Overexpression of BDNF and uPA Combined with the Suppression of Von Hippel–Lindau Tumor Suppressor Enhances the Neuroprotective Activity of the Secretome of Human Mesenchymal Stromal Cells in the Model of Intracerebral Hemorrhage
Abstract
1. Introduction
2. Results
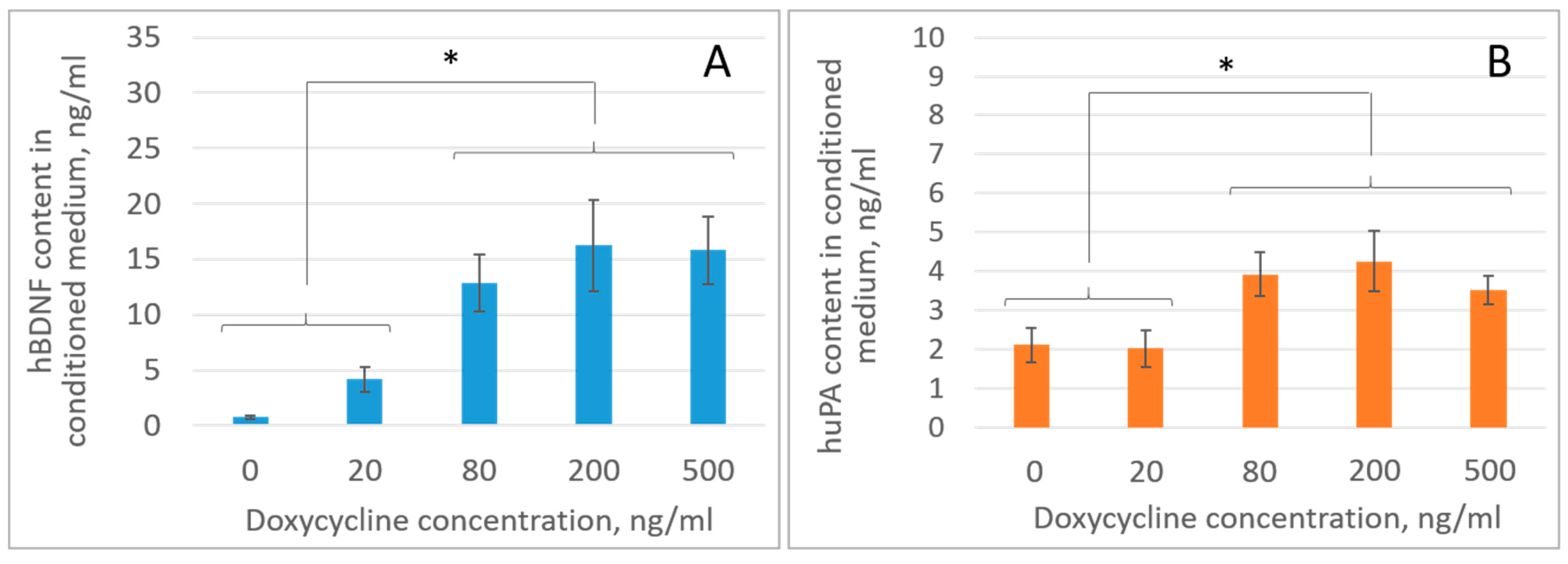
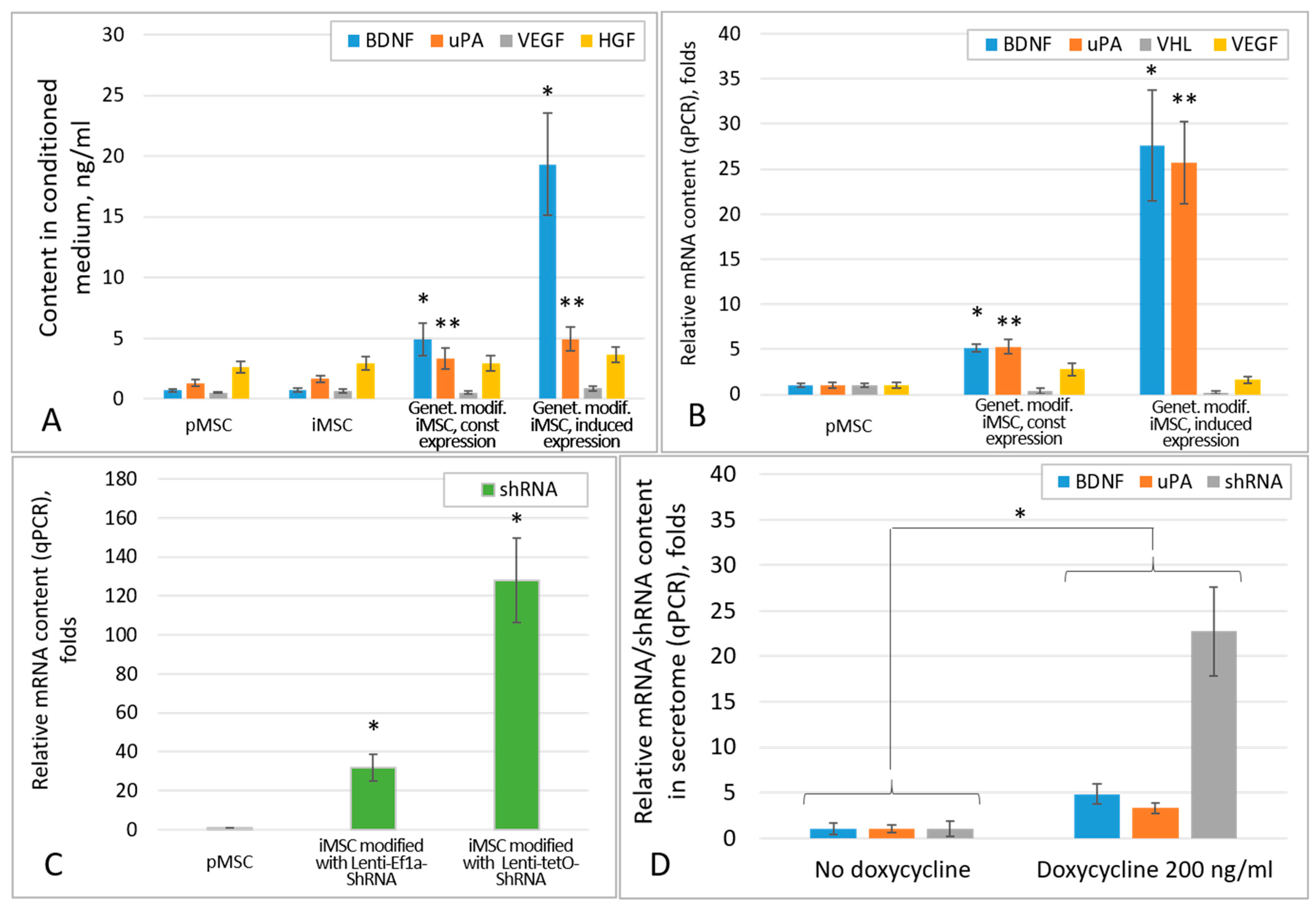
2.1. Genetic Modification of iMSCs Increases the Content of Growth Factors in Their Secretome
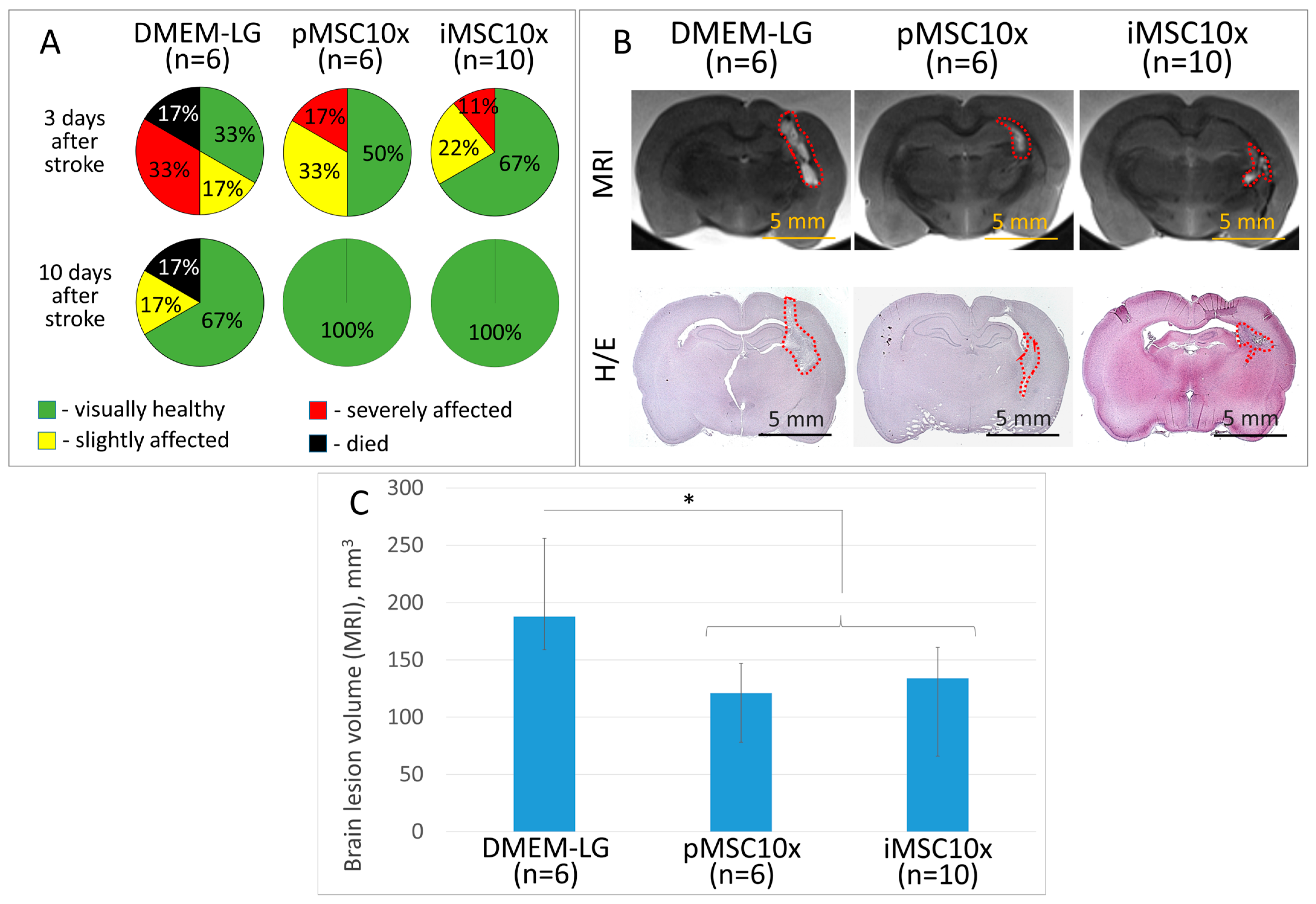
2.2. iMSC and pMSC Secretomes Reveal Equal Neuroprotective Activity in the ICH Model
2.3. Overexpression of BDNF and uPA Combined with the Suppression of VHL Enhances the Neuroprotective Activity of iMSC Secretome in the Model of Intracerebral Hemorrhage
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Animals
4.3. Assembly of Vectors for MSC Genetic Modification
4.4. Genetic Modification of MSC
4.5. Obtaining MSC Secretomes
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Reverse Transcription and qPCR
4.8. A Model of Intracerebral Hemorrhage
4.9. Assessment of Animal Survival and Neurological Status
4.10. MRI
4.11. Histologic Examination of Brain Slices
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulyaeva, N. Biochemical Mechanisms and Translational Relevance of Hippocampal Vulnerability to Distant Focal Brain Injury: The Price of Stress Response. Biochemistry (Moscow) 2019, 84, 1306–1328. [Google Scholar] [CrossRef] [PubMed]
- Bolshakov, A.; Tret’yakova, L.; Kvichansky, A.; Gulyaeva, N. Glucocorticoids: Dr. Jekyll and Mr. Hyde of Hippocampal Neuroinflammation. Biochemistry (Moscow) 2021, 86, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Buccilli, B.; Alan, A.; Baha’, A.; Shahzad, A.; Almealawy, Y.F.; Chisvo, N.S.; Ennabe, M.; Weinand, M. Neuroprotection strategies in traumatic brain injury: Studying the effectiveness of different clinical approaches. Surg. Neurol. Int. 2024, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Karagyaur, M.; Dzhauari, S.; Basalova, N.; Aleksandrushkina, N.; Sagaradze, G.; Danilova, N.; Malkov, P.; Popov, V.; Skryabina, M.; Efimenko, A.; et al. MSC Secretome as a Promising Tool for Neuroprotection and Neuroregeneration in a Model of Intracerebral Hemorrhage. Pharmaceutics 2021, 13, 2031. [Google Scholar] [CrossRef]
- Efimenko, A.; Starostina, E.; Kalinina, N.; Stolzing, A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 2011, 9, 10. [Google Scholar] [CrossRef]
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 4290. [Google Scholar] [CrossRef]
- Cui, L.; Golubczyk, D.; Tolppanen, A.; Boltze, J.; Jolkkonen, J. Cell therapy for ischemic stroke: Are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann. Neurol. 2019, 86, 5–16. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Hu, B.; Hong, Y.; Zhang, H.; Li, X.; Zhang, Y. Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Front. Cell. Neurosci. 2021, 15, 628940. [Google Scholar] [CrossRef]
- Dzhauari, S.; Basalova, N.; Primak, A.; Balabanyan, V.; Efimenko, A.; Skryabina, M.; Popov, V.; Velichko, A.; Bozov, K.; Akopyan, Z.; et al. The Secretome of Mesenchymal Stromal Cells in Treating Intracerebral Hemorrhage: The First Step to Bedside. Pharmaceutics 2023, 15, 1608. [Google Scholar] [CrossRef]
- Primak, A.; Kalinina, N.; Skryabina, M.; Usachev, V.; Chechekhin, V.; Vigovskiy, M.; Chechekhina, E.; Voloshin, N.; Kulebyakin, K.; Kulebyakina, M.; et al. Novel Immortalized Human Multipotent Mesenchymal Stromal Cell Line for Studying Hormonal Signaling. Int. J. Mol. Sci. 2024, 25, 2421. [Google Scholar] [CrossRef]
- Bischoff, D.S.; Makhijani, N.S.; Yamaguchi, D.T. Constitutive expression of human telomerase enhances the proliferation potential of human mesenchymal stem cells. Biores. Open Access 2012, 1, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Taylor, D.A.; Geng, Y.J.; De Caterina, R.; Shelat, H.; Perin, E.C.; Willerson, J.T. Transplantation of mesenchymal cells rejuvenated by the overexpression of telomerase and myocardin promotes revascularization and tissue repair in a murine model of hindlimb ischemia. Circ. Res. 2013, 113, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003, 27, 277–324. [Google Scholar] [CrossRef]
- Gomazkov, O.A. Neurotrophic Regulation and Brain Stem Cells; Publishing House “Icarus”: Auckland, New Zealand, 2006. [Google Scholar]
- Siconolfi, L.B.; Seeds, N.W. Induction of the plasminogen activator system accompanies peripheral nerve regeneration after sciatic nerve crush. J. Neurosci. 2001, 21, 4336–4347. [Google Scholar] [CrossRef]
- Pittman, R.I.; Ivins, J.K.; Buettner, H.M. Neuronal Plasminogen Activators: Cell Surface Binding Sites and Involvement in Neurite Outgrowth. J. Neurosci. 1989, 9, 4269–4286. [Google Scholar] [CrossRef] [PubMed]
- Dzhauari, S.; Litvinova, S.; Efimenko, A.; Aleksandrushkina, N.; Basalova, N.; Abakumov, M.; Danilova, N.; Malkov, P.; Balabanyan, V.; Bezuglova, T.; et al. Urokinase-Type Plasminogen Activator Enhances the Neuroprotective Activity of Brain-Derived Neurotrophic Factor in a Model of Intracerebral Hemorrhage. Biomedicines 2022, 10, 1346. [Google Scholar] [CrossRef]
- Tanimoto, K.; Makino, Y.; Pereira, T.; Poellinger, L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000, 19, 4298–4309. [Google Scholar] [CrossRef]
- Kapitsinou, P.P.; Haase, V.H. The VHL tumor suppressor and HIF: Insights from genetic studies in mice. Cell Death Differ. 2008, 15, 650–659. [Google Scholar] [CrossRef]
- Haase, V.H. The VHL tumor suppressor: Master regulator of HIF. Curr. Pharm. Des. 2009, 15, 3895–3903. [Google Scholar] [CrossRef]
- Meléndez-Rodríguez, F.; Roche, O.; Sanchez-Prieto, R.; Aragones, J. Hypoxia-Inducible Factor 2-Dependent Pathways Driving Von Hippel-Lindau-Deficient Renal Cancer. Front. Oncol. 2018, 8, 214. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Bakleh, M.Z.; Al Haj Zen, A. The Distinct Role of HIF-1α and HIF-2α in Hypoxia and Angiogenesis. Cells 2025, 14, 673. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, M.; Wang, Y.; Pasha, Z.; Li, T.; Ashraf, M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J. Mol. Cell. Cardiol. 2007, 42, 1036–1044. [Google Scholar] [CrossRef]
- Wang, X.; Bullock, A.J.; Zhang, L.; Wei, L.; Yu, D.; Mahagaokar, K.; Alsop, D.C.; Mier, J.W.; Atkins, M.B.; Coxon, A.; et al. The role of angiopoietins as potential therapeutic targets in renal cell carcinoma. Transl. Oncol. 2014, 7, 188–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Metelo, A.M.; Noonan, H.; Iliopoulos, O. HIF2a inhibitors for the treatment of VHL disease. Oncotarget 2015, 6, 23036–23037. [Google Scholar] [CrossRef]
- Hobson, M.I.; Green, C.J.; Terenghi, G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J. Anat. 2000, 197 Pt 4, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Storkebaum, E.; Carmeliet, P. VEGF: A critical player in neurodegeneration. J. Clin. Investig. 2004, 113, 14–18. [Google Scholar] [CrossRef]
- Maherali, N.; Ahfeldt, T.; Rigamonti, A.; Utikal, J.; Cowan, C.; Hochedlinger, K. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell 2008, 3, 340–345. [Google Scholar] [CrossRef]
- Lukavsky, P.J. Structure and function of HCV IRES domains. Virus Res. 2009, 139, 166–171. [Google Scholar] [CrossRef]
- Karagyaur, M.; Rostovtseva, A.; Semina, E.; Klimovich, P.; Balabanyan, V.; Makarevich, P.; Popov, V.; Stambolsky, D.; Tkachuk, V. A Bicistronic Plasmid Encoding Brain-Derived Neurotrophic Factor and Urokinase Plasminogen Activator Stimulates Peripheral Nerve Regeneration After Injury. J. Pharmacol. Exp. Ther. 2020, 372, 248–255. [Google Scholar] [CrossRef]
- Lee, H.H.; O’Malley, M.J.; Friel, N.A.; Chu, C.R. Effects of doxycycline on mesenchymal stem cell chondrogenesis and cartilage repair. Osteoarthr Cartil. 2013, 21, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, N.; Tyurin-Kuzmin, P.; Karagyaur, M.; Akopyan, Z.; Kulebyakin, K. Practical Use of Immortalized Cells in Medicine: Current Advances and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 12716. [Google Scholar] [CrossRef] [PubMed]
- Makridakis, M.; Roubelakis, M.G.; Vlahou, A. Stem cells: Insights into the secretome. Biochim. Biophys. Acta 2013, 1834, 2380–2384. [Google Scholar] [CrossRef]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015, 6, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Rezabakhsh, A.; Sokullu, E.; Rahbarghazi, R. Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res. Ther. 2021, 12, 521. [Google Scholar] [CrossRef]
- Giovannelli, L.; Bari, E.; Jommi, C.; Tartara, F.; Armocida, D.; Garbossa, D.; Cofano, F.; Torre, M.L.; Segale, L. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioact. Mater. 2023, 29, 16–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Mo, Y.; Chen, Z.; Chen, T.; Li, Y.; Zheng, Y.; Deng, S.; Xu, X.; Chen, H.; et al. Immortalized Mesenchymal Stem Cells: A Safe Cell Source for Cellular or Cell Membrane-Based Treatment of Glioma. Stem Cells Int. 2022, 2022, 6430565. [Google Scholar] [CrossRef]
- Primak, A.L.; Skryabina, M.N.; Dzhauari, S.S.; Tkachuk, V.A.; Karagyaur, M.N. The Secretome of Mesenchymal Stromal Cells as a New Hope in the Treatment of Acute Brain Tissue Injuries. Neurosci. Behav. Physiol. 2024, 54, 673–681. [Google Scholar] [CrossRef]
- Shahror, R.A.; Wu, C.C.; Chiang, Y.H.; Chen, K.Y. Genetically Modified Mesenchymal Stem Cells: The Next Generation of Stem Cell-Based Therapy for TBI. Int. J. Mol. Sci. 2020, 21, 4051. [Google Scholar] [CrossRef]
- Beegle, J.R.; Magner, N.L.; Kalomoiris, S.; Harding, A.; Zhou, P.; Nacey, C.; White, J.L.; Pepper, K.; Gruenloh, W.; Annett, G.; et al. Preclinical evaluation of mesenchymal stem cells overexpressing VEGF to treat critical limb ischemia. Mol. Ther. Methods Clin. Dev. 2016, 3, 16053. [Google Scholar] [CrossRef]
- Lopatina, T.; Kalinina, N.; Karagyaur, M.; Stambolsky, D.; Rubina, K.; Revischin, A.; Pavlova, G.; Parfyonova, Y.; Tkachuk, V. Correction: Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 2019, 14, e0219946. [Google Scholar] [CrossRef]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef]
- Cunningham, C.; Redondo-Castro, E.; Allan, S. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cerebr. Blood Flow Metab. 2018, 38, 1276–1292. [Google Scholar] [CrossRef]
- Kim, H.J.; Bayarsaikhan, D.; Lee, J.; Bayarsaikhan, G.; Lee, B. Brain-Derived Neurotrophic Factor Secreting Human Mesenchymal Stem Cells Improve Outcomes in Rett Syndrome Mouse Models. Front. Neurosci. 2021, 15, 725398. [Google Scholar] [CrossRef] [PubMed]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef] [PubMed]
- Guduric-Fuchs, J.; O’Connor, A.; Camp, B.; O’Neill, C.L.; Medina, R.J.; Simpson, D.A. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genom. 2012, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Ridder, K.; Sevko, A.; Heide, J.; Dams, M.; Rupp, A.K.; Macas, J.; Starmann, J.; Tjwa, M.; Plate, K.H.; Sültmann, H.; et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology 2015, 4, e1008371. [Google Scholar] [CrossRef]
- Basalova, N.A.; Dzhauari, S.S.; Yurshev, Y.A.; Primak, A.L.; Efimenko, A.Y.; Tkachuk, V.A.; Karagyaur, M.N. State-of-the-Art: The Use of Extracellular Vesicles and Preparations Based on Them for Neuroprotection and Stimulation of Brain Tissue Regeneration after Injury. Neurochem. J. 2023, 17, 560–570. [Google Scholar] [CrossRef]
- Alnoaman, H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Turkistani, A.; Allam, A.; Alexiou, A.; Papadakis, M.; Batiha, G.E. Dysregulation of proBDNF/p75NTR and BDNF/TrkB Signaling in Acute Ischemic Stroke: Different Sides of the Same Coins. Brain Res. Bull. 2025, 226, 111338. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Nie, Y.; Wei, W.; Xiong, W. proBDNF expression induces apoptosis and inhibits synaptic regeneration by regulating the RhoA-JNK pathway in an in vitro post-stroke depression model. Transl. Psychiatry 2021, 11, 578. [Google Scholar] [CrossRef]
- Montroull, L.E.; Rothbard, D.E.; Kanal, H.D.; D’Mello, V.; Dodson, V.; Troy, C.M.; Zanin, J.P.; Levison, S.W.; Friedman, W.J. Proneurotrophins Induce Apoptotic Neuronal Death After Controlled Cortical Impact Injury in Adult Mice. ASN Neuro 2020, 12, 1759091420930865. [Google Scholar] [CrossRef] [PubMed]
- Mayhan, W.G. VEGF increases permeability of the blood-brain barrier via a nitric oxide synthase/cGMP-dependent pathway. Am. J. Physiol. 1999, 276, C1148–C1153. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Y.; Wang, T.; Jiao, L.; Luo, Y. VEGF, a Key Factor for Blood Brain Barrier Injury After Cerebral Ischemic Stroke. Aging Dis. 2022, 13, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Čechová, K.; Průša, R.; Hort, J. Amyloid beta soluble forms and plasminogen activation system in Alzheimer’s disease: Consequences on extracellular maturation of brain-derived neurotrophic factor and therapeutic implications. CNS Neurosci. Ther. 2019, 25, 303–313. [Google Scholar] [CrossRef]
- Gerenu, G.; Martisova, E.; Ferrero, H.; Carracedo, M.; Rantamäki, T.; Ramirez, M.J.; Gil-Bea, F.J. Modulation of BDNF cleavage by plasminogen-activator inhibitor-1 contributes to Alzheimer’s neuropathology and cognitive deficits. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal Stem versus Stromal Cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Tyurin-Kuzmin, P.A.; Karagyaur, M.N.; Kulebyakin, K.Y.; Dyikanov, D.T.; Chechekhin, V.I.; Ivanova, A.M.; Skryabina, M.N.; Arbatskiy, M.S.; Sysoeva, V.Y.; Kalinina, N.I.; et al. Functional Heterogeneity of Protein Kinase A Activation in Multipotent Stromal Cells. Int. J. Mol. Sci. 2020, 21, 4442. [Google Scholar] [CrossRef]
- Kumar, M.; Keller, B.; Makalou, N.; Sutton, R.E. Systematic determination of the packaging limit of lentiviral vectors. Hum. Gene Ther. 2001, 12, 1893–1905. [Google Scholar] [CrossRef]
- Sweeney, N.P.; Vink, C.A. The impact of lentiviral vector genome size and producer cell genomic to gag-pol mRNA ratios on packaging efficiency and titre. Mol. Ther. Methods Clin. Dev. 2021, 21, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef] [PubMed]
- Makarenko, A.N.; Kositsyn, N.S.; Pasikova, N.V.; Svinov, M.M. Method of modeling local hemorrhage in various structures of the brain in experimental animals. IP Pavlov. J. Higher Nerv. Act. 2002, 52, 765–768. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th Edition; Academic Press: Cambridge, UK, 2007. [Google Scholar]
- McGraw, C.P.; Pashayan, A.G.; Wendel, O.T. Cerebral infarction in the Mongolian gerbil exacerbated by phenoxyben-zamine treatment. Stroke 1976, 7, 485–488. [Google Scholar] [CrossRef]
- Gannushkina, I.V. Brain blood circulation in different types of cerebral circulatory hypoxia. Bull. Russ. Acad. Med. Sci. 2000, 9, 22–27. [Google Scholar]

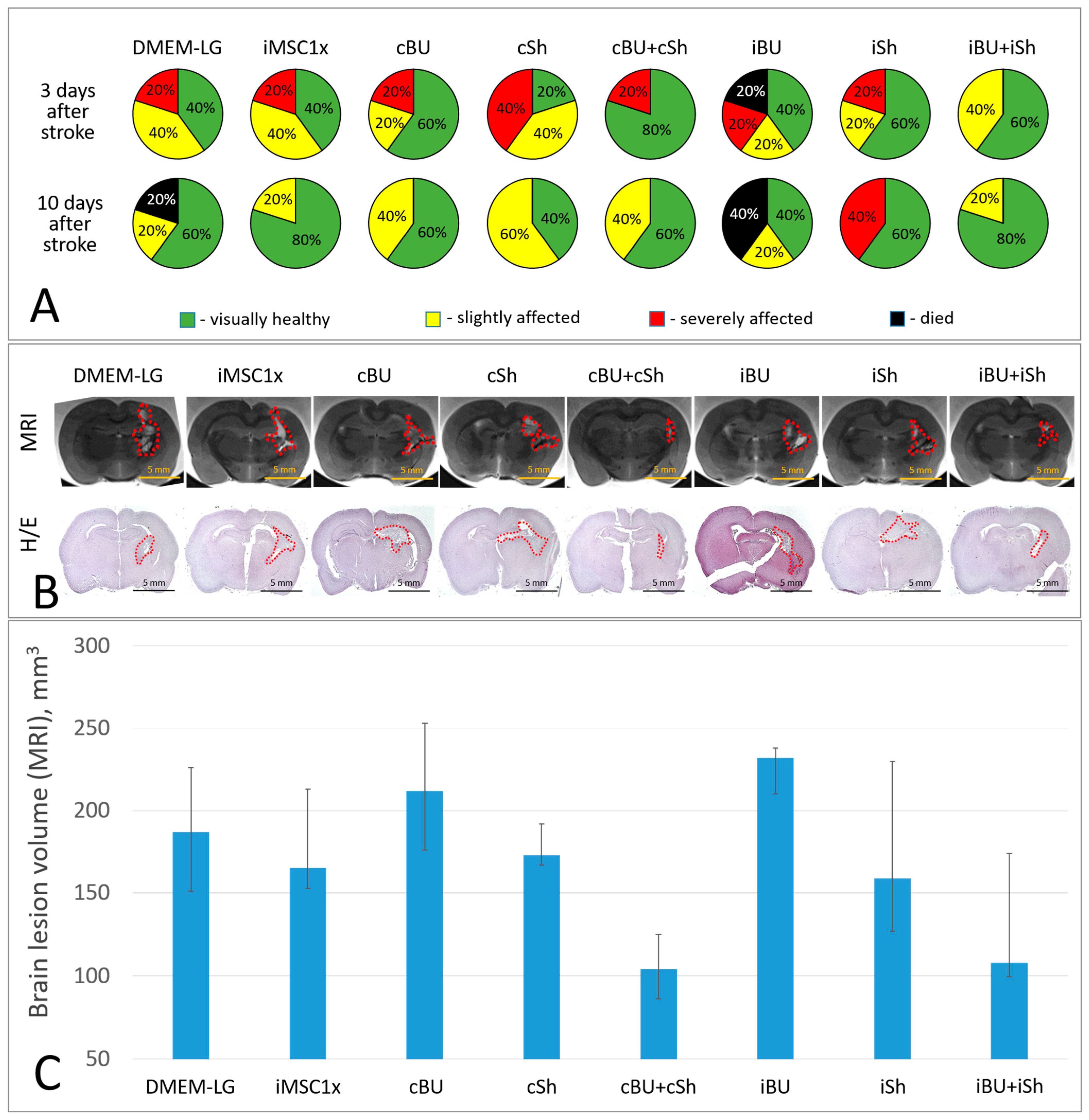
| pMSC | iMSC | cBU | cSh | cBU + cSh | iBU | iSh | iBU + iSh | |
|---|---|---|---|---|---|---|---|---|
| BDNF | 0.70 ± 0.16 | 0.73 ± 0.16 | 4.90 ± 1.31 | 0.81 ± 0.18 | 3.25 ± 0.45 | 19.32 ± 4.2 | 0.68 ± 0.15 | 9.43 ± 1.41 |
| uPA | 1.30 ± 0.28 | 1.65 ± 0.29 | 3.33 ± 0.84 | 1.39 ± 0.33 | 2.28 ± 0.40 | 4.94 ± 0.99 | 1.61 ± 0.34 | 2.97 ± 0.45 |
| VEGF | 0.53 ± 0.06 | 0.66 ± 0.19 | 0.71 ± 0.17 | 0.53 ± 0.11 | 0.59 ± 0.15 | 0.65 ± 0.19 | 0.86 ± 0.18 | 0.76 ± 0.11 |
| HGF | 2.62 ± 0.49 | 2.93 ± 0.57 | 2.38 ± 0.56 | 2.96 ± 0.63 | 2.61 ± 0.31 | 2.67 ± 0.59 | 3.61 ± 0.63 | 3.07 ± 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhauari, S.S.; Primak, A.L.; Basalova, N.A.; Kalinina, N.I.; Monakova, A.O.; Bozov, K.D.; Velichko, A.Y.; Illarionova, M.E.; Grigorieva, O.A.; Akopyan, Z.A.; et al. Overexpression of BDNF and uPA Combined with the Suppression of Von Hippel–Lindau Tumor Suppressor Enhances the Neuroprotective Activity of the Secretome of Human Mesenchymal Stromal Cells in the Model of Intracerebral Hemorrhage. Int. J. Mol. Sci. 2025, 26, 6697. https://doi.org/10.3390/ijms26146697
Dzhauari SS, Primak AL, Basalova NA, Kalinina NI, Monakova AO, Bozov KD, Velichko AY, Illarionova ME, Grigorieva OA, Akopyan ZA, et al. Overexpression of BDNF and uPA Combined with the Suppression of Von Hippel–Lindau Tumor Suppressor Enhances the Neuroprotective Activity of the Secretome of Human Mesenchymal Stromal Cells in the Model of Intracerebral Hemorrhage. International Journal of Molecular Sciences. 2025; 26(14):6697. https://doi.org/10.3390/ijms26146697
Chicago/Turabian StyleDzhauari, Stalik S., Alexandra L. Primak, Nataliya A. Basalova, Natalia I. Kalinina, Anna O. Monakova, Kirill D. Bozov, Arkadiy Ya. Velichko, Maria E. Illarionova, Olga A. Grigorieva, Zhanna A. Akopyan, and et al. 2025. "Overexpression of BDNF and uPA Combined with the Suppression of Von Hippel–Lindau Tumor Suppressor Enhances the Neuroprotective Activity of the Secretome of Human Mesenchymal Stromal Cells in the Model of Intracerebral Hemorrhage" International Journal of Molecular Sciences 26, no. 14: 6697. https://doi.org/10.3390/ijms26146697
APA StyleDzhauari, S. S., Primak, A. L., Basalova, N. A., Kalinina, N. I., Monakova, A. O., Bozov, K. D., Velichko, A. Y., Illarionova, M. E., Grigorieva, O. A., Akopyan, Z. A., Popov, V. S., Malkov, P. G., Efimenko, A. Y., Tkachuk, V. A., & Karagyaur, M. N. (2025). Overexpression of BDNF and uPA Combined with the Suppression of Von Hippel–Lindau Tumor Suppressor Enhances the Neuroprotective Activity of the Secretome of Human Mesenchymal Stromal Cells in the Model of Intracerebral Hemorrhage. International Journal of Molecular Sciences, 26(14), 6697. https://doi.org/10.3390/ijms26146697







