Collagen-Based Drug Delivery Agents for Glioblastoma Multiforme Treatment
Abstract
1. Introduction
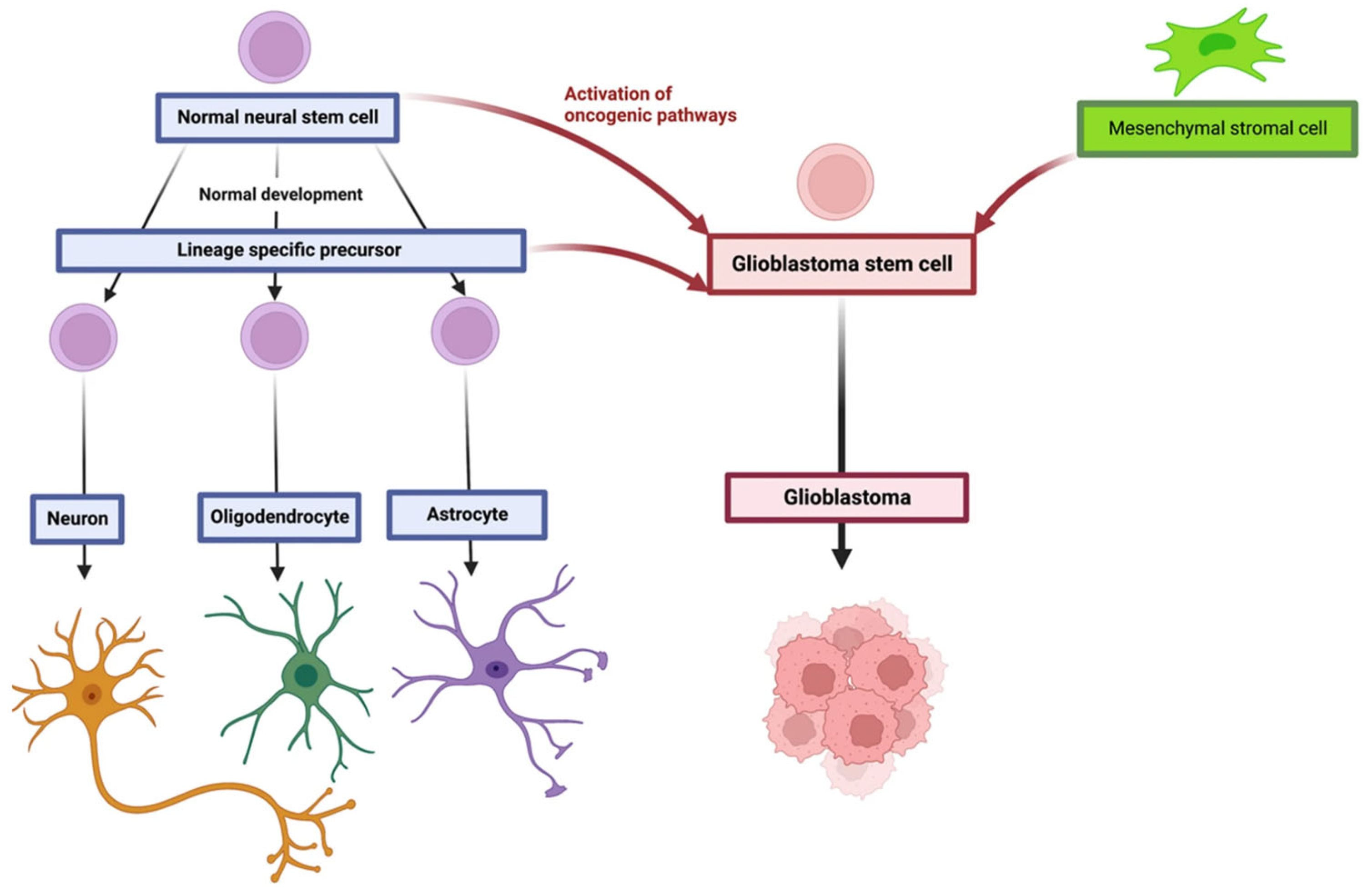
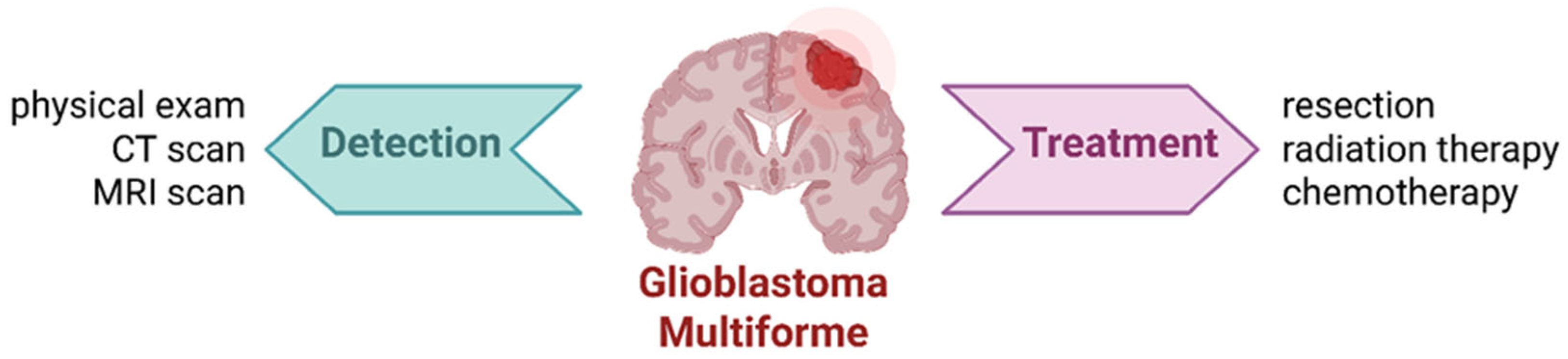
2. Glioblastoma Multiforme
3. Role of Proteins in the Brain
4. Collagen
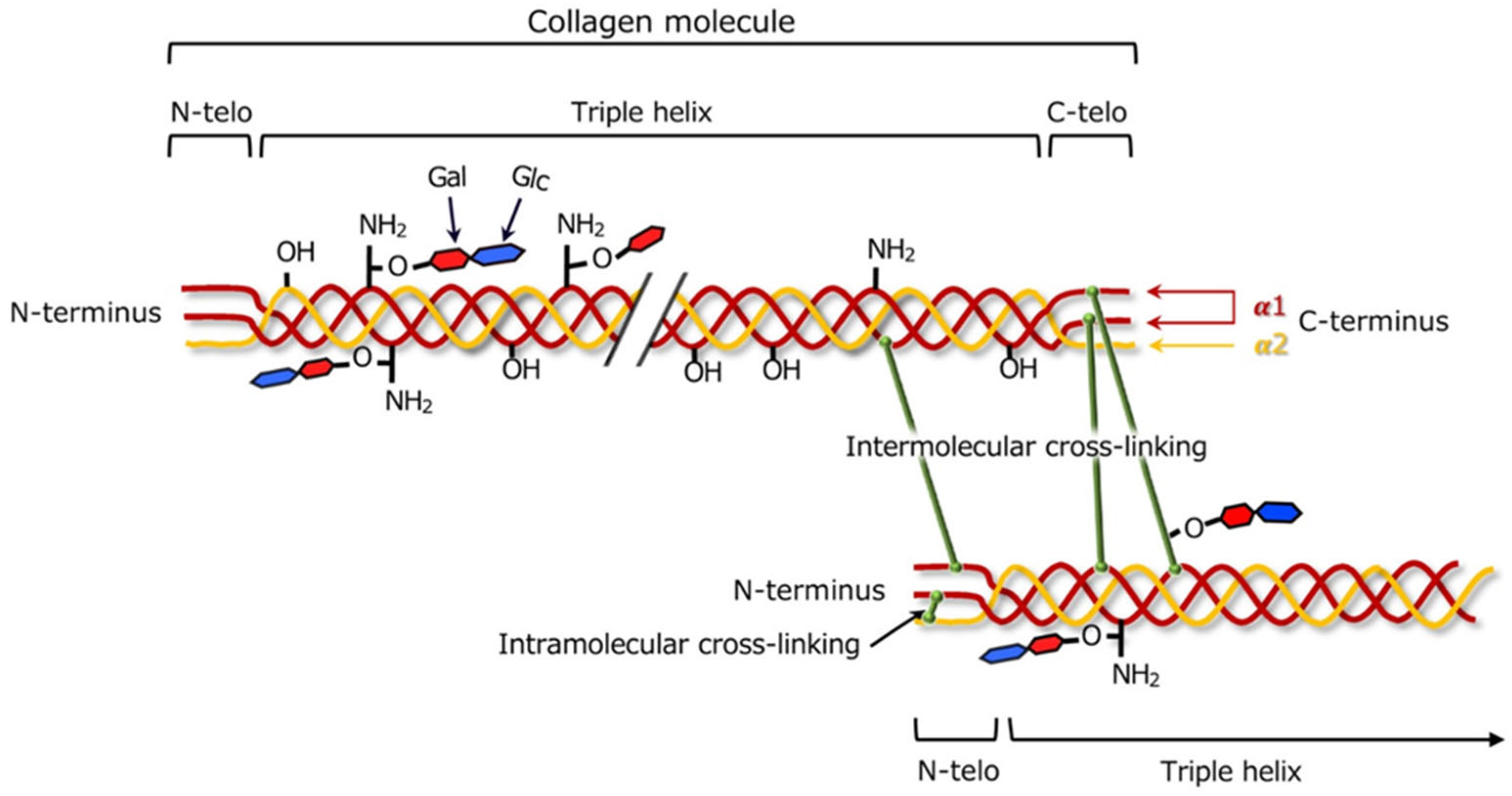
4.1. Chemistry of Collagen
4.2. Cross-Linking of Collagen
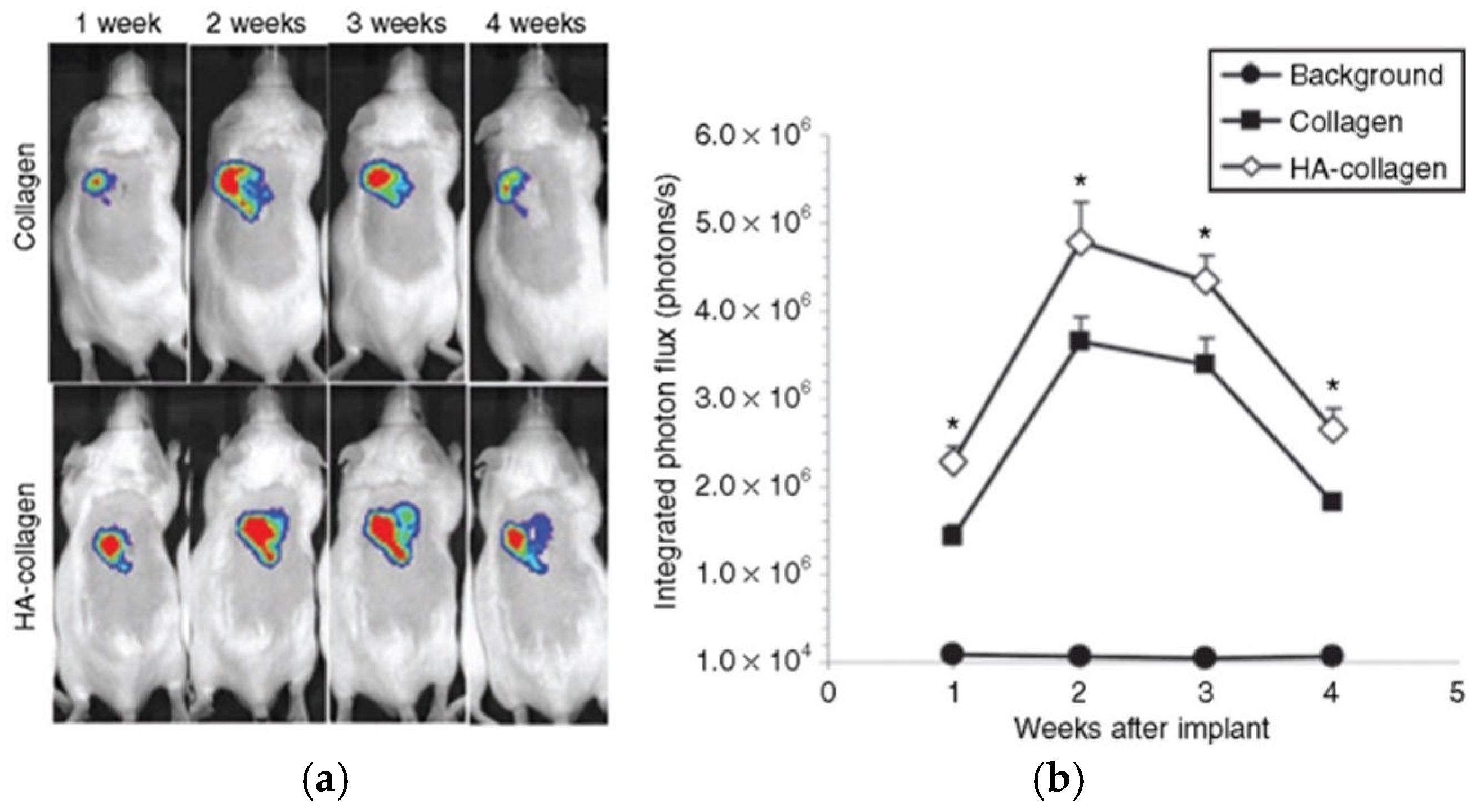
4.3. Collagen as a Drug Carrier
4.4. Stimuli-Responsive Properties of Collagen Carriers
5. Collagen-Based Drug Carriers for Glioblastoma
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Urbanska, K.; Sokolowska, J.; Szmidt, M.; Sysa, P. Glioblastoma Multiforme—An Overview. Contemp. Oncol./Współczesna Onkol. 2014, 18, 307–312. [Google Scholar] [CrossRef]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastomas. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, R.; Yu, Y.; Liu, J.; Luo, T.; Fan, F. Glioblastoma Treatment Modalities besides Surgery. J. Cancer 2019, 10, 4793. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jin, J.; Lu, Y.; Hang, D.; Zhang, X.; Zhao, G.; Wang, Q.; Li, Y.; Liu, H. Collagen IV/Laminin-1 Coated Temozolomide-Prodrug Hydrogel for Recruiting and Eradicating Residual Glioma Cells. Chem. Eng. J. 2025, 503, 158616. [Google Scholar] [CrossRef]
- Maryam, S.; Krukiewicz, K. Sweeten the Pill: Multi-Faceted Polysaccharide-Based Carriers for Colorectal Cancer Treatment. Int. J. Biol. Macromol. 2024, 282, 136696. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Wirsching, H.G.; Weller, M. Glioblastoma. In Malignant Brain Tumors: State-Of-The-Art Treatment; Moliterno Gunel, J., Piepmeier, J., Baehring, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 265–288. [Google Scholar] [CrossRef]
- Ah-Pine, F.; Khettab, M.; Bedoui, Y.; Slama, Y.; Daniel, M.; Doray, B.; Gasque, P. On the Origin and Development of Glioblastoma: Multifaceted Role of Perivascular Mesenchymal Stromal Cells. Acta Neuropathol. Commun. 2023, 11, 104. [Google Scholar] [CrossRef]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; Exon Publications: Brisbane, Australia, 2017; pp. 143–153. [Google Scholar] [CrossRef]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical Presentation, Diagnosis, and Management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef]
- Rodgers, L.T.; Villano, J.L.; Hartz, A.M.S.; Bauer, B. Glioblastoma Standard of Care: Effects on Tumor Evolution and Reverse Translation in Preclinical Models. Cancers 2024, 16, 2638. [Google Scholar] [CrossRef]
- Karcher, S.; Steiner, H.H.; Ahmadi, R.; Zoubaa, S.; Vasvari, G.; Bauer, H.; Unterberg, A.; Herold-Mende, C. Different Angiogenic Phenotypes in Primary and Secondary Glioblastomas. Int. J. Cancer 2006, 118, 2182–2189. [Google Scholar] [CrossRef]
- Chang, J.E.; Khuntia, D.; Robins, H.I.; Mehta, M.P. Radiotherapy and Radiosensitizers in the Treatment of Glioblastoma Multiforme. Clin. Adv. Hematol. Oncol. 2007, 5, 894–902+907. [Google Scholar] [PubMed]
- Simpson, J.R.; Horton, J.; Scott, C.; Curran, W.J.; Rubin, P.; Fischbach, J.; Isaacson, S.; Rotman, M.; Asbell, S.O.; Nelson, J.S.; et al. Influence of Location and Extent of Surgical Resection on Survival of Patients with Glioblastoma Multiforme: Results of Three Consecutive Radiation Therapy Oncology Group (RTOG) Clinical Trials. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 239–244. [Google Scholar] [CrossRef]
- Kislin, K.L.; McDonough, W.S.; Eschbacher, J.M.; Armstrong, B.A.; Berens, M.E. NHERF-1: Modulator of Glioblastoma Cell Migration and Invasion. Neoplasia 2009, 11, 377-IN7. [Google Scholar] [CrossRef]
- Cho, D.Y.; Yang, W.K.; Lee, H.C.; Hsu, D.M.; Lin, H.L.; Lin, S.Z.; Chen, C.C.; Harn, H.J.; Liu, C.L.; Lee, W.Y.; et al. Adjuvant Immunotherapy with Whole-Cell Lysate Dendritic Cells Vaccine for Glioblastoma Multiforme: A Phase II Clinical Trial. World Neurosurg. 2012, 77, 736–744. [Google Scholar] [CrossRef]
- Li, M.; Mukasa, A.; del-Mar Inda, M.; Zhang, J.; Chin, L.; Cavenee, W.; Furnari, F. Guanylate Binding Protein 1 Is a Novel Effector of EGFR-Driven Invasion in Glioblastoma. J. Exp. Med. 2011, 208, 2657–2673. [Google Scholar] [CrossRef]
- Guo, D.; Wang, B.; Han, F.; Lei, T. RNA Interference Therapy for Glioblastoma. Expert Opin. Biol. Ther. 2010, 10, 927–936. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Luksik, A.S.; Yazigi, E.; Shah, P.; Jackson, C.M. CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. Cancers 2023, 15, 1414. [Google Scholar] [CrossRef] [PubMed]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Soles, A.; Selimovic, A.; Sbrocco, K.; Ghannoum, F.; Hamel, K.; Moncada, E.L.; Gilliat, S.; Cvetanovic, M. Extracellular Matrix Regulation in Physiology and in Brain Disease. Int. J. Mol. Sci. 2023, 24, 7049. [Google Scholar] [CrossRef]
- Novak, U.; Kaye, A.H. Extracellular Matrix and the Brain: Components and Function. J. Clin. Neurosci. 2000, 7, 280–290. [Google Scholar] [CrossRef]
- Ucar, B.; Humpel, C. Collagen for Brain Repair: Therapeutic Perspectives. Neural Regen. Res. 2018, 13, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B. Natural Biomaterials in Brain Repair: A Focus on Collagen. Neurochem. Int. 2021, 146, 105033. [Google Scholar] [CrossRef]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like Materials—Biomaterials for Applications in Regenerative Medicine and Cancer Therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Guo, L.; Xiang, W.; Pan, Z.; Gu, H.; Jiang, X. Post-Translational Modifications of Collagen and Its Related Diseases in Metabolic Pathways. Acta Pharm Sin B 2025, 15, 1773–1795. [Google Scholar] [CrossRef]
- Sephel, G.; Woodward, S. Repair, Regeneration, and Fibrosis. In Rubin’s Pathology: Clinicopathologic Foundations of Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Yeung, C.Y.C.; Svensson, R.B.; Mogensen, N.M.B.; Merkel, M.F.R.; Schjerling, P.; Jokipii-Utzon, A.; Zhang, C.; Carstensen, H.; Buhl, R.; Kjaer, M. Mechanical Properties, Collagen and Glycosaminoglycan Content of Equine Superficial Digital Flexor Tendons Are Not Affected by Training. J. Anat. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Kew, S.J.; Gwynne, J.H.; Enea, D.; Brookes, R.; Rushton, N.; Best, S.M.; Cameron, R.E. Synthetic Collagen Fascicles for the Regeneration of Tendon Tissue. Acta Biomater. 2012, 8, 3723–3731. [Google Scholar] [CrossRef]
- Wan, C.; Hao, Z.; Wen, S.; Leng, H. A Quantitative Study of the Relationship between the Distribution of Different Types of Collagen and the Mechanical Behavior of Rabbit Medial Collateral Ligaments. PLoS ONE 2014, 9, e103363. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, B.; Wang, C.; Yan, Z.; Yang, F.; Su, H. Collagen Remodeling-Mediated Signaling Pathways and Their Impact on Tumor Therapy. J. Biol. Chem. 2025, 301, 108330. [Google Scholar] [CrossRef]
- Jessen, H.; Hoyer, N.; Prior, T.S.; Frederiksen, P.; Karsdal, M.A.; Leeming, D.J.; Bendstrup, E.; Sand, J.M.B.; Shaker, S.B. Turnover of Type I and III Collagen Predicts Progression of Idiopathic Pulmonary Fibrosis. Respir. Res. 2021, 22, 205. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, K.; Kasamatsu, A.; Yamauchi, M. Collagen Cross-Linking in Oral Cancer. Oral Sci. Int. 2024, 21, 3–14. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current Methods of Collagen Cross-Linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Eyre, D.R.; Weis, M.A.; Wu, J.J. Advances in Collagen Cross-Link Analysis. Methods 2008, 45, 65–74. [Google Scholar] [CrossRef]
- Yang, C. Enhanced Physicochemical Properties of Collagen by Using EDC/NHS-Crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kulka-Kamińska, K.; Brudzyńska, P.; Lewandowska, K.; Piwowarski, Ł. The Influence of Various Crosslinking Conditions of EDC/NHS on the Properties of Fish Collagen Film. Mar. Drugs 2024, 22, 194. [Google Scholar] [CrossRef]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV Irradiation as a Binding Site Conserving Method for Crosslinking Collagen-Based Scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 14. [Google Scholar] [CrossRef]
- Mohamed, F.; Bradley, D.A.; Winlove, C.P. Effects of Ionizing Radiation on Extracellular Matrix. Nucl. Instrum. Methods Phys. Res. A 2007, 580, 566–569. [Google Scholar] [CrossRef]
- Verma, A.K. Collagen-Based Biomaterial as Drug Delivery Module. In Collagen Biomaterials; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Paul, G.R.; Attenburrow, G. Cross-Linking of Extruded Collagen Fibers—A Biomimetic Three-Dimensional Scaffold for Tissue Engineering Applications. J. Biomed. Mater. Res. A 2009, 89A, 895–908. [Google Scholar] [CrossRef]
- Robins, S.P. Biochemistry and Functional Significance of Collagen Cross-Linking. Biochem. Soc. Trans. 2007, 35, 849–852. [Google Scholar] [CrossRef]
- Herchenhan, A.; Uhlenbrock, F.; Eliasson, P.; Weis, M.; Eyre, D.; Kadler, K.E.; Magnusson, S.P.; Kjaer, M. Lysyl Oxidase Activity Is Required for Ordered Collagen Fibrillogenesis by Tendon Cells. J. Biol. Chem. 2015, 290, 16440–16450. [Google Scholar] [CrossRef]
- Voicu, G.; Geanaliu-Nicolae, R.E.; Pîrvan, A.A.; Andronescu, E.; Iordache, F. Synthesis, Characterization and Bioevaluation of Drug-Collagen Hybrid Materials for Biomedical Applications. Int. J. Pharm. 2016, 510, 474–484. [Google Scholar] [CrossRef]
- Idu, A.A.; Albu Kaya, M.G.; Rău, I.; Radu, N.; Dinu-Pîrvu, C.E.; Ghica, M.V. Novel Collagen Membrane Formulations with Irinotecan or Minocycline for Potential Application in Brain Cancer. Materials 2024, 17, 3510. [Google Scholar] [CrossRef]
- Yue, W.; Wang, T.; Xie, L.; Shen, J. An Injectable in Situ Hydrogel Platform for Sustained Drug Release against Glioblastoma. J. Drug Deliv. Sci. Technol. 2024, 95, 105527. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.; Chen, J. Effect of PH on the Structure, Functional Properties and Rheological Properties of Collagen from Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Skin. Mar. Drugs 2024, 22, 45. [Google Scholar] [CrossRef]
- Setoyama, S.; Haraguchi, R.; Aoki, S.; Oishi, Y.; Narita, T. PH-Responsive Collagen Hydrogels Prepared by UV Irradiation in the Presence of Riboflavin. Int. J. Mol. Sci. 2024, 25, 10439. [Google Scholar] [CrossRef]
- Knapp, J.P.; Kakish, J.E.; Bridle, B.W.; Speicher, D.J. Tumor Temperature: Friend or Foe of Virus-Based Cancer Immunotherapy. Biomedicines 2022, 10, 2024. [Google Scholar] [CrossRef] [PubMed]
- Ohmes, J.; Saure, L.M.; Schütt, F.; Trenkel, M.; Seekamp, A.; Scherließ, R.; Adelung, R.; Fuchs, S. Injectable Thermosensitive Chitosan-Collagen Hydrogel as A Delivery System for Marine Polysaccharide Fucoidan. Mar. Drugs 2022, 20, 402. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Seker-Polat, F.; Degirmenci, N.P.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- van Solinge, T.S.; Nieland, L.; Chiocca, E.A.; Broekman, M.L.D. Advances in Local Therapy for Glioblastoma—Taking the Fight to the Tumour. Nat. Rev. Neurol. 2022, 18, 221. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Shin, S.; Shea, L.D. Lentivirus Immobilization to Nanoparticles for Enhanced and Localized Delivery from Hydrogels. Mol. Ther. 2010, 18, 700–706. [Google Scholar] [CrossRef]
- Jain, A.; Betancur, M.; Patel, G.D.; Valmikinathan, C.M.; Mukhatyar, V.J.; Vakharia, A.; Pai, S.B.; Brahma, B.; MacDonald, T.J.; Bellamkonda, R.V. Guiding Intracortical Brain Tumour Cells to an Extracortical Cytotoxic Hydrogel Using Aligned Polymeric Nanofibres. Nat. Mater. 2014, 13, 308–316. [Google Scholar] [CrossRef]
- Pinnaduwage, D.; Sorensen, S.P.; Youssef, E.F.; Srivastava, S.P.; Yan, X.; Jani, S. Dosimetric Impact of Cesium 131 Seed Migration in Collagen Carrier Brain Brachytherapy Implants. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e310–e311. [Google Scholar] [CrossRef]
- Pinnaduwage, D.S.; Srivastava, S.P.; Yan, X.; Jani, S.; Brachman, D.G.; Sorensen, S.P. Dosimetric Impacts of Source Migration, Radioisotope Type, and Decay with Permanent Implantable Collagen Tile Brachytherapy for Brain Tumors. Technol. Cancer Res. Treat. 2022, 21, 1–14. [Google Scholar] [CrossRef]
- Youssef, E.; Nakaji, P.; Thomas, T.; McBride, H.; Fram, E.; Brachman, D. SCDT-36. Novel Modular, Permanently Implanted Collagen-Based Device for Intraoperative Brachytherapy in Patients with Central Nervous System Tumors. Neuro Oncol. 2017, 19, vi272. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, F.; Ali, H.; Lathia, J.D.; Chen, P. Immunotherapy for Glioblastoma: Current State, Challenges, and Future Perspectives. Cell. Mol. Immunol. 2024, 21, 1354–1375. [Google Scholar] [CrossRef]
- Liu, X.; Ma, L.; Liang, J.; Zhang, B.; Teng, J.; Gao, C. RNAi Functionalized Collagen-Chitosan/Silicone Membrane Bilayer Dermal Equivalent for Full-Thickness Skin Regeneration with Inhibited Scarring. Biomaterials 2013, 34, 2038–2048. [Google Scholar] [CrossRef]
- Talebloo, N.; Bernal, M.A.O.; Kenyon, E.; Mallett, C.L.; Fazleabas, A.; Moore, A. Detection of Endometriosis Lesions Using Gd-Based Collagen I Targeting Probe in Murine Models of Endometriosis. Mol. Imaging Biol. 2023, 25, 833–843. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Zhao, M.; Matsuura, Y.; Laurent, S.; Yang, P.C.; Bernstein, D.; Ruiz-Lozano, P.; Serpooshan, V. Infection-Resistant MRI-Visible Scaffolds for Tissue Engineering Applications. Bioimpacts 2016, 6, 111. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Siefen, T.; Bjerregaard, S.; Borglin, C.; Lamprecht, A. Assessment of Joint Pharmacokinetics and Consequences for the Intraarticular Delivery of Biologics. J. Control. Release 2022, 348, 745–759. [Google Scholar] [CrossRef]
- Brem, H.; Gabikian, P. Biodegradable Polymer Implants to Treat Brain Tumors. J. Control. Release 2001, 74, 63–67. [Google Scholar] [CrossRef]

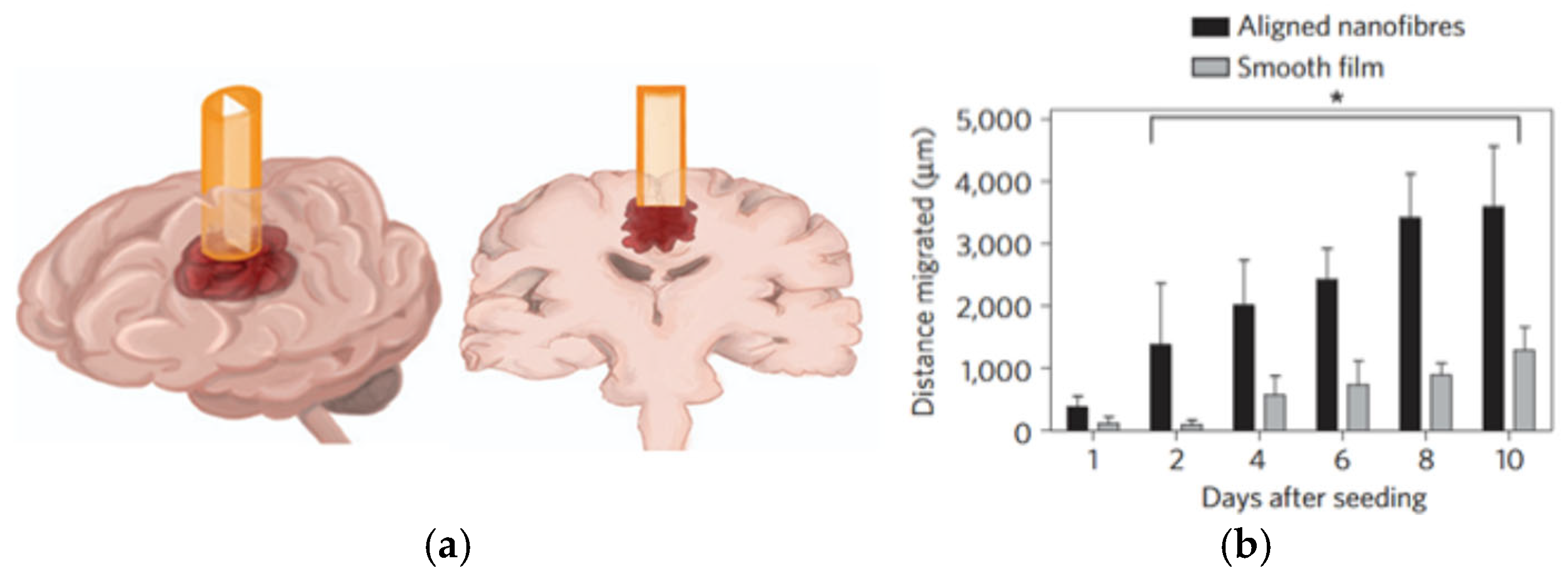
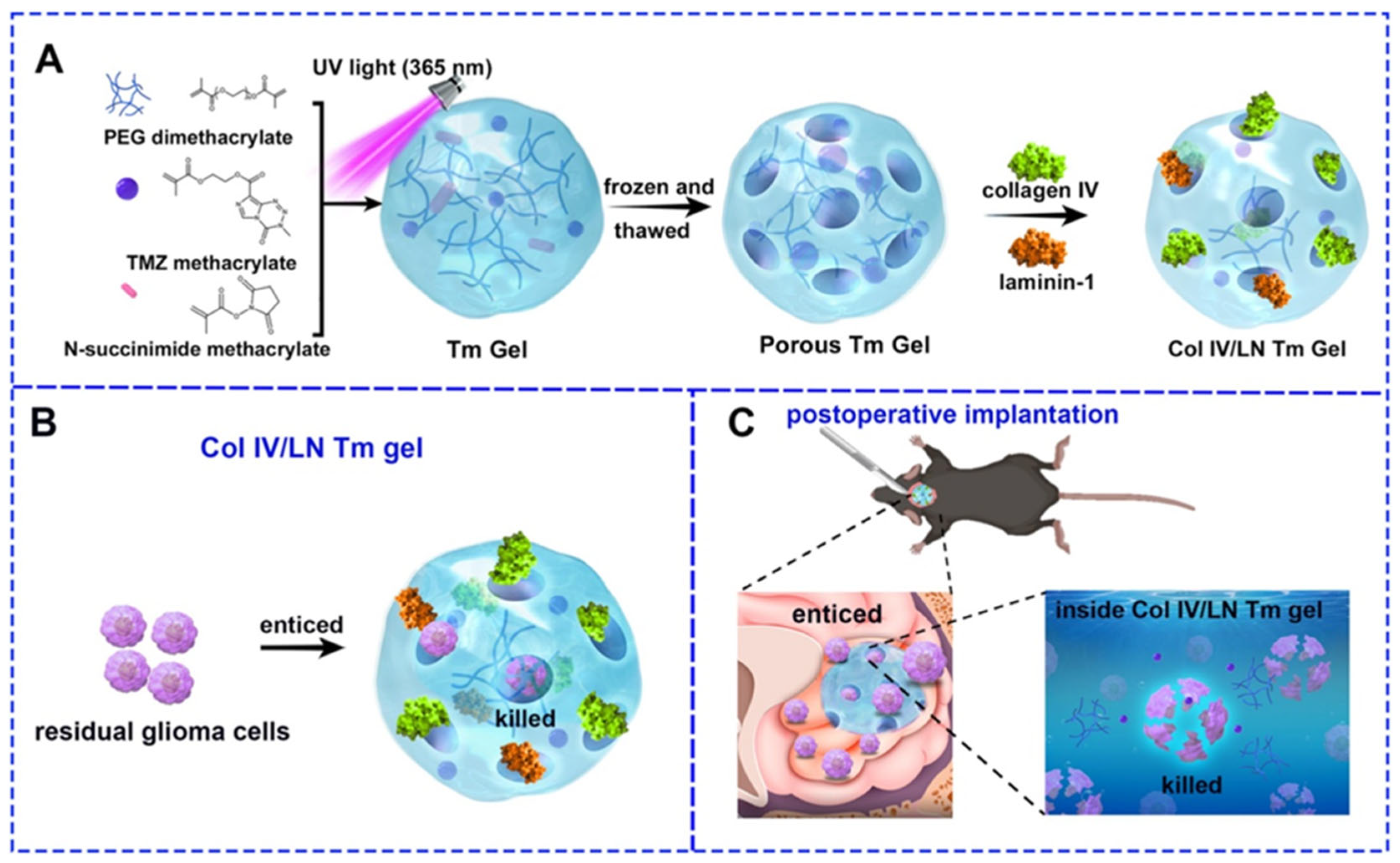
| Cross-Linking Method | Cross-Linking Agents | Mechanism | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Chemical | Glutaraldehyde, EDC/NHS | Covalent bonding via amino/acid groups | Strong bonds, tunable stiffness | Residual toxicity (e.g., glutaraldehyde) | [40,41] |
| Physical | Dehydrothermal treatment, mechanical forces | Hydrogen bonding, chain entanglement | No chemical reagents, low cost | Weak stability, uncontrolled cross-linking | [39,45] |
| Enzymatic | Lysyl oxidase, transglutaminase | Enzyme-mediated covalent cross-links | Biocompatible, residue-free, ECM-like networks | Sensitive to environment, cost | [47,49] |
| Collagen Form | Anticancer Agent | Implantation Method | Release Characteristics | Reference |
|---|---|---|---|---|
| Hydrogel | Lentivirus | Subcutaneous implantation | Stable release over 4 weeks | [60] |
| Cyclopamine | Intracortical conduit implant | >10 days sustained release | [61] | |
| Temozolomide | Gelation in resection cavity | Up to 30 days, 60% total release | [5] | |
| Membrane | Irinotecan, Minocycline | Implantation at tumor site | 1–2 days | [50] |
| Injectable gel | Temozolomide-loaded liposomes | In situ gelling (37 °C) | Controlled release over 1 month | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzdek, B.; Fołta, K.; Staniek, N.; Stolarczyk, M.; Krukiewicz, K. Collagen-Based Drug Delivery Agents for Glioblastoma Multiforme Treatment. Int. J. Mol. Sci. 2025, 26, 6513. https://doi.org/10.3390/ijms26136513
Guzdek B, Fołta K, Staniek N, Stolarczyk M, Krukiewicz K. Collagen-Based Drug Delivery Agents for Glioblastoma Multiforme Treatment. International Journal of Molecular Sciences. 2025; 26(13):6513. https://doi.org/10.3390/ijms26136513
Chicago/Turabian StyleGuzdek, Barbara, Kaja Fołta, Natalia Staniek, Magdalena Stolarczyk, and Katarzyna Krukiewicz. 2025. "Collagen-Based Drug Delivery Agents for Glioblastoma Multiforme Treatment" International Journal of Molecular Sciences 26, no. 13: 6513. https://doi.org/10.3390/ijms26136513
APA StyleGuzdek, B., Fołta, K., Staniek, N., Stolarczyk, M., & Krukiewicz, K. (2025). Collagen-Based Drug Delivery Agents for Glioblastoma Multiforme Treatment. International Journal of Molecular Sciences, 26(13), 6513. https://doi.org/10.3390/ijms26136513





