A Systems Biology Approach to Memory Health: Integrating Network Pharmacology, Gut Microbiota, and Multi-Omics for Health Functional Foods
Abstract
1. Introduction
2. Biomarkers in Memory Impairment: From Clinical Diagnostics to HFF
2.1. Established Clinical Biomarkers for Memory Impairment
2.1.1. Neuroimaging Biomarkers
2.1.2. Cerebrospinal Fluid and Blood-Based Biomarkers
2.1.3. Memory Assessments and Digital Biomarkers
2.2. Emerging Biomarkers Amenable to Modulation
2.2.1. Inflammatory and Oxidative Stress Biomarkers
2.2.2. Metabolic Dysfunction Biomarkers
2.2.3. Neuroplasticity and Neurotrophic Markers
2.2.4. Gut–Brain Axis Biomarkers
2.2.5. Epigenetic and Genetic Biomarkers
2.3. Critical Evaluation of Current Biomarker Limitations and Integrative Approaches for HFF Validation
3. Systems-Based Framework for Biomarker Discovery and Functional Food Design
3.1. Network Pharmacology as the Computational Foundation
3.1.1. Strategic Role Within the Integrated Framework
3.1.2. Methodological Workflow for Memory Health Applications
- Stage 1: Compound-Target Interaction Analysis. Bioactive food components including flavonoids, anthocyanins, alkaloids, and fatty acids are screened using databases such as TCMSP, PubChem, and SwissTargetPrediction based on pharmacokinetic properties like oral bioavailability (OB) and drug-likeness (DL). It ensures identified targets are nutritionally accessible and neurologically relevant.
- Stage 2: Disease-Target Network Construction. Rather than focusing exclusively on Alzheimer’s disease, memory-specific genes linked to diverse impairments are curated from GeneCards, DISGENET, Online Mendelian Inheritance in Man (OMIM), and AlzGene, then intersected with compound targets using visualization tools like Cytoscape (version 3.9.1, 25 March 2025). The broad approach avoids single-condition bias by encompassing age-related decline, vascular cognitive impairment, stress-induced dysfunction, and post-infectious symptoms.
- Stage 3: Pathway Enrichment Analysis. Tools such as Database for Annotation, Visualization, and Integrated Discovery (DAVID), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Metascape highlight critical biological pathways amenable to dietary intervention, including PI3K-Akt, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), MAPK, BDNF/TrkB, nuclear factor erythroid 2-related factor 2 (Nrf2), and toll-like receptor signaling. The methodology aids in identifying potential biomarkers such as BDNF, IL-6, tau, and SCFAs for subsequent validation [82,83].
3.1.3. Evidence-Based Applications and Validation
3.1.4. Integration with Gut Microbiota and Multi-Omics
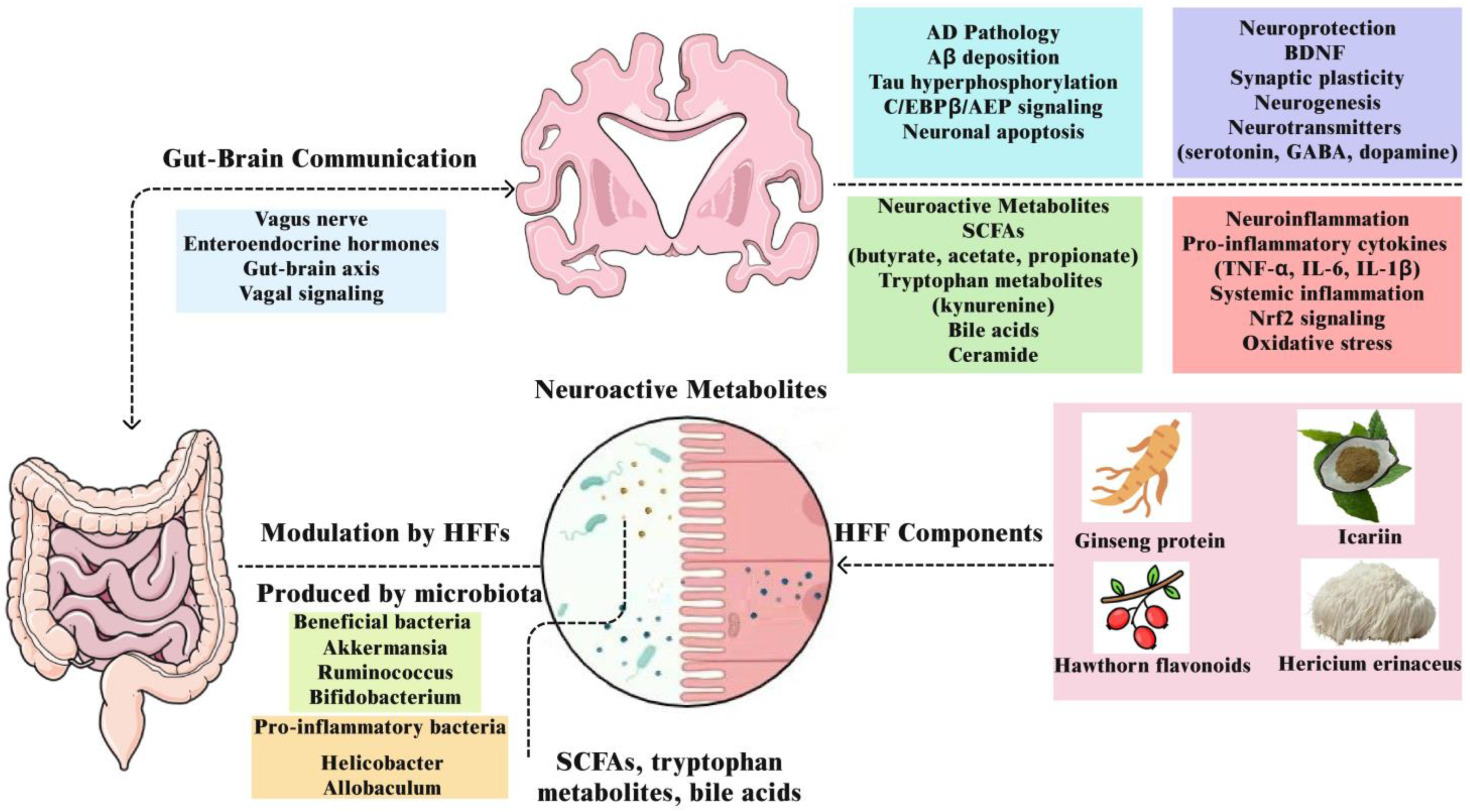
3.2. Gut Microbiota and the Gut–Brain Axis
3.2.1. Microbiome Composition and Memory-Related Signatures
3.2.2. Host–Microbiome–Genotype Interactions and Precision HFF Strategies
3.2.3. Neuroactive Microbial Metabolites: SCFAs, Bile Acids, and Tryptophan Pathways
3.2.4. Gut–Brain Communication via the Vagus Nerve and Hormonal Signals
3.2.5. Gut Barrier Integrity and Systemic Inflammation
3.2.6. Neurotransmitter Modulation by Gut Microbiota
3.2.7. Microbiota-Mediated Conversion of HFF Components
3.3. Integration of Multi-Omics for Functional Food Design to Prevent Memory Impairment
3.3.1. Transcriptomic Analyses
3.3.2. Proteomics Approaches
3.3.3. Metabolomic Profiling
3.3.4. Epigenomics and MicroRNAs
3.3.5. Genomics and Nutritional Precision
3.3.6. Cross-Validation Strategies for HFF Memory Studies
4. Key Mechanistic Pathways and Biomarkers Modulated by HFF
4.1. Neuroinflammation and Oxidative Stress: NF-κB, Nrf2, and Cytokines
4.2. Synaptic Plasticity and Neurogenesis: BDNF-TrkB, CREB, Wnt/β-Catenin
4.3. Gut–Brain Axis and Metabolite Signaling: SCFAs, Tryptophan, Bile Acids
5. Regulatory Framework and Clinical Development of HFFs for Memory Impairment
5.1. Current Regulations for Memory/Cognitive Function Claims in Major Markets
5.2. Evidence Requirements for Claims
5.3. Prevention vs. Improvement Claims
5.4. HFFs for Memory Function Available in the Market
5.5. Safety Considerations
6. Clinical Translation of HFFs
- (1)
- Formulation Design and Bioavailability Enhancement: Unlike single-compound therapeutics, HFFs contain complex mixtures of bioactive compounds (polyphenols, alkaloids, flavonoids) with varying physicochemical properties and inherently poor bioavailability. To overcome these limitations, advanced delivery systems have been developed including nano-emulsification, liposomal encapsulation, and microencapsulation technologies. The approaches significantly enhance gastrointestinal stability and enable targeted delivery of memory health compounds such as curcumin, ginsenosides, and flavonoid glycosides. Particularly promising are formulations designed for blood–brain barrier penetration, utilizing lipid-based carriers and surface modifications that facilitate central nervous system access [165]. Additionally, co-administration strategies using bioavailability enhancers (e.g., piperine, phospholipid complexation) and timing optimization relative to meals can further improve compound absorption and therapeutic efficacy.
- (2)
- Standardization and Quality Control: Natural product variability due to cultivation conditions, genotype, harvest timing, and processing methods creates significant batch consistency challenges. Effective quality control solutions require dual approaches combining HPLC fingerprinting with bioassay-based functional verification to ensure both chemical consistency and biological activity. While these rigorous methods can create industrial-scale production bottlenecks, implementing automated analytical systems and establishing standardized operating procedures with qualified suppliers can streamline quality assurance processes without compromising product integrity [166].
- (3)
- Product Stability and Shelf-Life Optimization: Cognitive health compounds are often sensitive to oxidation, hydrolysis, and photodegradation [167]. Comprehensive stability solutions include accelerated and real-time stability testing protocols to identify optimal protective excipients (antioxidants, chelating agents), packaging materials (light-protective, moisture-barrier), and storage conditions. Advanced packaging technologies such as blister packs with desiccants, nitrogen-flushed containers, and temperature-indicating labels ensure efficacy maintenance throughout shelf life—particularly crucial for products targeting aging populations with longer storage periods.
- (4)
- Patient Compliance and Adherence Solutions: Memory disorder patients face unique compliance challenges that significantly impact treatment outcomes. Memory impairment can lead to forgotten doses, confusion about dosing schedules, and difficulty distinguishing between different medications or supplements. Evidence-based formulation strategies to improve compliance include developing once-daily sustained-release formulations, creating distinctive packaging with clear labeling and integrated reminder systems, optimizing palatability through taste-masking technologies and preferred delivery formats (liquids, soft gels, chewable tablets), and incorporating digital adherence monitoring tools. Patient-centered design approaches, including smart packaging with dose tracking, mobile app reminders, and simplified administration protocols, are essential for ensuring therapeutic benefits reach the intended population. Healthcare provider education about compliance monitoring and structured family caregiver involvement protocols becomes crucial for successful long-term treatment outcomes.
- (5)
- Regulatory Compliance and Market Access: Marketing memory support products requires careful navigation between permissible structure-function claims and prohibited disease-prevention claims [168]. Successful regulatory strategies include early engagement with regulatory agencies, robust clinical documentation supporting safety and efficacy claims, and development of clear labeling that communicates benefits within approved frameworks. Digital marketing environments require particular attention to claim substantiation and appropriate targeting, especially for products serving vulnerable populations [169]. International market access demands understanding varying regulatory requirements across jurisdictions and adapting product positioning accordingly while maintaining scientific integrity.
7. Next-Generation HFF Formula Development: Testable Hypotheses and Conceptual Frameworks
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, J.H. Dementia Epidemiology Fact Sheet 2022. Ann. Rehabil. Med. 2022, 46, 53–59. [Google Scholar] [CrossRef]
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E.; et al. The worldwide costs of dementia in 2019. Alzheimer’s Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef]
- Anand, S.; Schoo, C. Mild Cognitive Impairment. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sharma, S.; Rahate, K.; Kumar, R. Novel Emerging Targets Identification in Reducing Risk of Alzheimer’s Disease. Central Nerv. Syst. Agents Med. Chem. 2024, 25, 454–474. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Kaushik, P.; Parvez, S. Memory related molecular signatures: The pivots for memory consolidation and Alzheimer’s related memory decline. Ageing Res. Rev. 2022, 76, 101577. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Yu, H.-J.; Koh, S.-H. Revolutionizing Alzheimer’s Diagnosis and Management: The Dawn of Biomarker-Based Precision Medicine. Dement. Neurocogn. Disord. 2024, 23, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Cutrona, C.; Leodori, G.; Malimpensa, L.; D’aNtonio, F.; Conte, A.; Belvisi, D. Exploring easily accessible neurophysiological biomarkers for predicting Alzheimer’s disease progression: A systematic review. Alzheimer’s Res. Ther. 2024, 16, 244. [Google Scholar] [CrossRef]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef]
- Atlante, A.; Amadoro, G.; Bobba, A.; Latina, V. Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease. Cells 2020, 9, 2347. [Google Scholar] [CrossRef]
- Chen, D. Biomarkers navigate drug development: Pharmacology, effectiveness and safety. Med. Drug Discov. 2024, 21, 100174. [Google Scholar] [CrossRef]
- Kaštelan, S.; Antunica, A.G.; Puzović, V.; Pavičić, A.D.; Čanović, S.; Kovačević, P.; Vučemilović, P.A.F.; Konjevoda, S. Non-Invasive Retinal Biomarkers for Early Diagnosis of Alzheimer’s Disease. Biomedicines 2025, 13, 283. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, M.; Stiles, W.R.; Choi, H.S. Neuroimaging Modalities in Alzheimer’s Disease: Diagnosis and Clinical Features. Int. J. Mol. Sci. 2022, 23, 6079. [Google Scholar] [CrossRef]
- Ereira, S.; Waters, S.; Razi, A.; Marshall, C.R. Early detection of dementia with default-mode network effective connectivity. Nat. Ment. Health 2024, 2, 787–800. [Google Scholar] [CrossRef]
- Maschio, C.; Ni, R. Amyloid and Tau Positron Emission Tomography Imaging in Alzheimer’s Disease and Other Tauopathies. Front. Aging Neurosci. 2022, 14, 838034. [Google Scholar] [CrossRef]
- Pawlowski, M.; Meuth, S.G.; Duning, T. Cerebrospinal Fluid Biomarkers in Alzheimer’s Disease—From Brain Starch to Bench and Bedside. Diagnostics 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Wattmo, C.; Blennow, K.; Hansson, O. Cerebro-spinal fluid biomarker levels: Phosphorylated tau (T) and total tau (N) as markers for rate of progression in Alzheimer’s disease. BMC Neurol. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Benedet, A.L.; Pascoal, T.A.; Karikari, T.K.; Lantero-Rodriguez, J.; Brum, W.S.; Mathotaarachchi, S.; Therriault, J.; Savard, M.; Chamoun, M.; et al. Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer’s disease. EBioMedicine 2022, 76, 103836. [Google Scholar] [CrossRef]
- Varesi, A.; Carrara, A.; Pires, V.G.; Floris, V.; Pierella, E.; Savioli, G.; Prasad, S.; Esposito, C.; Ricevuti, G.; Chirumbolo, S.; et al. Blood-Based Biomarkers for Alzheimer’s Disease Diagnosis and Progression: An Overview. Cells 2022, 11, 1367. [Google Scholar] [CrossRef]
- Schraen-Maschke, S.; Duhamel, A.; Vidal, J.S.; Ramdane, N.; Vaudran, L.; Dussart, C.; Buée, L.; Sablonnière, B.; Delaby, C.; Allinquant, B.; et al. The free plasma amyloid Aβ1–42/Aβ1–40 ratio predicts conversion to dementia for subjects with mild cognitive impairment with performance equivalent to that of the total plasma Aβ1–42/Aβ1–40 ratio. The BALTAZAR study. Neurobiol. Dis. 2024, 193, 106459. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, F.; Kac, P.R.; Brum, W.S.; Zetterberg, H.; Blennow, K.; Karikari, T.K. Plasma phospho-tau in Alzheimer’s disease: Towards diagnostic and therapeutic trial applications. Mol. Neurodegener. 2023, 18, 18. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. The duality of amyloid-β: Its role in normal and Alzheimer’s disease states. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef]
- Mekhora, C.; Lamport, D.J.; Spencer, J.P.E. An overview of the relationship between inflammation and cognitive function in humans, molecular pathways and the impact of nutraceuticals. Neurochem. Int. 2024, 181, 105900. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Estrella, A.; Hakim, O.; Milazzo, P.; Patel, S.; Pintagro, C.; Li, D.; Zhao, R.; Vance, D.E.; Li, W.; et al. Mini-Mental State Examination and Montreal Cognitive Assessment as Tools for Following Cognitive Changes in Alzheimer’s Disease Neuroimaging Initiative Participants. J. Alzheimer’s Dis. 2022, 90, 263–270. [Google Scholar] [CrossRef]
- Kueper, J.K.; Speechley, M.; Montero-Odasso, M. The Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): Modifications and Responsiveness in Pre-Dementia Populations. A Narrative Review. J. Alzheimer’s Dis. 2018, 63, 423–444. [Google Scholar] [CrossRef] [PubMed]
- DuBord, A.Y.; Paolillo, E.W.; Staffaroni, A.M. Remote Digital Technologies for the Early Detection and Monitoring of Cognitive Decline in Patients With Type 2 Diabetes: Insights From Studies of Neurodegenerative Diseases. J. Diabetes Sci. Technol. 2024, 18, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef]
- Kara, S.P.; Altunan, B.; Unal, A. Investigation of the peripheral inflammation (neutrophil–lymphocyte ratio) in two neurodegenerative diseases of the central nervous system. Neurol. Sci. 2022, 43, 1799–1807. [Google Scholar] [CrossRef]
- Uto, T.; Suangkaew, N.; Morinaga, O.; Kariyazono, H.; Oiso, S.; Shoyama, Y. Eriobotryae folium extract suppresses LPS-Induced iNOS and COX-2 expression by inhibition of NF-κB and MAPK activation in murine macrophages. Am. J. Chin. Med. 2010, 38, 985–994. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Jian, T.-Y.; Lv, H.; Ding, X.-Q.; Zuo, Y.-Y.; Ren, B.-R.; Chen, J.; Li, W.-L. Antitussive and expectorant properties of growing and fallen leaves of loquat (Eriobotrya japonica). Braz. J. Pharmacogn. 2018, 28, 239–242. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.R.; Kim, M.; Park, J.M.; Kang, M.; Oh, J.; Lee, C.J.; Park, S.; Kang, S.-M.; Manabe, I.; et al. Common and differential effects of docosahexaenoic acid and eicosapentaenoic acid on helper T-cell responses and associated pathways. BMB Rep. 2021, 54, 278. [Google Scholar] [CrossRef]
- Lamantia, V.; Bissonnette, S.; Beaudry, M.; Cyr, Y.; Rosiers, C.D.; Baass, A.; Faraj, M. EPA and DHA inhibit LDL-induced upregulation of human adipose tissue NLRP3 inflammasome/IL-1β pathway and its association with diabetes risk factors. Sci. Rep. 2024, 14, 27146. [Google Scholar] [CrossRef]
- Luo, Q.; Luo, L.; Zhao, J.; Wang, Y.; Luo, H. Biological potential and mechanisms of Tea’s bioactive compounds: An Updated review. J. Adv. Res. 2024, 65, 345–363. [Google Scholar] [CrossRef]
- Nguyen, V.T.T.; Slotos, R.S.; Guilherme, M.D.S.; Nguyen, T.T.; Weisenburger, S.; Lehner, M.D.; Endres, K. Ginkgo biloba extract EGb 761® ameliorates cognitive impairment and alleviates TNFα response in 5xFAD Alzheimer‘s disease model mice. Phytomedicine 2025, 136, 156327. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, K.J.; Chei, S.; Seo, Y.J.; Lee, K.; Lee, B.Y. Korean Red Ginseng and Korean black ginseng extracts, JP5 and BG1, prevent hepatic oxidative stress and inflammation induced by environmental heat stress. J. Ginseng Res. 2020, 44, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Adam, R.H.I.; Bansal, R.; Broersen, K. A Review of Oxidative Stress Products and Related Genes in Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 83, 977–1001. [Google Scholar] [CrossRef]
- Nantachai, G.; Vasupanrajit, A.; Tunvirachaisakul, C.; Solmi, M.; Maes, M. Oxidative stress and antioxidant defenses in mild cognitive impairment: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 79, 101639. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Cho, M.L.; Kim, D.B.; Shin, G.H.; Lee, J.H.; Lee, J.S.; Shin, H.M.; Lee, O.-H. The antioxidant activity and their major antioxidant compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281–13295. [Google Scholar] [CrossRef]
- Bailly, C. The traditional Chinese medicine WuJiaPi (Acanthopanacis cortex) and its main anti-inflammatory terpenoids. Longhua Chin. Med. 2021, 4, 14–25. [Google Scholar] [CrossRef]
- Ji, X.; Zou, W.; Fan, L.; Zhou, Z.; Zhu, X.; Li, X. Insulin resistance-related features are associated with cognitive decline: A cross-sectional study in adult patients with type 1 diabetes. Diabetol. Metab. Syndr. 2024, 16, 13. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S.; Moon, N.R. β-Amyloid-induced cognitive dysfunction impairs glucose homeostasis by increasing insulin resistance and decreasing β-cell mass in non-diabetic and diabetic rats. Metabolism 2013, 62, 1749–1760. [Google Scholar] [CrossRef]
- Ronchetti, S.; Labombarda, F.; Del Core, J.; Roig, P.; De Nicola, A.F.; Pietranera, L. The phytoestrogen genistein improves hippocampal neurogenesis and cognitive impairment and decreases neuroinflammation in an animal model of metabolic syndrome. J. Neuroendocr. 2025, 37, e13480. [Google Scholar] [CrossRef]
- Kim, D.S.; Kang, S.; Moon, N.R.; Shin, B.K.; Park, S. Zeaxanthin and Lutein Ameliorate Alzheimer’s Disease-like Pathology: Modulation of Insulin Resistance, Neuroinflammation, and Acetylcholinesterase Activity in an Amyloid-β Rat Model. Int. J. Mol. Sci. 2024, 25, 9828. [Google Scholar] [CrossRef]
- Park, S.; Moon, N.R.; Kang, S.; Kim, D.S. Ferulic acid and vinpocetine intake improves memory function by enhancing insulin sensitivity and reducing neuroinflammation and oxidative stress in type 2 diabetic animals with induced Alzheimer’s disease. J. Funct. Foods 2022, 95, 105180. [Google Scholar] [CrossRef]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. BioFactors 2021, 47, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Hu, M.; Huang, J.; Yu, S.; Zeng, H.; Mao, L. EPA and DHA differentially improve insulin resistance by reducing adipose tissue inflammation—Targeting GPR120/PPARγ pathway. J. Nutr. Biochem. 2024, 130, 109648. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. The Role of Brain-Derived Neurotrophic Factor as an Essential Mediator in Neuronal Functions and the Therapeutic Potential of Its Mimetics for Neuroprotection in Neurologic and Psychiatric Disorders. Molecules 2025, 30, 848. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S.; Kim, H.J. The combination of luteolin and l-theanine improved Alzheimer disease—Like symptoms by potentiating hippocampal insulin signaling and decreasing neuroinflammation and norepinephrine degradation in amyloid-β-infused rats. Nutr. Res. 2018, 60, 116–131. [Google Scholar] [CrossRef]
- Zhao, W.N.; Hylton, N.K.; Wang, J.; Chindavong, P.S.; Alural, B.; Kurtser, I.; Subramanian, A.; Mazitschek, R.; Perlis, R.H.; Haggarty, S.J. Activation of WNT and CREB signaling pathways in human neuronal cells in response to the Omega-3 fatty acid docosahexaenoic acid (DHA). Mol. Cell. Neurosci. 2019, 99, 103386. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Wang, L.; Wang, F.; Zhang, J. Resveratrol improved mitochondrial biogenesis by activating SIRT1/PGC-1α signal pathway in SAP. Sci. Rep. 2024, 14, 26216. [Google Scholar] [CrossRef]
- Davinelli, S.; Medoro, A.; Ali, S.; Passarella, D.; Intrieri, M.; Scapagnini, G. Dietary Flavonoids and Adult Neurogenesis: Potential Implications for Brain Aging. Curr. Neuropharmacol. 2023, 21, 651–668. [Google Scholar] [CrossRef]
- Bae, H.J.; Kim, J.; Jeon, S.J.; Kim, J.; Goo, N.; Jeong, Y.; Cho, K.; Cai, M.; Jung, S.Y.; Kwon, K.J.; et al. Green tea extract containing enhanced levels of epimerized catechins attenuates scopolamine-induced memory impairment in mice. J. Ethnopharmacol. 2020, 258, 112923. [Google Scholar] [CrossRef]
- Han, X.; Zhang, M.; Liu, Y.; Huang, Y.; Yang, X.; Wang, R.; Sun, W.-Y.; So, K.-F.; Chiu, K.; He, R.-R.; et al. Lycium barbarum extract improves brain and visual functions in mice models of Alzheimer’s disease through activating WNT pathway. Phytomedicine 2025, 139, 156523. [Google Scholar] [CrossRef] [PubMed]
- Kearns, R. Gut–Brain Axis and Neuroinflammation: The Role of Gut Permeability and the Kynurenine Pathway in Neurological Disorders. Cell. Mol. Neurobiol. 2024, 44, 64. [Google Scholar] [CrossRef]
- Park, S.; Bae, J.H. Probiotics for weight loss: A systematic review and meta-analysis. Nutr. Res. 2015, 35, 566–575. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Ryu, M.S.; Yang, H.J.; Park, S. γ-PGA-Rich Chungkookjang, Short-Term Fermented Soybeans: Prevents Memory Impairment by Modulating Brain Insulin Sensitivity, Neuro-Inflammation, and the Gut-Microbiome-Brain Axis. Foods 2021, 10, 221. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Probiotics for Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Foods 2021, 10, 1672. [Google Scholar] [CrossRef]
- Mahboobi, S.; Ghasvarian, M.; Ghaem, H.; Alipour, H.; Alipour, S.; Eftekhari, M.H. Effects of probiotic and magnesium co-supplementation on mood, cognition, intestinal barrier function and inflammation in individuals with obesity and depressed mood: A randomized, double-blind placebo-controlled clinical trial. Front. Nutr. 2022, 9, 1018357. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Carbia, C.; Cryan, J.F. Going with 1672grain: Fiber, cognition, and the microbiota-gut-brain-axis. Exp. Biol. Med. 2021, 246, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Leyrolle, Q.; Cserjesi, R.; Mulders, D.G.H.M.; Zamariola, G.; Hiel, S.; Gianfrancesco, M.A.; Portheault, D.; Amadieu, C.; Bindels, L.B.; Leclercq, S.; et al. Prebiotic effect on mood in obese patients is determined by the initial gut microbiota composition: A randomized, controlled trial. Brain Behav. Immun. Integr. 2021, 94, 289–298. [Google Scholar] [CrossRef]
- Kanimozhi, N.V.; Muthusamy, S.; Sukumar, M. Aging through the lens of the gut microbiome: Challenges and therapeutic opportunities. Arch. Gerontol. Geriatr. Plus 2025, 2, 100142. [Google Scholar]
- Boehme, M.; Guzzetta, K.E.; Wasén, C.; Cox, L.M. The gut microbiota is an emerging target for improving brain health during ageing. Gut Microbiome 2023, 4, e2. [Google Scholar] [CrossRef]
- Xiao, J.; Katsumata, N.; Bernier, F.; Ohno, K.; Yamauchi, Y.; Odamaki, T.; Yoshikawa, K.; Ito, K.; Kaneko, T. Probiotic Bifidobacterium breve in Improving Cognitive Functions of Older Adults with Suspected Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimer’s Dis. 2020, 77, 139–147. [Google Scholar] [CrossRef]
- Signal, B.; Pérez Suárez, T.G.; Taberlay, P.C.; Woodhouse, A. Cellular specificity is key to deciphering epigenetic changes underlying Alzheimer’s disease. Neurobiol. Dis. 2023, 186, 106284. [Google Scholar] [CrossRef] [PubMed]
- Borsoi, F.T.; Neri-Numa, I.A.; de Oliveira, W.Q.; de Araújo, F.F.; Pastore, G.M. Dietary polyphenols and their relationship to the modulation of non-communicable chronic diseases and epigenetic mechanisms: A mini-review. Food Chem. 2023, 6, 100155. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.; Kaliszewska, A.; Uceda, S.; Reiriz, M.; Arias, N. Targeting DNA Methylation in the Adult Brain through Diet. Nutrients 2021, 13, 3979. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Falla, D.; Vélez, J.I.; Acosta-Baena, N.; Baena, A.; Moreno, S.; Krasemann, S.; Lopera, F.; Mastronardi, C.A.; Arcos-Burgos, M. Genetic modifiers of cognitive decline in PSEN1 E280A Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 2873–2885. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, E.; Lane, H.Y. Genetic Biomarkers on Age-Related Cognitive Decline. Front. Psychiatry 2017, 8, 247. [Google Scholar] [CrossRef]
- Giuliani, A.; Gaetani, S.; Sorgentoni, G.; Agarbati, S.; Laggetta, M.; Matacchione, G.; Gobbi, M.; Rossi, T.; Galeazzi, R.; Piccinini, G.; et al. Circulating Inflamma-miRs as Potential Biomarkers of Cognitive Impairment in Patients Affected by Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 647015. [Google Scholar] [CrossRef]
- Kumari, A.; Rahaman, A.; Zeng, X.-A.; Baloch, Z. Therapeutic potential and microRNA regulating properties of phytochemicals in Alzheimer’s disease. Mol. Ther. Nucleic Acids 2025, 36, 102439. [Google Scholar] [CrossRef]
- An, Y.; Feng, L.; Zhang, X.; Wang, Y.; Wang, Y.; Tao, L.; Qin, Z.; Xiao, R. Dietary intakes and biomarker patterns of folate, vitamin B(6), and vitamin B(12) can be associated with cognitive impairment by hypermethylation of redox-related genes NUDT15 and TXNRD1. Clin. Epigenetics 2019, 11, 139. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Michaelson, D.M.; Hartmann, T. Omega-3 fatty acids, lipids, and apoE lipidation in Alzheimer’s disease: A rationale for multi-nutrient dementia prevention. J. Lipid Res. 2017, 58, 2083–2101. [Google Scholar] [CrossRef]
- Yassine, H.N. Personalized Nutrition for APOE4 carriers: Mechanisms and biomarkers that can inform future trials. Alzheimer’s Dement. 2023, 19, e077701. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Ramos-Lopez, O.; Pérusse, L.; Kato, H.; Ordovas, J.M.; Martínez, J.A. Precision nutrition: A review of current approaches and future endeavors. Trends Food Sci. Technol. 2022, 128, 253–264. [Google Scholar] [CrossRef]
- Tingö, L.; Bergh, C.; Rode, J.; Rubio, M.F.R.; Persson, J.; Johnson, L.B.; Smit, L.H.; Hutchinson, A.N. The Effect of Whole-Diet Interventions on Memory and Cognitive Function in Healthy Older Adults—A Systematic Review. Adv. Nutr. Int. Rev. J. 2024, 15, 100291. [Google Scholar] [CrossRef] [PubMed]
- Rustichelli, S.; Lanni, C.; Zarà, M.; Guidetti, G.F.; Torti, M.; Canobbio, I. Curcumin Modulates Platelet Activation and ROS Production Induced by Amyloid Peptides: New Perspectives in Attenuating Prothrombotic Risk in Alzheimer’s Disease Patients. Nutrients 2024, 16, 4419. [Google Scholar] [CrossRef] [PubMed]
- Rouch, L.; Giudici, K.V.; Cantet, C.; Guyonnet, S.; Delrieu, J.; Legrand, P.; Catheline, D.; Andrieu, S.; Weiner, M.; Barreto, P.D.S.; et al. Associations of erythrocyte omega-3 fatty acids with cognition, brain imaging and biomarkers in the Alzheimer’s disease neuroimaging initiative: Cross-sectional and longitudinal retrospective analyses. Am. J. Clin. Nutr. 2022, 116, 1492–1506. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Q.; Zhang, Z.; Yu, Z.; Wang, X.; Lu, Q. Exploring AI in metasurface structures with forward and inverse design. iScience 2025, 28, 111995. [Google Scholar] [CrossRef]
- Zhou, J.; Kim, Y.K.; Li, C.; Park, S. Natural compounds for Alzheimer’s prevention and treatment: Integrating SELFormer-based computational screening with experimental validation. Comput. Biol. Med. 2025, 185, 109523. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Yang, H.-J.; Zhang, T.; Kim, M.-J.; Hur, H.-J.; Wu, X.; Jang, D.-J.; Park, S. Efficacy and Mechanism of Schisandra chinensis Fructus Water Extract in Alzheimer’s Disease: Insights from Network Pharmacology and Validation in an Amyloid-β Infused Animal Model. Nutrients 2024, 16, 3751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, C.; Yue, Y.; Yang, H.-J.; Ryu, M.S.; Wu, X.; Jeong, D.Y.; Park, S. Fermented red pepper paste (kochujang) modulates glucose metabolism and gut microbiota in parasympathetic suppression: Network pharmacology and In vivo study. Food Biosci. 2024, 61, 104531. [Google Scholar] [CrossRef]
- Ye, X.-w.; Wang, H.-l.; Cheng, S.-q.; Xia, L.-j.; Xu, X.-f.; Li, X.-r. Network pharmacology-based strategy to investigate the pharmacologic mechanisms of coptidis rhizoma for the treatment of alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 890046. [Google Scholar] [CrossRef]
- Zeng, P.; Wang, X.-M.; Ye, C.-Y.; Su, H.-F.; Tian, Q. The main alkaloids in Uncaria rhynchophylla and their anti-Alzheimer’s disease mechanism determined by a network pharmacology approach. Int. J. Mol. Sci. 2021, 22, 3612. [Google Scholar] [CrossRef] [PubMed]
- Martiz, R.M.; Patil, S.M.; Abdulaziz, M.; Babalghith, A.; Al-Areefi, M.; Al-Ghorbani, M.; Kumar, J.M.; Prasad, A.; Nagalingaswamy, N.P.M.; Ramu, R. Defining the role of isoeugenol from Ocimum tenuiflorum against diabetes mellitus-linked Alzheimer’s disease through network pharmacology and computational methods. Molecules 2022, 27, 2398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef]
- Yang, D.; Li, W.; Chen, Q.; Liu, S.; Peng, C.; Deng, F.; Meng, Y.; Yang, Y.; Yan, P.; Ao, H.; et al. Gut-Brain Axis-Based Polygala Tenuifolia and Magnolia Officinalis Improve D-gal-Induced Cognitive Impairment in Mice Through cAMP and NF-κB Signaling Pathways. Drug Des. Dev. Ther. 2025, 19, 1869–1894. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, Z.; Liao, W.; Zhang, R.; Chen, G.; Ma, L.; Yu, H. The Medicinal Species of the Lycium Genus (Goji Berries) in East Asia: A Review of Its Effect on Cell Signal Transduction Pathways. Plants 2024, 13, 1531. [Google Scholar] [CrossRef]
- Ryuk, J.A.; Ko, B.S.; Moon, N.R.; Park, S. Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis. Antioxidants 2022, 11, 168. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Sun, Z.; Chen, M.; Han, Y.; Li, Y.; Dong, X.; Ding, S.; Fang, Z.; Li, W.; et al. Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J. Ginseng Res. 2021, 45, 665–675. [Google Scholar] [CrossRef]
- Guan, Y.; Shi, D.; Wang, S.; Sun, Y.; Song, W.; Liu, S.; Wang, C. Hericium coralloides Ameliorates Alzheimer’s Disease Pathologies and Cognitive Disorders by Activating Nrf2 Signaling and Regulating Gut Microbiota. Nutrients 2023, 15, 3799. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Bacigalupe, R.; Wang, J.; Rühlemann, M.C.; Tito, R.Y.; Falony, G.; Joossens, M.; Vieira-Silva, S.; Henckaerts, L.; Rymenans, L.; et al. Genome-wide associations of human gut microbiome variation and implications for causal inference analyses. Nat. Microbiol. 2020, 5, 1079–1087. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Pepke, M.L.; Hansen, S.B.; Limborg, M.T. Unraveling host regulation of gut microbiota through the epigenome–microbiome axis. Trends Microbiol. 2024, 32, 1229–1240. [Google Scholar] [CrossRef]
- Kim, D.S.; Ting, Z.; Park, S. Protective effects of Forsythiae fructus and Cassiae semen water extract against memory deficits through the gut-microbiome-brain axis in an Alzheimer’s disease model. Pharm. Biol. 2022, 60, 212–224. [Google Scholar] [CrossRef]
- Zhang, T.; Kim, M.J.; Kim, M.J.; Wu, X.; Yang, H.J.; Yuan, H.; Huang, S.; Yoon, S.M.; Kim, K.-N.; Park, S. Long-Term Effect of Porcine Brain Enzyme Hydrolysate Intake on Scopolamine-Induced Memory Impairment in Rats. Int. J. Mol. Sci. 2022, 23, 3361. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. Role of Microbiota-Modified Bile Acids in the Regulation of Intracellular Organelles and Neurodegenerative Diseases. Genes 2023, 14, 825. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Yue, Y.; Wu, X. Effects of Bile Acid Modulation by Dietary Fat, Cholecystectomy, and Bile Acid Sequestrant on Energy, Glucose, and Lipid Metabolism and Gut Microbiota in Mice. Int. J. Mol. Sci. 2022, 23, 5935. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Huang, W.; Selwyn, F.P.; Klaassen, C.D. Dose-response effect of berberine on bile acid profile and gut microbiota in mice. BMC Complement. Altern. Med. 2016, 16, 394. [Google Scholar] [CrossRef]
- Lin, L.; Li, C.; Zhang, D.; Yuan, M.; Chen, C.H.; Li, M. Synergic Effects of Berberine and Curcumin on Improving Cognitive Function in an Alzheimer’s Disease Mouse Model. Neurochem. Res. 2020, 45, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yue, Y.; Li, C.; Wu, X.; Park, S. Vagus Nerve Suppression in Ischemic Stroke by Carotid Artery Occlusion: Implications for Metabolic Regulation, Cognitive Function, and Gut Microbiome in a Gerbil Model. Int. J. Mol. Sci. 2024, 25, 7831. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Y.; Wang, H.; Alam, A.; Kang, S.S.; Ahn, E.H.; Liu, X.; Jia, J.; Ye, K. Gut inflammation triggers C/EBPβ/δ-secretase-dependent gut-to-brain propagation of Aβ and Tau fibrils in Alzheimer’s disease. EMBO J. 2021, 40, e106320. [Google Scholar] [CrossRef]
- Murphy, A.J.; O’NEal, A.G.; Cohen, R.A.; Lamb, D.G.; Porges, E.C.; Bottari, S.A.; Ho, B.; Trifilio, E.; DeKosky, S.T.; Heilman, K.M.; et al. The Effects of Transcutaneous Vagus Nerve Stimulation on Functional Connectivity Within Semantic and Hippocampal Networks in Mild Cognitive Impairment. Neurotherapeutics 2022, 20, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, X.; Huang, S.; Feng, X.; Zhang, X.; Xiang, D. A monomeric polysaccharide from Polygonatum sibiricum improves cognitive functions in a model of Alzheimer’s disease by reshaping the gut microbiota. Int. J. Biol. Macromol. 2022, 213, 404–415. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Liu, M.; Zhang, Z.; Ji, Y.; Xu, L.; Liu, Y. Polysaccharide from Polygala tenuifolia alleviates cognitive decline in Alzheimer’s disease mice by alleviating Aβ damage and targeting the ERK pathway. J. Ethnopharmacol. 2024, 321, 117564. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.J.; Cha, M.G.; Kwon, G.H.; Han, S.H.; Yoon, S.J.; Lee, S.K.; Ahn, M.E.; Won, S.-M.; Ahn, E.H.; Suk, K.T. Akkermansia muciniphila improve cognitive dysfunction by regulating BDNF and serotonin pathway in gut-liver-brain axis. Microbiome 2024, 12, 181. [Google Scholar] [CrossRef]
- Tian, P.; Zou, R.; Wang, L.; Chen, Y.; Qian, X.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Wang, G.; et al. Multi-Probiotics ameliorate Major depressive disorder and accompanying gastrointestinal syndromes via serotonergic system regulation. J. Adv. Res. 2022, 45, 117–125. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Ran, Z.; Ju, B.; Cao, L.; Hou, Q.; Wen, L.; Geng, R.; Liao, Y.; Hu, J.; Yang, J. Microbiome–metabolomics analysis reveals the potential effect of verbascoside in alleviating cognitive impairment in db/db mice. Food Funct. 2023, 14, 3488–3508. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.M.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.; Kim, M.J.; Yang, H.J.; Kang, S.; Park, S. Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybeans. Arch. Microbiol. 2019, 201, 1009–1017. [Google Scholar] [CrossRef]
- Lin, M.; Guo, J.; Gu, Z.; Tang, W.; Tao, H.; You, S.; Jia, D.; Sun, Y.; Jia, P. Machine learning and multi-omics integration: Advancing cardiovascular translational research and clinical practice. J. Transl. Med. 2025, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, S.; Dong, X. Plant-derived bioactive compounds and their novel role in central nervous system disorder treatment via ATF4 targeting: A systematic literature review. Biomed. Pharmacother. 2024, 176, 116811. [Google Scholar] [CrossRef]
- Hussein, R.M.; Youssef, A.M.; Magharbeh, M.K.; Al-Dalaen, S.M.; Al-Jawabri, N.A.; Al-Nawaiseh, T.N.; Al-Jwanieh, A.; Al-Ani, F.S. Protective effect of Portulaca oleracea extract against lipopolysaccharide-induced neuroinflammation, memory decline, and oxidative stress in mice: Potential role of miR-146a and miR-let 7. J. Med. Food 2022, 25, 807–817. [Google Scholar] [CrossRef]
- Yuan, F.-Y.; Ju, C.; Zang, C.-X.; Liu, H.; Shang, M.-Y.; Ning, J.-W.; Yang, Y.; Ma, J.-W.; Li, G.; Yao, X.-S.; et al. Gardenia jasminoides extract GJ-4 alleviates memory deficiency of vascular dementia in rats through PERK-mediated endoplasmic reticulum stress pathway. Am. J. Chin. Med. 2023, 51, 53–72. [Google Scholar] [CrossRef]
- Maugeri, A.; Russo, C.; Patanè, G.T.; Barreca, D.; Mandalari, G.; Navarra, M. The inhibition of mitogen-activated protein kinases (MAPKs) and NF-κB underlies the neuroprotective capacity of a cinnamon/curcumin/turmeric spice blend in Aβ-exposed THP-1 cells. Molecules 2023, 28, 7949. [Google Scholar] [CrossRef]
- Zhao, Z.; Yuan, Y.; Li, S.; Wang, X.; Yang, X. Natural compounds from herbs and nutraceuticals as glycogen synthase kinase-3β inhibitors in Alzheimer’s disease treatment. CNS Neurosci. Ther. 2024, 30, e14885. [Google Scholar] [CrossRef]
- Li, D.-D.; Zheng, C.-Q.; Zhang, F.; Shi, J.-S. Potential neuroprotection by Dendrobium nobile Lindl alkaloid in Alzheimer’s disease models. Neural Regen. Res. 2022, 17, 972–977. [Google Scholar]
- Janik, R.; Thomason, L.A.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 2016, 125, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Lejri, I.; Grimm, A.; Eckert, A. Ginkgo biloba extract increases neurite outgrowth and activates the Akt/mTOR pathway. PLoS ONE 2019, 14, e0225761. [Google Scholar] [CrossRef]
- Cao, G.; Su, P.; Zhang, S.; Guo, L.; Zhang, H.; Liang, Y.; Qin, C.; Zhang, W. Ginsenoside Re reduces Aβ production by activating PPARγ to inhibit BACE1 in N2a/APP695 cells. Eur. J. Pharmacol. 2016, 793, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zeng, M.; Hao, F.; Hao, Z.; Liang, X.; Zhang, Z.; Wu, Y.; Zhang, Y.; Wang, R.; Feng, W.; et al. Cornus officinalis Sieb. Et Zucc. attenuates Aβ25–35-induced mitochondrial damage and neuroinflammation in mice by modulating the ERK pathway. Phytomedicine 2024, 129, 155709. [Google Scholar] [CrossRef]
- Xia, B.; Huang, X.; Sun, G.; Tao, W. Iridoids from Gardeniae fructus ameliorates depression by enhancing synaptic plasticity via AMPA receptor-mTOR signaling. J. Ethnopharmacol. 2021, 268, 113665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xie, M.; Wei, D.; Wang, L.; Hu, M.; Zhang, Q.; He, Z.; Peng, W.; Wu, C. Hydroxy-α-sanshool isolated from Zanthoxylum bungeanum attenuates learning and memory impairments in scopolamine-treated mice. Food Funct. 2019, 10, 7315–7324. [Google Scholar] [CrossRef] [PubMed]
- Sung, N.S.; Uhm, S.H.; Kang, H.B.; Lee, N.S.; Jeong, Y.-G.; Kim, D.K.; Kim, D.-S.; Yoo, Y.C.; Han, S.Y. Rubus fruticosus leaf extract inhibits vascular dementia-induced memory impairment and neuronal loss by attenuating neuroinflammation. Anat. Cell Biol. 2023, 56, 494–507. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, F.; Xing, Z.; Chen, J.; Peng, C.; Li, D. Beneficial effects of natural flavonoids on neuroinflammation. Front. Immunol. 2022, 13, 1006434. [Google Scholar] [CrossRef]
- Jager, P.L.D.; Srivastava, G.; Lunnon, K.; Burgess, J.; Schalkwyk, L.C.; Yu, L.; Eaton, M.L.; Keenan, B.T.; Ernst, J.; McCabe, C.; et al. Alzheimer’s disease: Early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat. Neurosci. 2014, 17, 1156–1163. [Google Scholar] [CrossRef]
- Lukiw, W.J. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport 2007, 18, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Kuiperij, H.B.; Claassen, J.A.; Küsters, B.; Verbeek, M.M. MicroRNAs in Alzheimer’s disease: Differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol. Aging 2014, 35, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Van Giau, V.; An, S.S. Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer’s disease. J. Neurol. Sci. 2016, 360, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, J.; Huang, L.; Chen, X.; Li, D.; Wang, T.; Hu, C.; Xu, J.; Zhang, C.; Zen, K.; et al. Serum MicroRNA Profiles Serve as Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Dis. Markers 2015, 2015, 625659. [Google Scholar] [CrossRef]
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 2006, 63, 168–174. [Google Scholar] [CrossRef]
- Tanzi, R.E. The genetics of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006296. [Google Scholar] [CrossRef]
- Miyashita, A.; Kikuchi, M.; Hara, N.; Ikeuchi, T. Genetics of Alzheimer’s disease: An East Asian perspective. J. Hum. Genet. 2023, 68, 115–124. [Google Scholar] [CrossRef]
- Park, S.; Wu, X. Modulation of the Gut Microbiota in Memory Impairment and Alzheimer’s Disease via the Inhibition of the Parasympathetic Nervous System. Int. J. Mol. Sci. 2022, 23, 13574. [Google Scholar] [CrossRef]
- Petre, M.L.; Tsichla, H.; Kontouli-Pertesi, A.N.; Mpoulioglou, O.E.; Kouvela, M.; Vamvakaris, I.N.; Gkiozos, I.; Syrigos, K.N.; Anagnostopoulos, A.K. Precision nutrition: Is tailor-made dietary intervention a reality yet? (Review). Biomed. Rep. 2025, 22, 86. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bucci, L.R. Effects of Silk Fibroin Enzyme Hydrolysates on Memory and Learning: A Review. Molecules 2022, 27, 5407. [Google Scholar] [CrossRef]
- Newton, A.C.; Keranen, L.M. Phosphatidyl-L-serine is necessary for protein kinase C’s high-affinity interaction with diacylglycerol-containing membranes. Biochemistry 1994, 33, 6651–6658. [Google Scholar] [CrossRef]
- Huang, B.X.; Akbar, M.; Kevala, K.; Kim, H.Y. Phosphatidylserine is a critical modulator for Akt activation. J. Cell Biol. 2011, 192, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Baroni, M.; Mangialasche, F.; Mecocci, P. Vitamin E family: Role in the pathogenesis and treatment of Alzheimer’s disease. Alzheimer’s Dement. 2016, 2, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, Y.; Yao, H.; Imamura, Y.; Hashimoto, M.; Monji, A. Lower brain-derived neurotrophic factor levels are associated with age-related memory impairment in community-dwelling older adults: The Sefuri study. Sci. Rep. 2020, 10, 16442. [Google Scholar] [CrossRef] [PubMed]
- Konishi, K.; Cherkerzian, S.; Aroner, S.; Jacobs, E.G.; Rentz, D.M.; Remington, A.; Aizley, H.; Hornig, M.; Klibanski, A.; Goldstein, J.M. Impact of BDNF and sex on maintaining intact memory function in early midlife. Neurobiol. Aging 2019, 88, 137–149. [Google Scholar] [CrossRef]
- Gravesteijn, E.; Mensink, R.P.; Plat, J. Effects of nutritional interventions on BDNF concentrations in humans: A systematic review. Nutr. Neurosci. 2022, 25, 1425–1436. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Wang, F.; Ding, G.; Chen, W.; Fang, R.; Yao, Y.; Pang, M.; Lu, Z.-Q.; Liu, J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016, 1642, 70–78. [Google Scholar] [CrossRef]
- El Azab, E.F.; Abdulmalek, S. Amelioration of age-related multiple neuronal impairments and inflammation in high-fat diet-fed rats: The prospective multitargets of geraniol. Oxidative Med. Cell. Longev. 2022, 2022, 4812993. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.-D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Paley, E.L.; Merkulova-Rainon, T.; Faynboym, A.; Shestopalov, V.I.; Aksenoff, I. Geographical Distribution and Diversity of Gut Microbial NADH:Ubiquinone Oxidoreductase Sequence Associated with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Ren, S.; Qin, P.; Wang, G.; Yang, J. Anti-inflammatory and microbiota-regulating property of deficiency tonic medicines in edible traditional chinese medicine: A promising therapy for depressive disorder. J. Food Biochem. 2024, 2024, 4062632. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. Global regulatory frameworks for fermented foods: A review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef]
- Derbyshire, E. Nutrition for ADHD and Dyslexia: Unlocking the Potential for Learning and Wellbeing; Jessica Kingsley Publishers: London, UK, 2025. [Google Scholar]
- Kušar, A.; Žmitek, K.; Lähteenmäki, L.; Raats, M.M.; Pravst, I. Comparison of requirements for using health claims on foods in the European Union, the USA, Canada, and Australia/New Zealand. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1307–1332. [Google Scholar] [CrossRef] [PubMed]
- Welter, V.D.E.; Welter, N.G.E.; Großschedl, J. Experience and health-related behavior in times of the corona crisis in Germany: An exploratory psychological survey considering the identification of compliance-enhancing strategies. Int. J. Environ. Res. Public Health 2021, 18, 933. [Google Scholar] [CrossRef]
- Hadanny, A.; Zilberman-Itskovich, S.; Catalogna, M.; Elman-Shina, K.; Lang, E.; Finci, S.; Polak, N.; Shorer, R.; Parag, Y.; Efrati, S. Long term outcomes of hyperbaric oxygen therapy in post covid condition: Longitudinal follow-up of a randomized controlled trial. Sci. Rep. 2024, 14, 3604. [Google Scholar] [CrossRef]
- Biernacka, P.; Adamska, I.; Felisiak, K. The potential of Ginkgo biloba as a source of biologically active compounds—A review of the recent literature and patents. Molecules 2023, 28, 3993. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Kimura, K.; Saito, N.; Nakamura, H.; Takeda, Y. The effect of casein hydrolysate intake on cerebral neural regulation during cognitive tasks in the elderly. Exp. Gerontol. 2022, 165, 111853. [Google Scholar] [CrossRef]
- Schächtle, M.A.; Rosshart, S.P. The microbiota-gut-brain axis in health and disease and its implications for translational research. Front. Cell. Neurosci. 2021, 15, 698172. [Google Scholar] [CrossRef] [PubMed]
- Hock, S.C.; Siang, T.K.; Wah, C.L. Continuous manufacturing versus batch manufacturing: Benefits, opportunities and challenges for manufacturers and regulators. GaBI J. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Gray, N.E.; Farina, M.; Tucci, P.; Saso, L. The role of the NRF2 pathway in maintaining and improving cognitive function. Biomedicines 2022, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Day, P.; Twiddy, J.; Dubljević, V. Present and emerging ethical issues with tDCS use: A summary and review. Neuroethics 2023, 16, 1. [Google Scholar] [CrossRef]
- Narang, U.; Yadav, M.S.; Rindfleisch, A. The “idea advantage”: How content sharing strategies impact engagement in online learning platforms. J. Mark. Res. 2022, 59, 61–78. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Beck, T.; Boyle, P.; Dhana, K.; Desai, P.; Evans, D.A.; Rajan, K.B. APOE4, Blood Neurodegenerative Biomarkers, and Cognitive Decline in Community-Dwelling Older Adults. JAMA Netw. Open 2025, 8, e258903. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Kinney, J. Biomarkers for Alzheimer’s disease: Context of use, qualification, and roadmap for clinical implementation. Medicina 2022, 58, 952. [Google Scholar] [CrossRef]
- Sánchez-Ortí, J.V.; Correa-Ghisays, P.; Balanzá-Martínez, V.; Selva-Vera, G.; Vila-Francés, J.; Magdalena-Benedito, R.; San-Martin, C.; Victor, V.M.; Escribano-Lopez, I.; Hernandez-Mijares, A.; et al. Inflammation and lipid metabolism as potential biomarkers of memory impairment across type 2 diabetes mellitus and severe mental disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 127, 110817. [Google Scholar] [CrossRef]
- Brunt, V.E.; LaRocca, T.J.; Bazzoni, A.E.; Sapinsley, Z.J.; Miyamoto-Ditmon, J.; Gioscia-Ryan, R.A.; Neilson, A.P.; Link, C.D.; Seals, D.R. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 2021, 43, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Aubry, G.; Lee, H.J.; Lu, H. Advances in microfluidics: Technical innovations and applications in diagnostics and therapeutics. Anal. Chem. 2023, 95, 444–467. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Ortega-Santos, C.P.; Whisner, C.M.; Klein-Seetharaman, J.; Jasbi, P. Navigating challenges and opportunities in multi-omics integration for personalized healthcare. Biomedicines 2024, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Kirby, T.O.; Sapp, P.A.; Gonzalez, A.M.; Marshall, T.M.; Esposito, R. Nutrient synergy: Definition, evidence, and future directions. Front. Nutr. 2023, 10, 1279925. [Google Scholar] [CrossRef]
- McEwen, S.C.; Merrill, D.A.; Bramen, J.; Porter, V.; Panos, S.; Kaiser, S.; Hodes, J.; Ganapathi, A.; Bell, L.; Bookheimer, T.; et al. A systems-biology clinical trial of a personalized multimodal lifestyle intervention for early Alzheimer’s disease. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2021, 7, e12191. [Google Scholar] [CrossRef]

| Category | Established Clinical Biomarkers | Detection Method | Emerging Nutritionally Modulatable Biomarkers | Role in HFF Development |
|---|---|---|---|---|
| Neuroimaging | Hippocampal atrophy (MRI) Aβ/tau deposition (PET) | Structural/Molecular imaging | Default mode network activity (fMRI) | Early functional changes before structural damage |
| Cerebrospinal Fluid | Aβ42, p-tau, t-tau (AT(N) system) | Lumbar puncture | Neuroinflammatory cytokines (e.g., IL-1β, TNF-α) | Monitor neuroinflammation response to dietary interventions |
| Blood-Based | Plasma p-tau, Aβ42/40 ratio Neurofilament light chain | Immunoassays | BDNF, miRNA profiles (e.g., miR-132, miR-146a) | Non-invasive tracking of synaptic plasticity and epigenetic regulation |
| Cognitive Assessment | MMSE, MoCA, ADAS-Cog | Behavioral tests | Digital biomarkers (wearable devices) | Real-world cognitive monitoring |
| Gut–Brain Axis | - | - | SCFAs (butyrate), gut permeability (zonulin), microbiota diversity | Key targets for prebiotics/probiotics efficacy validation |
| Oxidative Stress | CSF F2-isoprostanes | Mass spectrometry | Urinary 8-OHdG, serum MDA | Directly responsive to antioxidant-rich HFFs (e.g., berries, turmeric) |
| Metabolic Dysfunction | - | - | HOMA-IR, AGEs, carnitine profiles | Link metabolic health to cognitive decline; modulated by polyphenols |
| Health Functional Food | Active Ingredients | Therapeutic Targets | Model Organisms | Ref. | ||
|---|---|---|---|---|---|---|
| In vitro | In vivo | Human/review | ||||
| Phosphatidylserine | Phosphatidylserine | Akt/PKC signaling activation | human | [143,144] | ||
| Fibroin extract | Silk fibroin | ERK/JNK/NF-κB pathway inhibition | human | [142] | ||
| Ginkgo biloba leaf extract | Flavonoids, terpenoids | Akt/mTOR pathway activation; NF-κB inhibition | human | [125] | ||
| EPA and DHA | Omega-3 fatty acids | GPR120/PPARγ pathway modulation | human | [30,31,45] | ||
| Green tea extract (L-theanine) | L-theanine | NF-κB pathway inhibition | human | [32] | ||
| Ginseng and Acanthopanax mixture | Saponins, lignans | NLRP3 inflammasome inhibition | N2a/APP695 cell | APP/PS1 mouse | human | [34,37,38] |
| Lycium chinense extract | Polysaccharides, alkaloids | Wnt/NF-κB pathway modulation; PI3K-AKT-mTOR activation | human | [52,89] | ||
| BT-11 (Polygala tenuifolia) | Tenuifolin, polygalaxanthone III | AChE inhibition; BDNF/TrkB signaling upregulation | human | [88] | ||
| High-temp. fermented green tea | Gallocatechin gallate | PKA/NF-κB/MAPK pathway inhibition | human | [51] | ||
| Pomegranate-derived metabolites | Urolithin A (microbial metabolite) | Mitochondrial biogenesis enhancement | human | [117] | ||
| Bifidobacterium breve | – | Tryptophan metabolism modulation (↑ 5-HTP/5-HT) | human | [110] | ||
| Hericium erinaceus | Not specified | Redox balance, Nrf2 activation | APP/PS1 mouse | [92] | ||
| Forsythia suspensa | Not specified | SCFA production, cognitive function | Aβ25–35–treated SD-rat | [96] | ||
| Cassiae Semen | Not specified | SCFA production, cognitive function | Aβ25–35–treated SD-rat | [96] | ||
| Polygonatum sibiricum | Polysaccharides | Gut microbiota modulation, neuroinflammation | 5xFAD mice | [106] | ||
| Schisandra chinensis | Gomisin A, Schisandrin | Neuroinflammation, synaptic function | Aβ25–35–treated SD-rat | Review | [82] | |
| Cornus officinalis | Polysaccharides, Iridoid glycosides | Synaptic plasticity, BDNF-TrkB signaling | Aβ25–35-treated mice | [127] | ||
| Zanthoxylum bungeanum | Hydroxy-α-sanshool | CREB/BDNF axis | scopolamine-treated mice | [129] | ||
| Rubus idaeus | Flavonoids | Cholinergic function, oxidative stress | VD (Vascular demented) SD rat | Review | [130] | |
| Portulaca oleracea | Purslane amide E | Oxidative stress, neurotoxicity | LPS-treated mice | [119] | ||
| Gardenia jasminoides | Total flavonoids | Cholinergic system, PERK pathway | VD rat model | [120] | ||
| Porcine brain enzyme hydrolysate | Leucine, lysine, phenylalanine, tripeptides, or tetrapeptides | Gut–brain axis, memory function | scopolamine-treated SD-rat | [83] | ||
| Dendrobium nobile | Alkaloids (DNLA) | Tau hyperphosphorylation, Aβ neurotoxicity | Review | [123] | ||
| Panax notoginseng | Ginsenosides | PI3K/Akt/mTOR pathway, cholinergic neurotransmission | N2a/APP695 cell | [125] | ||
| Rubus fruticosus | Extract | Memory deficits in vascular dementia | VD(Vascular demented) SD rat | [130] | ||
| Cinnamon | Cinnamaldehyde | Mitochondrial dynamics, Aβ accumulation | THP-1 cell | [121] | ||
| Inulin | - | Neurotransmitter production, cognitive function | Aβ25–35–treated SD-rat | [95] | ||
| Luteolin | - | Insulin resistance, neuroinflammation | Aβ25–35–treated SD-rat | Review | [44,47] | |
| Resveratrol | - | SIRT1/PGC-1α pathway, mitochondrial biogenesis | severe acute pancreatitis (SAP) SD-rat | [49] | ||
| Ferulic Acid | - | Insulin sensitivity, neuroinflammation | Px and Aβ25–35 treated SD-rat | [69] | ||
| Berberine | - | Bile acid metabolism, neuroprotection | C57BL/6 mice, Aβ1-42 treated mice | [101,102] | ||
| Curcumin | - | Bile acid metabolism, neuroprotection | Aβ1-42-treated mice | [102] | ||
| Akkermansia muciniphila | - | Gut barrier function, neuroinflammation | APP/PS1 mice | [108] | ||
| Lactobacillus | - | Dopaminergic signaling, cognitive function | HT22 nerve cell | Tg-APP/PS1 mice | [124] | |
| Health Functional Food | Dosage (mg/day) | Active Compounds (s) | Targeted Pathways | Modulated Biomarkers | Ref. |
|---|---|---|---|---|---|
| Phosphatidylserine | 300~800 | Phosphatidylserine | Akt, protein kinase C (PKC), and Raf-1 signaling activation | Phosphatidylserine | [150,151,166,167] |
| Fibroin extract | 200~400 | Silk fibroin | ERK signaling activation, JNK signaling pathway activation, NF-κB signaling pathway activation | IL-1β ↓, IL-6 ↓, TNF-α ↓, brain ACh level ↑ | [130,147] |
| Eriobotrya folium extract | 1500 | Flavonoids, quercetin, triterpenoid acids, sesquiterpene glycosides | iNOS expression inhibition, COX-2 expression inhibition, NF-κB binding activity inhibition | NO ↓, PGE2 ↓, iNOS ↓, COX-2 ↓, NF-κB ↓, MAPK phosphorylation ↓ | [28,29] |
| BT-11 (Polygala tenuifolia Willd.) extract | 300 | Tenuifolin, senegenin, polygalacic acid, polygalaxanthone III, 3.6′-disinapoyl sucrose, polygalacic acid | Acetylcholine enzyme inhibition, ERK, cAMP, NF-κB, BDNF/TrkB signaling pathway | TNF-α ↓, IL-1β ↓, IL-6 ↓, IFN-γ ↓, LPS ↓, SOD ↑, GSH level ↑ | [89] |
| EPA and DHA | 900~2000 | Omega-3 fatty acid | GPR120/PPARγ pathway inhibition, IFN-γ secretion inhibition, WAT NLRP3 inflammasome/IL-1β pathway upregulation | TNF-α ↓, IFN-γ ↓ | [30,31,45] |
| Green tea extract theanine compounds | 1680 | L-theanine | NF-κB pathway inhibition | IL-23 ↓, IL-1β ↓, TNF-α ↓, COX-2 ↓, IL-17A ↓ | [32] |
| High temperature treated green tea extract | 900 | Gallocatechin gallate, catechin | PKA Pathway modulation, NF-κB and MAPK pathways inhibition | Improve glucose tolerance, insulin sensitivity | [51] |
| Ginkgo leaf extract | 120 | Flavonoids(quercetin, kaempferol, isorhamnetin), Terpenoids(ginkgolides, bilobalide) | Activates the Akt/mTOR pathway, PI3K/AKT signaling pathway upregulation, modulates AMPK-mTOR Pathway, and NF-κB pathway inhibition | IL-1β ↓, TNF-α ↓, IL-6 ↓, SIRT-1 expression ↑ | [113,128] |
| Ginseng and Acanthopanax Koreanum mixture | 5200 | Proanthocyanidin, triterpenoid saponins, lignans, coumarins, flavones, phenolic compounds, acankoreosides | NF-κB pathway inhibition, NLRP3, and dopaminergic pathways, tumor necrosis factor alpha-α inhibition | Antioxidant activities (ABTS, FRAP, reducing power, ORAC), NLRP3 inflammasome ↓ | [34,37,38,92] |
| Lycium chinense extract | 1425 | Polysaccharides, alkaloids, flavonoids | WNT pathway activation, aberrant NF-κB activation inhibition, PI3K-AKT-mTOR signaling activation, BCL2-Associated X (Bax)/B-cell lymphoma-2 (Bcl-2) downregulation | BDNF expression ↑, amyloid-beta (Aβ) deposits ↓, tau phosphorylation ↓, TNF-α ↓, iNOS ↓, IL-1β ↓, COX-2 ↓ | [52,90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Zhou, J.; Li, H.; Kang, S.; Park, S. A Systems Biology Approach to Memory Health: Integrating Network Pharmacology, Gut Microbiota, and Multi-Omics for Health Functional Foods. Int. J. Mol. Sci. 2025, 26, 6698. https://doi.org/10.3390/ijms26146698
Yuan H, Zhou J, Li H, Kang S, Park S. A Systems Biology Approach to Memory Health: Integrating Network Pharmacology, Gut Microbiota, and Multi-Omics for Health Functional Foods. International Journal of Molecular Sciences. 2025; 26(14):6698. https://doi.org/10.3390/ijms26146698
Chicago/Turabian StyleYuan, Heng, Junyu Zhou, Hongbao Li, Suna Kang, and Sunmin Park. 2025. "A Systems Biology Approach to Memory Health: Integrating Network Pharmacology, Gut Microbiota, and Multi-Omics for Health Functional Foods" International Journal of Molecular Sciences 26, no. 14: 6698. https://doi.org/10.3390/ijms26146698
APA StyleYuan, H., Zhou, J., Li, H., Kang, S., & Park, S. (2025). A Systems Biology Approach to Memory Health: Integrating Network Pharmacology, Gut Microbiota, and Multi-Omics for Health Functional Foods. International Journal of Molecular Sciences, 26(14), 6698. https://doi.org/10.3390/ijms26146698









