From Adipose to Action: Reprogramming Stem Cells for Functional Neural Progenitors for Neural Regenerative Therapy
Abstract
1. Introduction
2. Characteristics of NSCs and ADSCs
2.1. Sources
2.1.1. NSCs
2.1.2. ADSCs
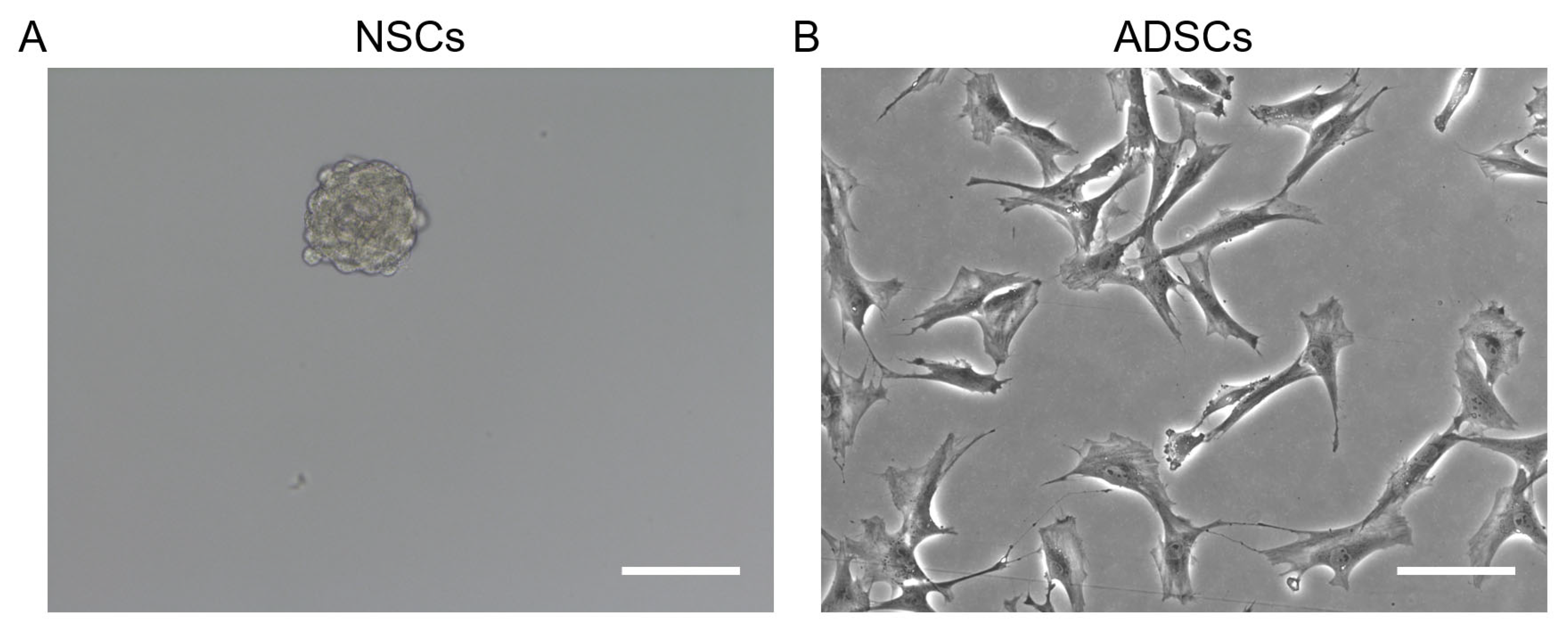
2.2. Morphological Characteristics
2.2.1. NSCs
2.2.2. ADSCs
2.3. Cell Surface Markers
2.3.1. NSCs
2.3.2. ADSCs
2.4. Functional Properties
2.4.1. NSCs
2.4.2. ADSCs
| Characteristics | NSCs | ADSCs | References |
|---|---|---|---|
| Sources | Embryonic NSCs (neural tube region); Adult NSCs (hippocampus, SVZ); ESCs; iPSCs | Adipose tissue (subcutaneous fat) | [17,49] |
| Morphological Characteristics | Form neurospheres in specific culture medium; Spindle-shaped or multi-protrusion morphology | Fibroblast-like, spindle-shaped morphology | [30,57] |
| Differentiation Potential | Neurons; Astrocytes; Oligodendrocytes | Adipocytes; Osteoblasts; Chondrocytes; Hepatic lineage; Neural cells | [34,58] |
| Surface Markers | Nestin; Sox2; CD133; Musashi-1 | CD9, CD10, CD13, CD29, CD73, CD90, CD105, CD271; Do not express HSCs markers (CD31, CD45, CD11B) | [41,59,60] |
| Neurotrophic factors | NGF, BDNF, GDNF, IGF-1; TGF-β; IGF1 | VEGF, EGF, HGF, IGF1, PGDF, FGF, TGF-β, BDNF, GDNF, NGF | [52,61,62,63,64] |
| Proliferation Capacity | Self-renewal through symmetric and asymmetric division | Self-renewal and long-term proliferation capacity in vitro | [18,56] |
| Immunogenicity | Allogeneic transplantation may trigger immune responses | Suitable for autologous transplantation | [65,66] |
| Special effect | - | Secreting cytokines and exosomes; EVs | [54,55,56,67,68] |
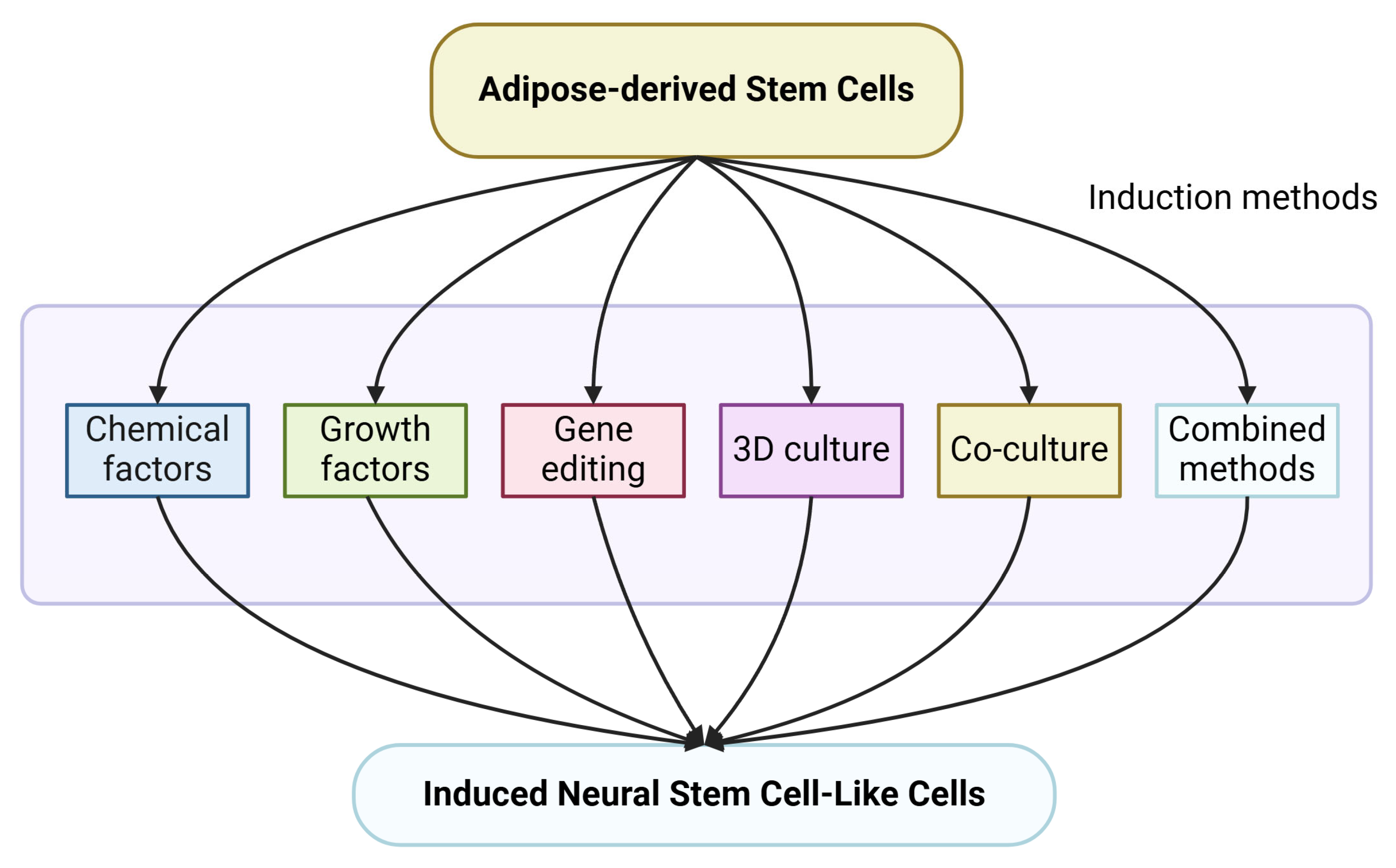
3. Research Methods of ADSCs-to-iNSCs Induction Process
3.1. Chemical Induction
3.2. Growth Factors
3.3. Gene Editing Technology
3.4. Three-Dimensional (3D) Culture System
3.5. Co-Culture Induction
3.6. Combined Induction
| Induction Methods | Key Factors/Techniques | Advantages | Challenges | References |
|---|---|---|---|---|
| Chemical Factor | RA; BME; Forskolin; Sertraline; VPA; VPA + butylated hydroxyanisole + insulin + hydrocortisone; LC; BMP4 | Cost-effective; Easy to implement | Limited specificity; Potential off-target effects | [50,70,72,73,74,75] |
| Growth Factor | BDNF; GDNF; EGF; bFGF; NGF; TGF-β; N2; B27; Ghrelin; FGF2 | High specificity and efficacy | High cost and potential instability of growth factors | [81,85,86,87,88] |
| Gene Editing | Sox2; CGRP; OCT4; KLF4; SOX2; and c-MYC | High precision; Long-lasting effects | Ethical concerns, off-target effects; Technical complexity | [89,94,103] |
| 3D Culture System | Fibrin matrix microenvironment; Hydrogel scaffold; PEG-Based 3D Matrix | Better mimics in vivo conditions | Complex setup; Potential variability | [95,96,104] |
| Co-Culture Induction | Direct contact co-culture; No-contact co-culture with ESCs; Chitosan co-culture Systems | Utilizes natural signaling mechanisms | Requires access to other cells | [16,99,105] |
| Combined Induction | Melatonin + CM; Indomethacin + Insulin + IBMX + PBM; Sox1 Activation + CM; bFGF + forskolin; BDNF + RA; SB431542 + noggin + LDN193289 + EGF + bFGF; 3D hydrogels + B27 + C1; Insoluble fibrin supported adhesion matrix + growth factors; | Maximizes induction efficiency/outcomes | Increased complexity and cost | [81,100,101,102,106,107,108,109] |
4. Molecular Signal Pathways of ADSCs-to-iNSCs Induction Process
4.1. Notch Signaling Pathway
4.2. Wnt/β-Catenin Signaling Pathway
4.3. Akt/mTOR Signaling Pathway
4.4. Calcium Signaling and Redox Regulation
4.5. Multi-Pathway Crosstalk
| Signaling Pathway | Source | Induction Methods | Description | References |
|---|---|---|---|---|
| Notch | hADSCs | Biomimetic niche | Maintains the undifferentiated state of iNSCs | [107] |
| Wnt/β-catenin | hADSCs | Biomimetic niche | Induces cell proliferation | [109] |
| rADSCs | Ghrelin | Promotes neural differentiation | [92] | |
| rADSCs | LC | Promotes neural differentiation | [78] | |
| rADSCs | CGRP gene-editing | Promotes neural differentiation | [94] | |
| Calcium (Ca2+) and ROS | rADSCs | VPA | Promotes neural differentiation | [75] |
| iNOS-NO-sGC | rADSCs | VPA | Promotes neural differentiation | [74] |
| Akt/mTOR | rADSCs | Ghrelin | Promotes neural differentiation | [92] |
| PKA | rADSCs | LC | Promotes neural differentiation | [78] |
5. Application Prospects
5.1. Therapeutic Potential in Parkinson’s Disease
5.2. Drug and Neurotoxicity Assessment
5.3. Neural Tissue Engineering
| Application | Key Findings | References |
|---|---|---|
| Dopaminergic neuron replacement | ADSCs differentiate into TH-positive neurons and improve motor deficits in PD models | [126,127] |
| Neuroprotection | ADSCs suppress neuroinflammation and preserve dopaminergic neurons | [123,125,137] |
| Drug assessment | ADSCs and iNSCs provide a platform for identifying neuroprotective compounds and drug delivery | [57,133] |
| Neural tissue engineering | iNSCs combined with biomaterials promote nerve regeneration and axonal regrowth | [136] |
6. Research Challenges and Future Prospects
6.1. Technical Bottlenecks
6.2. Clinical Translation Challenges
6.3. Future Directions
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADSCs | adipose-derived stem cells |
| ASCs | adult stem cells |
| BDNF | brain-derived neurotrophic factor |
| BME | β-mercaptoethanol |
| bFGF | basic fibroblastic growth factor |
| C1 | CultureOne |
| Ca2+ | calcium |
| CGRP | calcitonin gene-related peptide |
| CNS | central nervous system |
| CNTF | ciliary neurotrophic factor |
| CM | conditioned medium |
| EGF | epidermal growth factor |
| ESCs | embryonic stem cells |
| EVs | extracellular vesicles |
| FGF-2 | fibroblast growth factor 2 |
| GDNF | glial-derived neurotrophic factor |
| hADSCs | human ADSCs |
| HGF | hepatic growth factor |
| HPL | human platelet lysate |
| hPSCs | human pluripotent stem cells |
| IBMX | 3-Isobutyl-1-methylxanthine |
| iNSCs | induced neural stem cell-like cells |
| iPSCs | induced pluripotent stem cells |
| LC | L-carnitine |
| mRNA | messenger RNA |
| MSCs | mesenchymal stem cells |
| NGF | nerve growth factor |
| NICD | Notch intracellular domain |
| NSCs | neural stem cells |
| NT-3 | neurotrophin-3 |
| NTN | neurturin |
| OCD | obsessive-compulsive disorder |
| PBM | photobiomodulation |
| PD | Parkinson’s disease |
| PDGF | platelet-derived growth factor |
| PKA | protein kinase A |
| qPCR | quantitative polymerase chain reaction |
| RA | retinoic acid |
| RARs | RA receptors |
| RNA | ribonucleic acid |
| SCI | spinal cord injury |
| scRNA-seq | single-cell RNA sequencing |
| SSRIs | selective serotonin reuptake inhibitors |
| SVF | stromal vascular fraction |
| SVZ | subventricular zone |
| TGF-β | transforming growth factor beta |
| VEGF | vascular endothelial growth factor |
| VPA | Valproic Acid |
| Wnt | Wingless-related integration site |
References
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Arachchige, A.S.P.M. Depletion of Dopamine in Parkinson’s Disease and Relevant Therapeutic Options: A Review of the Literature. AIMS Neurosci. 2023, 10, 200–231. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, H.; Liu, G.; Zhao, L.; Dai, C.; Liang, Y.; Du, J.; Zhou, X.; Mo, L.; Tan, C.; et al. A Review on Pathology, Mechanism, and Therapy for Cerebellum and Tremor in Parkinson’s Disease. Npj Park. Dis. 2022, 8, 82. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, J.; Wang, Y.; Fan, F.; Liu, S.; Wang, Y. Neural Stem/Progenitor Cell Transplantation in Parkinson’s Rodent Animals: A Meta-Analysis and Systematic Review. Stem Cells Transl. Med. 2022, 11, 383–393. [Google Scholar] [CrossRef]
- Kirkeby, A.; Nelander, J.; Hoban, D.B.; Rogelius, N.; Bjartmarz, H.; Novo Nordisk Cell Therapy R&D; Storm, P.; Fiorenzano, A.; Adler, A.F.; Vale, S.; et al. Preclinical Quality, Safety, and Efficacy of a Human Embryonic Stem Cell-Derived Product for the Treatment of Parkinson’s Disease, STEM-PD. Cell Stem Cell 2023, 30, 1299–1314.e9. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced Pluripotent Stem Cells (iPSCs): Molecular Mechanisms of Induction and Applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef]
- Alió Del Barrio, J.L.; De la Mata, A.; De Miguel, M.P.; Arnalich-Montiel, F.; Nieto-Miguel, T.; El Zarif, M.; Cadenas-Martín, M.; López-Paniagua, M.; Galindo, S.; Calonge, M.; et al. Corneal Regeneration Using Adipose-Derived Mesenchymal Stem Cells. Cells 2022, 11, 2549. [Google Scholar] [CrossRef]
- Umezawa, A.; Fukuda, A.; Horikawa, R.; Uchida, H.; Enosawa, S.; Oishi, Y.; Nakamura, N.; Sasaki, K.; Yanagi, Y.; Shimizu, S.; et al. First-in-Human Clinical Study of an Embryonic Stem Cell Product for Urea Cycle Disorders. Stem Cell Res. Ther. 2025, 16, 120. [Google Scholar] [CrossRef]
- Soltani, A.; Moradi, M.; Nejad, A.R.; Moradi, S.; Javandoost, E.; Nazari, H.; Jafarian, A. Adipose-Derived Stem Cells: Potentials, Availability and Market Size in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2023, 18, 347–379. [Google Scholar] [CrossRef]
- Qin, Y.; Ge, G.; Yang, P.; Wang, L.; Qiao, Y.; Pan, G.; Yang, H.; Bai, J.; Cui, W.; Geng, D. An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity. Adv. Sci. 2023, 10, 2207334. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Cryopreservation of Whole Adipose Tissue for Future Use in Regenerative Medicine. J. Surg. Res. 2014, 187, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shen, X.-M.; Wang, Z.-F.; Li, K.; Wang, W. The Notch Signalling Pathway and miRNA Regulation Play Important Roles in the Differentiation of Schwann Cells from Adipose-Derived Stem Cells. Lab. Investig. 2022, 102, 320–328. [Google Scholar] [CrossRef]
- Pelegri, N.G.; Milthorpe, B.K.; Gorrie, C.A.; Santos, J. Neurogenic Marker Expression in Differentiating Human Adipose Derived Adult Mesenchymal Stem Cells. Stem Cell Investig. 2023, 10, 7. [Google Scholar] [CrossRef]
- Kaminska, A.; Radoszkiewicz, K.; Rybkowska, P.; Wedzinska, A.; Sarnowska, A. Interaction of Neural Stem Cells (NSCs) and Mesenchymal Stem Cells (MSCs) as a Promising Approach in Brain Study and Nerve Regeneration. Cells 2022, 11, 1464. [Google Scholar] [CrossRef]
- Mannino, G.; Russo, C.; Maugeri, G.; Musumeci, G.; Vicario, N.; Tibullo, D.; Giuffrida, R.; Parenti, R.; Lo Furno, D. Adult Stem Cell Niches for Tissue Homeostasis. J. Cell. Physiol. 2022, 237, 239–257. [Google Scholar] [CrossRef]
- Tang, X.; Deng, P.; Li, L.; He, Y.; Wang, J.; Hao, D.; Yang, H. Advances in Genetically Modified Neural Stem Cell Therapy for Central Nervous System Injury and Neurological Diseases. Stem Cell Res. Ther. 2024, 15, 482. [Google Scholar] [CrossRef]
- David-Bercholz, J.; Kuo, C.T.; Deneen, B. Astrocyte and Oligodendrocyte Responses from the Subventricular Zone After Injury. Front. Cell. Neurosci. 2021, 15, 797553. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Manthou, M.E.; Meditskou, S.; Tremblay, M.-È.; Petratos, S.; Zoupi, L.; Boziki, M.; Kesidou, E.; Simeonidou, C.; Theotokis, P. Origin and Emergence of Microglia in the CNS—An Interesting (Hi)Story of an Eccentric Cell. Curr. Issues Mol. Biol. 2023, 45, 2609–2628. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Zheng, Y.; Wen, H.; Han, X.; Zhang, M.; Guan, W. Differentiation Potential of Neural Stem Cells Derived from Fetal Sheep. Anim. Cells Syst. 2017, 21, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Iltis, A.S.; Koster, G.; Reeves, E.; Matthews, K.R.W. Ethical, Legal, Regulatory, and Policy Issues Concerning Embryoids: A Systematic Review of the Literature. Stem Cell Res. Ther. 2023, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Konno, M.; Hamabe, A.; Hasegawa, S.; Ogawa, H.; Fukusumi, T.; Nishikawa, S.; Ohta, K.; Kano, Y.; Ozaki, M.; Noguchi, Y.; et al. Adipose-Derived Mesenchymal Stem Cells and Regenerative Medicine. Dev. Growth Differ. 2013, 55, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Ouji-Sageshima, N.; Hiyama, A.; Kumamoto, M.; Kitabatake, M.; Hara, A.; Furukawa, R.; Hontsu, S.; Kawaguchi, T.; Sawabata, N.; Muro, S.; et al. Adipose-Derived Mesenchymal Stem Cells (ADSCs) Have Anti-Fibrotic Effects on Lung Fibroblasts from Idiopathic Pulmonary Fibrosis (IPF) Patients. Cells 2024, 13, 2050. [Google Scholar] [CrossRef]
- Farahmand, Y.; Nabiuni, M.; Vafaei Mastanabad, M.; Sheibani, M.; Mahmood, B.S.; Obayes, A.M.; Asadi, F.; Davallou, R. The Exo-microRNA (miRNA) Signaling Pathways in Pathogenesis and Treatment of Stroke Diseases: Emphasize on Mesenchymal Stem Cells (MSCs). Cell Biochem. Funct. 2024, 42, e3917. [Google Scholar] [CrossRef]
- Sharma, S.; Muthu, S.; Jeyaraman, M.; Ranjan, R.; Jha, S.K. Translational Products of Adipose Tissue-Derived Mesenchymal Stem Cells: Bench to Bedside Applications. World J. Stem Cells 2021, 13, 1360–1381. [Google Scholar] [CrossRef]
- Czerwiec, K.; Zawrzykraj, M.; Deptuła, M.; Skoniecka, A.; Tymińska, A.; Zieliński, J.; Kosiński, A.; Pikuła, M. Adipose-Derived Mesenchymal Stromal Cells in Basic Research and Clinical Applications. Int. J. Mol. Sci. 2023, 24, 3888. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.-H.; Kim, H.-S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef]
- Pan, H.; Bao, L.; Ji, M.; Lyu, Z.; Qi, N.; Wu, Y. A Human Embryonic Stem Cell-Derived Neural Stem Cell Senescence Model Triggered by Oxidative Stress. Curr. Stem Cell Res. Ther. 2025. [Google Scholar] [CrossRef]
- Ou, Y.; Che, M.; Peng, J.; Zhou, M.; Wu, G.; Gong, H.; Li, K.; Wang, X.; Niu, P.; Qi, S.; et al. An Efficient Method for the Isolation and Cultivation of Hypothalamic Neural Stem/Progenitor Cells from Mouse Embryos. Front. Neuroanat. 2022, 16, 711138. [Google Scholar] [CrossRef]
- Vishwakarma, S.K.; Bardia, A.; Tiwari, S.K.; Paspala, S.A.B.; Khan, A.A. Current Concept in Neural Regeneration Research: NSCs Isolation, Characterization and Transplantation in Various Neurodegenerative Diseases and Stroke: A Review. J. Adv. Res. 2014, 5, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-C.; Peng, B.-Y.; Chen, M.-S.; Thalib, B.; Ruslin, M.; Tung, T.D.X.; Chou, H.-H.; Ou, K.-L. The Potential of the Stem Cells Composite Hydrogel Wound Dressings for Promoting Wound Healing and Skin Regeneration: In Vitro and In Vivo Evaluation. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Lee, J.H.; Oh, S.C.; Lee, M.Y.; Lim, N.K. Human Fibroblast Growth Factor-Treated Adipose-Derived Stem Cells Facilitate Wound Healing and Revascularization in Rats with Streptozotocin-Induced Diabetes Mellitus. Cells 2023, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Combellack, E.J.; Griffin, M.; Sedaghati, T.; Javed, M.; Findlay, M.W.; Wallace, C.G.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M.; et al. The Regenerative Role of Adipose-Derived Stem Cells (ADSC) in Plastic and Reconstructive Surgery. Int. Wound J. 2017, 14, 112–124. [Google Scholar] [CrossRef]
- Ababneh, N.A.; Al-Kurdi, B.; Jamali, F.; Awidi, A. A Comparative Study of the Capability of MSCs Isolated from Different Human Tissue Sources to Differentiate into Neuronal Stem Cells and Dopaminergic-like Cells. PeerJ 2022, 10, e13003. [Google Scholar] [CrossRef]
- Hemmati, H.D.; Nakano, I.; Lazareff, J.A.; Masterman-Smith, M.; Geschwind, D.H.; Bronner-Fraser, M.; Kornblum, H.I. Cancerous Stem Cells Can Arise from Pediatric Brain Tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 15178–15183. [Google Scholar] [CrossRef]
- Baba, Y.; Onishi-Sakamoto, S.; Ide, K.; Nishifuji, K. Nestin Is a Marker of Unipotent Embryonic and Adult Progenitors Differentiating into an Epithelial Cell Lineage of the Hair Follicles. Sci. Rep. 2022, 12, 17820. [Google Scholar] [CrossRef]
- Hagey, D.W.; Bergsland, M.; Muhr, J. SOX2 Transcription Factor Binding and Function. Dev. Camb. Engl. 2022, 149, dev200547. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Li, Q.; Liu, H.; Han, J.; Zhang, H.; Cheng, L.; Lin, G. Short C-Terminal Musashi-1 Proteins Regulate Pluripotency States in Embryonic Stem Cells. Cell Rep. 2023, 42, 113308. [Google Scholar] [CrossRef]
- Pleskač, P.; Fargeas, C.A.; Veselska, R.; Corbeil, D.; Skoda, J. Emerging Roles of Prominin-1 (CD133) in the Dynamics of Plasma Membrane Architecture and Cell Signaling Pathways in Health and Disease. Cell. Mol. Biol. Lett. 2024, 29, 41. [Google Scholar] [CrossRef]
- Khazaei, S.; Keshavarz, G.; Bozorgi, A.; Nazari, H.; Khazaei, M. Adipose Tissue-Derived Stem Cells: A Comparative Review on Isolation, Culture, and Differentiation Methods. Cell Tissue Bank. 2022, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Mo, J.; Dong, S.; Liao, Z.; Zhang, B.; Zhu, P. Integrinβ-1 in Disorders and Cancers: Molecular Mechanisms and Therapeutic Targets. Cell Commun. Signal. CCS 2024, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Mu, Z.; Chen, Y.; Wu, C.; Shi, J.; Bai, N. Therapeutic Potential of ADSCs in Diabetic Wounds: A Proteomics-Based Approach. Front. Cell Dev. Biol. 2024, 12, 1468220. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.-S.; Sheen, I.-S.; Lin, S.-S.; Leu, C.-M.; Chang, C.-F. The Role of Endoglin in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 3208. [Google Scholar] [CrossRef]
- Papadopoulos, K.S.; Piperi, C.; Korkolopoulou, P. Clinical Applications of Adipose-Derived Stem Cell (ADSC) Exosomes in Tissue Regeneration. Int. J. Mol. Sci. 2024, 25, 5916. [Google Scholar] [CrossRef]
- Llorente, V.; Velarde, P.; Desco, M.; Gómez-Gaviro, M.V. Current Understanding of the Neural Stem Cell Niches. Cells 2022, 11, 3002. [Google Scholar] [CrossRef]
- Matsubara, S.; Matsuda, T.; Nakashima, K. Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors. Cells 2021, 10, 1145. [Google Scholar] [CrossRef]
- De Gioia, R.; Biella, F.; Citterio, G.; Rizzo, F.; Abati, E.; Nizzardo, M.; Bresolin, N.; Comi, G.P.; Corti, S. Neural Stem Cell Transplantation for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 3103. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Pękała, M.; Serwin, N.; Gliźniewicz, M.; Grygorcewicz, B.; Michalczyk, A.; Heryć, R.; Budkowska, M.; Dołęgowska, B. The Use of Stem Cells as a Potential Treatment Method for Selected Neurodegenerative Diseases: Review. Cell. Mol. Neurobiol. 2023, 43, 2643–2673. [Google Scholar] [CrossRef]
- Setiawan, A.M.; Kamarudin, T.A.; Abd Ghafar, N. The Role of BMP4 in Adipose-Derived Stem Cell Differentiation: A Minireview. Front. Cell Dev. Biol. 2022, 10, 1045103. [Google Scholar] [CrossRef]
- Jankowski, M.; Stefańska, K.; Suchodolski, M.; Dompe, C.; Wąsiatycz, G.; Kempisty, B.; Nowicki, M.; Roszak, M. Differential Regulation of Apoptosis-Related Genes during Long-Term Culture and Differentiation of Canine Adipose-Derived Stem Cells —A Functional Bioinformatical Analysis. Front. Genet. 2024, 15, 1515778. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.; Fayoud, H.; Shaimardanova, A.; Sufianov, A.; Sufianova, G.; Solovyeva, V.; Rizvanov, A. Growth Factors and Their Application in the Therapy of Hereditary Neurodegenerative Diseases. Biomedicines 2024, 12, 1906. [Google Scholar] [CrossRef] [PubMed]
- Surico, P.L.; Scarabosio, A.; Miotti, G.; Grando, M.; Salati, C.; Parodi, P.C.; Spadea, L.; Zeppieri, M. Unlocking the Versatile Potential: Adipose-Derived Mesenchymal Stem Cells in Ocular Surface Reconstruction and Oculoplastics. World J. Stem Cells 2024, 16, 89–101. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Li, Y.; Wang, L.; Zhao, Y.; Yuan, R.; Yang, M.-M.; Chen, Y.; Zhang, H.; Zhou, F.-H.; Qian, Z.-R.; et al. Mesenchymal Stem Cell-Derived Exosomes Regulate Microglia Phenotypes: A Promising Treatment for Acute Central Nervous System Injury. Neural Regen. Res. 2023, 18, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chen, Q.; Zhang, Q.; Tian, W.; Chen, T.; Liu, Z. Therapeutic Potential of Adipose-Derived Stem Cell Extracellular Vesicles: From Inflammation Regulation to Tissue Repair. Stem Cell Res. Ther. 2024, 15, 249. [Google Scholar] [CrossRef]
- Ciervo, Y.; Gatto, N.; Allen, C.; Grierson, A.; Ferraiuolo, L.; Mead, R.J.; Shaw, P.J. Adipose-Derived Stem Cells Protect Motor Neurons and Reduce Glial Activation in Both In Vitro and In Vivo Models of ALS. Mol. Ther. Methods Clin. Dev. 2021, 21, 413–433. [Google Scholar] [CrossRef]
- Corrêa, N.C.R.; Kuligovski, C.; Paschoal, A.C.C.; Abud, A.P.R.; Rebelatto, C.L.K.; Leite, L.M.B.; Senegaglia, A.C.; Dallagiovanna, B.; de Aguiar, A.M. Human Adipose-Derived Stem Cells (ADSC) and Human Periodontal Ligament Stem Cells (PDLSC) as Cellular Substrates of a Toxicity Prediction Assay. Regul. Toxicol. Pharmacol. 2018, 92, 75–82. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, H.; Zheng, Q.; Yang, L.; Cao, G.; Yuan, J.; Hu, S.; Li, Z. Modulating Efficient Differentiation of Neural Stem Cells into Neurons by Using Plasmonic Nanoparticles and the NIR II Irradiation to Boost Therapy of Parkinson’s Disease. Nano Today 2024, 57, 102392. [Google Scholar] [CrossRef]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural Stem Cell Niche Heterogeneity. Semin. Cell Dev. Biol. 2019, 95, 42–53. [Google Scholar] [CrossRef]
- Salehi, H.; Amirpour, N.; Niapour, A.; Razavi, S. An Overview of Neural Differentiation Potential of Human Adipose Derived Stem Cells. Stem Cell Rev. Rep. 2016, 12, 26–41. [Google Scholar] [CrossRef]
- Rhode, S.C.; Beier, J.P.; Ruhl, T. Adipose Tissue Stem Cells in Peripheral Nerve Regeneration-In Vitro and In Vivo. J. Neurosci. Res. 2021, 99, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.E.; Blurton-Jones, M. Neural Stem Cell Therapy for Neurodegenerative Disorders: The Role of Neurotrophic Support. Neurochem. Int. 2017, 106, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Remmers, F.; Lutz, B.; Leschik, J. ESC-Derived BDNF-Overexpressing Neural Progenitors Differentially Promote Recovery in Huntington’s Disease Models by Enhanced Striatal Differentiation. Stem Cell Rep. 2016, 7, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.-L.; Zou, Y.; Shi, Y.; Zhang, P.; Zhang, R.-P.; Dai, X.-J.; Liu, B.; Wang, T.-H. Tree Shrew Neural Stem Cell Transplantation Promotes Functional Recovery of Tree Shrews with a Hemi-Sectioned Spinal Cord Injury by Upregulating Nerve Growth Factor Expression. Int. J. Mol. Med. 2018, 41, 3267–3277. [Google Scholar] [CrossRef]
- Moon, S.; Hong, J.; Go, S.; Kim, B.-S. Immunomodulation for Tissue Repair and Regeneration. Tissue Eng. Regen. Med. 2023, 20, 389–409. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, H.; Tang, R.; Zhang, S.; Chen, X.; Pei, L. Autologous Transplantation of Adipose-Derived Stromal Cells Combined with Sevoflurane Ameliorates Acute Lung Injury Induced by Cecal Ligation and Puncture in Rats. Sci. Rep. 2020, 10, 13760. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.O.; Gracheva, O.A.; Mukhutdinova, D.M.; Chelyshev, Y.A.; Rizvanov, A.A. Mesenchymal Stem Cells and the Neuronal Microenvironment in the Area of Spinal Cord Injury. Neural Regen. Res. 2019, 14, 227–237. [Google Scholar] [CrossRef]
- Shariati Najafabadi, S.; Amirpour, N.; Amini, S.; Zare, N.; Kazemi, M.; Salehi, H. Human Adipose Derived Stem Cell Exosomes Enhance the Neural Differentiation of PC12 Cells. Mol. Biol. Rep. 2021, 48, 5033–5043. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Duester, G. Retinoic Acid Signaling Pathways. Dev. Camb. Engl. 2019, 146, dev167502. [Google Scholar] [CrossRef]
- Brown, G. Retinoic Acid Receptor Regulation of Decision-Making for Cell Differentiation. Front. Cell Dev. Biol. 2023, 11, 1182204. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Ren, W.; Zhou, Y.; Ye, Z.; Tan, W.-S. β-Mercaptoethanol Promotes Osteogenesis of Human Mesenchymal Stem Cells via Sirt1-ERK Pathway. Cytotechnology 2020, 72, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Trentz, O.A.; Reddy, M.S.; Rela, M.; Kandasamy, M.; Sellathamby, S. In Vitro Transdifferentiation of Human Adipose Tissue-Derived Stem Cells to Neural Lineage Cells—A Stage-Specific Incidence. Adipocyte 2019, 8, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Bağcı, F.Ö.; Özgörgülü, A.; Çiçek, G.; Özen, E.U.; Duman, S.; Aktan, T.M.; Reisli, I.; Bagci, F.O.; Özgörgülü, A.; Çiçek, G.; et al. Evaluation of the Interaction Between Wharton’s Jelly-Derived Mesenchymal Stem Cells and β-Mercaptoethanol. Cureus 2025, 17, e83115. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Fujimoto, S.; Hayashi, D.; Suzuki, T.; Sakaue, M.; Miyazaki, Y.; Tanaka, K.; Usami, M.; Takizawa, T. Valproic Acid Promotes Mature Neuronal Differentiation of Adipose Tissue-Derived Stem Cells through iNOS-NO-sGC Signaling Pathway. Nitric Oxide Biol. Chem. 2019, 93, 1–5. [Google Scholar] [CrossRef]
- Satoh, A.; Fujimoto, S.; Irie, T.; Suzuki, T.; Miyazaki, Y.; Tanaka, K.; Usami, M.; Takizawa, T. Valproic Acid Promotes Differentiation of Adipose Tissue-Derived Stem Cells to Neuronal Cells Selectively Expressing Functional N-Type Voltage-Gated Ca2+ Channels. Biochem. Biophys. Res. Commun. 2022, 589, 55–62. [Google Scholar] [CrossRef]
- Liu, S.; Tian, H.; Niu, Y.; Yu, C.; Xie, L.; Jin, Z.; Niu, W.; Ren, J.; Fu, L.; Yao, Z. Combined Cell Grafting and VPA Administration Facilitates Neural Repair through Axonal Regeneration and Synaptogenesis in Traumatic Brain Injury. Acta Biochim. Biophys. Sin. 2022, 54, 1289–1300. [Google Scholar] [CrossRef]
- Guerreiro, G.; Deon, M.; Becker, G.S.; Dos Reis, B.G.; Wajner, M.; Vargas, C.R. Neuroprotective Effects of L-Carnitine towards Oxidative Stress and Inflammatory Processes: A Review of Its Importance as a Therapeutic Drug in Some Disorders. Metab. Brain Dis. 2025, 40, 127. [Google Scholar] [CrossRef]
- Fathi, E.; Farahzadi, R.; Charoudeh, H.N. L-Carnitine Contributes to Enhancement of Neurogenesis from Mesenchymal Stem Cells through Wnt/β-Catenin and PKA Pathway. Exp. Biol. Med. 2017, 242, 482–486. [Google Scholar] [CrossRef]
- Santos-Cruz, L.F.; Campos-Aguilar, M.; Castañeda-Partida, L.; Sigrist-Flores, S.C.; Heres-Pulido, M.E.; Dueñas-García, I.E.; Piedra-Ibarra, E.; Jiménez-Flores, R.; Ponciano-Gómez, A. Impact of Larval Sertraline Exposure on Alternative Splicing in Neural Tissue of Adult Drosophila Melanogaster. Int. J. Mol. Sci. 2025, 26, 563. [Google Scholar] [CrossRef]
- Razavi, S.; Jahromi, M.; Amirpour, N.; Khosravizadeh, Z. Effect of Sertraline on Proliferation and Neurogenic Differentiation of Human Adipose-Derived Stem Cells. Adv. Biomed. Res. 2014, 3, 97. [Google Scholar] [CrossRef]
- Feng, N.; Han, Q.; Li, J.; Wang, S.; Li, H.; Yao, X.; Zhao, R.C. Generation of Highly Purified Neural Stem Cells from Human Adipose-Derived Mesenchymal Stem Cells by Sox1 Activation. Stem Cells Dev. 2014, 23, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, S. Potential Role of Growth Factors Controlled Release in Achieving Enhanced Neuronal Trans-Differentiation from Mesenchymal Stem Cells for Neural Tissue Repair and Regeneration. Mol. Neurobiol. 2022, 59, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Guan, Y.; Zheng, J.; Wang, X.; Wang, M.; Zhu, Z.; Peng, Q.; Wang, H.-H.; Li, M. Development of Synthetic Modulator Enabling Long-Term Propagation and Neurogenesis of Human Embryonic Stem Cell-Derived Neural Progenitor Cells. Biol. Res. 2023, 56, 59. [Google Scholar] [CrossRef]
- Kang, S.K.; Putnam, L.A.; Ylostalo, J.; Popescu, I.R.; Dufour, J.; Belousov, A.; Bunnell, B.A. Neurogenesis of Rhesus Adipose Stromal Cells. J. Cell Sci. 2004, 117 Pt 18, 4289–4299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, N.; Tang, Y.; Yang, E.; Dong, S.; Huang, M.; Pan, C.; Zhang, Y.; Zhang, P.; Chen, H.; et al. Efficient Generation of Neural Stem Cell-like Cells from Rat Adipose Derived Stem Cells after Lentiviral Transduction with Green Fluorescent Protein. Mol. Neurobiol. 2014, 50, 647–654. [Google Scholar] [CrossRef]
- Darvishi, M.; Tiraihi, T.; Mesbah-Namin, S.A.; Delshad, A.; Taheri, T. Motor Neuron Transdifferentiation of Neural Stem Cell from Adipose-Derived Stem Cell Characterized by Differential Gene Expression. Cell. Mol. Neurobiol. 2017, 37, 275–289. [Google Scholar] [CrossRef]
- Yang, E.; Liu, N.; Tang, Y.; Hu, Y.; Zhang, P.; Pan, C.; Dong, S.; Zhang, Y.; Tang, Z. Generation of Neurospheres from Human Adipose-Derived Stem Cells. BioMed Res. Int. 2015, 2015, 743714. [Google Scholar] [CrossRef]
- Lim, J.-H.; Koh, S.; Thomas, R.; Breen, M.; Olby, N.J. Evaluation of Gene Expression and DNA Copy Number Profiles of Adipose Tissue-Derived Stromal Cells and Consecutive Neurosphere-like Cells Generated from Dogs with Naturally Occurring Spinal Cord Injury. Am. J. Vet. Res. 2017, 78, 371–380. [Google Scholar] [CrossRef]
- Kruminis-Kaszkiel, E.; Osowski, A.; Bejer-Oleńska, E.; Dziekoński, M.; Wojtkiewicz, J. Differentiation of Human Mesenchymal Stem Cells from Wharton’s Jelly Towards Neural Stem Cells Using a Feasible and Repeatable Protocol. Cells 2020, 9, 739. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-Derived Stem Cells Differentiate into a Schwann Cell Phenotype and Promote Neurite Outgrowth In Vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-B.; Pan, Y.-M.; Liu, Y.-S.; Hu, J.-H.; Zhang, X.-D.; Zhang, D.-W.; Wang, Y.; Feng, Y.-K.; Yu, J.-B.; Cheng, Y.-X. Ghrelin Promotes Neural Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cell via AKT/mTOR and β-Catenin Signaling Pathways. Kaohsiung J. Med. Sci. 2020, 36, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhou, C.; Wang, N.; Yang, H.; Gao, W.-Q. Conversion of Adipose Tissue-Derived Mesenchymal Stem Cells to Neural Stem Cell-Like Cells by a Single Transcription Factor, Sox2. Cell. Reprogramming 2015, 17, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Du, X.; Fang, Z.; Xiong, W.; Li, G.; Liao, H.; Xiao, J.; Wang, G.; Li, F. Effect of Calcitonin Gene-Related Peptide on the Neurogenesis of Rat Adipose-Derived Stem Cells In Vitro. PLoS ONE 2014, 9, e86334. [Google Scholar] [CrossRef]
- Chandrababu, K.; Sreelatha, H.V.; Sudhadevi, T.; Anil, A.; Arumugam, S.; Krishnan, L.K. In Vivo Neural Tissue Engineering Using Adipose-Derived Mesenchymal Stem Cells and Fibrin Matrix. J. Spinal Cord. Med. 2023, 46, 262–276. [Google Scholar] [CrossRef]
- Gomila Pelegri, N.; Stanczak, A.M.; Bottomley, A.L.; Milthorpe, B.K.; Gorrie, C.A.; Padula, M.P.; Santos, J. Adipose-Derived Stem Cells Spontaneously Express Neural Markers When Grown in a PEG-Based 3D Matrix. Int. J. Mol. Sci. 2023, 24, 12139. [Google Scholar] [CrossRef]
- Liu, R.; Meng, X.; Yu, X.; Wang, G.; Dong, Z.; Zhou, Z.; Qi, M.; Yu, X.; Ji, T.; Wang, F. From 2D to 3D Co-Culture Systems: A Review of Co-Culture Models to Study the Neural Cells Interaction. Int. J. Mol. Sci. 2022, 23, 13116. [Google Scholar] [CrossRef]
- Lo Furno, D.; Mannino, G.; Pellitteri, R.; Zappalà, A.; Parenti, R.; Gili, E.; Vancheri, C.; Giuffrida, R. Conditioned Media from Glial Cells Promote a Neural-Like Connexin Expression in Human Adipose-Derived Mesenchymal Stem Cells. Front. Physiol. 2018, 9, 1742. [Google Scholar] [CrossRef]
- Bahmani, L.; Taha, M.F.; Javeri, A. Coculture with Embryonic Stem Cells Improves Neural Differentiation of Adipose Tissue-Derived Stem Cells. Neuroscience 2014, 272, 229–239. [Google Scholar] [CrossRef]
- Gomila Pelegri, N.; Stanczak, A.M.; Bottomley, A.L.; Cummins, M.L.; Milthorpe, B.K.; Gorrie, C.A.; Padula, M.P.; Santos, J. Neural Marker Expression in Adipose-Derived Stem Cells Grown in PEG-Based 3D Matrix Is Enhanced in the Presence of B27 and CultureOne Supplements. Int. J. Mol. Sci. 2023, 24, 16269. [Google Scholar] [CrossRef]
- Romano, I.R.; D’Angeli, F.; Gili, E.; Fruciano, M.; Lombardo, G.A.G.; Mannino, G.; Vicario, N.; Russo, C.; Parenti, R.; Vancheri, C.; et al. Melatonin Enhances Neural Differentiation of Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 4891. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, N.; Lee, J.; Choe, E.K.; Kim, M.K.; Lee, J.; Byun, M.S.; Chon, M.-W.; Kim, S.W.; Lee, C.J.; et al. Small Molecule-Based Lineage Switch of Human Adipose-Derived Stem Cells into Neural Stem Cells and Functional GABAergic Neurons. Sci. Rep. 2017, 7, 10166. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Chwalek, K.; Moore, Y.E.; Kelley, M.R.; Abbott, R.D.; Moss, S.; Kaplan, D.L. Expandable and Rapidly Differentiating Human Induced Neural Stem Cell Lines for Multiple Tissue Engineering Applications. Stem Cell Rep. 2016, 7, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered Neural Tissue with Aligned, Differentiated Adipose-Derived Stem Cells Promotes Peripheral Nerve Regeneration across a Critical Sized Defect in Rat Sciatic Nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef]
- Lo Furno, D.; Mannino, G.; Giuffrida, R.; Gili, E.; Vancheri, C.; Tarico, M.S.; Perrotta, R.E.; Pellitteri, R. Neural Differentiation of Human Adipose-Derived Mesenchymal Stem Cells Induced by Glial Cell Conditioned Media. J. Cell. Physiol. 2018, 233, 7091–7100. [Google Scholar] [CrossRef]
- Abrahamse, H.; Crous, A. Photobiomodulation Effects on Neuronal Transdifferentiation of Immortalized Adipose-Derived Mesenchymal Stem Cells. Lasers Med. Sci. 2024, 39, 257. [Google Scholar] [CrossRef]
- Jang, S.; Cho, H.-H.; Cho, Y.-B.; Park, J.-S.; Jeong, H.-S. Functional Neural Differentiation of Human Adipose Tissue-Derived Stem Cells Using bFGF and Forskolin. BMC Cell Biol. 2010, 11, 25. [Google Scholar] [CrossRef]
- Anghileri, E.; Marconi, S.; Pignatelli, A.; Cifelli, P.; Galié, M.; Sbarbati, A.; Krampera, M.; Belluzzi, O.; Bonetti, B. Neuronal Differentiation Potential of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 909–916. [Google Scholar] [CrossRef]
- Chandrababu, K.; Senan, M.; Krishnan, L.K. Exploitation of Fibrin-Based Signaling Niche for Deriving Progenitors from Human Adipose-Derived Mesenchymal Stem Cells towards Potential Neural Engineering Applications. Sci. Rep. 2020, 10, 7116. [Google Scholar] [CrossRef]
- Schäffler, A.; Büchler, C. Concise Review: Adipose Tissue-Derived Stromal Cells—Basic and Clinical Implications for Novel Cell-Based Therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Zhang, H.; Wang, Z.; Nan, G.; Li, Y.; Zhang, F.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. The Evolving Roles of Canonical WNT Signaling in Stem Cells and Tumorigenesis: Implications in Targeted Cancer Therapies. Lab. Investig. J. Tech. Methods Pathol. 2016, 96, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch Signaling Pathway: Architecture, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.; Kareta, M.S.; Chanda, S.; Ahlenius, H.; Perotti, N.; Zhou, B.; Grieder, S.D.; Ge, X.; Drake, S.; Euong Ang, C.; et al. Myt1l Safeguards Neuronal Identity by Actively Repressing Many Non-Neuronal Fates. Nature 2017, 544, 245–249. [Google Scholar] [CrossRef]
- Borghese, L.; Dolezalova, D.; Opitz, T.; Haupt, S.; Leinhaas, A.; Steinfarz, B.; Koch, P.; Edenhofer, F.; Hampl, A.; Brüstle, O. Inhibition of Notch Signaling in Human Embryonic Stem Cell–Derived Neural Stem Cells Delays G1/S Phase Transition and Accelerates Neuronal Differentiation In Vitro and In Vivo. Stem Cells 2010, 28, 955–964. [Google Scholar] [CrossRef]
- Leung, R.W.H.; Lee, T.K.W. Wnt/β-Catenin Signaling as a Driver of Stemness and Metabolic Reprogramming in Hepatocellular Carcinoma. Cancers 2022, 14, 5468. [Google Scholar] [CrossRef]
- Yu, M.; Qin, K.; Fan, J.; Zhao, G.; Zhao, P.; Zeng, W.; Chen, C.; Wang, A.; Wang, Y.; Zhong, J.; et al. The Evolving Roles of Wnt Signaling in Stem Cell Proliferation and Differentiation, the Development of Human Diseases, and Therapeutic Opportunities. Genes Dis. 2024, 11, 101026. [Google Scholar] [CrossRef]
- Kang, D.E.; Soriano, S.; Xia, X.; Eberhart, C.G.; Strooper, B.D.; Zheng, H.; Koo, E.H. Presenilin Couples the Paired Phosphorylation of β-Catenin Independent of Axin: Implications for β-Catenin Activation in Tumorigenesis. Cell 2002, 110, 751–762. [Google Scholar] [CrossRef]
- Austin, S.H.L.; Gabarró-Solanas, R.; Rigo, P.; Paun, O.; Harris, L.; Guillemot, F.; Urbán, N. Wnt/β-Catenin Signalling Is Dispensable for Adult Neural Stem Cell Homeostasis and Activation. Dev. Camb. Engl. 2021, 148, dev199629. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Sun, F.; Li, Y.; Wu, J.; Zou, Q. The Nerve-Induced Adipose Stem Cells Promote Nerve Repair in Stress Urinary Incontinence by Regulating Schwann Cell Repair Phenotype Conversion Through Activation of the Notch Pathway. Mol. Neurobiol. 2025, 62, 7330–7344. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.; Shi, G.; Lei, X.; Huang, Y.; Bai, L.; Qin, C. Effectiveness and Mechanisms of Adipose-Derived Stem Cell Therapy in Animal Models of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Transl. Neurodegener. 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.K.; Martinez, T.N.; Ruhn, K.A.; Wrage, P.C.; Keefer, E.W.; Botterman, B.R.; Tansey, K.E.; Tansey, M.G. Autologous Transplants of Adipose-Derived Adult Stromal (ADAS) Cells Afford Dopaminergic Neuroprotection in a Model of Parkinson’s Disease. Exp. Neurol. 2008, 210, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Tambe, P.; Undale, V.; Sanap, A.; Bhonde, R.; Mante, N. The Prospective Role of Mesenchymal Stem Cells in Parkinson’s Disease. Park. Relat. Disord. 2024, 127, 107087. [Google Scholar] [CrossRef]
- Feng, N.; Huang, X.; Jia, Y. Small Extracellular Vesicles from Adipose Derived Stem Cells Alleviate Microglia Activation and Improve Motor Deficit of Parkinson’s Disease via miR-100-5p/DTX3L/STAT1 Signaling Axis. Exp. Neurol. 2025, 389, 115250. [Google Scholar] [CrossRef]
- Faghih, H.; Javeri, A.; Amini, H.; Taha, M.F. Directed Differentiation of Human Adipose Tissue-Derived Stem Cells to Dopaminergic Neurons in Low-Serum and Serum-Free Conditions. Neurosci. Lett. 2019, 708, 134353. [Google Scholar] [CrossRef]
- Hamedi, H.; Ghorbanian, S.; Mirzaeian, L.; Abrari, K.; Mozdziak, P.; Ghorbanian, M.T. Intravenous Transplantation of Adipose-Derived Mesenchymal Stem Cells Promoted the Production of Dopaminergic Neurons and Improved Spatial Memory in A Rat Model of Parkinson’s Disease. Cell J. Yakhteh 2023, 25, 317–326. [Google Scholar] [CrossRef]
- Jankowski, M.; Dompe, C.; Sibiak, R.; Wąsiatycz, G.; Mozdziak, P.; Jaśkowski, J.M.; Antosik, P.; Kempisty, B.; Dyszkiewicz-Konwińska, M. In Vitro Cultures of Adipose-Derived Stem Cells: An Overview of Methods, Molecular Analyses, and Clinical Applications. Cells 2020, 9, 1783. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Shi, L.; Fang, L.; Xu, L.; Cao, Y. Neural Stemness Unifies Cell Tumorigenicity and Pluripotent Differentiation Potential. J. Biol. Chem. 2022, 298, 102106. [Google Scholar] [CrossRef]
- Chi, K.; Fu, R.-H.; Huang, Y.-C.; Chen, S.-Y.; Hsu, C.-J.; Lin, S.-Z.; Tu, C.-T.; Chang, L.-H.; Wu, P.-A.; Liu, S.-P. Adipose-Derived Stem Cells Stimulated with n-Butylidenephthalide Exhibit Therapeutic Effects in a Mouse Model of Parkinson’s Disease. Cell Transplant. 2018, 27, 456–470. [Google Scholar] [CrossRef]
- Takahashi, H.; Ishikawa, H.; Tanaka, A. Regenerative Medicine for Parkinson’s Disease Using Differentiated Nerve Cells Derived from Human Buccal Fat Pad Stem Cells. Hum. Cell 2017, 30, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, M.; Li, H.; Yan, M.; He, Z.; Wang, W.; Wang, W.; Lu, S. Recovery of Behavioral Symptoms in Hemi-Parkinsonian Rhesus Monkeys through Combined Gene and Stem Cell Therapy. Cytotherapy 2013, 15, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Herea, D.-D.; Labusca, L.; Radu, E.; Chiriac, H.; Grigoras, M.; Panzaru, O.D.; Lupu, N. Human Adipose-Derived Stem Cells Loaded with Drug-Coated Magnetic Nanoparticles for In-Vitro Tumor Cells Targeting. Mater. Sci. Eng. C 2019, 94, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Juberg, D.R.; Fox, D.A.; Forcelli, P.A.; Kacew, S.; Lipscomb, J.C.; Saghir, S.A.; Sherwin, C.M.; Koenig, C.M.; Hays, S.M.; Kirman, C.R. A Perspective on In Vitro Developmental Neurotoxicity Test Assay Results: An Expert Panel Review. Regul. Toxicol. Pharmacol. 2023, 143, 105444. [Google Scholar] [CrossRef]
- Wu, H.; Fan, Y.; Zhang, M. Advanced Progress in the Role of Adipose-Derived Mesenchymal Stromal/Stem Cells in the Application of Central Nervous System Disorders. Pharmaceutics 2023, 15, 2637. [Google Scholar] [CrossRef]
- Nakajima, T.; Tada, K.; Nakada, M.; Matsuta, M.; Tsuchiya, H. Facilitatory Effects of Artificial Nerve Filled with Adipose-Derived Stem Cell Sheets on Peripheral Nerve Regeneration: An Experimental Study. J. Orthop. Sci. 2021, 26, 1113–1118. [Google Scholar] [CrossRef]
- Berg, J.; Roch, M.; Altschüler, J.; Winter, C.; Schwerk, A.; Kurtz, A.; Steiner, B. Human Adipose-Derived Mesenchymal Stem Cells Improve Motor Functions and Are Neuroprotective in the 6-Hydroxydopamine-Rat Model for Parkinson’s Disease When Cultured in Monolayer Cultures but Suppress Hippocampal Neurogenesis and Hippocampal Memory Function When Cultured in Spheroids. Stem Cell Rev. Rep. 2015, 11, 133–149. [Google Scholar] [CrossRef]
- Isaković, J.; Šerer, K.; Barišić, B.; Mitrečić, D. Mesenchymal Stem Cell Therapy for Neurological Disorders: The Light or the Dark Side of the Force? Front. Bioeng. Biotechnol. 2023, 11, 1139359. [Google Scholar] [CrossRef]
- Câmara, D.A.D.; Shibli, J.A.; Müller, E.A.; De-Sá-Junior, P.L.; Porcacchia, A.S.; Blay, A.; Lizier, N.F. Adipose Tissue-Derived Stem Cells: The Biologic Basis and Future Directions for Tissue Engineering. Materials 2020, 13, 3210. [Google Scholar] [CrossRef]
- Saffari, T.M.; Saffari, S.; Vyas, K.S.; Mardini, S.; Shin, A.Y. Role of Adipose Tissue Grafting and Adipose-Derived Stem Cells in Peripheral Nerve Surgery. Neural Regen. Res. 2022, 17, 2179–2184. [Google Scholar] [CrossRef]
- Dong, L.; Li, X.; Leng, W.; Guo, Z.; Cai, T.; Ji, X.; Xu, C.; Zhu, Z.; Lin, J. Adipose Stem Cells in Tissue Regeneration and Repair: From Bench to Bedside. Regen. Ther. 2023, 24, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Farhana, S.; Kai, Y.C.; Kadir, R.; Sulaiman, W.A.W.; Nordin, N.A.; Nasir, N.A.M. The Fate of Adipose Tissue and Adipose-Derived Stem Cells in Allograft. Cell Tissue Res. 2023, 394, 269–292. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, W.; Liu, Q.; Ou, Y.; Li, J.; Yan, Q.; Zhang, P. Single-Cell RNA-Seq Reveals the Pseudo-Temporal Dynamic Evolution Characteristics of ADSCs to Neuronal Differentiation. Cell. Mol. Neurobiol. 2024, 45, 5. [Google Scholar] [CrossRef] [PubMed]
- Toyserkani, N.M.; Jørgensen, M.G.; Tabatabaeifar, S.; Jensen, C.H.; Sheikh, S.P.; Sørensen, J.A. Concise Review: A Safety Assessment of Adipose-Derived Cell Therapy in Clinical Trials: A Systematic Review of Reported Adverse Events. Stem Cells Transl. Med. 2017, 6, 1786–1794. [Google Scholar] [CrossRef]
- Merrell, A.J.; Stanger, B.Z. Adult Cell Plasticity In Vivo: Trans-Differentiation Is Back in Style. Nat. Rev. Mol. Cell Biol. 2016, 17, 413–425. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Zhao, L.; Liu, Y.; Su, Y.; Gong, X.; Liu, F.; Zhang, L. The Heterogeneity of Mesenchymal Stem Cells: An Important Issue to Be Addressed in Cell Therapy. Stem Cell Res. Ther. 2023, 14, 381. [Google Scholar] [CrossRef]
- Cremona, M.; Gallazzi, M.; Rusconi, G.; Mariotta, L.; Gola, M.; Soldati, G. State of the Art in the Standardization of Stromal Vascular Fraction Processing. Biomolecules 2025, 15, 199. [Google Scholar] [CrossRef]
- van Strien, M.E.; Sluijs, J.A.; Reynolds, B.A.; Steindler, D.A.; Aronica, E.; Hol, E.M. Isolation of Neural Progenitor Cells from the Human Adult Subventricular Zone Based on Expression of the Cell Surface Marker CD271. Stem Cells Transl. Med. 2014, 3, 470–480. [Google Scholar] [CrossRef]
- Fayazi, M.; Salehnia, M.; Ziaei, S. Differentiation of Human CD146-Positive Endometrial Stem Cells to Adipogenic-, Osteogenic-, Neural Progenitor-, and Glial-like Cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 408–414. [Google Scholar] [CrossRef]
- Figiel-Dabrowska, A.; Radoszkiewicz, K.; Rybkowska, P.; Krzesniak, N.E.; Sulejczak, D.; Sarnowska, A. Neurogenic and Neuroprotective Potential of Stem/Stromal Cells Derived from Adipose Tissue. Cells 2021, 10, 1475. [Google Scholar] [CrossRef]
- Bydon, M. Phase I Clinical Trial of Autologous Adipose Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury; Clinical Trial Registration NCT03308565; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/study/NCT03308565 (accessed on 31 March 2025).
- Ahmed, M.; Muffat, J.; Li, Y. Understanding Neural Development and Diseases Using CRISPR Screens in Human Pluripotent Stem Cell-Derived Cultures. Front. Cell Dev. Biol. 2023, 11, 1158373. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E. Stem Cell Therapy: A Revolutionary Cure or a Pandora’s Box. Stem Cell Res. Ther. 2025, 16, 255. [Google Scholar] [CrossRef] [PubMed]
- Magaz, A.; Faroni, A.; Gough, J.E.; Reid, A.J.; Li, X.; Blaker, J.J. Bioactive Silk-Based Nerve Guidance Conduits for Augmenting Peripheral Nerve Repair. Adv. Healthc. Mater. 2018, 7, e1800308. [Google Scholar] [CrossRef] [PubMed]
- Poongodi, R.; Chen, Y.-L.; Yang, T.-H.; Huang, Y.-H.; Yang, K.D.; Lin, H.-C.; Cheng, J.-K. Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair. Int. J. Mol. Sci. 2021, 22, 13347. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Zhang, Z.; Li, M.; Yung, K.K.L.; Cheung, K.-h. From Adipose to Action: Reprogramming Stem Cells for Functional Neural Progenitors for Neural Regenerative Therapy. Int. J. Mol. Sci. 2025, 26, 6599. https://doi.org/10.3390/ijms26146599
Peng J, Zhang Z, Li M, Yung KKL, Cheung K-h. From Adipose to Action: Reprogramming Stem Cells for Functional Neural Progenitors for Neural Regenerative Therapy. International Journal of Molecular Sciences. 2025; 26(14):6599. https://doi.org/10.3390/ijms26146599
Chicago/Turabian StylePeng, Junjie, Zhu Zhang, Min Li, Ken Kin Lam Yung, and King-ho Cheung. 2025. "From Adipose to Action: Reprogramming Stem Cells for Functional Neural Progenitors for Neural Regenerative Therapy" International Journal of Molecular Sciences 26, no. 14: 6599. https://doi.org/10.3390/ijms26146599
APA StylePeng, J., Zhang, Z., Li, M., Yung, K. K. L., & Cheung, K.-h. (2025). From Adipose to Action: Reprogramming Stem Cells for Functional Neural Progenitors for Neural Regenerative Therapy. International Journal of Molecular Sciences, 26(14), 6599. https://doi.org/10.3390/ijms26146599







