The Seminal Role of the Proinflammatory Cytokine IL-1β and Its Signaling Cascade in Glioblastoma Pathogenesis and the Therapeutic Effect of Interleukin-1β Receptor Antagonist (IL-1RA) and Tolcapone †
Abstract
1. Introduction
2. Results
2.1. IL-1β Induced Morphological Changes in Human Glioblastoma Cancer Cells Following Treatment with Either IL-1RA or Tolcapone
2.2. IL-1β Treatment Elevates GFAP Expression in Human Glioblastoma Cancer Cells
2.3. Cytotoxic Effects of IL-1RA and Tolcapone on Human Glioblastoma Cancer Cells
2.4. IL-1RA and Tolcapone Inhibited the Formation of U87 Colonies and Cancer Invasiveness
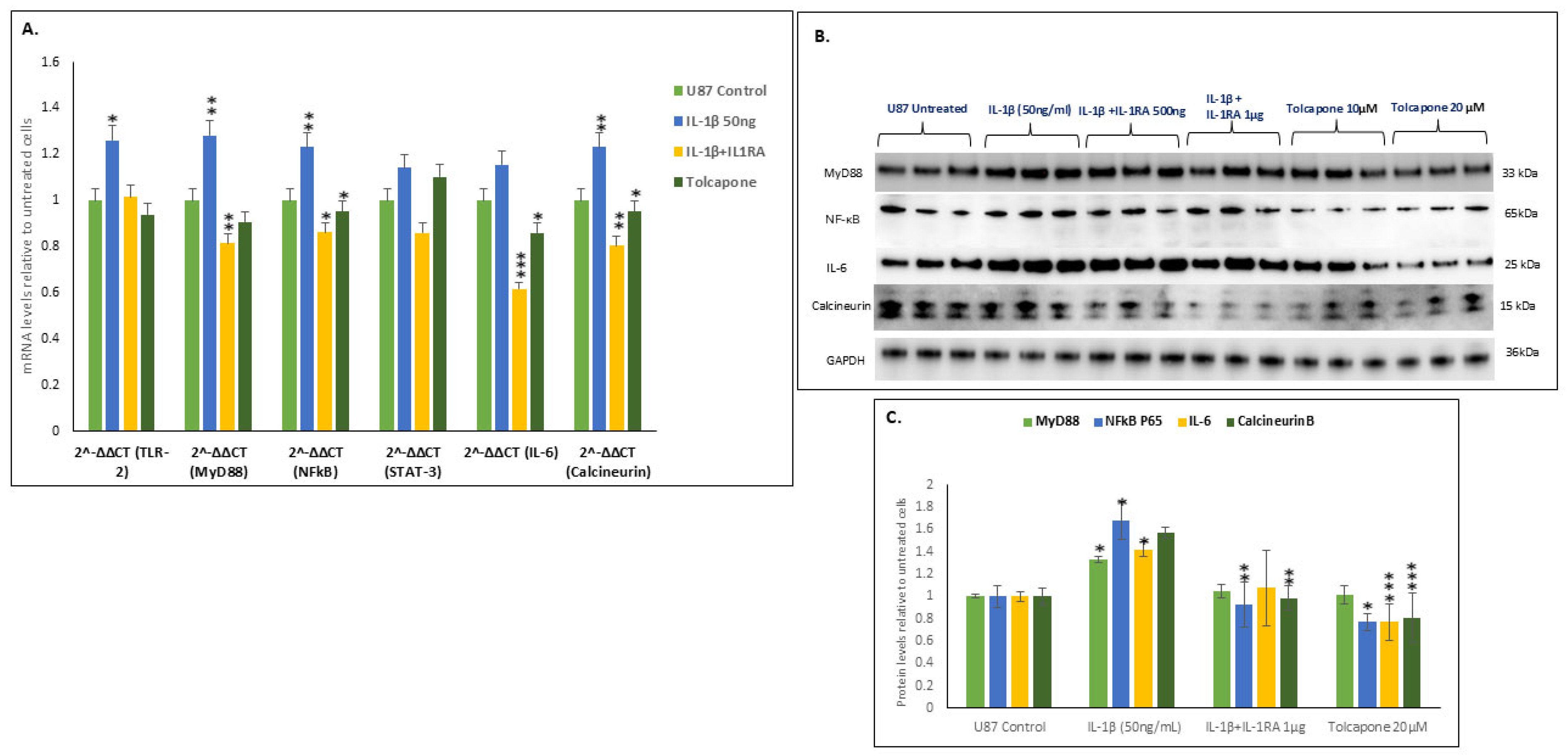
2.5. IL-1β Increased the Transcription and Translation of the MyD88-NFĸB-IL-6-Calcineurin Pathway
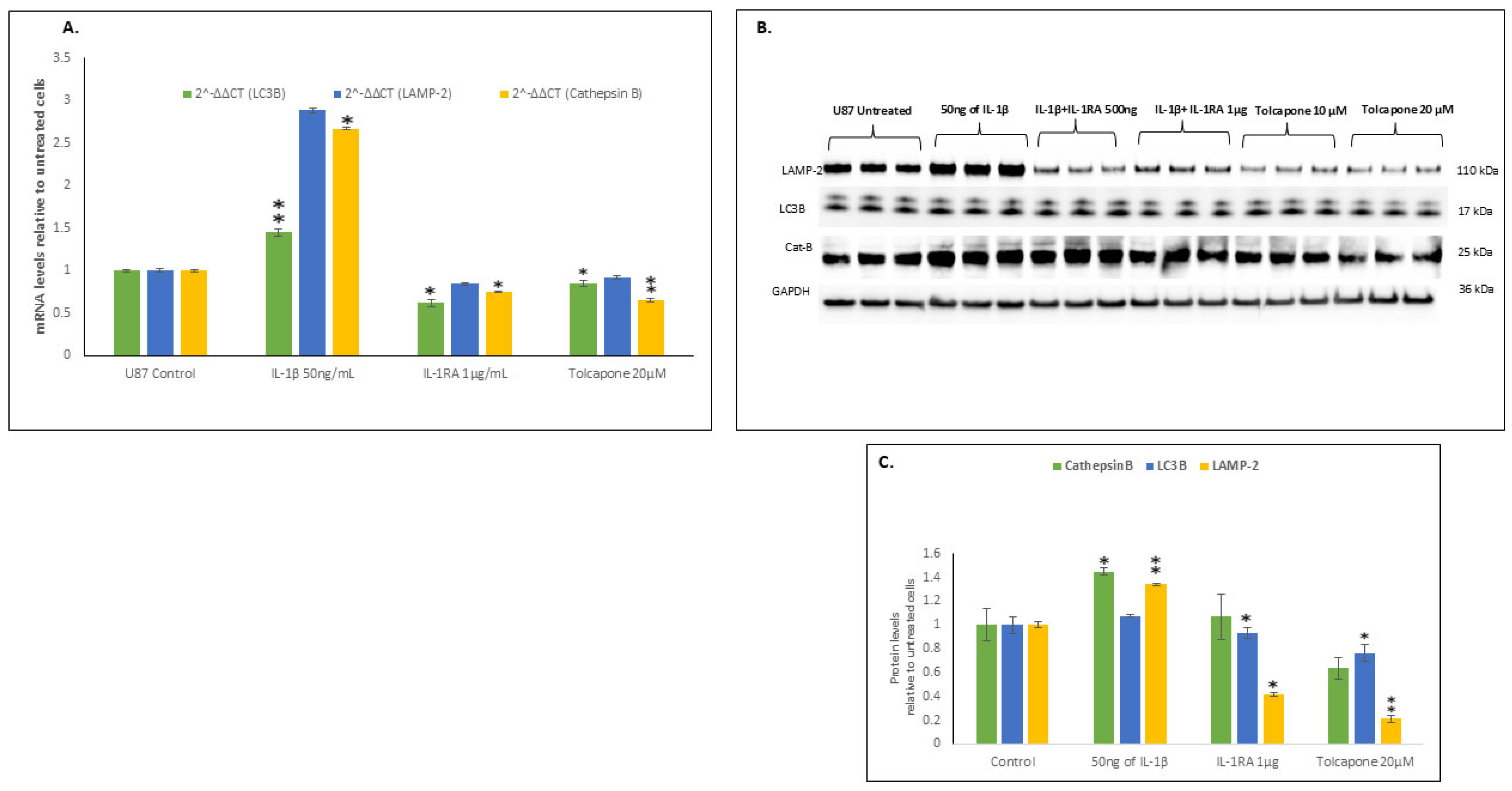
2.6. IL-1β Increased the Transcription and Translation of Components of Autophagy
2.7. IL-1β Down Regulates Apoptosis Resulting in U87 Cancer Cell Survival and Metastasis
3. Discussion
4. Materials and Methods
4.1. MTT Assay
4.2. Immunofluorescence Microscopy
4.3. Clonogenic Assay
4.4. Scratch Wound Healing Assay
4.5. RT-PCR Amplification
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S.; Stanley, L.C.; Ling, C.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L.; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Barger, S.W.; Griffin, W.S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci. 2003, 23, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, M.; Parcon, P.A.; Bose, C.; Liu, L.; Jones, R.A.; Farlow, M.R.; Mrak, R.E.; Barger, S.W.; Griffin, W.S.T. Interleukin-1β drives NEDD8 nuclear-to-cytoplasmic translocation, fostering parkin activation via NEDD8 binding to the P-ubiquitin activating site. J. Neuroinflamm. 2019, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ottewell, P.D. The role of IL-1B in breast cancer bone metastasis. J. Bone Oncol. 2024, 46, 100608. [Google Scholar] [CrossRef] [PubMed]
- Whitley, S.K.; Balasubramani, A.; Zindl, C.L.; Sen, R.; Shibata, Y.; Crawford, G.E.; Weathington, N.M.; Hatton, R.D.; Weaver, C.T. IL-1R signaling promotes STAT3 and NF-κB factor recruitment to distal cis-regulatory elements that regulate Il17a/f transcription. J. Biol. Chem. 2018, 293, 15790–15800. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Grumont, R.J.; Rourke, I.J.; Gerondakis, S. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev. 1999, 13, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Verzella, D.; Tessitore, A.; Alesse, E.; Capalbo, C.; Zazzeroni, F. Cancer secretome and inflammation: The bright and the dark sides of NF-κB. Semin. Cell Dev. Biol. 2018, 78, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Roderick, H.L.; Cook, S.J. Ca2+ signalling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Hurmath, K.F.; Ramaswamy, P.; Nandakumar, D.N. IL-1β microenvironment promotes proliferation, migration, and invasion of human glioma cells. Cell Biol. Int. 2014, 38, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Coelho, M.A.; Soares-da-Silva, P. Effects of tolcapone upon soluble and membrane-bound brain and liver catechol-O-methyltransferase. Brain Res. 1999, 821, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kurth, M.C.; Adler, C.H.; St Hilaire, M.; Singer, C.; Waters, C.; LeWitt, P.; Chernik, D.A.; Dorflinger, E.E.; Yoo, K. Tolcapone improves motor function and reduces levodopa requirement in patients with Parkinson’s disease experiencing motor fluctuations: A multicenter, double-blind, randomized, placebo-controlled trial. Neurology 1997, 48, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Maser, T.; Rich, M.; Hayes, D.; Zhao, P.; Nagulapally, A.B.; Bond, J.; Saulnier Sholler, G. Tolcapone induces oxidative stress leading to apoptosis and inhibition of tumor growth in Neuroblastoma. Cancer Med. 2017, 6, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Narasimhappagari, J.; Liu, L.; Balasubramaniam, M.; Ayyadevara, S.S.; Aboud, O.; Griffin, W.S.T. The role of the proinflammatory cytokine IL-1β and its signaling cascade in glioblastoma pathogenesis and the therapeutic effect of IL-1RA and Tolcapone as anticancer agents [abstract]. In Proceedings of the American Association for Cancer Research Annual Meeting 2025; Part 2 (Late-Breaking, Clinical Trial, and Invited Abstracts), Chicago, IL, USA, 25–30 April 2025. [Google Scholar]
- Dutta, D.; Jana, M.; Majumder, M.; Mondal, S.; Roy, A.; Pahan, K. Selective targeting of the TLR2/MyD88/NF-κB pathway reduces α-synuclein spreading in vitro and in vivo. Nat. Commun. 2021, 12, 5382. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, L.; Ronellenfitsch, M.W.; Antonietti, P.; Ilina, E.I.; Jung, J.; Stadel, D.; Flohr, L.M.; Zinke, J.; von Renesse, J.; Drott, U.; et al. Diagnostic and clinical relevance of the autophago-lysosomal network in human gliomas. Oncotarget 2016, 7, 20016. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, N.; Belur, S.; Chachadi, V.B.; Roy, S.; Inamdar, S.R. Aspergillus niger lectin elicits MyD88 dependent proliferation and apoptosis at lower and higher doses in immortalized human corneal epithelial cells leading to pathogenesis. Int. J. Biol. Macromol. 2020, 165, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, N.; Belur, S.; Ballal, S.; Roy, S.; Inamdar, S.R. Cephalosporium curvulum lectin causes mycotic keratitis by initiating infection through MyD88 dependent cellular proliferation and apoptosis in human corneal epithelial cells. Glycoconj. J. 2021, 38, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Y.; Wang, L.; Li, L. Role of TLR4/MyD88/NF-κB signaling in heart and liver-related complications in a rat model of type 2 diabetes mellitus. J. Int. Med. Res. 2021, 49, 300060521997590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, K.; Lee, O.Y.; Park, Y.; Seo, M.W.; Lee, D.S. IL-1β induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer 2016, 16, 724. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Brun, M.; Godbout, R. Activation of calcineurin in cancer: Many paths, one hub. Transl. Cancer Res. 2016, 5. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Thompson, J.C.; Griesinger, A.M.; Amani, V.; Donson, A.M.; Birks, D.K.; Morgan, M.J.; Mirsky, D.M.; Handler, M.H.; Foreman, N.K.; et al. Autophagy inhibition improves chemosensitivity in BRAFV600E brain tumors. Cancer Discov. 2014, 4, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, M.; Narasimhappagari, J.; Liu, L.; Ganne, A.; Ayyadevara, S.; Atluri, R.; Ayyadevara, H.; Caldwell, G.; Reis, R.J.S.; Barger, S.W.; et al. Rescue of ApoE4-related lysosomal autophagic failure in Alzheimer’s disease by targeted small molecules. Commun. Biol. 2024, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Chang, O.; Cheon, S.; Semenova, N.; Azad, N.; Iyer, A.K.; Yakisich, J.S. Prolonged Low-Dose Administration of FDA-Approved Drugs for Non-Cancer Conditions: A Review of Potential Targets in Cancer Cells. Int. J. Mol. Sci. 2025, 26, 2720. [Google Scholar] [CrossRef] [PubMed]
- Hensley, P.; Mishra, M.; Kyprianou, N. Targeting caspases in cancer therapeutics. Biol. Chem. 2013, 394, 831–843. [Google Scholar] [CrossRef] [PubMed]




| Gene [Direction] | Sequence | Species |
|---|---|---|
| TLR-2 | [F]5′-AAG GGC AGC TCA GGA TCT TT-3′ [R]5′-AGA CTG CCC AGG GAA GAA AA-3′ | Human |
| MyD88 | [F]5′-CCA GCA TTG AGG AGG ATT GC-3′ [R]5′-GCT CTG CTG TCC GTG GGA-3′ | Human |
| NF-κB p65 | [F]5′-AGA TAC CAC CAA GAC CCA CC-3′ [R]5′-CTG TCC CTG GTC CTG TGT AG-3′ | Human |
| GFAP | [F]5′-GAGAGGGACAATCTGGCACA-3′ [R]5′-GGC TTC ATC TGC TTC CTG TCT-3′ | Human |
| PPP3 | [F]5′-AGA GGC AAA GGG TTT GGA TAG-3′ [R]5′-ATG TGC GGT GTT CAG AGA AT-3′ | Human |
| IL-6 | [F]5′-TGA AAG CAG CAA AGA GGC AC-3′ [R]5′-TCA CCA GGC AAG TCT CCT CAT-3′ | Human |
| Cathepsin-B | [F]5′-TCT CTG ACC GGA TCT GCA TC-3′ [R]5′-TCA CAG GGA ATG GAG TA-3′ | Human |
| LC3B | [F]5′-GTT ACG GAA AGC AGC AGT GTA-3′ [R]5′-CAG AAG GGA GTG TGT CTG AAT G-3′ | Human |
| Lamp2 | [F]5′-GAA ATG CCA GTG TGT CCT AGA-3′ [R]5′-TCC CAA AGT GCT GGG ATT AC-3′ | Human |
| 18S | [F]5′-TTC GGA CGT CTG CCC TAT CAA-3′ [R]5′-ATG GTA GGC ACG GCG ACT A-3′ | Human |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narasimhappagari, J.; Liu, L.; Balasubramaniam, M.; Ayyadevara, S.; Aboud, O.; Griffin, W.S.T. The Seminal Role of the Proinflammatory Cytokine IL-1β and Its Signaling Cascade in Glioblastoma Pathogenesis and the Therapeutic Effect of Interleukin-1β Receptor Antagonist (IL-1RA) and Tolcapone. Int. J. Mol. Sci. 2025, 26, 6893. https://doi.org/10.3390/ijms26146893
Narasimhappagari J, Liu L, Balasubramaniam M, Ayyadevara S, Aboud O, Griffin WST. The Seminal Role of the Proinflammatory Cytokine IL-1β and Its Signaling Cascade in Glioblastoma Pathogenesis and the Therapeutic Effect of Interleukin-1β Receptor Antagonist (IL-1RA) and Tolcapone. International Journal of Molecular Sciences. 2025; 26(14):6893. https://doi.org/10.3390/ijms26146893
Chicago/Turabian StyleNarasimhappagari, Jagadeesh, Ling Liu, Meenakshisundaram Balasubramaniam, Srinivas Ayyadevara, Orwa Aboud, and W. Sue T. Griffin. 2025. "The Seminal Role of the Proinflammatory Cytokine IL-1β and Its Signaling Cascade in Glioblastoma Pathogenesis and the Therapeutic Effect of Interleukin-1β Receptor Antagonist (IL-1RA) and Tolcapone" International Journal of Molecular Sciences 26, no. 14: 6893. https://doi.org/10.3390/ijms26146893
APA StyleNarasimhappagari, J., Liu, L., Balasubramaniam, M., Ayyadevara, S., Aboud, O., & Griffin, W. S. T. (2025). The Seminal Role of the Proinflammatory Cytokine IL-1β and Its Signaling Cascade in Glioblastoma Pathogenesis and the Therapeutic Effect of Interleukin-1β Receptor Antagonist (IL-1RA) and Tolcapone. International Journal of Molecular Sciences, 26(14), 6893. https://doi.org/10.3390/ijms26146893






