Melatonin as the Missing Link Between Sleep Deprivation and Immune Dysregulation: A Narrative Review
Abstract
1. Introduction
2. Results
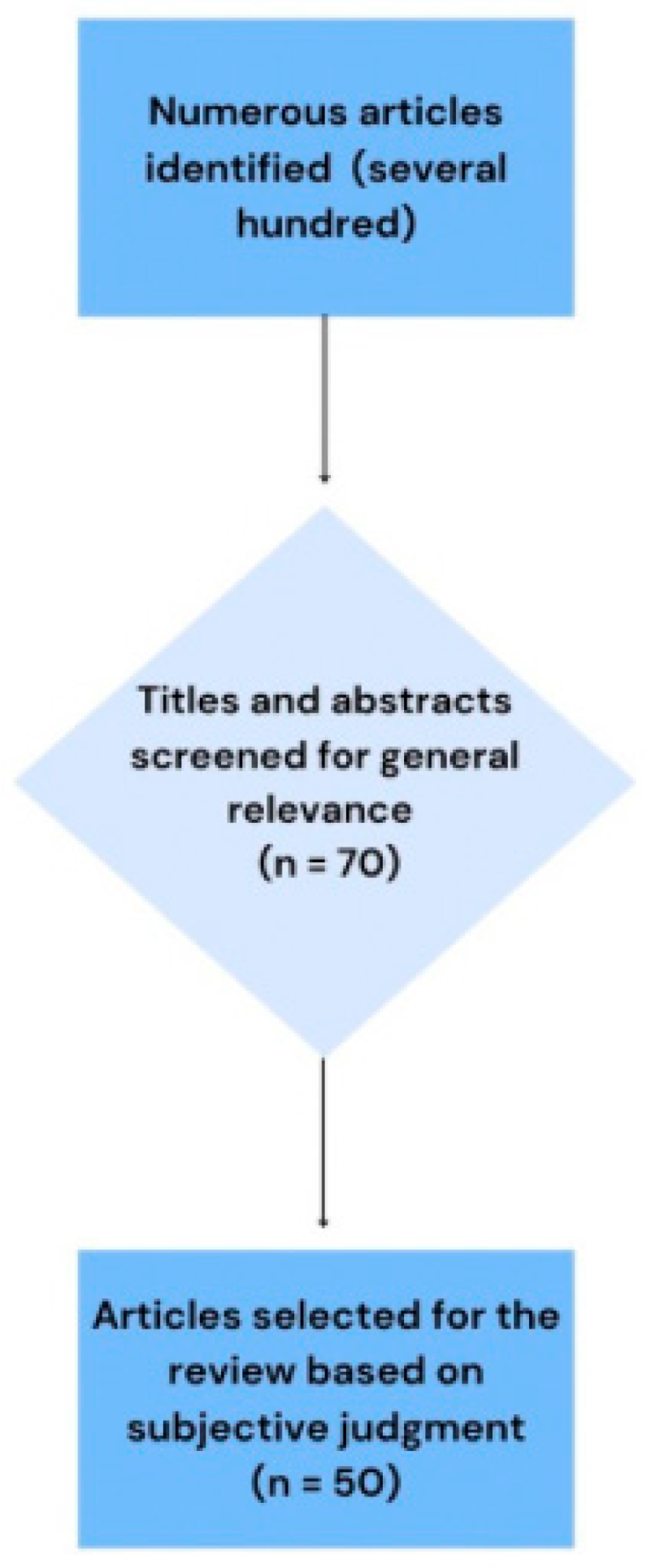
2.1. Study Selection
- Insufficient clarity regarding the study population or sampling method;
- Absence of a control or comparison group;
- Lack of clearly defined outcomes;
- Very small sample size with no justification of power.
2.2. Study Characteristic
2.3. Results of Individual Studies
2.4. Certainty of Evidence
3. Discussion
3.1. Interpretation of the Results in the Context of the Other Evidence
3.2. Limitations of the Evidence Included in the Review
3.3. Limitations of the Review Process Used
3.4. Implications for Practice, Policy, and Future Research
4. Materials and Methods
- Insufficient clarity regarding the study population or sampling method;
- Absence of a control or comparison group;
- Lack of clearly defined outcomes;
- Very small sample size with no justification of power.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, K.K.; Ghosh, S.; Bhola, A.; Verma, P.; Amist, A.D.; Sharma, H.; Sachdeva, P.; Sinha, J.K. Sleep and Immune System Crosstalk: Implications for Inflammatory Homeostasis and Disease Pathogenesis. Ann. Neurosci. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Shneider, A.; Kudriavtsev, A.; Vakhrusheva, A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020, 39, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Pires, A.S.; Tan, V.; Chidambaram, S.B.; Guillemin, G.J. Effects of Sleep Deprivation on the Tryptophan Metabolism. Int. J. Tryptophan Res. 2020, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Esquifino, A.I. Sleep and the Immune System. Curr. Immunol. Rev. 2012, 8, 50–62. [Google Scholar] [CrossRef]
- A Rossignol, D.; E Frye, R. Melatonin in autism spectrum disorders: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Markus, R.P.; A Fernandes, P.; Kinker, G.S.; Cruz-Machado, S.d.S.; Marçola, M. Immune-pineal axis – acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 2017, 175, 3239–3250. [Google Scholar] [CrossRef]
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 116960. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin alleviates oxidative stress in sleep-deprived mice. Int. Immunopharmacol. 2020, 78, 106041. [Google Scholar] [CrossRef]
- Kuna, K.; Szewczyk, K.; Gabryelska, A.; Białasiewicz, P.; Ditmer, M.; Strzelecki, D.; Sochal, M. Potential Role of Sleep Deficiency in Inducing Immune Dysfunction. Biomedicines 2022, 10, 2159. [Google Scholar] [CrossRef]
- Irwin, M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav. Immun. 2002, 16, 503–512. [Google Scholar] [CrossRef]
- Kwon, K.; Lee, E.; Kim, M.; Jeon, S.; Choi, Y.; Shin, C.; Han, S.-H. The potential role of melatonin on sleep deprivation-induced cognitive impairments: Implication of FMRP on cognitive function. Neuroscience 2015, 301, 403–414. [Google Scholar] [CrossRef]
- Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Esquifino, A.I.; Pandi-Perumal, S.R.; Miller, S.C. Melatonin, immune function and aging. Immun. Ageing 2005, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Esquifino, A.I.; Srinivasan, V.; Pandi-Perumal, S.R. Melatonin and the Immune System in Aging. Neuroimmunomodulation 2008, 15, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Sleep in Elite Athletes and Nutritional Interventions to Enhance Sleep. Sports Med. 2014, 44 (Suppl. S1), 13–23. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.-H.; Li, S.-X.; He, Z.-M.; Zhu, W.-L.; Ji, Y.-B.; Wang, Z.; Zhu, X.-M.; Yuan, K.; Bao, Y.-P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.; Zheng, C.; Liu, F.; Wu, L.; Tang, Y.; Wu, Y.; Lv, D.; Chen, H.; Qian, L.; Wu, X.; et al. Relationship between circadian genes and memory impairment caused by sleep deprivation. PeerJ 2022, 10, e13165. [Google Scholar] [CrossRef]
- Moradkhani, F.; Moloudizargari, M.; Fallah, M.; Asghari, N.; Khoei, H.H.; Asghari, M.H. Immunoregulatory role of melatonin in cancer. J. Cell. Physiol. 2019, 235, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Ragnoli, B.; Pochetti, P.; Pignatti, P.; Barbieri, M.; Mondini, L.; Ruggero, L.; Trotta, L.; Montuschi, P.; Malerba, M. Sleep deprivation, immune suppression and SARS-CoV-2 infection. Int. J. Environ. Res. Public Health 2022, 19, 904. [Google Scholar] [CrossRef]
- Huggard, D.; Kelly, L.; Worrall, A.; Gallagher, E.; Fallah, L.; Yoo, L.L.; McGrane, F.; Lagan, N.; Roche, E.; Balfe, J.; et al. Melatonin as an immunomodulator in children with Down syndrome. Pediatr. Res. 2021, 91, 1812–1820. [Google Scholar] [CrossRef]
- Hurtado-Alvarado, G.; Pavón, L.; Castillo-García, S.A.; Hernández, M.E.; Domínguez-Salazar, E.; Velázquez-Moctezuma, J.; Gómez-González, B. Sleep loss as a factor to induce inflammatory variations. J. Immunol. Res. 2013, 2013, 801341. [Google Scholar]
- Faraut, B.; Boudjeltia, K.Z.; Vanhamme, L.; Kerkhofs, M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med. Rev. 2012, 16, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Nobari, H.; Banihashemi, M.; Saedmocheshi, S.; Prieto-González, P.; Oliveira, R. Overview of the impact of sleep monitoring on optimal performance, immune system function and injury risk reduction in athletes: A narrative review. Sci. Prog. 2023, 106, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef]
- De Mello, M.T.; Silva, A.; de Carvalho Guerreiro, R.; da-Silva, F.R.; Esteves, A.M.; Poyares, D.; Piovezan, R.; Treptow, E.; Starling, M.; Rosa, D.S.; et al. Sleep and COVID-19: Considerations about immunity, pathophysiology, and treatment. Sleep Sci. 2020, 13, 199–209. [Google Scholar]
- Reiter, R.J. Melatonin: Clinical relevance. Best Pr. Res. Clin. Endocrinol. Metab. 2003, 17, 273–285. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S4–S16. [Google Scholar] [CrossRef]
- Molina-Carballo, A.; Palacios-López, R.; Jerez-Calero, A.; Augustín-Morales, M.C.; Agil, A.; Muñoz-Hoyos, A.; Muñoz-Gallego, A. Protective Effect of Melatonin Administration against SARS-CoV-2 Infection: A Systematic Review. Curr. Issues Mol. Biol. 2021, 44, 31–45. [Google Scholar] [CrossRef]
- Paredes, S.D.; Barriga, C.; Reiter, R.J.; Rodríguez, A.B. Role of tryptophan in aged sleep–wake cycle and immune function: Streptopelia risoria model. Int. J. Tryptophan Res. 2009, 2, 23–36. [Google Scholar] [CrossRef]
- Pivonello, C.; Negri, M.; Patalano, R.; Amatrudo, F.; Montò, T.; Liccardi, A.; Graziadio, C.; Muscogiuri, G.; Pivonello, R.; Colao, A. The role of melatonin in metaflammation and infections in obesity. Obes. Rev. 2022, 23, e13390. [Google Scholar] [CrossRef]
- Mozaffari, S.; Rahimi, R.; Abdollahi, M. Implications of melatonin therapy in irritable bowel syndrome: A systematic review. Curr. Pharm. Des. 2010, 16, 3646–3655. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. Sleep and sleep disturbances: Biological basis and clinical implications. Cell. Mol. Life Sci. 2007, 64, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef]
- Palma, B.D.; Tiba, P.A.; Machado, R.B.; Tufik, S.; Suchecki, D. Immune outcomes of sleep disorders: The hypothalamicpituitary-adrenal axis as a modulatory factor. Braz. J. Psychiatry 2007, 29 (Suppl. S1), s33–s38. [Google Scholar] [CrossRef] [PubMed]
- Ganz, F.D. Sleep and Immune Function. Crit. Care Nurse 2012, 32, e19–e25. [Google Scholar] [CrossRef]
- Bondy, S.C.; Campbell, A. Melatonin and Regulation of Immune Function: Impact on Numerous Diseases. Curr. Aging Sci. 2020, 13, 92–101. [Google Scholar] [CrossRef]
- Blask, D.E. Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev. 2009, 13, 257–264. [Google Scholar] [CrossRef]
- Pandiperumal, S.; Trakht, I.; Srinivasan, V.; Spence, D.; Maestroni, G.; Zisapel, N.; Cardinali, D. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Melatonin-Mediated Colonic Microbiota Metabolite Butyrate Prevents Acute Sleep Deprivation-Induced Colitis in Mice. Int. J. Mol. Sci. 2021, 22, 11894. [Google Scholar] [CrossRef]
- Al-Abri, M.A.; Al-Yaarubi, S.; Said, E.A. Circadian Rhythm, Sleep, and Immune Response and the Fight against COVID-19. Oman Med J. 2023, 38, e477. [Google Scholar] [CrossRef]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2020, 11, e01960. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflügers Arch. Eur. J. Physiol. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Innvær, K.S.; Myklatun, S.H. Sleep Deprivation and Its Consequences on Health—With Emphasis on Metabolic and Immunological Functions. Bachelor’s Thesis, Høgskulen på Vestlandet, Bergen, Norway, 2020. [Google Scholar]
- Pohanka, M. Impact of melatonin on immunity: A review. Open Med. 2013, 8, 369–376. [Google Scholar] [CrossRef]
- Bryant, P.A.; Trinder, J.; Curtis, N. Sick and tired: Does sleep have a vital role in the immune system? Nat. Rev. Immunol. 2004, 4, 457–467. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Asif, N.; Iqbal, R.; Nazir, C.F. Human immune system during sleep. Am. J. Clin. Exp. Immunol. 2017, 6, 92–96. [Google Scholar] [PubMed]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocr. 2014, 35, 76–88. [Google Scholar] [CrossRef]
- Horodincu, L.; Solcan, C. Influence of different light spectra on melatonin synthesis and immune modulation in poultry. Animals 2023, 13, 2095. [Google Scholar] [CrossRef]
| Clinical Condition/Population | Observation Regarding Melatonin | Effect on the Immune System | Type of Evidence | References |
|---|---|---|---|---|
| SARS-CoV-2 Infection | Melatonin acts as an adjuvant; reduces cytokine storm | Immunomodulatory, antioxidant, and anti-inflammatory actions; inhibition of Mpro and CD147-mediated viral entry. | Summarizes both clinical and experimental evidence | [19,29] |
| Down Syndrome (DS) | Melatonin decreases the expression of CD11b, TLR4, MyD88, and NLRP3 | Suppression of inflammatory signaling; cytokine modulation. | Experimental ex vivo study | [20] |
| ASD (Autism Spectrum Disorder) | Reduced melatonin levels in children with ASD | Increased inflammatory markers; potential anti-inflammatory effect of melatonin. | Summarizes both clinical and experimental evidence | [5] |
| Irritable Bowel Syndrome (IBS) | Disruption of endogenous melatonin levels; improvement with supplementation | Antinociceptive and anti-inflammatory effects; regulation of gastrointestinal motility. | Summarizes both clinical and experimental evidence | [32] |
| Obesity | Low melatonin levels associated with inflammation | Modulation of innate and adaptive immunity; reduced infection susceptibility. | Summarizes both clinical and experimental evidence | [31] |
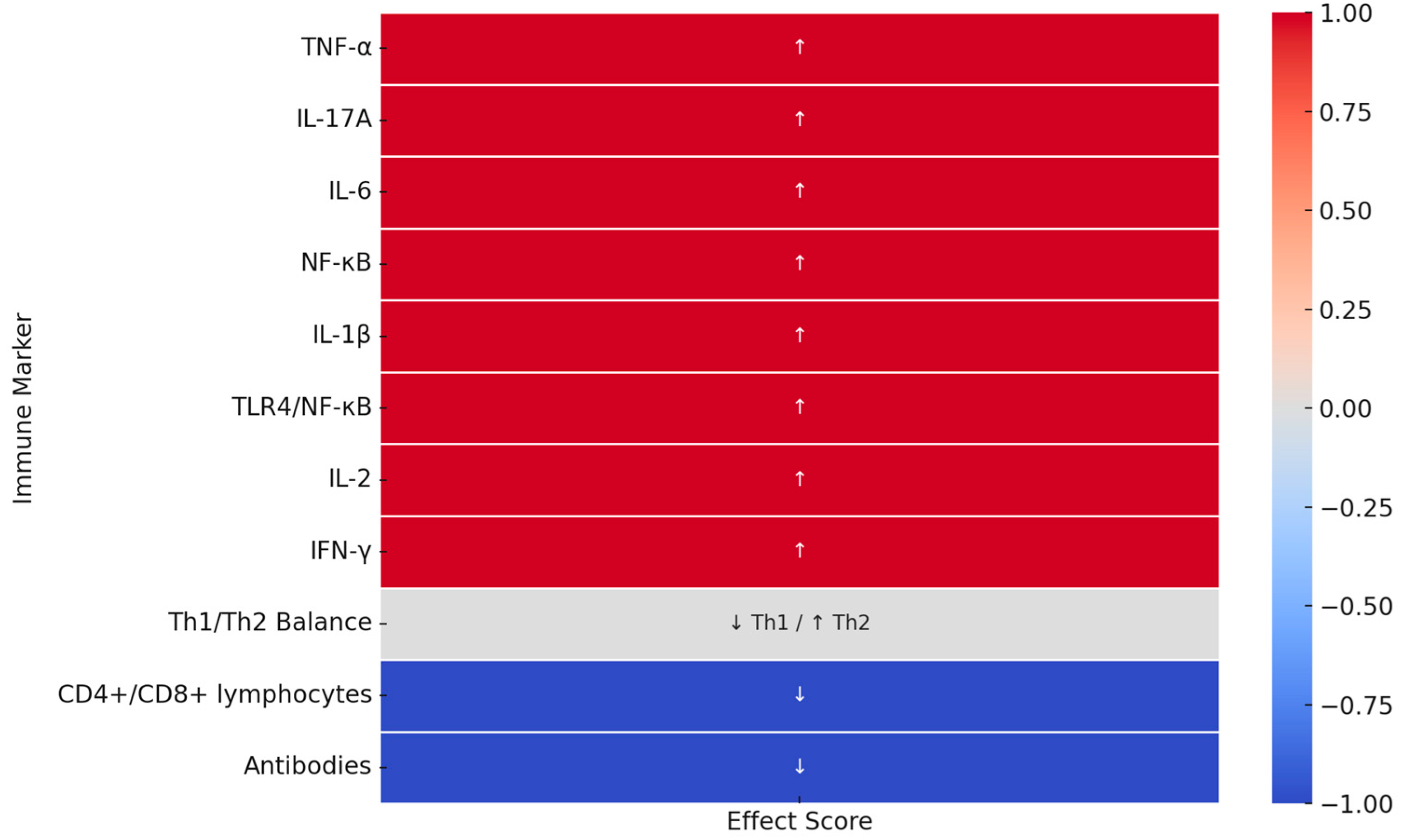
| Immunological Marker | Effect of Low Melatonin Levels/Sleep Deprivation | Type of Evidence | References |
|---|---|---|---|
| TNF-α | Elevated TNF-α expression in adipose and brain tissue, worsening inflammation. | Preclinical | [10] |
| IL-17A | Sustained increase even after REM deprivation ends (7 days post-experiment), suggesting long-term proinflammatory effects. | Preclinical | [10] |
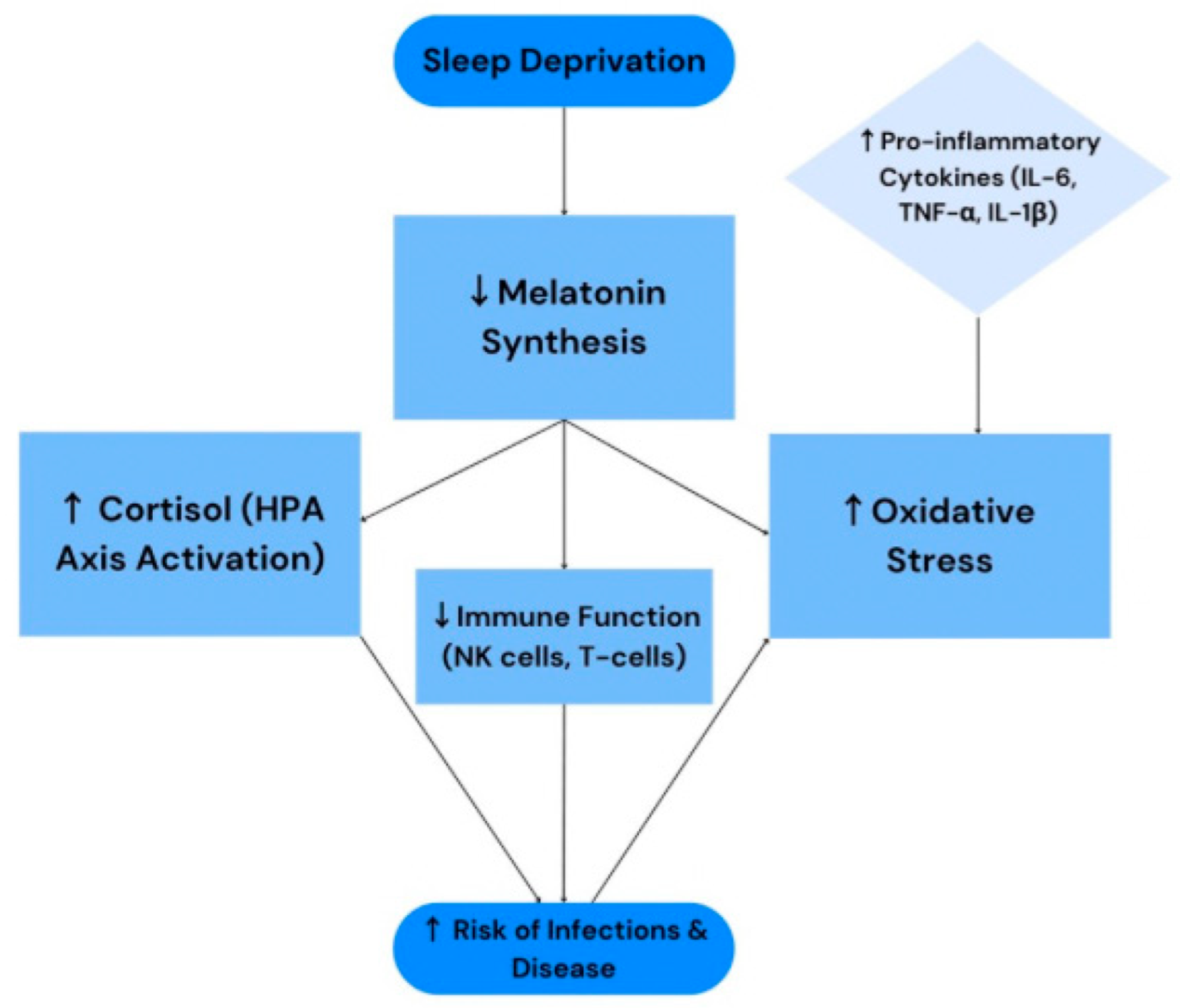
| Th1/Th2 Balance; Cortisol (HPA axis) | Sleep deprivation enhances HPA axis activity and cortisol levels, exerting immunosuppressive effects and shifting immunity from Th1 to Th2. | Clinical and Preclinical | [10,35,36] |
| CD4+ and CD8+ lymphocytes | Decrease in CD3+, CD4+, and CD8+ cell count in insomnia, secondary to HPA axis and cortisol effects. | Clinical | [10,35] |
| Antibodies (Humoral Response) | Individuals sleeping < 7 h exhibit significantly weaker vaccine responses. | Clinical and Preclinical | [10,36] |
| IL-6 | Increased IL-6 levels as a result of sleep deprivation, leading to an inflammatory state. | Preclinical | [16] |
| NF-κB | Activation of transcription factor promoting proinflammatory cytokine expression. | Preclinical | [16] |
| IL-1β | Increased expression in response to REM deprivation, persisting for several days post-deprivation. | Preclinical | [16] |
| TLR4/NF-κB activation | Activation of the signaling pathway associated with microbiota and neuroinflammation following microbiota transplantation. | Preclinical | [16] |
| IL-2 | Increased IL-2 levels post-deprivation. | Preclinical | [36] |
| IFN-γ | Increased IFN-γ levels post-deprivation. | Preclinical | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szataniak, I.; Packi, K. Melatonin as the Missing Link Between Sleep Deprivation and Immune Dysregulation: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 6731. https://doi.org/10.3390/ijms26146731
Szataniak I, Packi K. Melatonin as the Missing Link Between Sleep Deprivation and Immune Dysregulation: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(14):6731. https://doi.org/10.3390/ijms26146731
Chicago/Turabian StyleSzataniak, Ida, and Kacper Packi. 2025. "Melatonin as the Missing Link Between Sleep Deprivation and Immune Dysregulation: A Narrative Review" International Journal of Molecular Sciences 26, no. 14: 6731. https://doi.org/10.3390/ijms26146731
APA StyleSzataniak, I., & Packi, K. (2025). Melatonin as the Missing Link Between Sleep Deprivation and Immune Dysregulation: A Narrative Review. International Journal of Molecular Sciences, 26(14), 6731. https://doi.org/10.3390/ijms26146731






