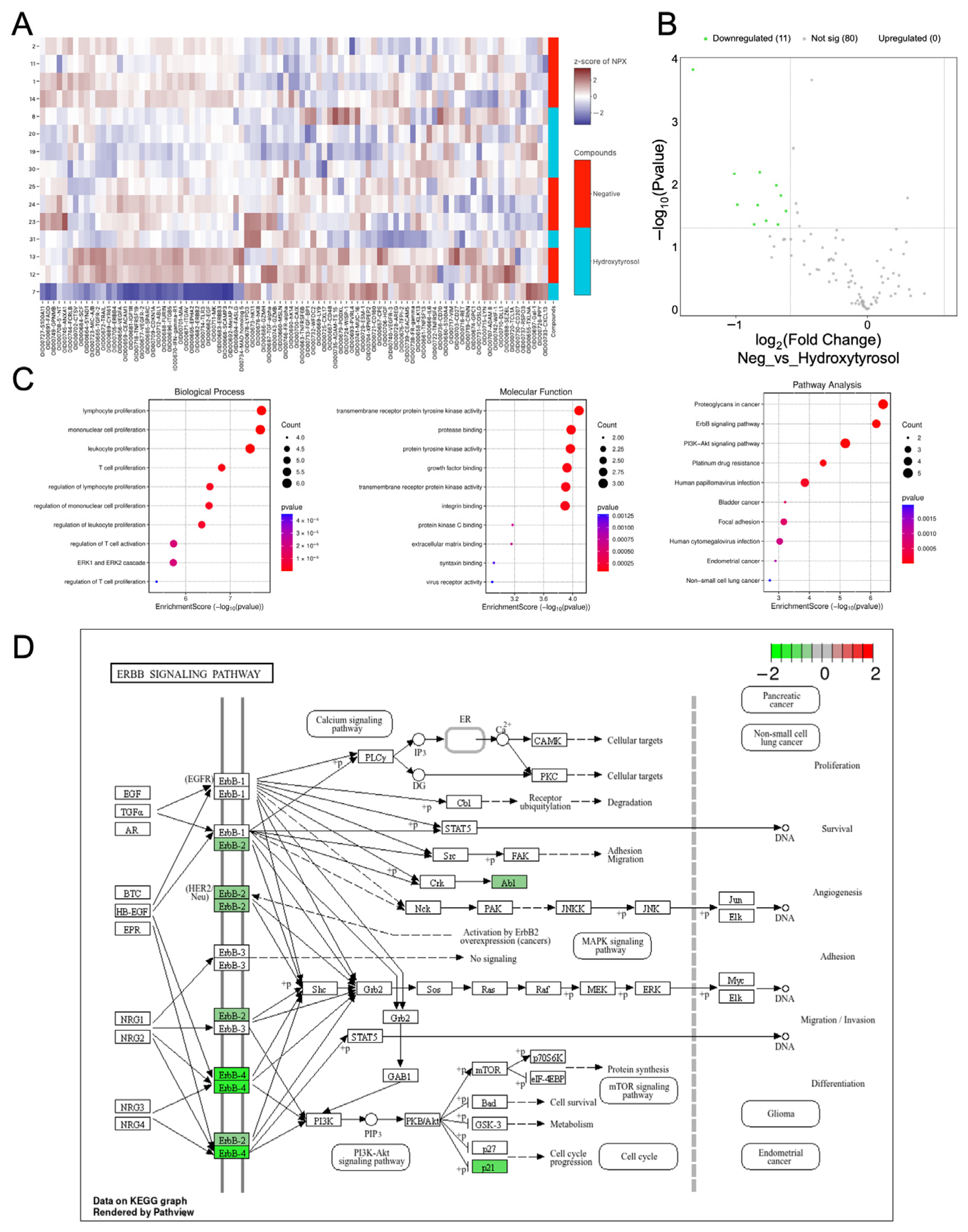
2.8. Hydroxytyrosol Downregulates Pro-Tumorigenic Protein Networks in C32 Spheroids
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Spheroid Formation
4.2. Cell Viability and Dose–Response Assays
4.3. Spheroid Growth and Morphometric Analysis
4.4. Spheroid Migration Assay
4.5. Spheroid Invasion Analysis
4.6. Flow Cytometric and Phenotypic Markers
4.7. Cell Cycle Analysis and Apoptosis Assay
4.8. Protein Extraction and Proteomic Profiling
4.9. Bioinformatic and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naik, P.P. Current Trends of Immunotherapy in the Treatment of Cutaneous Melanoma: A Review. Dermatol. Ther. 2021, 11, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kasakovski, D.; Skrygan, M.; Gambichler, T.; Susok, L. Advances in Targeting Cutaneous Melanoma. Cancers 2021, 13, 2090. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Santinon, F.; Flores González, R.E.; Del Rincón, S.V. Melanoma Plasticity: Promoter of Metastasis and Resistance to Therapy. Front. Oncol. 2021, 11, 756001. [Google Scholar] [CrossRef] [PubMed]
- Bhandaru, M.; Rotte, A. Monoclonal Antibodies for the Treatment of Melanoma: Present and Future Strategies. In Human Monoclonal Antibodies: Methods and Protocols; Steinitz, M., Ed.; Springer: New York, NY, USA, 2019; pp. 83–108. [Google Scholar]
- Malissen, N.; Grob, J.-J. Metastatic melanoma: Recent therapeutic progress and future perspectives. Drugs 2018, 78, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Mattia, G.; Puglisi, R.; Ascione, B.; Malorni, W.; Carè, A.; Matarrese, P. Cell death-based treatments of melanoma: Conventional treatments and new therapeutic strategies. Cell Death Dis. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, U.; Yap, J.; Sim, Y.R.M.; Qin, S.; Hu, J. Drug resistance in targeted cancer therapies with RAF inhibitors. Cancer Drug Resist. 2021, 4, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Imoto, H.; Rauch, N.; Neve, A.J.; Khorsand, F.; Kreileder, M.; Alexopoulos, L.G.; Rauch, J.; Okada, M.; Kholodenko, B.N.; Rukhlenko, O.S. A Combination of Conformation-Specific RAF Inhibitors Overcome Drug Resistance Brought about by RAF Overexpression. Biomolecules 2023, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Parra, D.; Zammit-Mangion, M. Comparative Analysis of the Effect of the BRAF Inhibitor Dabrafenib in 2D and 3D Cell Culture Models of Human Metastatic Melanoma Cells. In Vivo 2024, 38, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Vilain, R.E.; Menzies, A.M.; Wilmott, J.S.; Kakavand, H.; Madore, J.; Guminski, A.; Liniker, E.; Kong, B.Y.; Cooper, A.J.; Howle, J.R. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin. Cancer Res. 2017, 23, 5024–5033. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021, 12, 628690. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Bertinato, L.; Staniszewska, M.; Pancer, K.; Wieczorek, M.; Salmaso, S.; Caliceti, P.; Garofalo, M. From conventional therapies to immunotherapy: Melanoma treatment in review. Cancers 2020, 12, 3057. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef] [PubMed]
- Rager, T.; Eckburg, A.; Patel, M.; Qiu, R.; Gantiwala, S.; Dovalovsky, K.; Fan, K.; Lam, K.; Roesler, C.; Rastogi, A.; et al. Treatment of Metastatic Melanoma with a Combination of Immunotherapies and Molecularly Targeted Therapies. Cancers 2022, 14, 3779. [Google Scholar] [CrossRef] [PubMed]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Lia, F.; Zammit-Mangion, M.; Farrugia, C. A first description of the phenolic profile of EVOOs from the Maltese islands using SPE and HPLC: Pedo-climatic conditions modulate genetic factors. Agriculture 2019, 9, 107. [Google Scholar] [CrossRef]
- Lopez-Bascon, M.A.; Moscoso-Ruiz, I.; Quirantes-Pine, R.; Del Pino-Garcia, R.; Lopez-Gamez, G.; Justicia-Rueda, A.; Verardo, V.; Quiles, J.L. Characterization of Phenolic Compounds in Extra Virgin Olive Oil from Granada (Spain) and Evaluation of Its Neuroprotective Action. Int. J. Mol. Sci. 2024, 25, 4878. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Aprile, A.; Luvisi, A.; Nicoli, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; Sabella, E.; De Bellis, L. Phenolic Profile and Antioxidant Activity of Italian Monovarietal Extra Virgin Olive Oils. Antioxidants 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Del Saz-Lara, A.; López de Las Hazas, M.C.; Visioli, F.; Dávalos, A. Nutri-Epigenetic Effects of Phenolic Compounds from Extra Virgin Olive Oil: A Systematic Review. Adv. Nutr. 2022, 13, 2039–2060. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Vella, N.; Rosignoli, P. Epigenetic Modifications Induced by Olive Oil and Its Phenolic Compounds: A Systematic Review. Molecules 2021, 26, 273. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Ercelik, M.; Tekin, C.; Tezcan, G.; Ak Aksoy, S.; Bekar, A.; Kocaeli, H.; Taskapilioglu, M.O.; Eser, P.; Tunca, B. Leaf Phenolics Oleuropein, Hydroxytyrosol, Tyrosol, and Rutin Induce Apoptosis and Additionally Affect Temozolomide against Glioblastoma: In Particular, Oleuropein Inhibits Spheroid Growth by Attenuating Stem-like Cell Phenotype. Life 2023, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Z.; Yang, X.; Liu, L.; Ahn, K.S. An updated review on the potential antineoplastic actions of oleuropein. Phytother. Res. 2022, 36, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.; Aiello, A.; Rodríguez-Pérez, M.; Accardi, G.; Burgos-Ramos, E.; Silva, P. Olive Oil Components as Novel Antioxidants in Neuroblastoma Treatment: Exploring the Therapeutic Potential of Oleuropein and Hydroxytyrosol. Nutrients 2024, 16, 818. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.; Garcia-Gaipo, L.; Casar, B.; Gandarillas, A. DNA damage signalling histone H2AX is required for tumour growth. Cell Death Discov. 2024, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. Slas. Discov. Adv. Life Sci. RD 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Delorme, S.; Privat, M.; Sonnier, N.; Rouanet, J.; Witkowski, T.; Kossai, M.; Mishellany, F.; Radosevic-Robin, N.; Juban, G.; Molnar, I.; et al. New insight into the role of ANXA1 in melanoma progression: Involvement of stromal expression in dissemination. Am. J. Cancer Res. 2021, 11, 1600–1615. [Google Scholar] [PubMed]
- McCarthy, M.M.; DiVito, K.A.; Sznol, M.; Kovacs, D.; Halaban, R.; Berger, A.J.; Flaherty, K.T.; Camp, R.L.; Lazova, R.; Rimm, D.L.; et al. Expression of tumor necrosis factor--related apoptosis-inducing ligand receptors 1 and 2 in melanoma. Clin. Cancer Res. 2006, 12, 3856–3863. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Gini, B.; Wykosky, J.; Zanca, C.; Mischel, P.S.; Furnari, F.B.; Cavenee, W.K. A tale of two approaches: Complementary mechanisms of cytotoxic and targeted therapy resistance may inform next-generation cancer treatments. Carcinogenesis 2013, 34, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Phadke, M.; Remsing Rix, L.L.; Smalley, I.; Bryant, A.T.; Luo, Y.; Lawrence, H.R.; Schaible, B.J.; Chen, Y.A.; Rix, U.; Smalley, K.S.M.; et al. Dabrafenib inhibits the growth of BRAF-WT cancers through CDK16 and NEK9 inhibition. Mol. Oncol. 2018, 12, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Ouanouki, A.; Beliveau, R.; Desrosiers, R.R. Olive oil compounds inhibit vascular endothelial growth factor receptor-2 phosphorylation. Exp. Cell Res. 2014, 322, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Castañeda, L.D.; Nova, J.A.; Tovar-Parra, J.D. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: A systemic review. Melanoma Res. 2020, 30, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Geng, R.; Lin, E.; Zhao, P.; Chen, Y. ERBB1/2/3 Expression, Prognosis, and Immune Infiltration in Cutaneous Melanoma. Front. Genet. 2021, 12, 602160. [Google Scholar] [CrossRef] [PubMed]
- Shteinman, E.R.; Vergara, I.A.; Rawson, R.V.; Lo, S.N.; Maeda, N.; Koyama, K.; da Silva, I.P.; Long, G.V.; Scolyer, R.A.; Wilmott, J.S.; et al. Molecular and clinical correlates of HER3 expression highlights its potential role as a therapeutic target in melanoma. Pathology 2023, 55, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Yajima, I.; Kumasaka, M.Y.; Thang, N.D.; Goto, Y.; Takeda, K.; Yamanoshita, O.; Iida, M.; Ohgami, N.; Tamura, H.; Kawamoto, Y.; et al. RAS/RAF/MEK/ERK and PI3K/PTEN/AKT Signaling in Malignant Melanoma Progression and Therapy. Dermatol. Res. Pract. 2012, 2012, 354191. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-X.; Qi, X.-Y. Targeting RAS/RAF/MEK/ERK signaling in metastatic melanoma. IUBMB Life 2013, 65, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Di Sano, C.; Barbieri, G. The Hydroxytyrosol Induces the Death for Apoptosis of Human Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 8047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Sommariva, M.; Anselmi, M.; Bianchi, F.; Limonta, P.; Gagliano, N. Differentiation States of Phenotypic Transition of Melanoma Cells Are Revealed by 3D Cell Cultures. Cells 2024, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Shirley, C.A.; Chhabra, G.; Amiri, D.; Chang, H.; Ahmad, N. Immune escape and metastasis mechanisms in melanoma: Breaking down the dichotomy. Front. Immunol. 2024, 15, 1336023. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.D.Z.; Pal, A.; Barrett, T.F.; Heft Neal, M.E.; Puram, S.V. Epithelial-Mesenchymal Plasticity in Tumor Immune Evasion. Cancer Res. 2022, 82, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lozano, M.; Gonzalez-Gonzalez, A.; Marchal, J.A.; Munoz-Muela, E.; Molina, M.P.; Cara, F.E.; Brown, A.M.; Garcia-Rivas, G.; Hernandez-Brenes, C.; Lorente, J.A.; et al. Hydroxytyrosol inhibits cancer stem cells and the metastatic capacity of triple-negative breast cancer cell lines by the simultaneous targeting of epithelial-to-mesenchymal transition, Wnt/beta-catenin and TGFbeta signaling pathways. Eur. J. Nutr. 2019, 58, 3207–3219. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovar-Parra, D.; Mangion, M.Z. Hydroxytyrosol Reprograms the Tumor Microenvironment in 3D Melanoma Models by Suppressing ERBB Family and Kinase Pathways. Int. J. Mol. Sci. 2025, 26, 6957. https://doi.org/10.3390/ijms26146957
Tovar-Parra D, Mangion MZ. Hydroxytyrosol Reprograms the Tumor Microenvironment in 3D Melanoma Models by Suppressing ERBB Family and Kinase Pathways. International Journal of Molecular Sciences. 2025; 26(14):6957. https://doi.org/10.3390/ijms26146957
Chicago/Turabian StyleTovar-Parra, David, and Marion Zammit Mangion. 2025. "Hydroxytyrosol Reprograms the Tumor Microenvironment in 3D Melanoma Models by Suppressing ERBB Family and Kinase Pathways" International Journal of Molecular Sciences 26, no. 14: 6957. https://doi.org/10.3390/ijms26146957
APA StyleTovar-Parra, D., & Mangion, M. Z. (2025). Hydroxytyrosol Reprograms the Tumor Microenvironment in 3D Melanoma Models by Suppressing ERBB Family and Kinase Pathways. International Journal of Molecular Sciences, 26(14), 6957. https://doi.org/10.3390/ijms26146957




