Inclusion Complex of a Cationic Mono-Choline-β-Cyclodextrin Derivative with Resveratrol: Preparation, Characterization, and Wound-Healing Activity
Abstract
1. Introduction
2. Results and Discussion
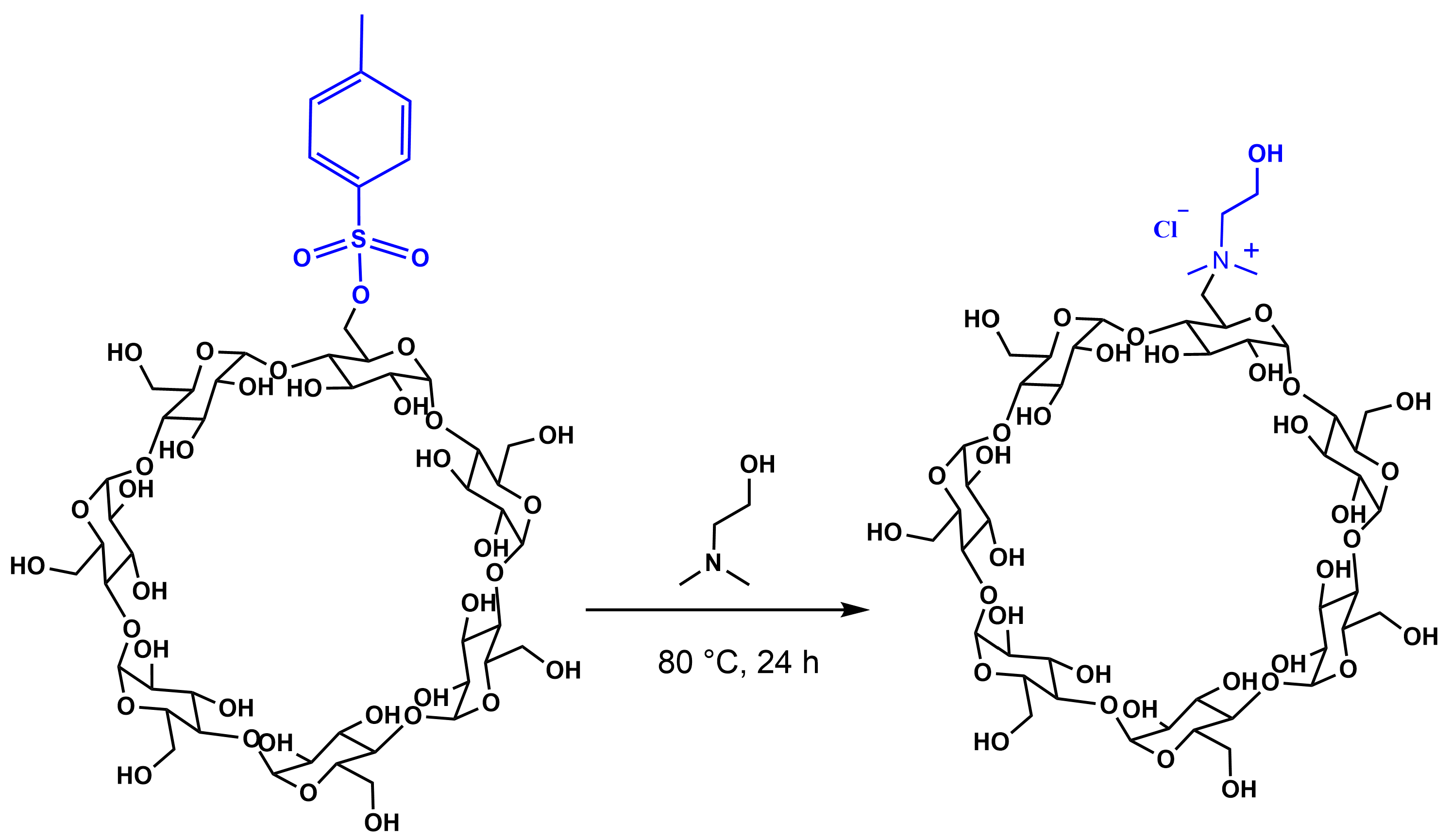
2.1. Synthesis of Mono-Choline-β-Cyclodextrin Derivative (β-CD-Chol)
2.2. Preparation and Characterization of the β-CD-Chol/RES Inclusion Complex
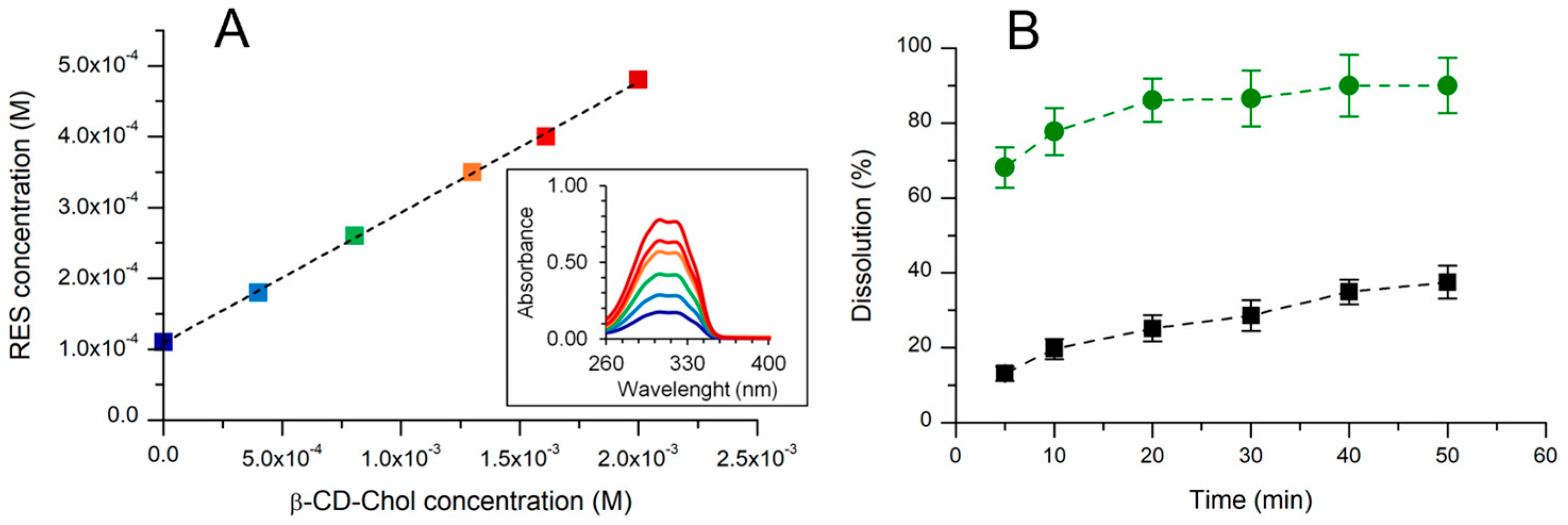
2.2.1. UV–Vis Characterization and Phase Solubility Analysis
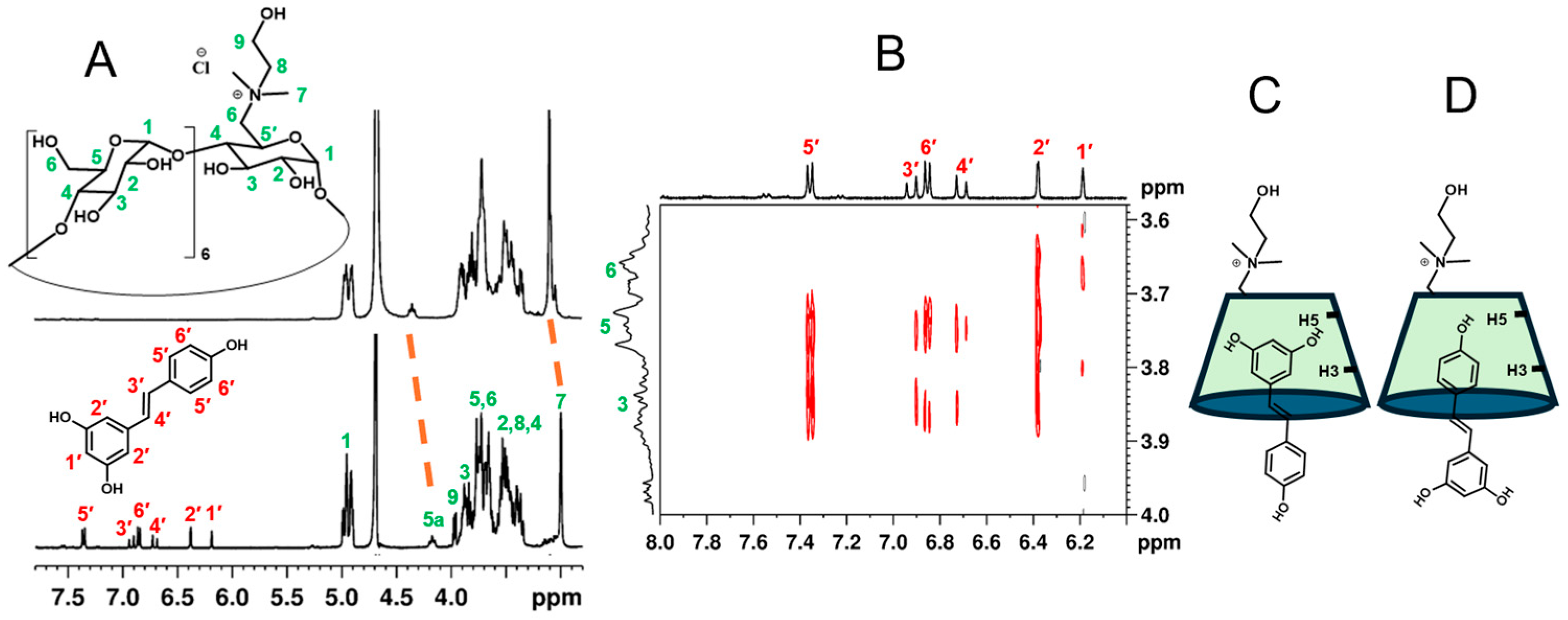
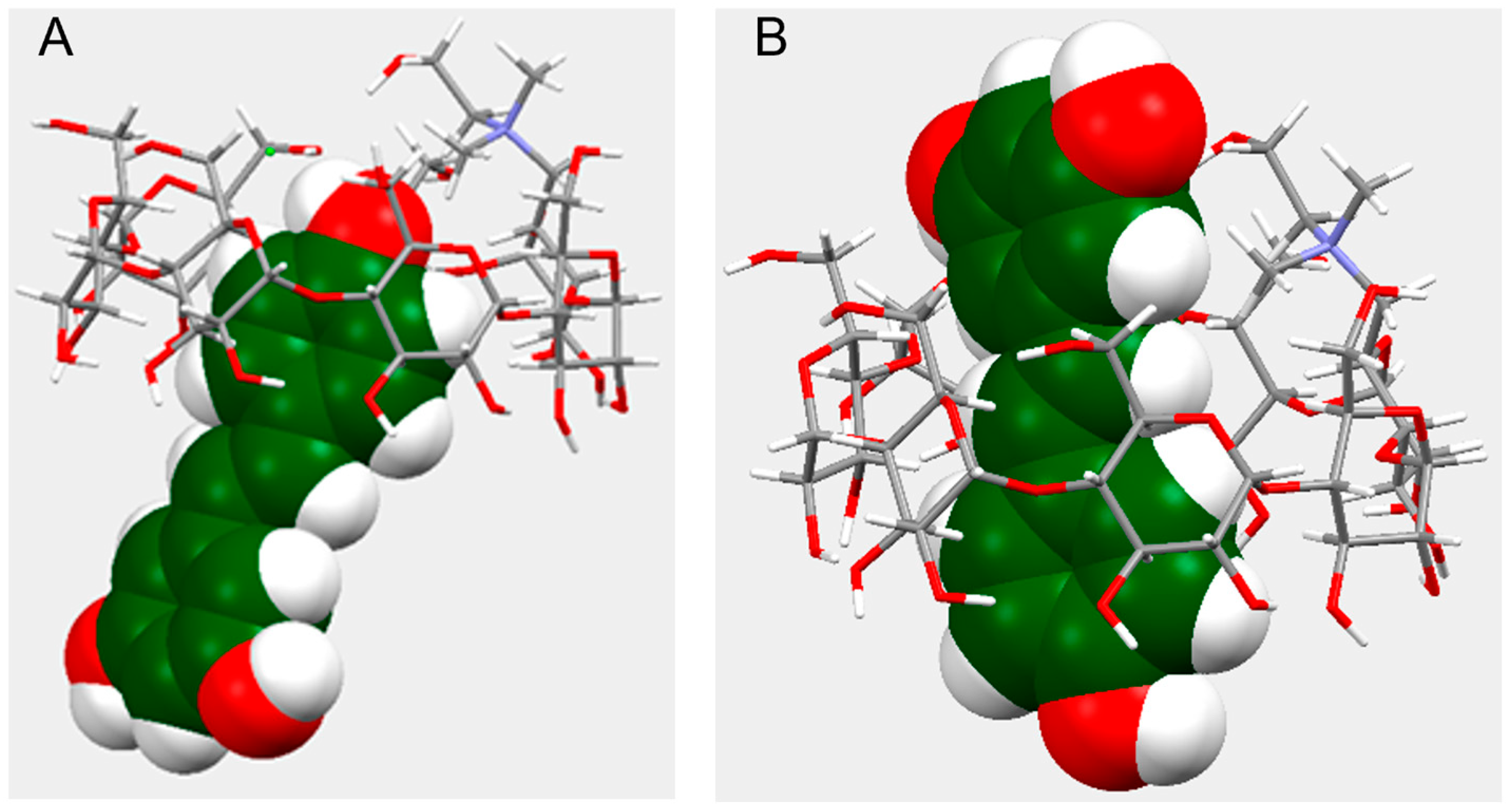
2.2.2. NMR Characterization of the Complex and Molecular Modeling Simulations
2.2.3. Radical Scavenging Activity and Photostability of RES in the Complex
2.3. Wound-Healing Activity
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of β-CD-Chol
3.4. Preparation of β-CD-Chol/RES Inclusion Complex
3.5. Phase Solubility Study
3.6. Water Solubility and Dissolution Rate Determination
3.7. Molecular Modeling Simulations
3.8. Evaluation of DPPH Radical Scavenging Activity
3.9. Photostability Study
3.10. Cell Cultures
3.11. Wound-Healing Assay
3.12. Statistical Analysis for Human Cell Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Mihis, A.G. Resveratrol as a privileged molecule with antioxidant activity. Food Chem. Adv. 2023, 3, 100539. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassawi, W.; Al-Sammak, M.A. Resveratrol as an Antiaging Drug: A Review of Articles. Iraqi J. Pharm. Sci. 2024, 33, 1–13. [Google Scholar] [CrossRef]
- Ribeiro, E.; Vale, N. The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens. Targets 2024, 2, 307–326. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, A.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From in Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Yen, F.-L.; Huang, H.-W.; Wu, T.-H.; Ko, H.-H.; Tzeng, W.-S.; Lin, C.-C. Resveratrol Nanoparticle System Improves Dissolution Properties and Enhances the Hepatoprotective Effect of Resveratrol through Antioxidant and Anti-Inflammatory Pathways. J. Agric. Food Chem. 2012, 60, 4662–4671. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.; Khan, H.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol. Adv. 2021, 53, 107844. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Abellán, C.; Fortea, I.; López-Nicolás, J.M.; Núñez-Delicado, E. Cyclodextrins as resveratrol carrier system. Food Chem. 2007, 104, 39–44. [Google Scholar] [CrossRef]
- Oliva, E.; Mathiron, D.; Bertaut, E.; Landy, D.; Cailleu, D.; Pilard, S.; Clément, C.; Courot, E.; Bonnet, V.; Djedaïni-Pilard, F. Physico-chemical studies of resveratrol, methyl-jasmonate and cyclodextrin interactions: An approach to resveratrol bioproduction optimization. RSC Adv. 2018, 8, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Trollope, L.; Cruickshank, D.L.; Noonan, T.; Bourne, S.A.; Sorrenti, M.; Catenacci, L.; Caira, M.R. Inclusion of trans-resveratrol in methylated cyclodextrins: Synthesis and solid-state structures. Beilstein J. Org. Chem. 2014, 10, 3136–3151. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Martinho, A.; Luís, Â.; Figueiras, A.; Oleastro, M.; Domingues, F.C.; Silva, F. Resveratrol encapsulation with methyl-β-cyclodextrin for antibacterial and antioxidant delivery applications. LWT Food Sci. Technol. 2015, 63, 1254–1260. [Google Scholar] [CrossRef]
- Yang, Z.; Argenziano, M.; Salamone, P.; Pirro, E.; Sprio, A.E.; Di Scipio, F.; Carere, M.E.; Quaglino, E.; Cavallo, F.; Cavalli, R.; et al. Preclinical pharmacokinetics comparison between resveratrol 2-hydroxypropyl-β-cyclodextrin complex and resveratrol suspension after oral administration. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 263–271. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Gerges, P.; Kfoury, M.; Landy, D.; Fourmentin, S.; Greige-Gerges, H. Cyclodextrin-based materials for pulmonary delivery: Insights and challenges. Carbohydr. Polym. 2025, 363, 123712. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tibi, I.P.-E.; Zaharieva, M.M.; Kaleva, M.; Najdenski, H.; Petrov, P.D.; Tzankova, V.; et al. Incorporation of Resveratrol-Hydroxypropyl-β-Cyclodextrin Complexes into Hydrogel Formulation for Wound Treatment. Gels 2024, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, W.; Guo, X.; Yao, P.; Xie, W. Resveratrol Accelerates Wound Healing by Attenuating Oxidative Stress-Induced Impairment of Cell Proliferation and Migration. Burns 2021, 47, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Hassanzadeh-Taheri, M.-M.; Zardast, M.; Moghaddam, A. The Angiogenetic Effect of Resveratrol on Dermal Wound Healing in Balb/C Mice. Mod. Care J. 2017, 14, e66118. [Google Scholar] [CrossRef]
- Pignet, A.-L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.-P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, S.; Li, Y.; Yuan, S. Molecular insights into the controlled release process of cyclodextrin-resveratrol inclusion complexes in the stratum corneum. Colloids Surf. B Biointerfaces 2025, 253, 114725. [Google Scholar] [CrossRef] [PubMed]
- Esteso, M.A.; Romero, C.M. Cyclodextrins: Properties and Applications. Int. J. Mol. Sci. 2024, 25, 4547. [Google Scholar] [CrossRef] [PubMed]
- Pedotti, S.; Ferreri, L.; Migliore, R.; Leotta, C.G.; Pitari, G.M.; D’Antona, N.; Petralia, S.; Aleo, D.; Sgarlata, C.; Consoli, G.M.L. A novel cationic β-cyclodextrin decorated with a choline-like pendant exhibits iodophor, mucoadhesive and bactericidal properties. Carbohydr. Polym. 2024, 28, 121698. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Consoli, G.M.L.; Granata, G.; Casili, G.; Ardizzone, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Topical Delivery of Curcumin by Choline-Calix[4]arene-based Nanohydrogel improves its therapeutic effect on a psoriasis mouse model. Int. J. Mol. Sci. 2020, 21, 5053. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y.; Kuang, Y.; An, S.; Ma, H.; Jiang, C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials 2013, 34, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.R.; Bondì, M.L.; Cavallaro, G.; Consoli, G.M.L.; Craparo, E.F.; Giammona, G.; Licciardi, M.; Pitarresi, G.; Granata, G.; Saladino, P.; et al. Nanostructured Formulations for the Delivery of Silibinin and Other Active Ingredients for Treating Ocular Diseases. PCT/IB2015/057732. WO 2016055976 A1, 14 April 2016. [Google Scholar]
- Granata, G.; Paterniti, I.; Geraci, C.; Cunsolo, F.; Esposito, E.; Cordaro, M.; Blanco, A.R.; Cuzzocrea, S.; Consoli, G.M.L. Potential Eye Drop based on a Calix[4]arene Nanoassembly for Curcumin Delivery: Enhanced Drug Solubility, Stability and Anti-Inflammatory Effect. Mol. Pharm. 2017, 14, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Venuti, V.; Cannavà, C.; Cristiano, M.C.; Fresta, M.; Majolino, D.; Paolino, D.; Stancanelli, R.; Tommasini, S.; Ventura, C.A. A characterization study of resveratrol/sulfobutyl ether-β-cyclodextrin inclusion complex and in vitro anticancer activity. Colloids Surf. B Biointerfaces 2014, 115, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Wischke, C.; Neffe, A.T.; Lendlein, A. Efficient synthesis of pure monotosylated beta-cyclodextrin and its dimers. Carbohyd. Res. 2013, 381, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Bertacche, V.; Lorenzi, N.; Nava, D.; Pini, E.; Sinico, C. Host–Guest interaction study of resveratrol with natural and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 279–287. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Troche-Pesqueira, E.; Pérez-Juste, I.; Navarro-Vázquez, A.; Cid, M.M. A β-cyclodextrin–resveratrol inclusion complex and the role of geometrical and electronic effects on its electronic induced circular dichroism. RSC Adv. 2013, 3, 10242–10250. [Google Scholar] [CrossRef]
- Iskineyeva, A.; Fazylov, S.; Bakirova, R.; Sarsenbekova, A.; Pustolaikina, I.; Seilkhanov, O.; Alsfouk, A.A.; Elkaeed, E.B.; Eissa, I.H.; Metwaly, A.M. Combined In Silico and Experimental Investigations of Resveratrol Encapsulation by Beta-Cyclodextrin. Plants 2022, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Carlotti, M.E.; Caron, G.; Ugazio, E.; Cavalli, R. In silico design, photostability and biological properties of the complex resveratrol/hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 171–180. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH• radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Oh, S.-J.; Liu, Y.; Lee, M.-Y. A Comparative Study of the Anti-Platelet Effects of cis- and trans-Resveratrol. Biomol. Ther. 2011, 19, 201–205. [Google Scholar] [CrossRef]
- Leischner, C.; Burkard, M.; Michel, A.; Berchtold, S.; Niessner, H.; Marongiu, L.; Busch, C.; Frank, J.; Lauer, U.M.; Venturelli, S. Comparative Analysis of the Antitumor Activity of Cis- and Trans-Resveratrol in Human Cancer Cells with Different p53 Status. Molecules 2021, 26, 5586. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F. Comparative Studies of the Antioxidant Effects of Cis- and Trans- Resveratrol. Curr. Med. Chem. 2006, 13, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Rius, C.; Abu-Taha, M.; Hermenegildo, C.; Piqueras, L.; Cerda-Nicolas, J.-M.; Issekutz, A.C.; Estañ, L.; Cortijo, J.; Morcillo, E.J.; Orallo, F.; et al. Trans- but not cis-resveratrol impairs angiotensin-II–mediated vascular inflammation through inhibition of NF-κB activation and peroxisome proliferator-activated receptor-γ upregulation. J. Immunol. 2010, 185, 3718–3727. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended tight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 2020, 11, e01493. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and Efficient Implicit Solvation Model for Fast Semiempirical Methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Accardo, P.; Consoli, G.M.L.; Paradisi, R.; Geraci, C. High vitamin E-loaded nanocapsules to fortify a fruit-based product. Food Biosci. 2025, 64, 105884. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedotti, S.; Ferreri, L.; Granata, G.; Gambera, G.; D’Antona, N.; Leotta, C.G.; Pitari, G.M.; Consoli, G.M.L. Inclusion Complex of a Cationic Mono-Choline-β-Cyclodextrin Derivative with Resveratrol: Preparation, Characterization, and Wound-Healing Activity. Int. J. Mol. Sci. 2025, 26, 6911. https://doi.org/10.3390/ijms26146911
Pedotti S, Ferreri L, Granata G, Gambera G, D’Antona N, Leotta CG, Pitari GM, Consoli GML. Inclusion Complex of a Cationic Mono-Choline-β-Cyclodextrin Derivative with Resveratrol: Preparation, Characterization, and Wound-Healing Activity. International Journal of Molecular Sciences. 2025; 26(14):6911. https://doi.org/10.3390/ijms26146911
Chicago/Turabian StylePedotti, Sonia, Loredana Ferreri, Giuseppe Granata, Giovanni Gambera, Nicola D’Antona, Claudia Giovanna Leotta, Giovanni Mario Pitari, and Grazia Maria Letizia Consoli. 2025. "Inclusion Complex of a Cationic Mono-Choline-β-Cyclodextrin Derivative with Resveratrol: Preparation, Characterization, and Wound-Healing Activity" International Journal of Molecular Sciences 26, no. 14: 6911. https://doi.org/10.3390/ijms26146911
APA StylePedotti, S., Ferreri, L., Granata, G., Gambera, G., D’Antona, N., Leotta, C. G., Pitari, G. M., & Consoli, G. M. L. (2025). Inclusion Complex of a Cationic Mono-Choline-β-Cyclodextrin Derivative with Resveratrol: Preparation, Characterization, and Wound-Healing Activity. International Journal of Molecular Sciences, 26(14), 6911. https://doi.org/10.3390/ijms26146911








