Receptor-Mediated Internalization of L-Asparaginase into Tumor Cells Is Suppressed by Polyamines
Abstract
1. Introduction
- Encapsulating L-ASNase within nanoparticles coated with polyamines or their analogs to improve its targeted delivery to tumor cells [15].
2. Results and Discussion
2.1. Article Structure
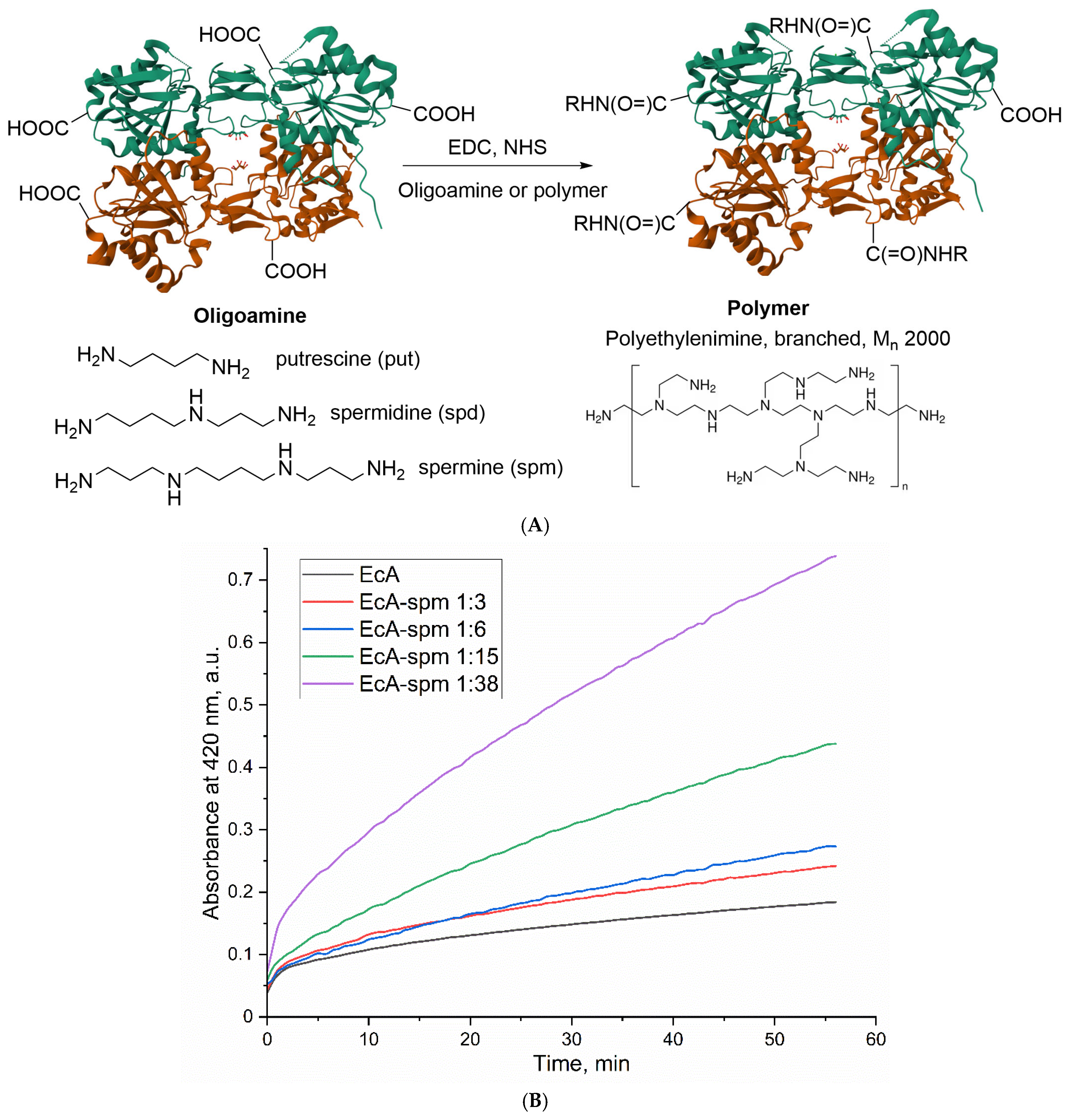
2.2. The Synthesis and Characterization of Polyamine-Conjugated L-ASNase Formulations
2.3. Biocatalytic Parameters of the Polyamine-Conjugated L-ASNases
2.3.1. Effect of Spermine Modification on the Catalytic Activity of L-ASNase
2.3.2. Effect of Spermidine Modification on the Catalytic Activity of L-ASNase
2.3.3. Effect of Putrescine Modification on the Catalytic Activity of L-ASNase
2.3.4. Effect of PEI2 Modification on the Catalytic Activity of L-ASNase
2.3.5. Comparative Analysis of Oligoamine and PEI2 Modification on L-ASNase Activity
2.4. The Visualization of the Interaction Between Polyamines and Lymphoma Cells
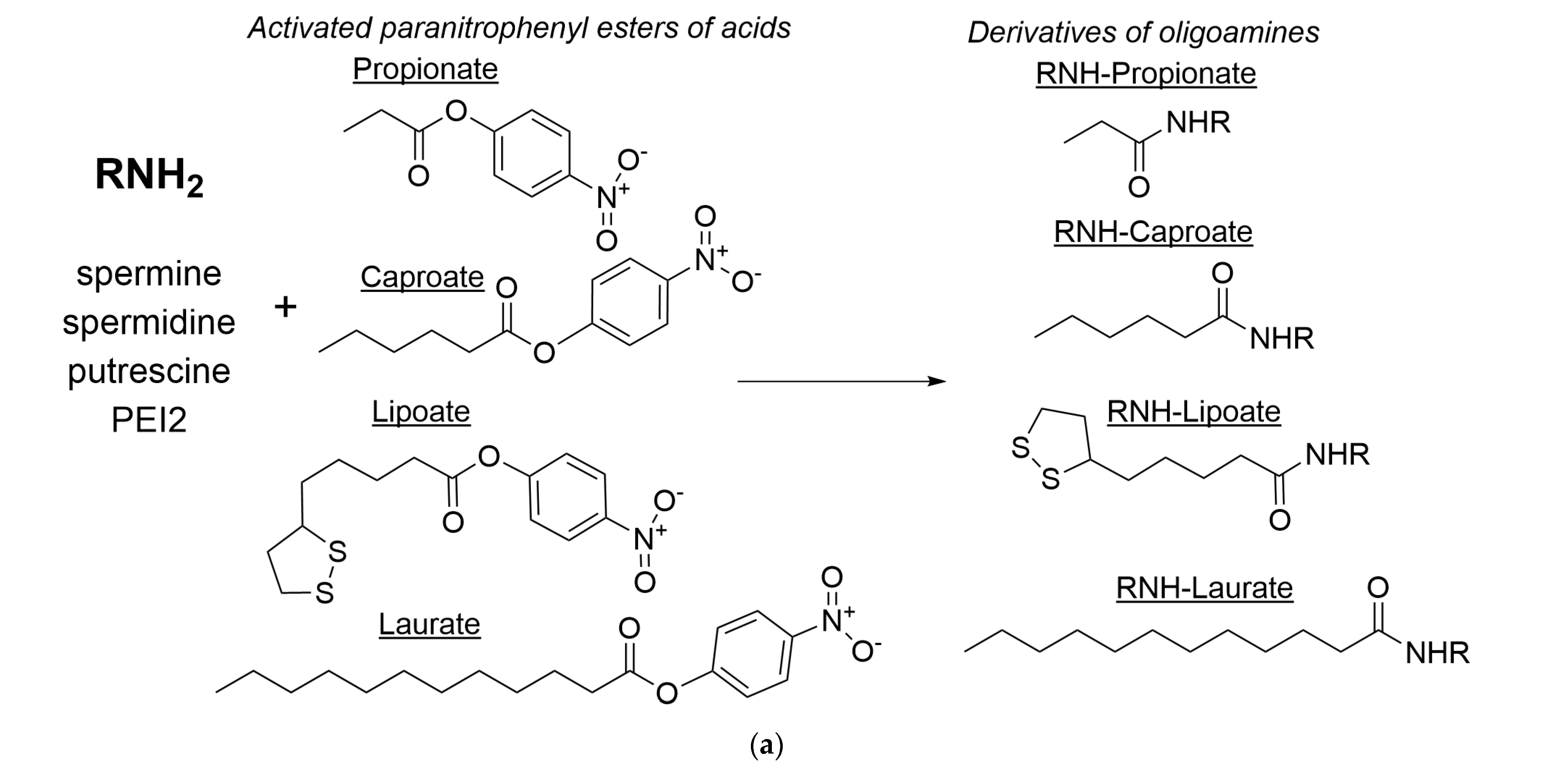
2.5. Synthesis and Characterization of Polyamine Derivatives as Potential PTS Inhibitors or Membrane Permeability Enhancers
2.6. Influence of Polyamine and Its Derivatives on L-ASNase Internalization into Eukariotic Cell and Cytotoxicity
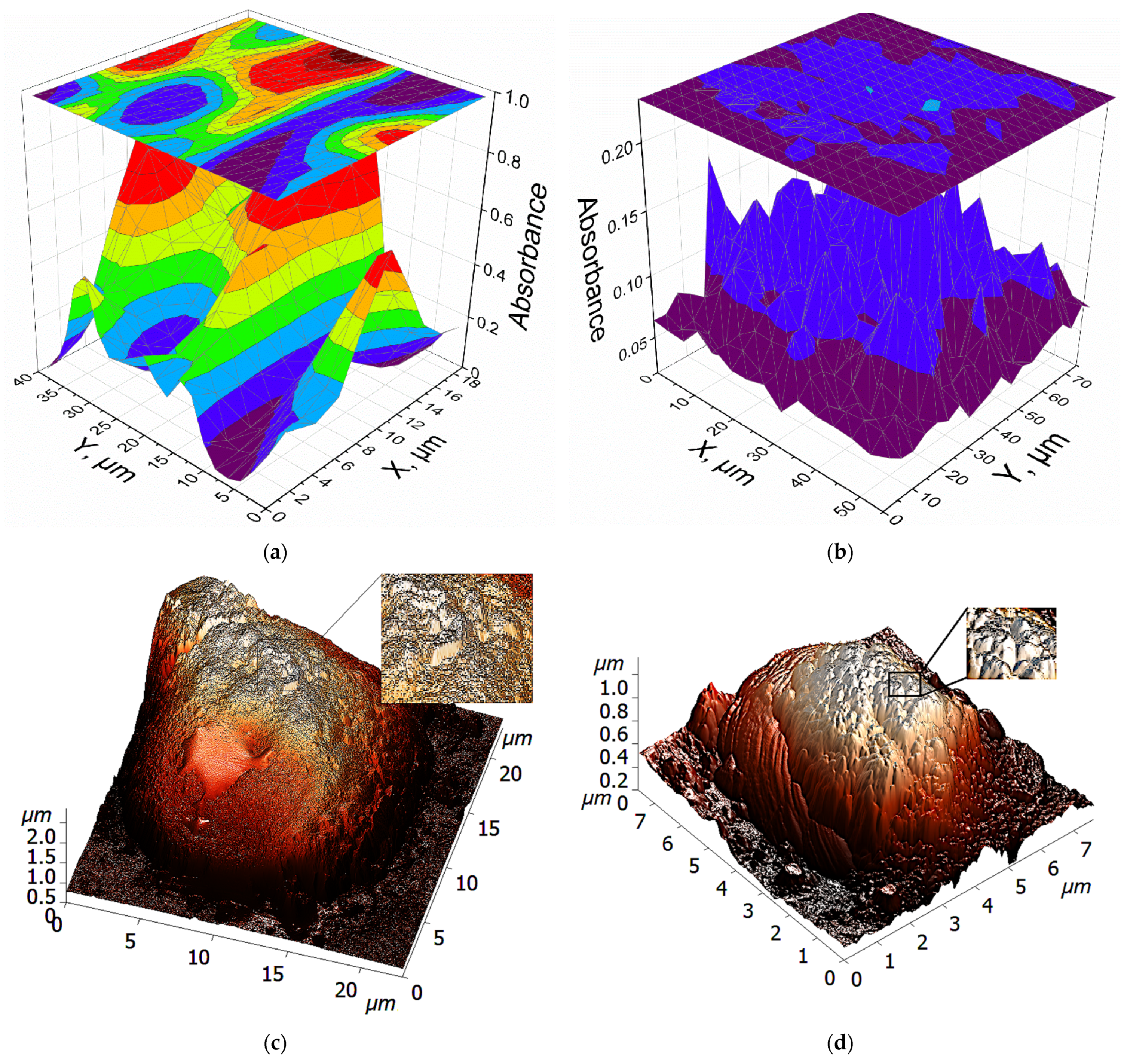
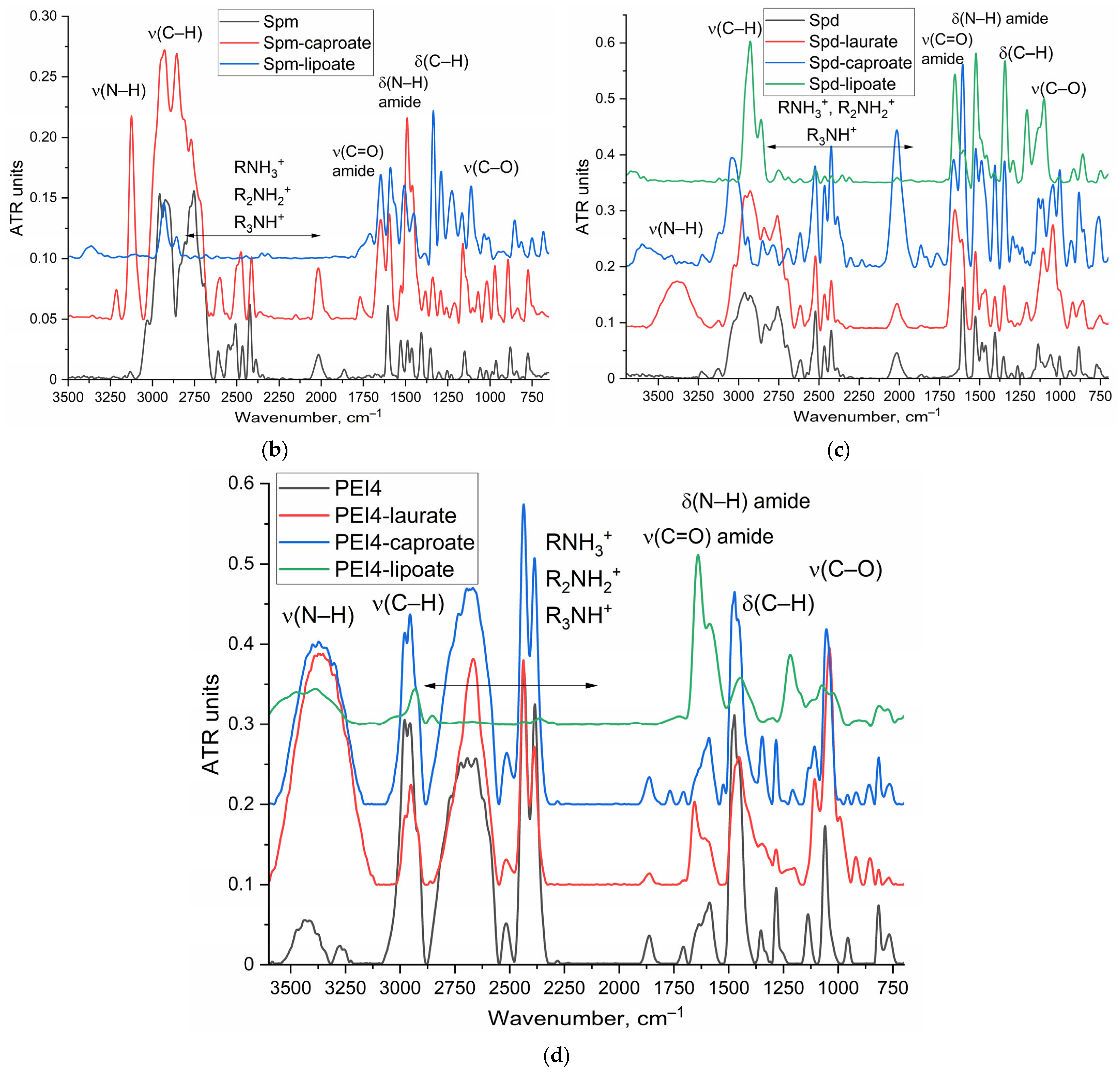
2.6.1. Fine Features of L-ASNase Interactions with Cancer Cells Studied Using FTIR Spectroscopy
2.6.2. Quantification of L-ASNase Interactions with Cancer Cells Studied Using Fluorimetry
2.6.3. The Effect of Polyamines and Their Derivatives on the Cytotoxicity of L-ASNase Towards Cancer Cells
2.6.4. Correlation of Binding Data with Cytotoxicity Data of L-ASNases
2.6.5. Mechanisms of Internalization of Native and Polyamine-Conjugated Asparaginases into Cancer Cells
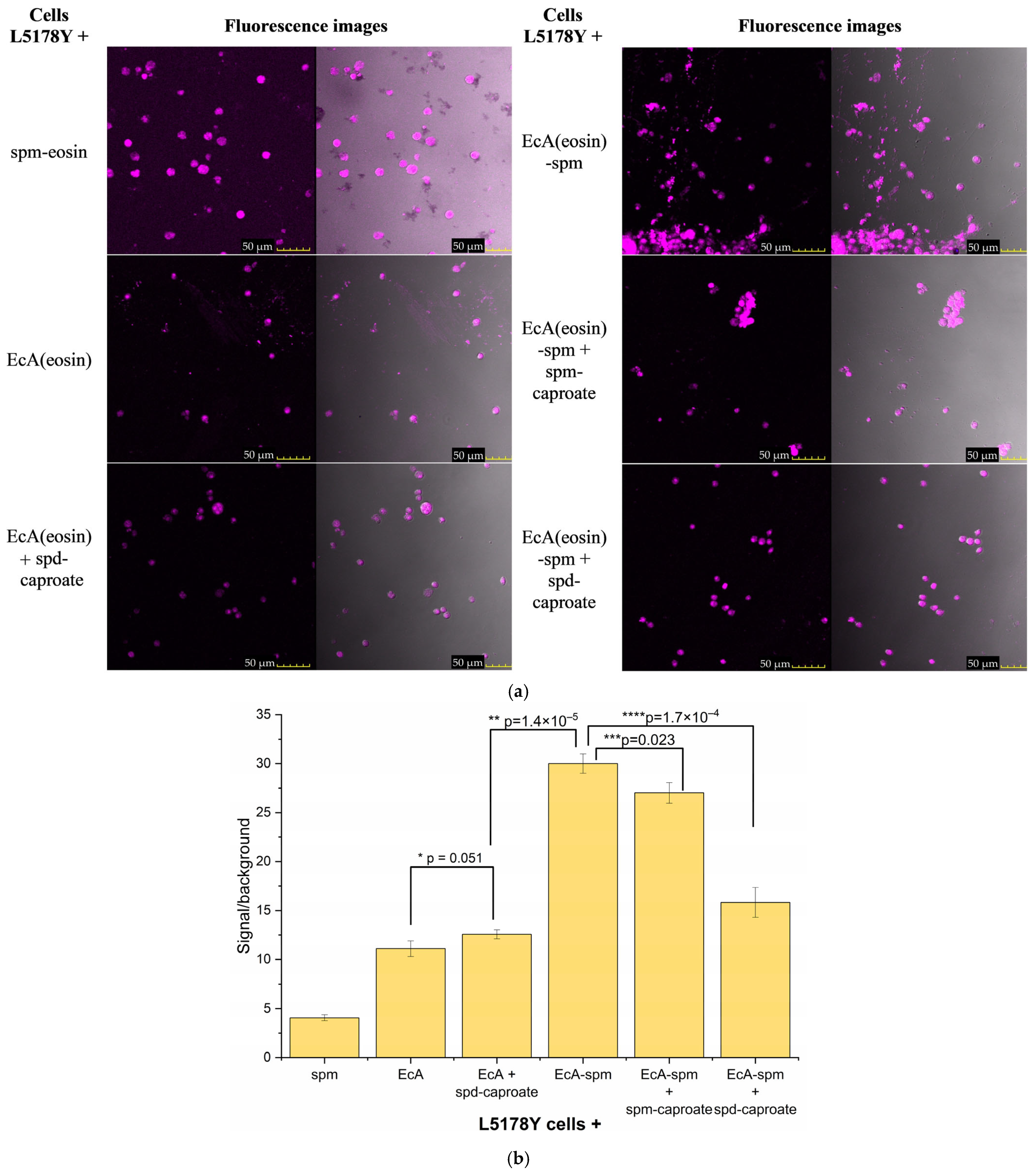
2.7. Visualization of the Polyamine Transport System’s Role in L-ASNase Delivery Using CLSM
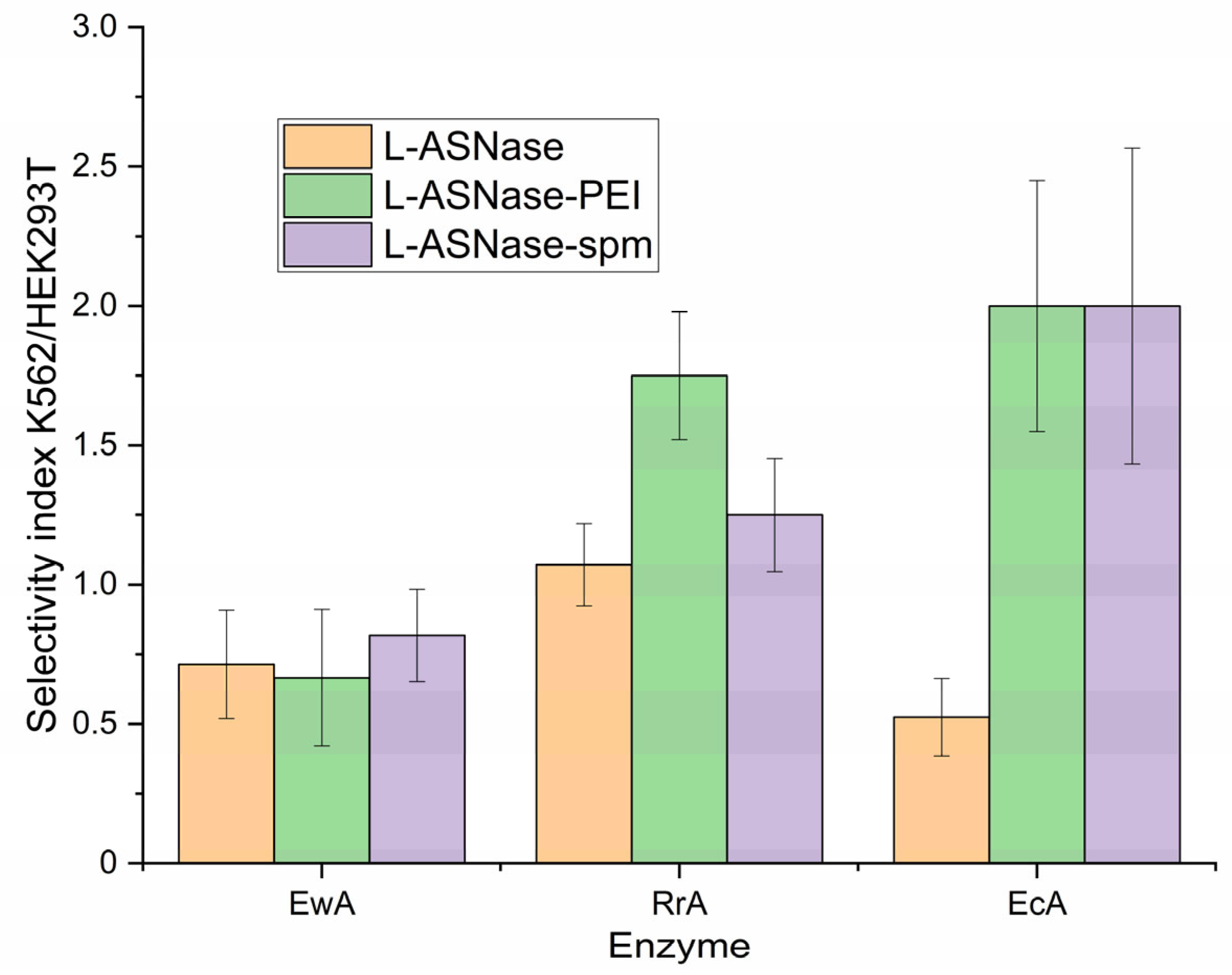
2.8. Selectivity of L-ASNases Against Cancerous Cells vs. Normal
3. Materials and Methods
3.1. Chemicals
3.2. L-Asparaginases
3.3. Synthesis of Polyamine-Conjugated L-ASNase
3.4. Synthesis of Polyamine Derivatives
3.5. FTIR Characterization of Polyamine-Conjugated L-ASNases and Polyamine Derivatives
3.6. Determination of L-ASNase Catalytic Activity
3.7. Cell Studies
3.7.1. Eukaryotic Cell Cultures
3.7.2. Fluorescence Determination of the Binding of L-Asparaginase Formulations to Cells in the Presence of Modulators
3.8. FTIR Spectroscopy for Studying Enzyme-Cell Interactions
3.9. Determination of Cell-Associated Enzyme Concentration
3.10. Cytotoxicity Assay
3.11. Investigation of the L-ASNase Interactions with Cancer Cells Using Confocal Laser Scanning Microscopy
3.12. Atomic Force Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Circular dichroism |
| CLSM | confocal laser scanning microscopy |
| EcA | Escherichia coli L-asparaginase |
| EwA | Erwinia carotowora L-asparaginase |
| FTIR | Fourier-transform infrared |
| L-ASNase | L-asparaginase |
| PEI | Polyethyleneimine |
| Put | Putrescine |
| RrA | Rhodospirillum rubrum L-asparaginase |
| Spd | Spermidine |
| Spm | Spermine |
| TNBS | Trinitrobenzene sulfonic acid |
References
- Pieters, R.; Hunger, S.P.; Boos, J.; Rizzari, C.; Silverman, L.; Baruchel, A.; Goekbuget, N.; Schrappe, M.; Pui, C. L-asparaginase Treatment in Acute Lymphoblastic Leukemia. Cancer 2011, 117, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Zokaee, H.; Sausville, E. a Asparaginase in the Treatment of Non-ALL Hematologic Malignancies. Cancer Chemother. Pharmacol. 2014, 73, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Van Trimpont, M.; Peeters, E.; De Visser, Y.; Schalk, A.M.; Mondelaers, V.; De Moerloose, B.; Lavie, A.; Lammens, T.; Goossens, S.; Van Vlierberghe, P. Novel Insights on the Use of L-Asparaginase as an Efficient and Safe Anti-Cancer Therapy. Cancers 2022, 14, 902. [Google Scholar] [CrossRef]
- Pritsa, A.A.; Papazisis, K.T.; Kortsaris, A.H.; Geromichalos, G.D.; Kyriakidis, D.A. Antitumor Activity of L-Asparaginase from Thermus Thermophilus. Anticancer Drugs 2001, 12, 137–142. [Google Scholar] [CrossRef]
- Abrar Mohammed, A.; Abdela, F.; Tasamma, A.T.; Shewangizaw, Y.K.; Weldeamanuel, M.T. Prevalence and Risk Factors of L-Asparaginase-Related Thrombosis Among Acute Lymphoblastic Lymphoma Patients in a Resource-Limited Setup of Sub-Saharan Region. Cancer Rep. 2025, 8, e70153. [Google Scholar] [CrossRef]
- Kawedia, J.D.; Rytting, M.E. Asparaginase in Acute Lymphoblastic Leukemia. Clin. Lymphoma. Myeloma Leuk. 2014, 14S, S14–S17. [Google Scholar] [CrossRef]
- Burke, M.J.; Zalewska-Szewczyk, B. Hypersensitivity Reactions to Asparaginase Therapy in Acute Lymphoblastic Leukemia: Immunology and Clinical Consequences. Futur. Oncol. 2022, 18, 1285–1299. [Google Scholar] [CrossRef]
- Salzer, W.; Bostrom, B.; Messinger, Y.; Perissinotti, A.J.; Marini, B. Asparaginase Activity Levels and Monitoring in Patients with Acute Lymphoblastic Leukemia. Leuk. Lymphoma 2018, 59, 1797–1806. [Google Scholar] [CrossRef]
- Nunes, J.C.F.; Cristóvão, R.O.; Freire, M.G.; Santos-Ebinuma, V.C.; Faria, J.L.; Silva, C.G.; Tavares, A.P.M. Recent Strategies and Applications for L-Asparaginase Confinement. Molecules 2020, 25, 5827. [Google Scholar] [CrossRef]
- Boos, J.; Werber, G.; Ahlke, E.; Schulze-Westhoff, P.; Nowak-Göttl, U.; Würthwein, G.; Verspohl, E.J.; Ritter, J.; Jürgens, H. Monitoring of Asparaginase Activity and Asparagine Levels in Children on Different Asparaginase Preparations. Eur. J. Cancer Part A 1996, 32, 1544–1550. [Google Scholar] [CrossRef]
- Hutson, R.G.; Kitoh, T.; Moraga Amador, D.A.; Cosic, S.; Schuster, S.M.; Kilberg, M.S. Amino Acid Control of Asparagine Synthetase: Relation to Asparaginase Resistance in Human Leukemia Cells. Am. J. Physiol.—Cell Physiol. 1997, 272, C1691–C1699. [Google Scholar] [CrossRef] [PubMed]
- Ottobre, M.; Colitto, L.; Marcone, M.I.; Krystal, E.; Moran, L.; Wittmund, L.E.; Gimenez, V.; Makiya, M. A Pediatric Laboratory Experience: Measurement of L-Asparaginase Activity in Patients with Acute Lymphoblastic Leukemia. a Multicentric Study. Blood 2024, 144, 2826. [Google Scholar] [CrossRef]
- Vadlamudi, S.; Krishna, B.; Subba Reddy, V.V.; Goldin, A. Schedule-Dependent Therapeutic Synergism for L-Asparaginase and Methotrexate in Leukemic (L5178Y) Mice. Cancer Res. 1973, 33, 2014–2019. [Google Scholar] [PubMed]
- Fonseca, M.H.G.; Fiúza, T.d.S.; Bath de Morais, S.; Souza, T.d.A.C.B.d.; Trevizani, R. Circumventing the Side Effects of L-Asparaginase. Biomed. Pharmacother. 2021, 139, 111616. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Kudryashova, E. V Targeted Polymeric Micelles System, Designed to Carry a Combined Cargo of L-Asparaginase and Doxorubicin, Shows Vast Improvement in Cytotoxic Efficacy. Polymers 2024, 16, 2132. [Google Scholar] [CrossRef]
- Hermanova, I.; Arruabarrena-Aristorena, A.; Valis, K.; Nuskova, H.; Alberich-Jorda, M.; Fiser, K.; Fernandez-Ruiz, S.; Kavan, D.; Pecinova, A.; Niso-Santano, M.; et al. Pharmacological Inhibition of Fatty-Acid Oxidation Synergistically Enhances the Effect of l-Asparaginase in Childhood ALL Cells. Leukemia 2016, 30, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Avramis, V.I.; Panosyan, E.H. Pharmacokinetic/Pharmacodynamic Relationships of Asparaginase Formulations: The Past, the Present and Recommendations for the Future. Clin. Pharmacokinet. 2005, 44, 367–393. [Google Scholar] [CrossRef]
- Oehrl, A.; Schötz, S.; Haag, R. Synthesis of PH-Degradable Polyglycerol-Based Nanogels by IEDDA-Mediated Crosslinking for Encapsulation of Asparaginase Using Inverse Nanoprecipitation. Colloid Polym. Sci. 2020, 298, 719–733. [Google Scholar] [CrossRef]
- Talluri, V.P.; Mutaliyeva, B.; Sharipova, A.; Ulaganathan, V.; Lanka, S.S.; Aidarova, S.; Suigenbayeva, A.; Tleuova, A. L-Asparaginase Delivery Systems Targeted to Minimize Its Side-Effects. Adv. Colloid Interface Sci. 2023, 316, 102915. [Google Scholar] [CrossRef]
- Dobryakova, N.V.; Zhdanov, D.D.; Sokolov, N.N.; Aleksandrova, S.S.; Pokrovskaya, M.V.; Kudryashova, E.V. Rhodospirillum Rubrum L-Asparaginase Conjugates with Polyamines of Improved Biocatalytic Properties as a New Promising Drug for the Treatment of Leukemia. Appl. Sci. 2023, 13, 3373. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Shishparyonok, A.N.; Pokrovskaya, M.V.; Alexandrova, S.S.; Zhdanov, D.D.; Kudryashova, E.V. Structural Features Underlying the Mismatch Between Catalytic and Cytostatic Properties in L-Asparaginase from Rhodospirillum Rubrum. Catalysts 2025, 15, 476. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Kudryashova, E.V. A Novel Approach for the Activity Assessment of L-Asparaginase Formulations When Dealing with Complex Biological Samples. Int. J. Mol. Sci. 2025, 26, 5227. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Polyamine Transport in Escherichia Coli. Amino Acids 1996, 10, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Muth, A. Design, Synthesis, and Biological Evaluation of Novel Polyamine Transport System Probes and Their Application to Human Cancers. Doctoral Dissertation, University of Central Florida, Orlando, FL, USA, 2012. [Google Scholar]
- Uemura, T.; Gerner, E.W. Polyamine Transport Systems in Mammalian Cells and Tissues. Methods Mol. Biol. 2011, 720, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.J.; Wallace, H.M. The Polyamine Transport System as a Target for Anticancer Drug Development. Amino Acids 2010, 38, 415–422. [Google Scholar] [CrossRef]
- Mohamad Reza Nazifi, S.; Sadeghi-aliabadi, H.; Fassihi, A.; Saghaie, L. Structure–Activity Relationship of Polyamine Conjugates for Uptake via Polyamine Transport System. Struct. Chem. 2019, 30, 175–184. [Google Scholar] [CrossRef]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and Membrane Transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef]
- Li, J.; Meng, Y.; Wu, X.; Sun, Y. Polyamines and Related Signaling Pathways in Cancer. Cancer Cell Int. 2020, 20, 539. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zeng, Y.; You, Y.Y.; Chen, Q.Y. Polyamine Metabolism and Anti-Tumor Immunity. Front. Immunol. 2025, 16, 1529337. [Google Scholar] [CrossRef]
- Sarah, V.V.; Dolores, I.; Kristina, S.; Justin, S.; Sivadasan, B.D.; Elke, A.; Hanne, D.; Nina, S.; Chris, V.D.H.; Joris, V.A.; et al. Astrocytic Polyamine Transport by ATP13A4 Tunes Excitatory Synaptic Transmission. medRxiv 2025. [Google Scholar] [CrossRef]
- van Veen, S.; Kourti, A.; Ausloos, E.; Van Asselberghs, J.; Van den Haute, C.; Baekelandt, V.; Eggermont, J.; Vangheluwe, P. ATP13A4 Upregulation Drives the Elevated Polyamine Transport System in the Breast Cancer Cell Line MCF7. Biomolecules 2023, 13, 918. [Google Scholar] [CrossRef]
- Liu, B.; Azfar, M.; Legchenko, E.; West, J.A.; Martin, S.; Van Den Haute, C.; Baekelandt, V.; Wharton, J.; Howard, L.; Wilkins, M.R.; et al. ATP13A3 Variants Promote Pulmonary Arterial Hypertension by Disrupting Polyamine Transport. Cardiovasc. Res. 2024, 120, 756–768. [Google Scholar] [CrossRef]
- Hamouda, N.N.; van den Haute, C.; Vanhoutte, R.; Sannerud, R.; Azfar, M.; Mayer, R.; Calabuig, Á.C.; Swinnen, J.V.; Agostinis, P.; Baekelandt, V.; et al. ATP13A3 Is a Major Component of the Enigmatic Mammalian Polyamine Transport System. J. Biol. Chem. 2021, 296, 100182. [Google Scholar] [CrossRef]
- Azfar, M.; Gao, W.; Van den Haute, C.; Xiao, L.; Karsa, M.; Pandher, R.; Ronca, E.; Bongers, A.; Karsa, A.; Spurling, D.; et al. The Polyamine Transporter ATP13A3 Mediates DFMO-Induced Polyamine Uptake in Neuroblastoma. bioRxiv 2024. [Google Scholar] [CrossRef]
- Belting, M.; Mani, K.; Jönsson, M.; Cheng, F.; Sandgren, S.; Jonsson, S.; Ding, K.; Delcros, J.G.; Fransson, L.Å. Glypican-1 Is a Vehicle for Polyamine Uptake in Mammalian Cells: A Pivotal Role for Nitrosothiol-Derived Nitric Oxide. J. Biol. Chem. 2003, 278, 47181–47189. [Google Scholar] [CrossRef]
- Cheng, F.; Fransson, L.-Å.; Mani, K. Common Traffic Routes for Imported Spermine and Endosomal Glypican-1-Derived Heparan Sulfate in Fibroblasts. Exp. Cell Res. 2018, 364, 133–142. [Google Scholar] [CrossRef]
- Cheng, F.; Mani, K.; van den Born, J.; Ding, K.; Belting, M.; Fransson, L.-Å. Nitric Oxide-Dependent Processing of Heparan Sulfate in Recycling S-Nitrosylated Glypican-1 Takes Place in Caveolin-1-Containing Endosomes. J. Biol. Chem. 2002, 277, 44431–44439. [Google Scholar] [CrossRef]
- van Veen, S.; Martin, S.; Haute, C.V.D.; Benoy, V.; Lyons, J.; Vanhoutte, R.; Kahler, J.P.; Decuypere, J.-P.; Gelders, G.; Lambie, E.; et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 2020, 578, 419–424. [Google Scholar] [CrossRef]
- Nachef, M.; Ali, A.K.; Almutairi, S.M.; Lee, S.H. Targeting SLC1A5 and SLC3A2/SLC7A5 as a Potential Strategy to Strengthen Anti-Tumor Immunity in the Tumor Microenvironment. Front. Immunol. 2021, 12, 624324. [Google Scholar] [CrossRef]
- Fredriksson, R.; Sreedharan, S.; Nordenankar, K.; Alsiö, J.; Lindberg, F.A.; Hutchinson, A.; Eriksson, A.; Roshanbin, S.; Ciuculete, D.M.; Klockars, A.; et al. The Polyamine Transporter Slc18b1(VPAT) Is Important for Both Short and Long Time Memory and for Regulation of Polyamine Content in the Brain. PLoS Genet. 2019, 15, e1008455. [Google Scholar] [CrossRef]
- Pochini, L. Involvement of Mammalian SoLute Carriers (SLC) in the Traffic of Polyamines. Front. Mol. Biosci. 2024, 11, 1452184. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, D.; Yang, L.; Wang, N.; Shen, J.; Wang, J.; Zhang, L.; Chen, L.; Gao, S.; Zong, W.-X.; et al. Activation of Polyamine Catabolism Promotes Glutamine Metabolism and Creates a Targetable Vulnerability in Lung Cancer. Proc. Natl. Acad. Sci. USA 2024, 121, e2319429121. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 Engages Polyamine Metabolism with P53-Mediated Ferroptotic Responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-A.; Stewart, T.M.; Casero, R.A. The Synergistic Benefit of Combination Strategies Targeting Tumor Cell Polyamine Homeostasis. Int. J. Mol. Sci. 2024, 25, 8173. [Google Scholar] [CrossRef]
- Cervelli, M.; Amendola, R.; Polticelli, F.; Mariottini, P. Spermine Oxidase: Ten Years After. Amino Acids 2012, 42, 441–450. [Google Scholar] [CrossRef]
- Ohkubo, S.; Mancinelli, R.; Miglietta, S.; Cona, A.; Angelini, R.; Canettieri, G.; Spandidos, D.A.; Gaudio, E.; Agostinelli, E. Maize Polyamine Oxidase in the Presence of Spermine/Spermidine Induces the Apoptosis of LoVo Human Colon Adenocarcinoma Cells. Int. J. Oncol. 2019, 54, 2080–2094. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, T.J. Polyamine Metabolism and Cancer. J. Cell. Mol. Med. 2003, 7, 113–126. [Google Scholar] [CrossRef]
- Thomas, T.J.; Thomas, T. Cellular and Animal Model Studies on the Growth Inhibitory Effects of Polyamine Analogues on Breast Cancer. Med. Sci. 2018, 6, 24. [Google Scholar] [CrossRef]
- Zanatta, J.M.; Acuña, S.M.; Bento, C.D.A.; Stolf, B.S.; Muxel, S.M. Polyamines Shift Expression of Macrophage L-Arginine Metabolism Related-Genes during Leishmania Amazonensis Infection. bioRxiv 2022, 2. [Google Scholar] [CrossRef]
- Wallace, H.M.; Fraser, A.V. Inhibitors of Polyamine Metabolism: Review Article. Amino Acids 2004, 26, 353–365. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kumar, V.; Burritt, D.J.; Fujita, M.; Mäkelä, P.S.A. Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783030274238. [Google Scholar]
- Ancheta, A.A. Putrescine-Mediated Changes in Mammalian Intracellular Polyamine Levels Increase Spermidine/Spermine-N1-Acetyltransferase Activity and Increase Gene Expression of Several Cell Cycle-Related Genes. Doctoral Dissertation, University of California, Riverside, Riverside, CA, USA, 2010. ISBN 9781626239777. [Google Scholar]
- Liu, C. The Role of Polyamine Acetylation in Regulating Adipose Tissue Metabolism; Temple University: Philadelphia, PA, USA, 2012. [Google Scholar]
- Porter, C.W. Activation of Polymine Catabolism as a Novel Strategy for Treating and/or Preventing Human Prostate Cancer; Final Report; Health Research, Inc.: Buffalo, NY, USA, 1 March 2006. [Google Scholar]
- Krasteva, G.; Pfeil, U.; Drab, M.; Kummer, W.; König, P. Caveolin-1 and -2 in Airway Epithelium: Expression and in Situ Association as Detected by FRET-CLSM. Respir. Res. 2006, 7, 108. [Google Scholar] [CrossRef]
- Mo, R.; Jin, X.; Li, N.; Ju, C.; Sun, M.; Zhang, C.; Ping, Q. The Mechanism of Enhancement on Oral Absorption of Paclitaxel by N-Octyl-O-Sulfate Chitosan Micelles. Biomaterials 2011, 32, 4609–4620. [Google Scholar] [CrossRef]
- König, P.; Krasteva, G.; Tag, C.; König, I.R.; Arens, C.; Kummer, W. FRET-CLSM and Double-Labeling Indirect Immunofluorescence to Detect Close Association of Proteins in Tissue Sections. Lab. Investig. 2006, 86, 853–864. [Google Scholar] [CrossRef]
- Yang, C.; Gao, S.; Dagnæs-Hansen, F.; Jakobsen, M.; Kjems, J. Impact of PEG Chain Length on the Physical Properties and Bioactivity of PEGylated Chitosan/SiRNA Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 12203–12216. [Google Scholar] [CrossRef]
- Plyasova, A.A.; Pokrovskaya, M.V.; Lisitsyna, O.M.; Pokrovsky, V.S.; Alexandrova, S.S.; Hilal, A.; Sokolov, N.N.; Zhdanov, D.D. Penetration into Cancer Cells via Clathrin-dependent Mechanism Allows L-asparaginase from Rhodospirillum Rubrum to Inhibit Telomerase. Pharmaceuticals 2020, 13, 286. [Google Scholar] [CrossRef]
- Zhang, M.; Asghar, S.; Jin, X.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. The Enhancing Effect of N-Acetylcysteine Modified Hyaluronic Acid-Octadecylamine Micelles on the Oral Absorption of Paclitaxel. Int. J. Biol. Macromol. 2019, 138, 636–647. [Google Scholar] [CrossRef]
- Luo, T.; Han, J.; Zhao, F.; Pan, X.; Tian, B.; Ding, X.; Zhang, J. Redox-Sensitive Micelles Based on Retinoic Acid Modified Chitosan Conjugate for Intracellular Drug Delivery and Smart Drug Release in Cancer Therapy. Carbohydr. Polym. 2019, 215, 8–19. [Google Scholar] [CrossRef]
- Weber, G.; Buhren, B.; Schrumpf, H.; Wohlrab, J.; Gerber, P. Therapeutic Enzymes: Function and Clinical Implications; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1148, ISBN 978-981-13-7708-2. [Google Scholar]
- Concepts, B.; Applications, T. Macrophage Targeted Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 9783030841638. [Google Scholar]
- Savchenko, I.V.; Zlotnikov, I.D.; Kudryashova, E.V. Biomimetic Systems Involving Macrophages and Their Potential for Targeted Drug Delivery. Biomimetics 2023, 8, 543. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef]
- Hucke, A.; Ciarimboli, G. The Role of Transporters in the Toxicity of Chemotherapeutic Drugs: Focus on Transporters for Organic Cations. J. Clin. Pharmacol. 2016, 56, S157–S172. [Google Scholar] [CrossRef]
- Huang, Y.; Anderle, P.; Bussey, K.J.; Barbacioru, C.; Shankavaram, U.; Dai, Z.; Reinhold, W.C.; Papp, A.; Weinstein, J.N.; Sadée, W. Membrane Transporters and Channels: Role of the Transportome in Cancer Chemosensitivity and Chemoresistance. Cancer Res. 2004, 64, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Conger, K. Identification of ASCT2 as the Primary Serine Transporter in Cancer. Doctoral Dissertation, University of Illinois at Chicago, Chicago, IL, USA, 2024. [Google Scholar]
- Sun, J.; Nagel, R.; Zaal, E.A.; Ugalde, A.P.; Han, R.; Proost, N.; Song, J.; Pataskar, A.; Burylo, A.; Fu, H.; et al. SLC 1A3 Contributes to L-asparaginase Resistance in Solid Tumors. EMBO J. 2019, 38, e102147. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, M.A.; Pokrovskaya, M.V.; Alexandrova, S.S.; Sokolov, N.N.; Kudryashova, E.V. Regulation of Catalytic Activity of Recombinant L-Asparaginase from Rhodospirillum Rubrum by Conjugation with a PEG-Chitosan Copolymer. Moscow Univ. Chem. Bull. 2018, 73, 185–191. [Google Scholar] [CrossRef]
- Sukhoverkov, K.V.; Sokolov, N.N.; Abakumova, O.Y.; Podobed, O.V.; Kudryashova, E.V. The Formation of Conjugates with PEG–Chitosan Improves the Biocatalytic Efficiency and Antitumor Activity of L-Asparaginase from Erwinia Carotovora. Moscow Univ. Chem. Bull. 2016, 71, 122–126. [Google Scholar] [CrossRef]
- Gao, W.; Karsa, M.; Xiao, L.; Spurling, D.; Karsa, A.; Ronca, E.; Bongers, A.; Guo, X.; Mayoh, C.; Azfar, M.; et al. Polyamine Depletion Limits Progression of Acute Leukaemia. Int. J. Cancer 2025, 156, 2360–2376. [Google Scholar] [CrossRef]
- Kay, K.E.; Lee, J.; Hong, E.S.; Beilis, J.; Dayal, S.; Wesley, E.R.; Mitchell, S.; Wang, S.Z.; Silver, D.J.; Volovetz, J.; et al. Tumor Cell-Derived Spermidine Promotes a pro-Tumorigenic Immune Microenvironment 2 in Glioblastoma via CD8+ T Cell Inhibition. J. Clin. Investig. 2025, 135, 1–13. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Wang, X.; Xiao, Z.; Ni, Q.; Yu, X.; Luo, G. Antitumor Potential of Polyamines in Cancer. Acta Biochim. Biophys. Sin. 2025. [Google Scholar] [CrossRef]
- Walters, J.D.; Wojcik, M.S. Polyamine Transport in Human Promyelocytic Leukemia Cells and Polymorphonuclear Leukocytes. Leuk. Res. 1994, 18, 703–708. [Google Scholar] [CrossRef]
- Vial, L.; Ludlow, R.F.; Leclaire, J.; Pérez-Fernández, R.; Otto, S. Controlling the Biological Effects of Spermine Using a Synthetic Receptor. J. Am. Chem. Soc. 2006, 128, 10253–10257. [Google Scholar] [CrossRef]
- Zhdanov, D.D.; Plyasova, A.A.; Gladilina, Y.A.; Pokrovsky, V.S.; Grishin, D.V.; Grachev, V.A.; Orlova, V.S.; Pokrovskaya, M.V.; Alexandrova, S.S.; Lobaeva, T.A.; et al. Inhibition of Telomerase Activity by Splice-Switching Oligonucleotides Targeting the MRNA of the Telomerase Catalytic Subunit Affects Proliferation of Human CD4+ T Lymphocytes. Biochem. Biophys. Res. Commun. 2019, 509, 790–796. [Google Scholar] [CrossRef]
- Krasotkina, J.; Borisova, A.A.; Gervaziev, Y.V.; Sokolov, N.N. One-Step Purification and Kinetic Properties of the Recombinant L -Asparaginase from Erwinia Carotovora. Biotech. Appl. Biochem. 2004, 39, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kudryashova, E.V.; Sukhoverkov, K.V. “Reagent-Free” l-Asparaginase Activity Assay Based on CD Spectroscopy and Conductometry. Anal. Bioanal. Chem. 2016, 408, 1183–1189. [Google Scholar] [CrossRef] [PubMed]


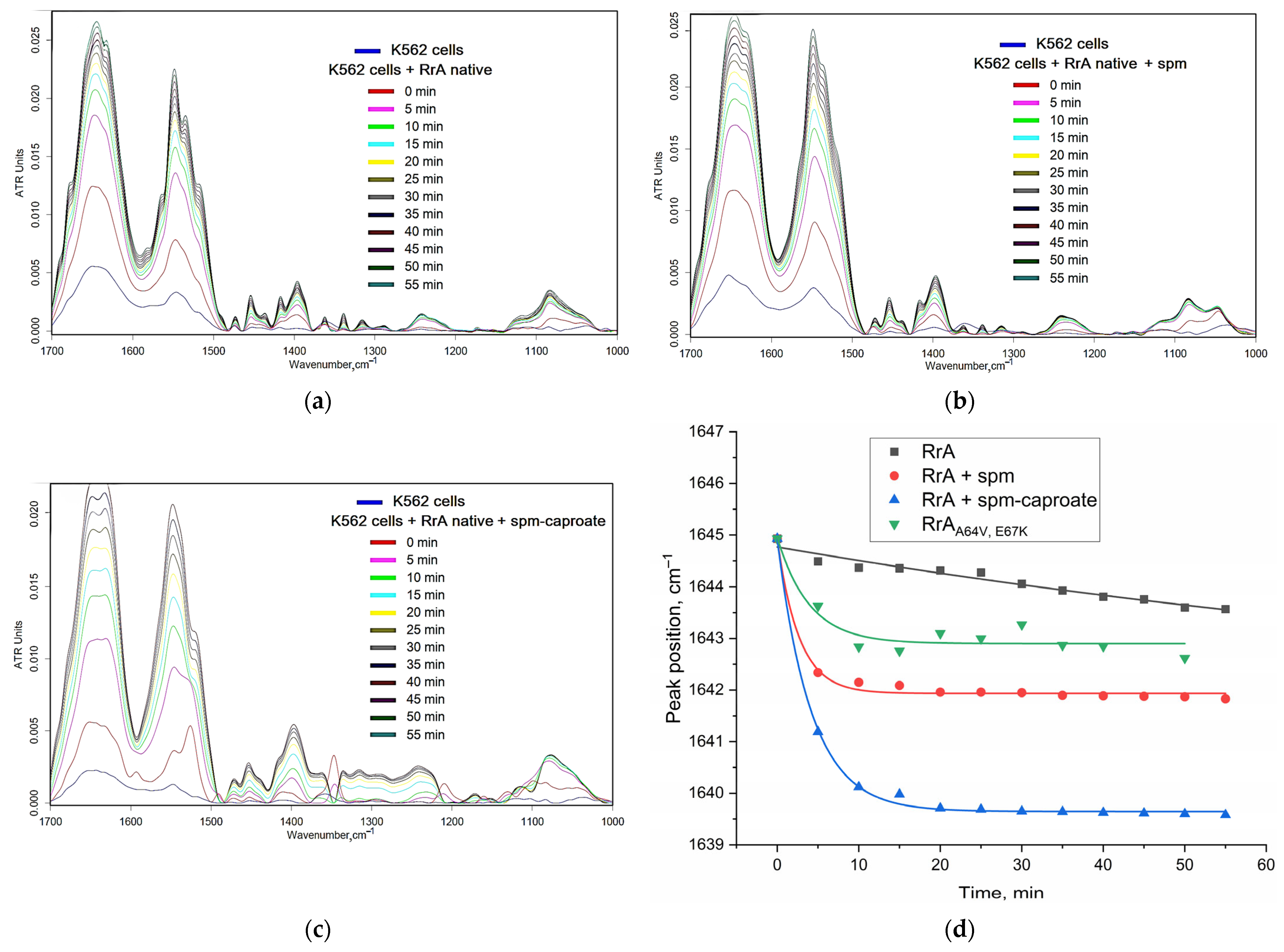
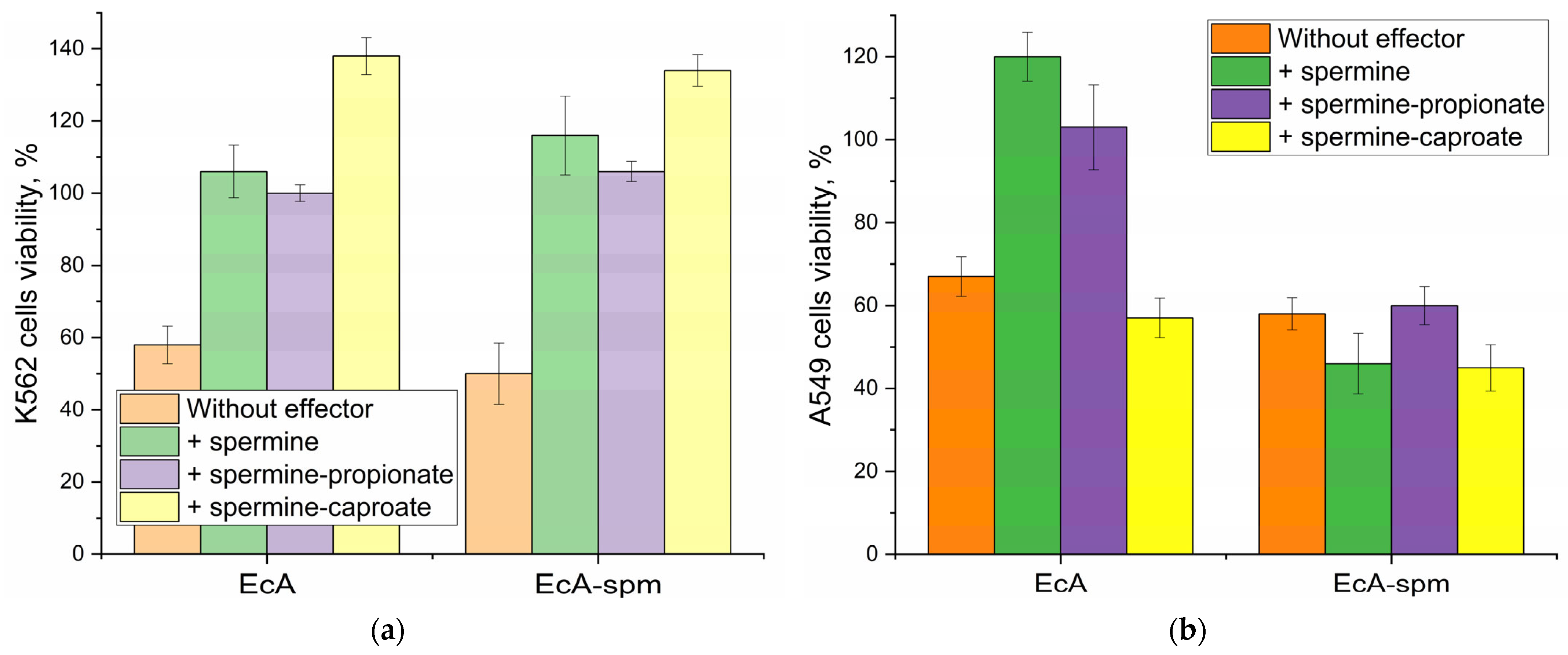
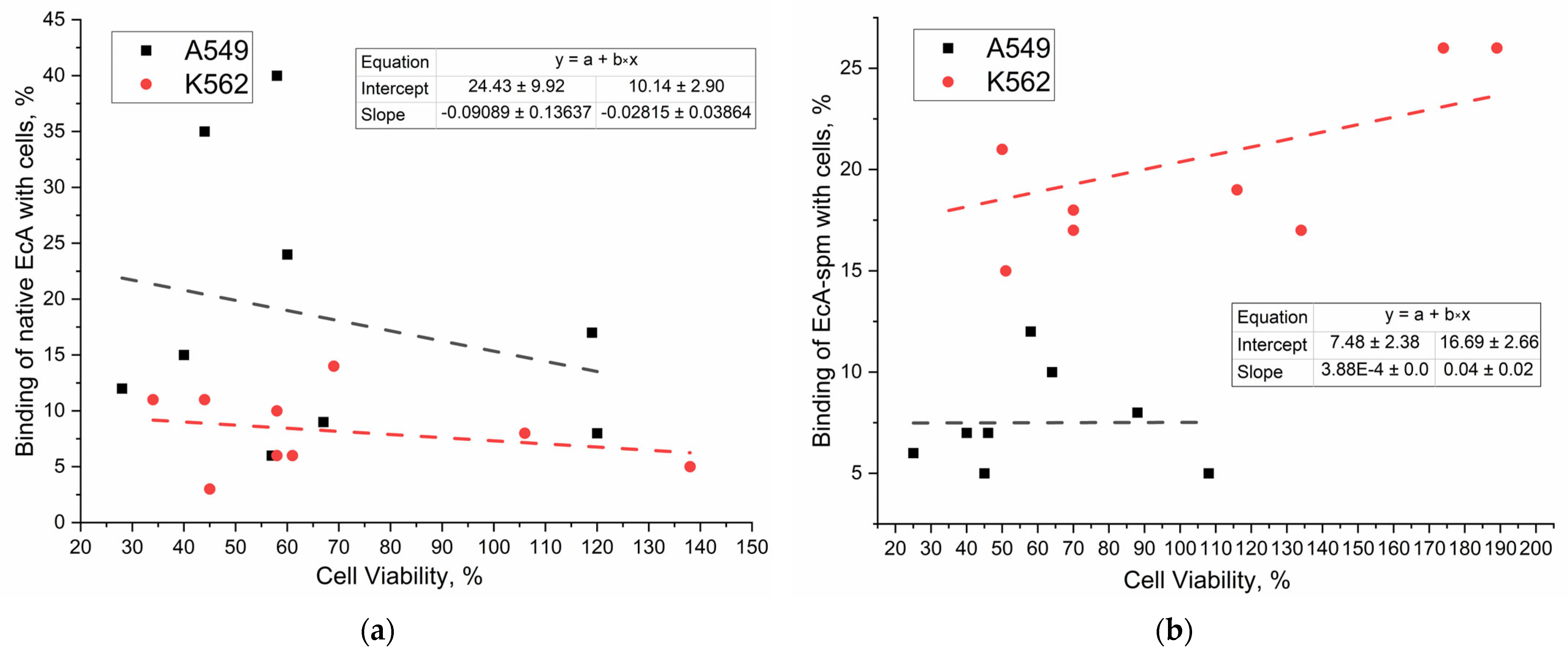
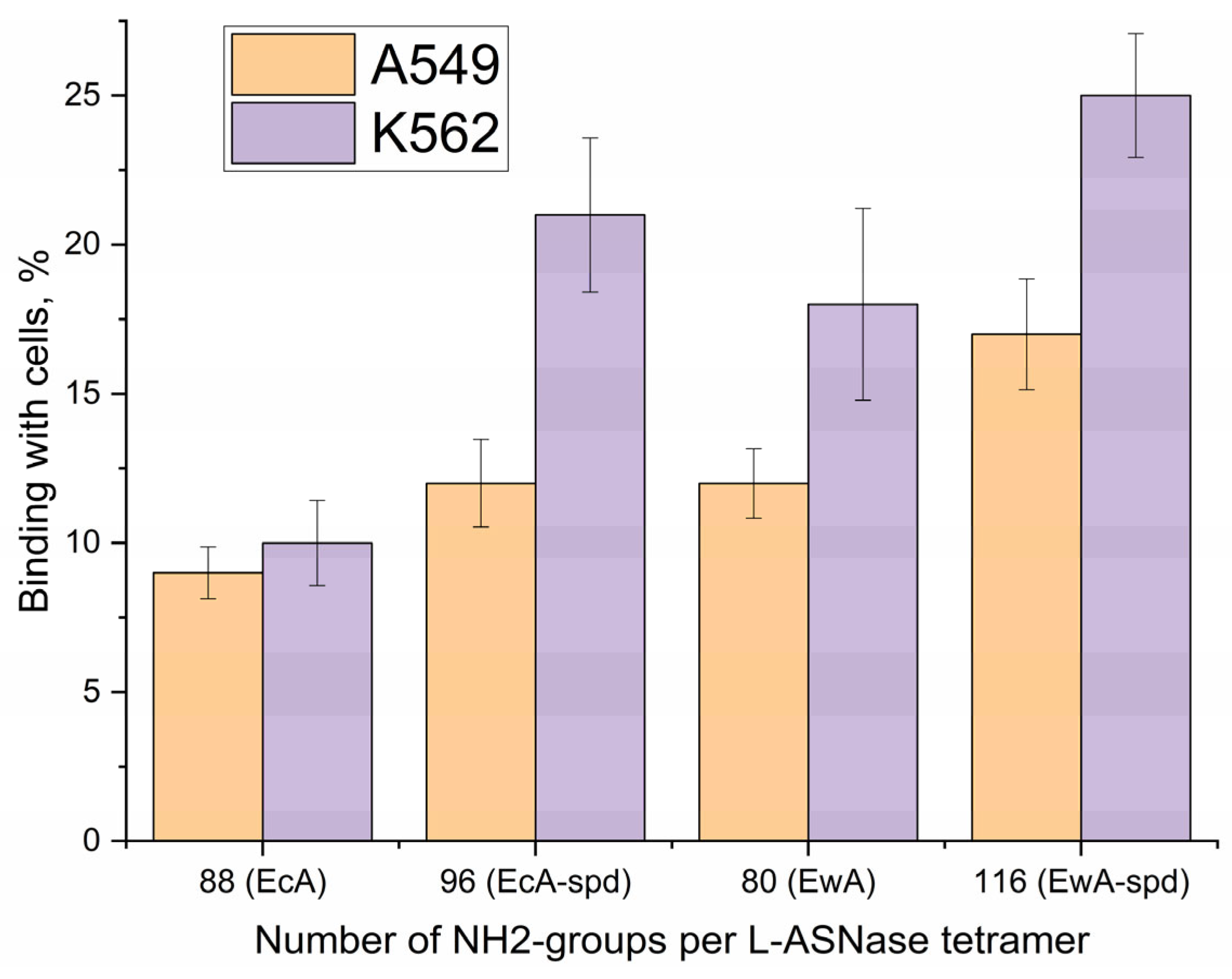
| Enzyme | Type | Quaternary Structure | MW (Monomer), kDa | pH-Optimum | Specific Activity of Native Enzyme, U/mg | Molar Ratios of Polyamine: Protein Tetramer Used in Synthesis and Determined Using TNBS Titration in Parentheses | |||
|---|---|---|---|---|---|---|---|---|---|
| Spermine (spm) | Spermidine (spd) | Putrescine (put) | PEI2 | ||||||
| RrA | Ia | Tetramer/dimer | 18 | 9.0–9.3 | 60 ± 6 | 2; 5; 10; 30 (2; 5; 9; 25) * | 2; 5; 10; 30 (2; 4; 9; 23) * | 2; 5; 10; 30 (2; 5; 8; 22) * | 0.2; 0.5; 2; 5 (0.2; 0.5; 1.8; 4) * |
| EwA | II | Tetramer | 34.2 | 8–9 | 370 ± 15 | 5; 10; 20; 50 (4; 8; 17; 42) * | 5; 10; 20; 50 (4; 9; 16; 36) * | 5; 10; 20; 50 (4; 8; 14; 35) * | 0.5; 1; 3; 5 (0.4; 0.8; 2.6; 4.5) * |
| EcA | II | Tetramer | 36.9 | 8–8.5 | 330 ± 20 | 5; 10; 20; 50 (3; 6; 15; 38) * | 5; 10; 20; 50 (4; 8; 17; 39) * | 5; 10; 20; 50 (4; 7; 18; 40) * | 0.5; 1; 3; 5 (0.5; 0.9; 2.5; 4.2) * |
| Enzyme or Polyamine | % Binding with A549 Cells * | % Binding with K562 Cells * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EcA | EcA-spd | EwA | EwA-spd | spm | PEI | EcA | EcA-spd | EwA | EwA-spd | spm | PEI | ||
| Typical Effect on the Enzyme (Polyamine) Binding with Cells ** | + | − | 0 | + | 0+ | 0 | 0− | 0 | 0− | 0− | + | 0 | |
| Effector | Without effector | 9 | 12 | 12 | 17 | 11 | 15 | 10 | 21 | 18 | 25 | 15 | 16 |
| Spm | 8 | 7 | 10 | 25 | 46 | 39 | 8 | 19 | 17 | 21 | 14 | 15 | |
| Spm-caproate | 6 | 5 | 7 | 21 | 6 | 12 | 5 | 17 | 8 | 13 | 11 | 13 | |
| Spm-lipoate | 24 | 5 | 7 | 23 | 8 | 17 | 6 | 18 | 9 | 17 | 13 | 20 | |
| Spd | 40 | 7 | 17 | 37 | 13 | 38 | 3 | 15 | 14 | 30 | 31 | 35 | |
| Spd-laurate | 35 | 10 | 11 | 20 | 13 | 12 | 11 | 24 | 18 | 24 | 22 | 16 | |
| Spd-caproate | 17 | 8 | 17 | 24 | 20 | 17 | 11 | 26 | 14 | 13 | 22 | 17 | |
| PEI4 | 15 | 6 | 11 | 21 | 16 | 17 | 6 | 17 | 11 | 20 | 20 | 16 | |
| PEI4-lipoate | 12 | 27 | 23 | 14 | 11 | 10 | 14 | 26 | 20 | 21 | 37 | 16 | |
| Cell Viability *, % | The Effect on Cell Growth | The Type of the Interaction with the Enzymes | A549 | K562 | ||||
|---|---|---|---|---|---|---|---|---|
| X = Spm | X = Spd | X = PEI | X = Spm | X = Spd | X = PEI | |||
| X | slight inhibition | - | 81 | 77 | 77 | 62 | 86 | 62 |
| X-propionate | inhibition in the case of spm | - | 38 | 90 | 90 | 66 | 87 | 104 |
| X-caproate | inhibition | - | 70 | 69 | 60 | 53 | 117 | 53 |
| X-lipoate | inhibition in the case of spd and PEI | - | 100 | 23 | 57 | 80 | 55 | 54 |
| EcA alone | cytostatic | - | 67 | 67 | 67 | 58 | 58 | 58 |
| EcA + X | mostly cytostatic | Synergy (except for spermine) | 120 (97) | 58 (45) | 40 (31) | 106 (66) | 45 (39) | 58 (36) |
| EcA + X-propionate | Synergy (except for spermine) | 103 (39) | 44 (40) | 50 (45) | 100 (66) | 44 (38) | 36 (37) | |
| EcA + X-caproate | Addition | 57 (40) | 119 (82) | 52 (31) | 138 (73) | 34 (40) | 70 (37) | |
| EcA + X-lipoate | Mainly Inhibition | 60 (60) | 283 (65) | 28 (16) | 61 (49) | 60 (33) | 69 (37) | |
| EcA-spm alone | cytostatic | - | 58 | 58 | 58 | 50 | 50 | 50 |
| EcA-spm + X | less efficient cytostatics | Addition for A549 Inhibition for K562 | 46 (37) | 40 (31) | 25 (19) | 116 (72) | 51 (44) | 71 (44) |
| EcA-spm + X-propionate | Inhibition for A549, Mainly Inhibition for K562 | 60 (42) | 64 (44) | 73 (44) | 106 (56) | 35 (41) | 77 (41) | |
| EcA-spm + X-caproate | Addition for A549 Mainly Inhibition for K562 | 45 (30) | 88 (59) | 28 (19) | 134 (78) | 174 (101) | 48 (28) | |
| EcA-spm + X-lipoate | Inhibition or indifferent | 108 (42) | 115 (46) | 58 (26) | 70 (46) | 71 (27) | 189 (70) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlotnikov, I.D.; Ezhov, A.A.; Kudryashova, E.V. Receptor-Mediated Internalization of L-Asparaginase into Tumor Cells Is Suppressed by Polyamines. Int. J. Mol. Sci. 2025, 26, 6749. https://doi.org/10.3390/ijms26146749
Zlotnikov ID, Ezhov AA, Kudryashova EV. Receptor-Mediated Internalization of L-Asparaginase into Tumor Cells Is Suppressed by Polyamines. International Journal of Molecular Sciences. 2025; 26(14):6749. https://doi.org/10.3390/ijms26146749
Chicago/Turabian StyleZlotnikov, Igor D., Alexander A. Ezhov, and Elena V. Kudryashova. 2025. "Receptor-Mediated Internalization of L-Asparaginase into Tumor Cells Is Suppressed by Polyamines" International Journal of Molecular Sciences 26, no. 14: 6749. https://doi.org/10.3390/ijms26146749
APA StyleZlotnikov, I. D., Ezhov, A. A., & Kudryashova, E. V. (2025). Receptor-Mediated Internalization of L-Asparaginase into Tumor Cells Is Suppressed by Polyamines. International Journal of Molecular Sciences, 26(14), 6749. https://doi.org/10.3390/ijms26146749








