The Dichloromethane Fraction of Sanguisorba tenuifolia Inhibits Inflammation in Cells Through Modulation of the p38/ERK/MAPK and NF-κB Signaling Pathway
Abstract
1. Introduction
2. Results
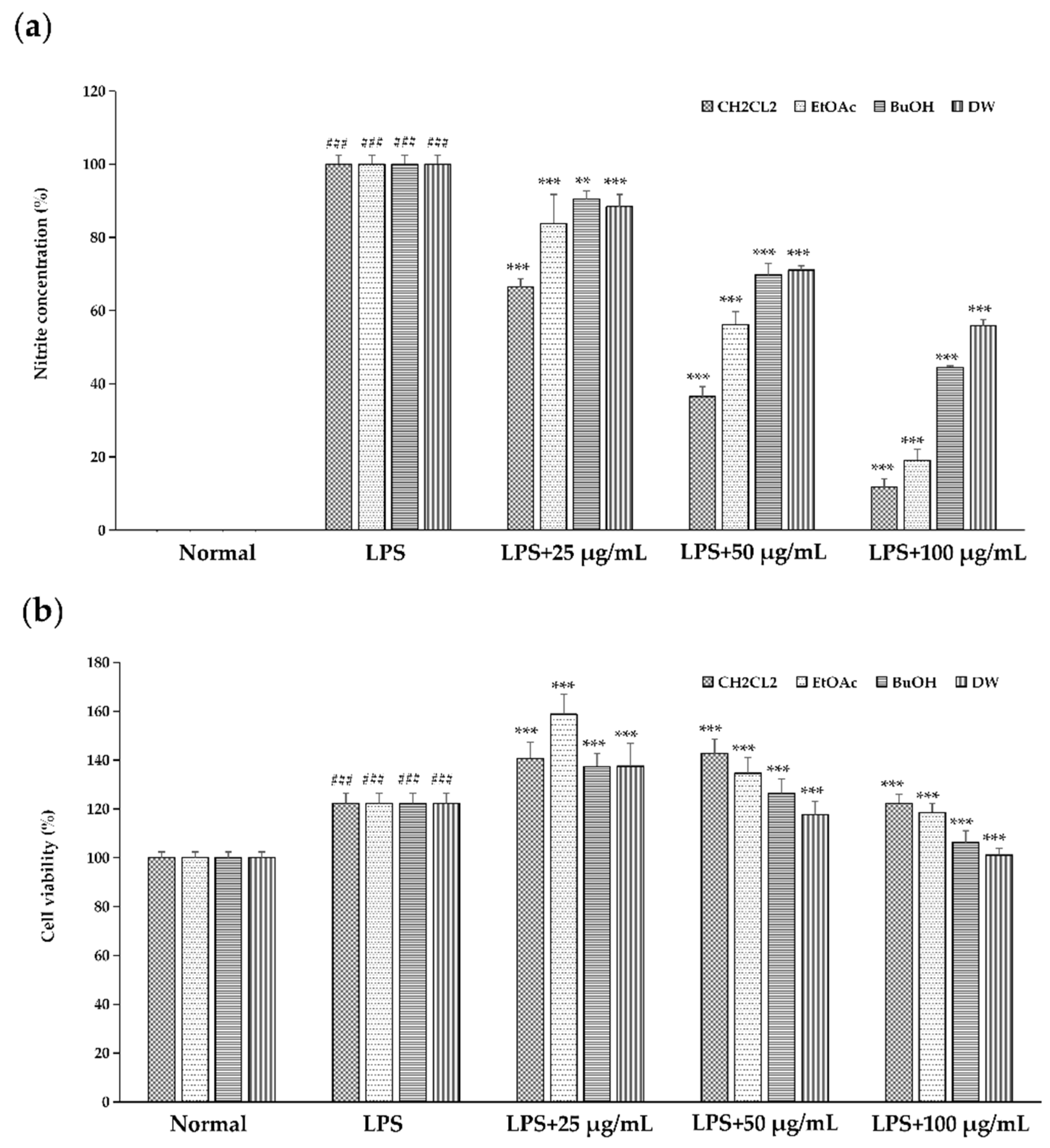
2.1. Effect of Different Solvent Fractionated Extracts of Sanguisorba tenuifolia on NO Production
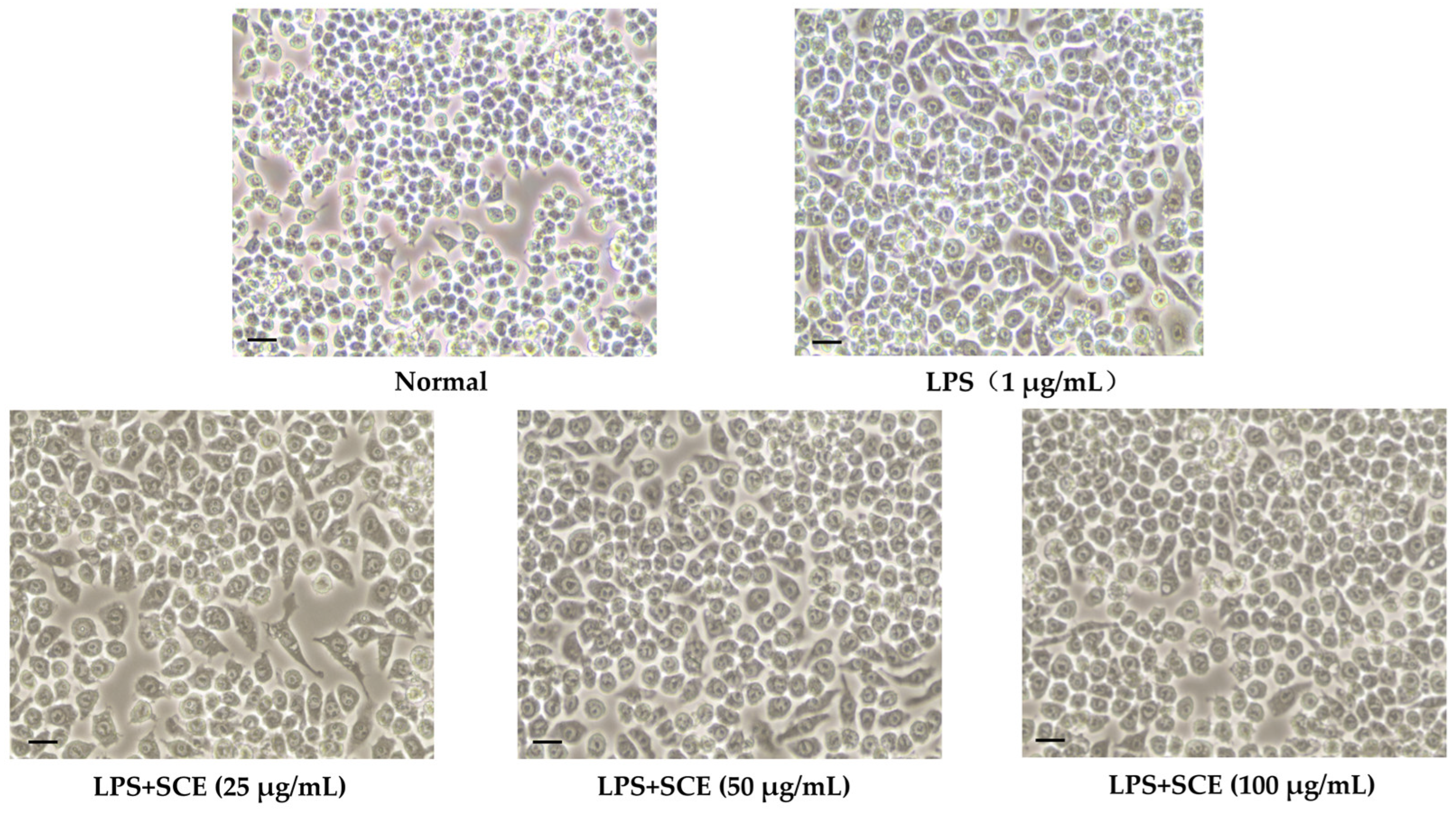
2.2. Effect of SCE on Cell Morphology Changes
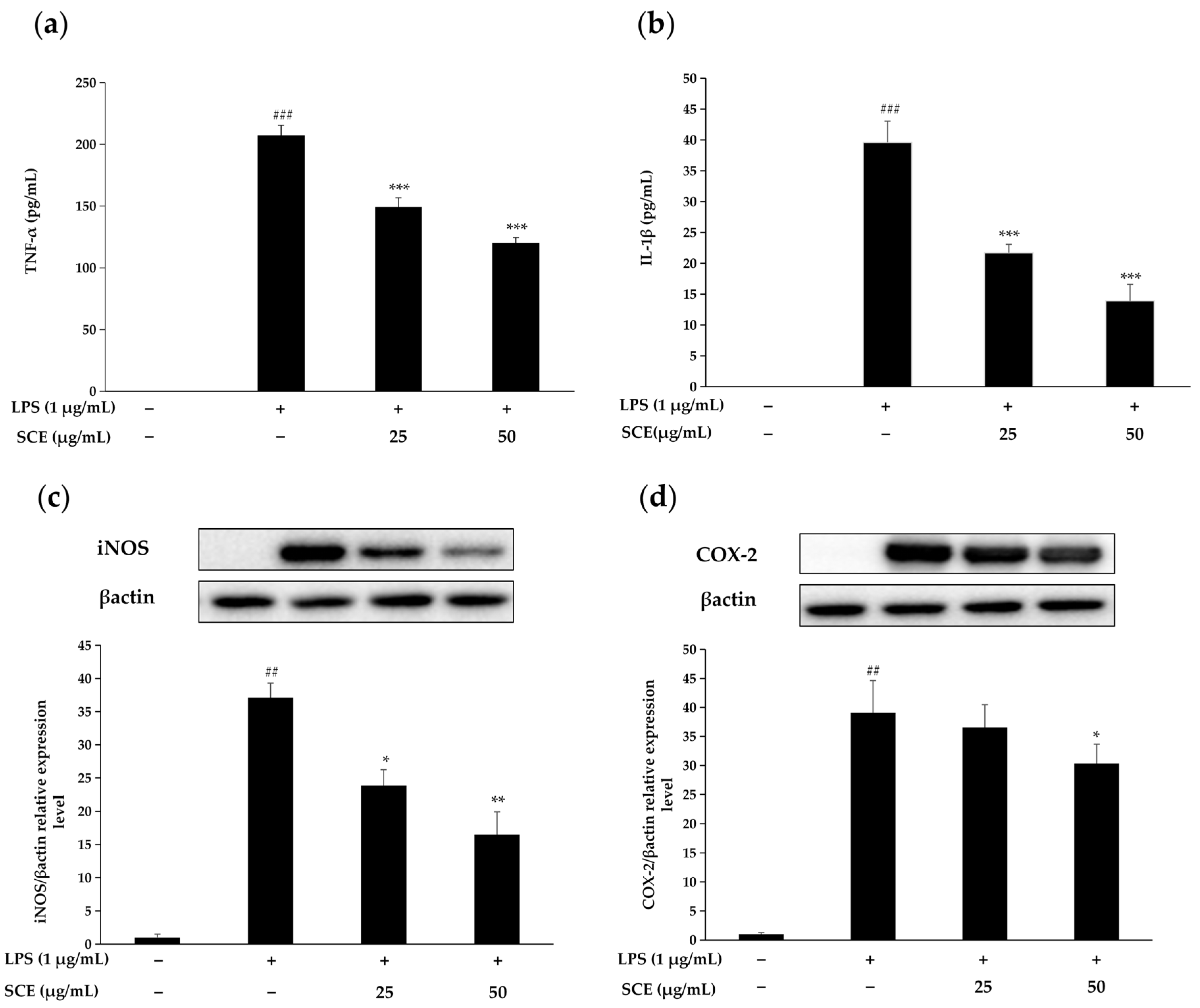
2.3. Effect of SCE on the Production of Inflammatory Cytokines and Inflammation-Related Proteins
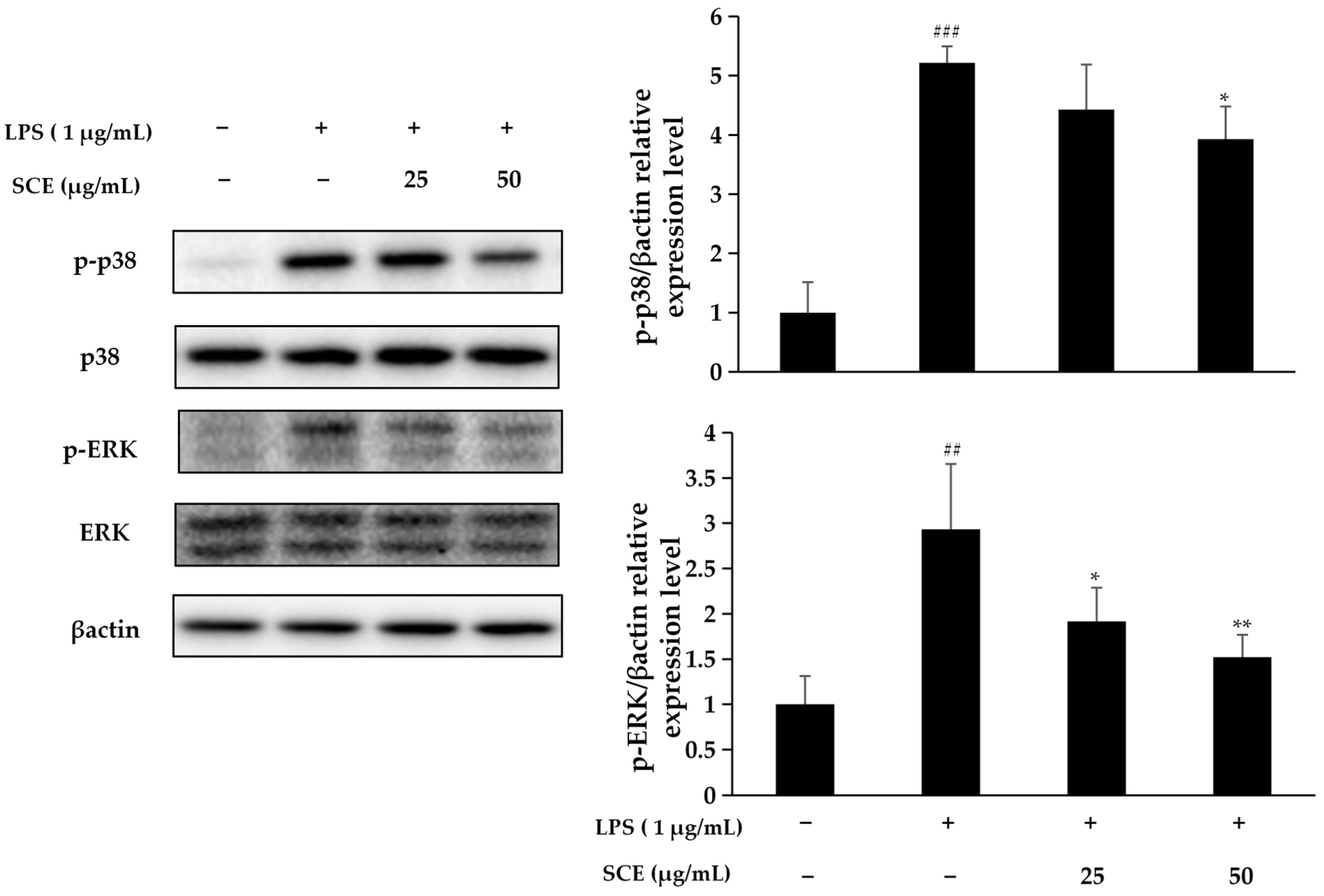
2.4. Effect of SCE on Activation of the MAPK Pathway
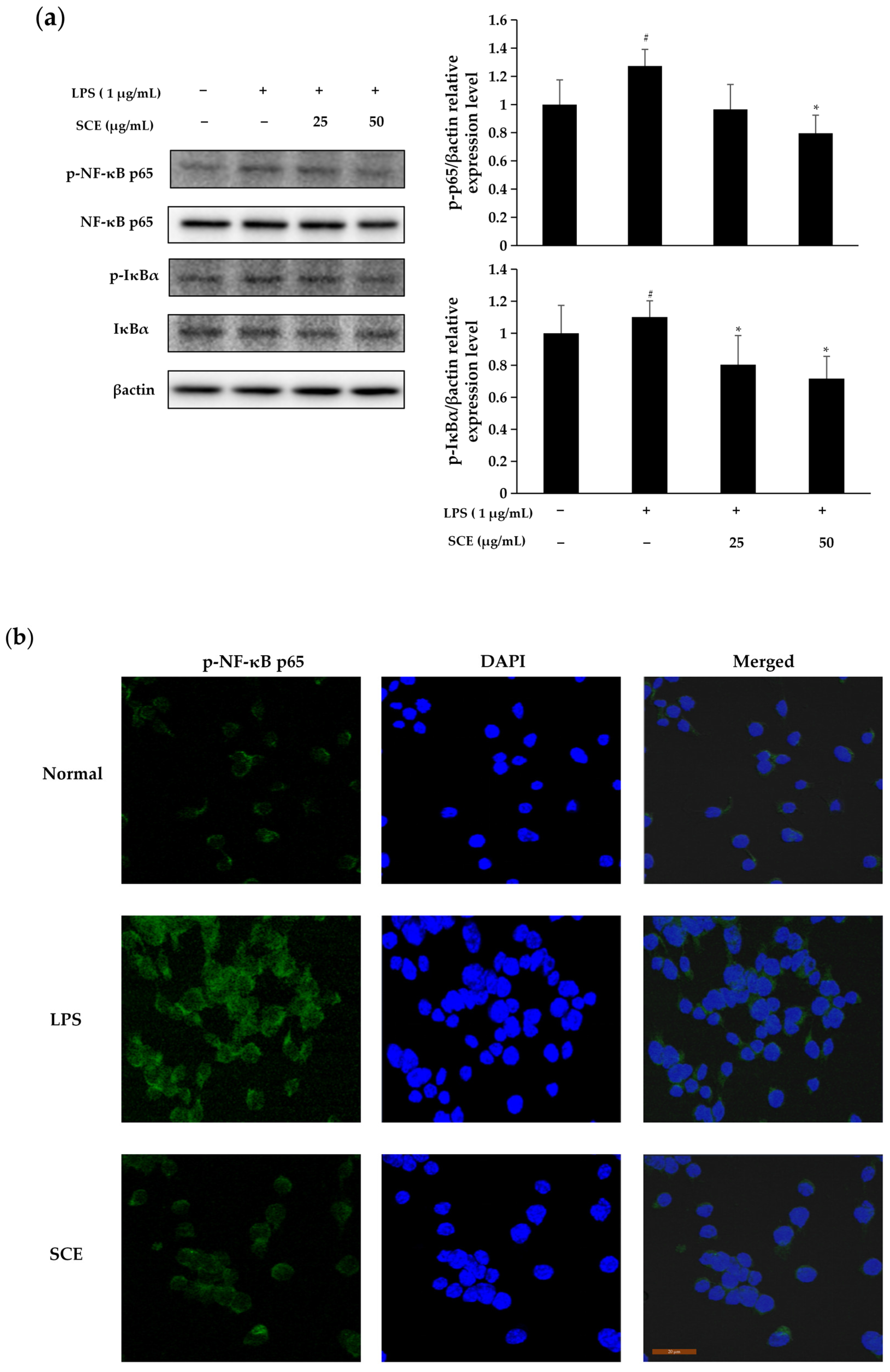
2.5. Effect of SCE on Activation of NF-κB Pathway
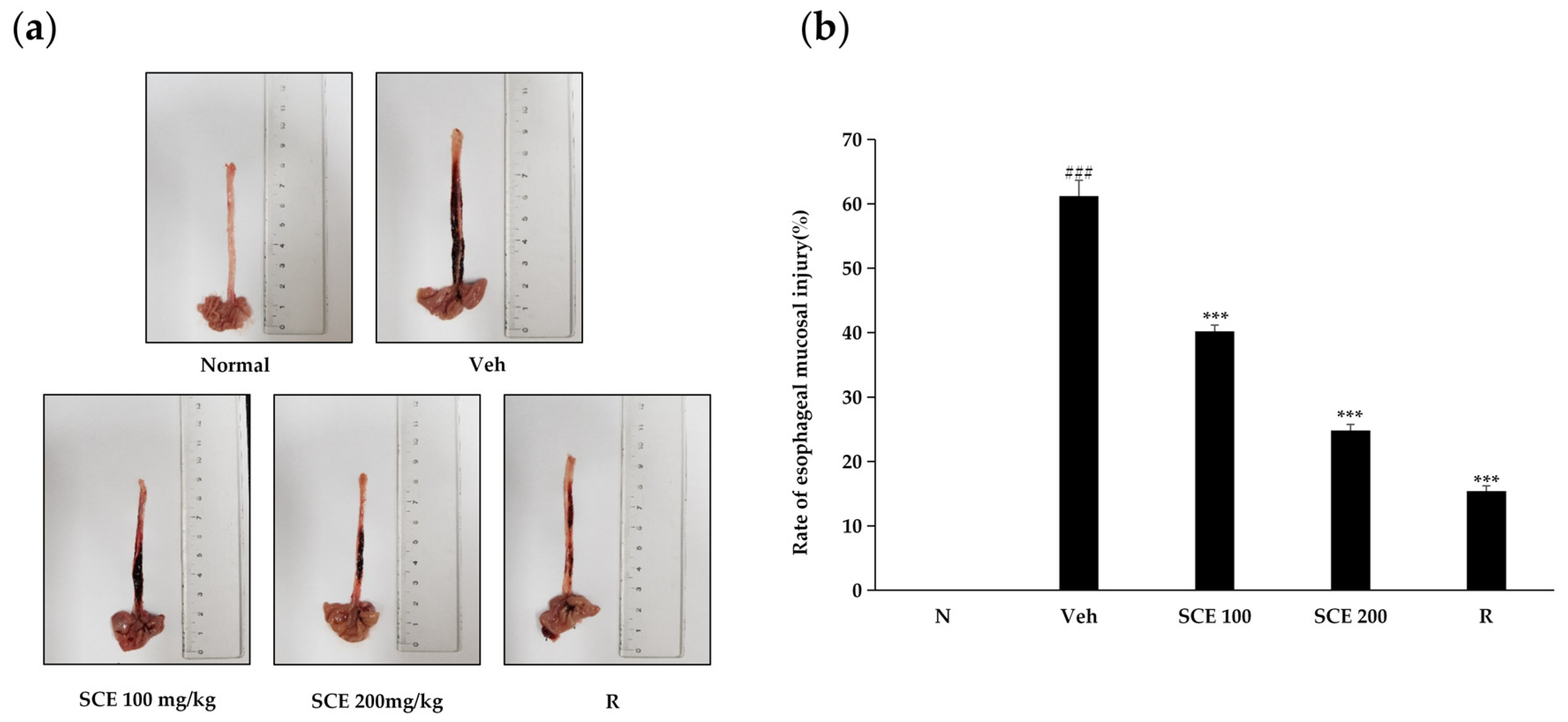
2.6. Effect of SCE on Esophageal Mucosal Injury in Rats
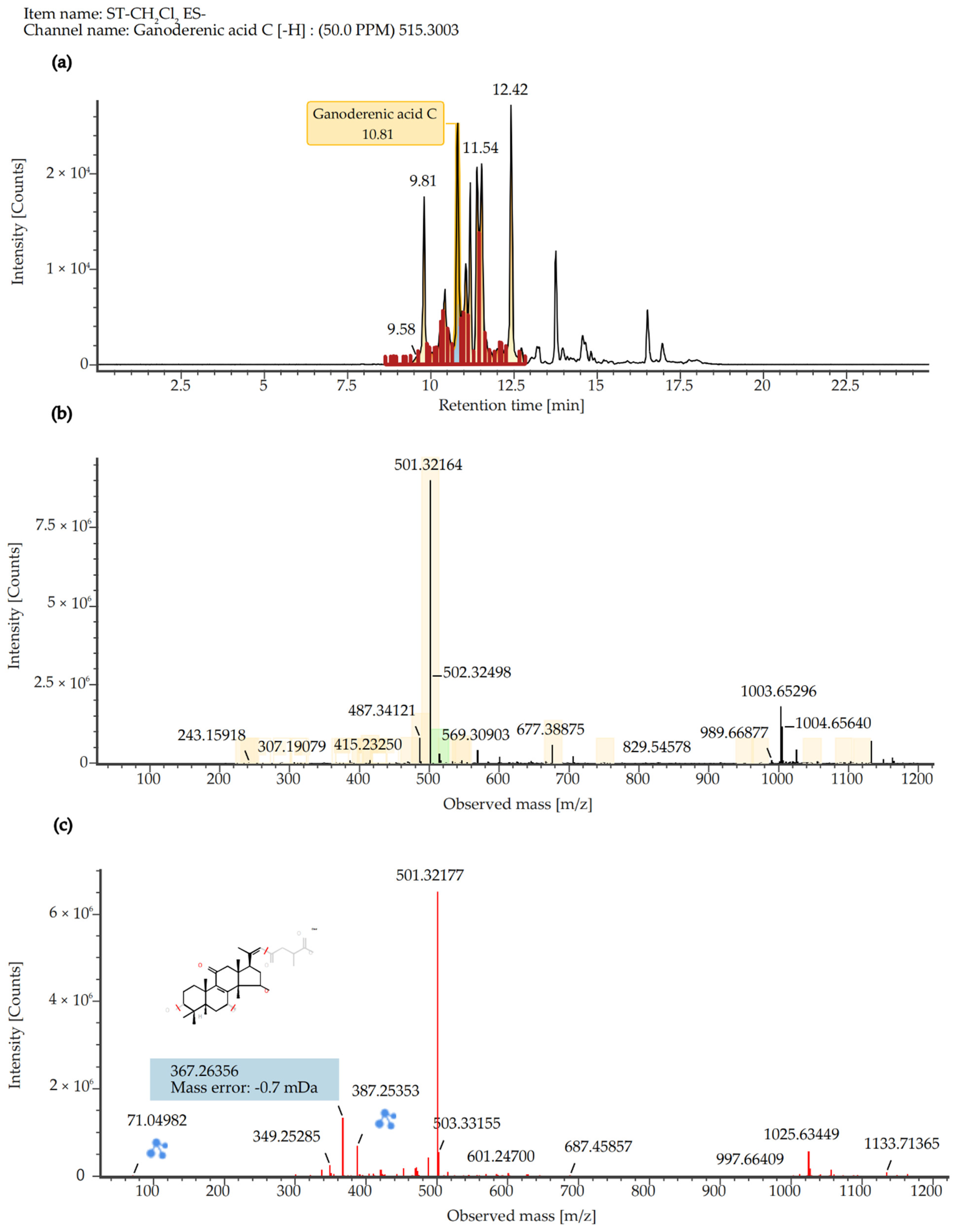
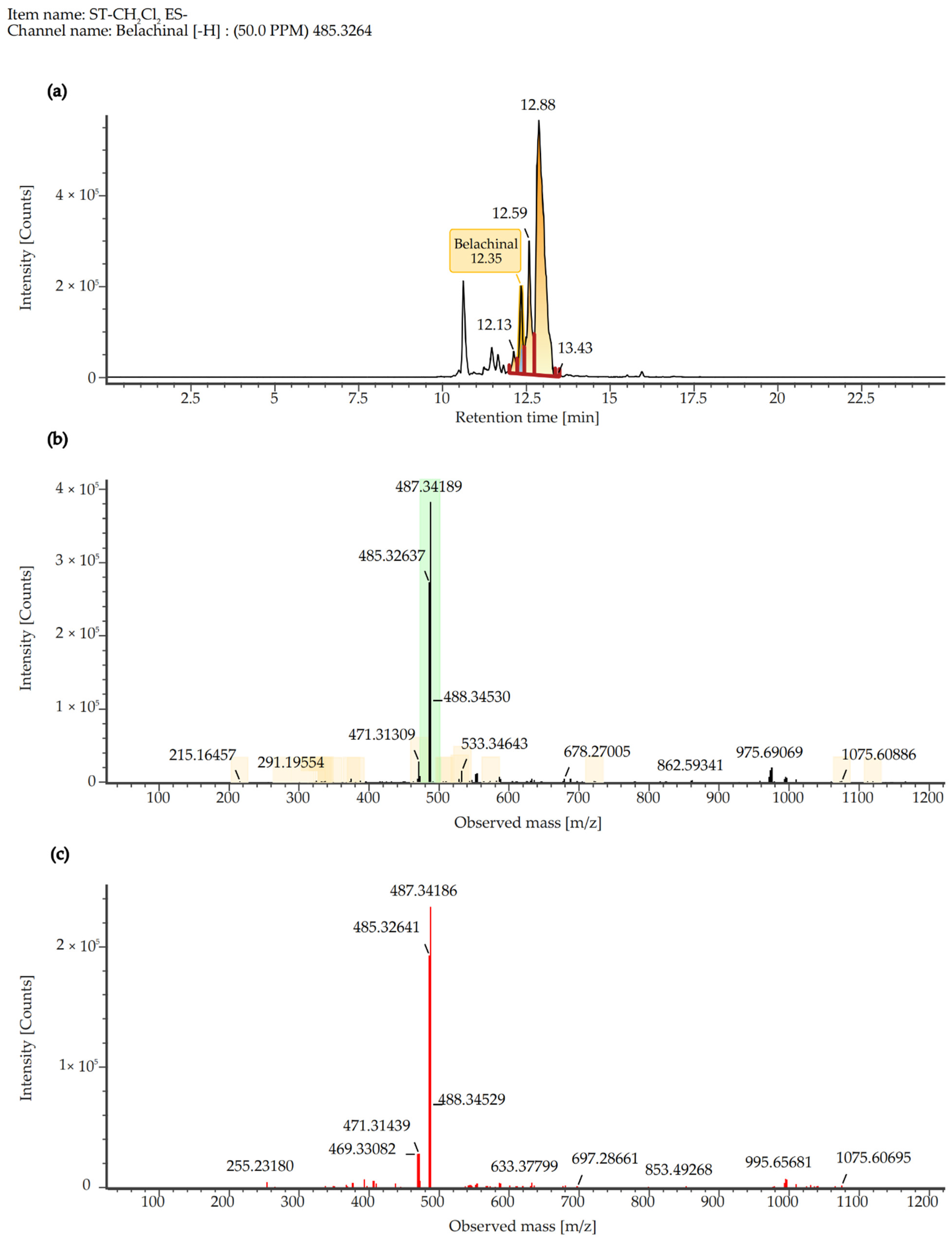
2.7. Quantitative Analysis of SCE Extract
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Preparation
4.3. Cell Culture
4.4. Determination of NO Content
4.5. Determination of Cell Viability and Observation of Morphological Changes
4.6. Determination of TNF-α and IL-1β Content
4.7. Immunofluorescence Assay
4.8. Protein Blotting to Determine Inflammatory Protein Expression
4.9. UPLC Mass Spectrometry Analysis and Conditions
4.10. Rat Reflux Esophagitis Test and Measurement of Esophageal Injury
4.11. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | Absorption, Distribution, Metabolism, Excretion and Toxicity |
| BuOH | N-butanol |
| CCK-8 | Cell counting kit-8 |
| CH2Cl2 | Dichloromethane |
| COX-2 | Cyclooxygenase-2 |
| DAPI | 4′,6′-diamidino-2-phenylindole |
| DEME | Dulbecco’s modified eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| DW | Distilled water |
| ELISA | Enzyme-linked immunosorbent assay |
| ESI | Electrospray ionization |
| EtOAc | Ethyl acetate |
| FBS | Fetal bovine serum |
| IL-1β | Interleukin-1β |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| PVDF | olyvinylidene fluoride |
| RE | Reflux esophagitis |
| SCE | Dichloromethane fraction of Sanguisorba tenuifolia |
| SPSS | Statistical product and service solutions |
| TLRs | Toll-like receptors |
| TNF-α | Tumor necrosis factor α |
| UPLC-MS | Ultra-performance liquid chromatography-tandem mass spectrometry |
References
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Schromm, A.B.; Brandenburg, K.; Loppnow, H.; Moran, A.P.; Koch, M.H.; Rietschel, E.T.; Seydel, U. Biological Activities of Lipopolysaccharides Are Determined by the Shape of Their Lipid A Portion. Eur. J. Biochem. 2000, 267, 2008–2013. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Wang, J.; Liu, Y.; Yan, P.; Meng, Q.; Yin, Y.; Wang, S. SIRT5-Related Desuccinylation Modification Contributes to Quercetin-Induced Protection against Heart Failure and High-Glucose-Prompted Cardiomyocytes Injury through Regulation of Mitochondrial Quality Surveillance. Oxid. Med. Cell. Longev. 2021, 2021, 5876841. [Google Scholar] [CrossRef] [PubMed]
- Korinek, M.; Tsai, Y.H.; El-Shazly, M.; Lai, K.H.; Backlund, A.; Wu, S.F.; Lai, W.C.; Wu, T.Y.; Chen, S.L.; Wu, Y.C.; et al. Anti-Allergic Hydroxy Fatty Acids from Typhonium blumei Explored through ChemGPS-NP. Front. Pharmacol. 2017, 8, 356. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.Q.; Kang, O.H.; Kwon, D.Y. Combination of Sanguisorbigenin and Conventional Antibiotic Therapy for Methicillin-Resistant Staphylococcus aureus: Inhibition of Biofilm Formation and Alteration of Cell Membrane Permeability. Int. J. Mol. Sci. 2022, 23, 4232. [Google Scholar] [CrossRef]
- Moţoc, T.C.A.; Ranga, F.; Teodorescu, A.G.; Pallag, A.; Vlad, A.M.; Bandici, L.; Vicas, S.I. Evaluation of Polyphenolic Composition and Antimicrobial Properties of Sanguisorba officinali L. and Sanguisorba minor Scop. Plants 2022, 11, 3561. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Chen, K.; Zhang, L.; Li, Y. Triterpenoids from the Roots of Sanguisorba officinalis and Their Nrf2 Stimulation Activity. Phytochemistry 2023, 214, 113803. [Google Scholar] [CrossRef]
- Yasueda, A.; Kayama, H.; Murohashi, M.; Nishimura, J.; Wakame, K.; Komatsu, K.I.; Ogino, T.; Miyoshi, N.; Takahashi, H.; Uemura, M.; et al. Sanguisorba officinalis L. Derived from Herbal Medicine Prevents Intestinal Inflammation by Inducing Autophagy in Macrophages. Sci. Rep. 2020, 10, 9972. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, Q.; Tamrakar, S.; Amen, Y.; Mori, Y.; Suhara, H.; Kaneko, S.; Kawashima, H.; Okuzono, K.; Inoue, Y.; et al. Changes in Content of Triterpenoids and Polysaccharides in Ganoderma lingzhi at Different Growth Stages. J. Nat. Med. 2018, 72, 734–744. [Google Scholar] [CrossRef]
- Li, S.; Hou, W.; Li, Y.; Liu, Z.; Yun, H.; Liu, Q.; Niu, H.; Liu, C.; Zhang, Y. Modeling and Optimization of the Protocol of Complex Chromatography Separation of Cyclooxygenase-2 Inhibitors from Ganoderma lucidum Spore. Phytochem. Anal. 2023, 34, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.S.; Tomescu, C.L.; Ionescu, A.M. Natural Bio-Compounds from Ganoderma lucidum and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, K.; Xu, J.; Yang, D.; Zhang, C.; Wang, Z.; Li, M. Belamcanda chinensis (L.) DC—An Ethnopharmacological, Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2016, 186, 1–13. [Google Scholar] [CrossRef]
- Gupta, M.K.; Vemula, S.; Donde, R.; Gouda, G.; Behera, L.; Vadde, R. In-Silico Approaches to Detect Inhibitors of the Human Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel. J. Biomol. Struct. Dyn. 2021, 39, 2617–2627. [Google Scholar] [CrossRef]
- Yin, Y.; Cai, J.; Zhou, L.; Xing, L.; Zhang, W. Dietary Oxidized Beef Protein Alters Gut Microbiota and Induces Colonic Inflammatory Damage in C57BL/6 Mice. Front. Nutr. 2022, 9, 980204. [Google Scholar] [CrossRef]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Kim, I.S.; Han, Y.S. Biosurfactants Induce Antimicrobial Peptide Production through the Activation of Tm Spatzles in Tenebrio molitor. Int. J. Mol. Sci. 2020, 21, 6090. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Zhang, J.; Luo, Y.; Han, W.; Wang, H.; Xia, H.; Chen, Z.; Yang, Y.; Chen, Q.; et al. Maternal Inflammation Exaggerates Offspring Susceptibility to Cerebral Ischemia-Reperfusion Injury via the COX-2/PGD2/DP2 Pathway Activation. Oxid. Med. Cell. Longev. 2022, 1571705. [Google Scholar] [CrossRef]
- Su, X.D.; Ali, I.; Arooj, M.; Koh, Y.S.; Yang, S.Y.; Kim, Y.H. Chemical constituents from Sanguisorba officinalis L. and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharmacal Res. 2018, 41, 497–505. [Google Scholar] [CrossRef]
- Moreira, R.; Jervis, P.J.; Carvalho, A.; Ferreira, P.M.T.; Martins, J.A.; Valentão, P.; Andrade, P.B.; Perreira, D.M. Biological Evaluation of Naproxen-Dehydrodipeptide Conjugates with Self-Hydrogelation Capacity as Dual LOX/COX Inhibitors. Pharmaceutics 2020, 12, 122. [Google Scholar] [CrossRef]
- Xie, J.; Wu, X.; Zheng, S.; Lin, K.; Su, J. Aligned Electrospun Poly(L-Lactide) Nanofibers Facilitate Wound Healing by Inhibiting Macrophage M1 Polarization via the JAK-STAT and NF-κB Pathways. J. Nanobiotechnol. 2022, 20, 342. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, H.; Chen, G.; Chen, X.; Hu, C.; Su, X.; Tan, F.; Zhao, X. Preventive Effect of Gonggan (Citrus reticulata Blanco Var. Gonggan) Peel Extract on Ethanol/HCl-Induced Gastric Injury in Mice via an Anti-Oxidative Mechanism. Front. Pharmacol. 2021, 12, 715306. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Tan, L.; Ju, F.; Kuang, Q.X.; Yang, T.L.; Deng, F.; Gu, Y.C.; Jiang, L.S.; Deng, Y.; Guo, D.L. Phenolic Glycosides from Sanguisorba officinalis and Their Anti-Inflammatory Effects. Nat. Prod. Res. 2022, 36, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.L.; Chen, J.F.; Tan, L.; Jin, M.Y.; Ju, F.; Cao, Z.X.; Deng, F.; Wang, L.N.; Gu, Y.C.; Deng, Y. Terpene Glycosides from Sanguisorba officinalis and Their Anti-Inflammatory Effects. Molecules 2019, 24, 2906. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xue, F.; Xiao, H.; Chen, L.; Zhang, Y. Bamboo Leaf Flavonoids Suppress Oxidative Stress-Induced Senescence of HaCaT Cells and UVB-Induced Photoaging of Mice through p38 MAPK and Autophagy Signaling. Nutrients 2022, 14, 793. [Google Scholar] [CrossRef]
- Lee, J.A.; Shin, J.Y.; Hong, S.S.; Cho, Y.R.; Park, J.H.; Seo, D.W.; Oh, J.S.; Kang, J.S.; Lee, J.H.; Ahn, E.K. Tetracera loureiri Extract Regulates Lipopolysaccharide-Induced Inflammatory Response Via Nuclear Factor-κB and Mitogen Activated Protein Kinase Signaling Pathways. Plants 2022, 11, 284. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Q.; Cao, Z.; Li, H.; Zhou, Y.; Zhang, P. Icariin Decreases Cell Proliferation and Inflammation of Rheumatoid Arthritis-Fibroblast-Like Synoviocytes via GAREM1/MAPK Signaling Pathway. Immunopharmacol. Immunotoxicol. 2024, 46, 86–92. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Liu, W.; Chu, Y.; Zhong, J.; Xie, Y.; Lou, X.; Ouyang, X. LOX-1 Regulates P. gingivalis-Induced Monocyte Migration and Adhesion to Human Umbilical Vein Endothelial Cells. Front. Cell Dev. Biol. 2020, 8, 596. [Google Scholar] [CrossRef]
- Shu, C.; Chen, J.; Lv, M.; Xi, Y.; Zheng, J.; Xu, X. Plumbagin Relieves Rheumatoid Arthritis through Nuclear Factor Kappa-B (NF-κB) Pathway. Bioengineered 2022, 13, 13632–13642. [Google Scholar] [CrossRef]
- Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. [Google Scholar] [CrossRef]
- Yu, T.; Lee, Y.J.; Yang, H.M.; Han, S.; Kim, J.H.; Lee, Y.; Kim, C.; Han, M.H.; Kim, M.Y.; Lee, J.; et al. Inhibitory Effect of Sanguisorba officinalis Ethanol Extract on NO and PGE2 Production Is Mediated by Suppression of NF-κB and AP-1 Activation Signaling Cascade. J. Ethnopharmacol. 2011, 134, 11–17. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H.; Fadda, A.M. Advanced Assessments on Innovative Methods to Improve the Bioaccessibility of Polyphenols in Wheat. Process Biochem. 2020, 88, 1–14. [Google Scholar] [CrossRef]
- Gou, W.; Luo, N.; Yu, B.; Wu, H.; Wu, S.; Tian, C.; Guo, J.; Ning, H.; Bi, C.; Wei, H.; et al. Ursolic Acid Derivative UA232 Promotes Tumor Cell Apoptosis by Inducing Endoplasmic Reticulum Stress and Lysosomal Dysfunction. Int. J. Biol. Sci. 2022, 18, 2639–2651. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.Q.; Su, X.W.; Liu, Y.F.; Tang, C.H.; Tang, Q.J.; Zhou, S.; Tan, Y.; Liu, L.P.; Zhang, J.S.; Feng, J. Effects of Oleic Acid Addition Methods on the Metabolic Flux Distribution of Ganoderic Acids R, S and T’s Biosynthesis. J. Fungi 2022, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Zakroysky, P.; Thai, W.E.; Deaño, R.C.; Basnet, S.; Onandia, Z.G.; Gandhi, S.; Tawakol, A.; Min, J.K.; Truong, Q.A. Steroid Exposure, Acute Coronary Syndrome, and Inflammatory Bowel Disease: Insights into the Inflammatory Milieu. Am. J. Med. 2015, 128, 303–311. [Google Scholar] [CrossRef]
- Jang, E.; Inn, K.S.; Jang, Y.P.; Lee, K.T.; Lee, J.H. Phytotherapeutic Activities of Sanguisorba officinalis and Its Chemical Constituents: A Review. Am. J. Chin. Med. 2018, 46, 299–318. [Google Scholar] [CrossRef]
- Nan, L.; Nam, H.H.; Park, B.Y.; Kim, B.T.; Choo, B.K. Ameliorative Effects of Magnolia sieboldii Buds Hexane Extract on Experimental Reflux Esophagitis. Phytother. Res. 2020, 34, 2385–2396. [Google Scholar] [CrossRef]

| No. | Component Name | Neutral Mass (Da) | Observe Neutral Mass (Da) | Detector Counts | Adducts | Isotope Match Intensity RMS Percent | Type of Compound |
|---|---|---|---|---|---|---|---|
| 1 | 2,3-(S)-Hexahydroxydiphenoyl- D-glucose | 482.0697 | 482.0673 | 6155 | -H | 960.52 | Tannin |
| 2 | Corilagin | 634.0806 | 634.0807 | 4466 | +HCOO | 48.89 | Ellagitannin |
| 3 | 3-O-Methyl ellagic acid | 316.0219 | 316.0222 | 139,306 | -H | 3.53 | Hydrolyzable tannins |
| 4 | 3,3′-Di-O-methylellagic acid | 330.0376 | 330.0377 | 61,897 | -H | 4.18 | Phenols |
| 5 | Ganoderenic acid C | 516.3075 | 515.3003 | 10,350 | -H | 33.07 | Triterpenoids |
| 6 | Belachinal | 486.3345 | 486.3337 | 95,911 | -H | 199.14 | Triterpenoids |
| 7 | Ergosta-4,6,8(14),22- tetraen-3-one | 392.3079 | 392.3058 | 6764 | +HCOO | 21.61 | Steroids |
| 8 | Poricoic acid B | 484.3189 | 484.3185 | 183,076 | -H, +HCOO | 365.91 | Triterpenoids |
| 9 | α-Boswellic acid | 456.3603 | 456.3598 | 942,668 | -H, +HCOO | 2.63 | Pentacyclic triterpene |
| 10 | 3β-O-trans-p-Coumaroyl alphitolic acid | 618.392 | 618.3914 | 190,687 | -H | 2.79 | Triterpenoids |
| 11 | Polyporusterone D | 460.3189 | 460.3194 | 5071 | -H | 1.82 | Steroids |
| 12 | α-Hydroxy tetracosanic acid | 384.3603 | 384.3593 | 34,746 | -H, +HCOO | 5.07 | Fatty acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lu, Y.; Niu, F.; Fa, S.; Nan, L.; Nam, H.H. The Dichloromethane Fraction of Sanguisorba tenuifolia Inhibits Inflammation in Cells Through Modulation of the p38/ERK/MAPK and NF-κB Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 6732. https://doi.org/10.3390/ijms26146732
Wang Y, Lu Y, Niu F, Fa S, Nan L, Nam HH. The Dichloromethane Fraction of Sanguisorba tenuifolia Inhibits Inflammation in Cells Through Modulation of the p38/ERK/MAPK and NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(14):6732. https://doi.org/10.3390/ijms26146732
Chicago/Turabian StyleWang, Yue, Yiming Lu, Fuao Niu, Siqi Fa, Li Nan, and Hyeon Hwa Nam. 2025. "The Dichloromethane Fraction of Sanguisorba tenuifolia Inhibits Inflammation in Cells Through Modulation of the p38/ERK/MAPK and NF-κB Signaling Pathway" International Journal of Molecular Sciences 26, no. 14: 6732. https://doi.org/10.3390/ijms26146732
APA StyleWang, Y., Lu, Y., Niu, F., Fa, S., Nan, L., & Nam, H. H. (2025). The Dichloromethane Fraction of Sanguisorba tenuifolia Inhibits Inflammation in Cells Through Modulation of the p38/ERK/MAPK and NF-κB Signaling Pathway. International Journal of Molecular Sciences, 26(14), 6732. https://doi.org/10.3390/ijms26146732






