Nanomedicine Strategies in the Management of Inflammatory Bowel Disease and Colorectal Cancer
Abstract
1. Introduction
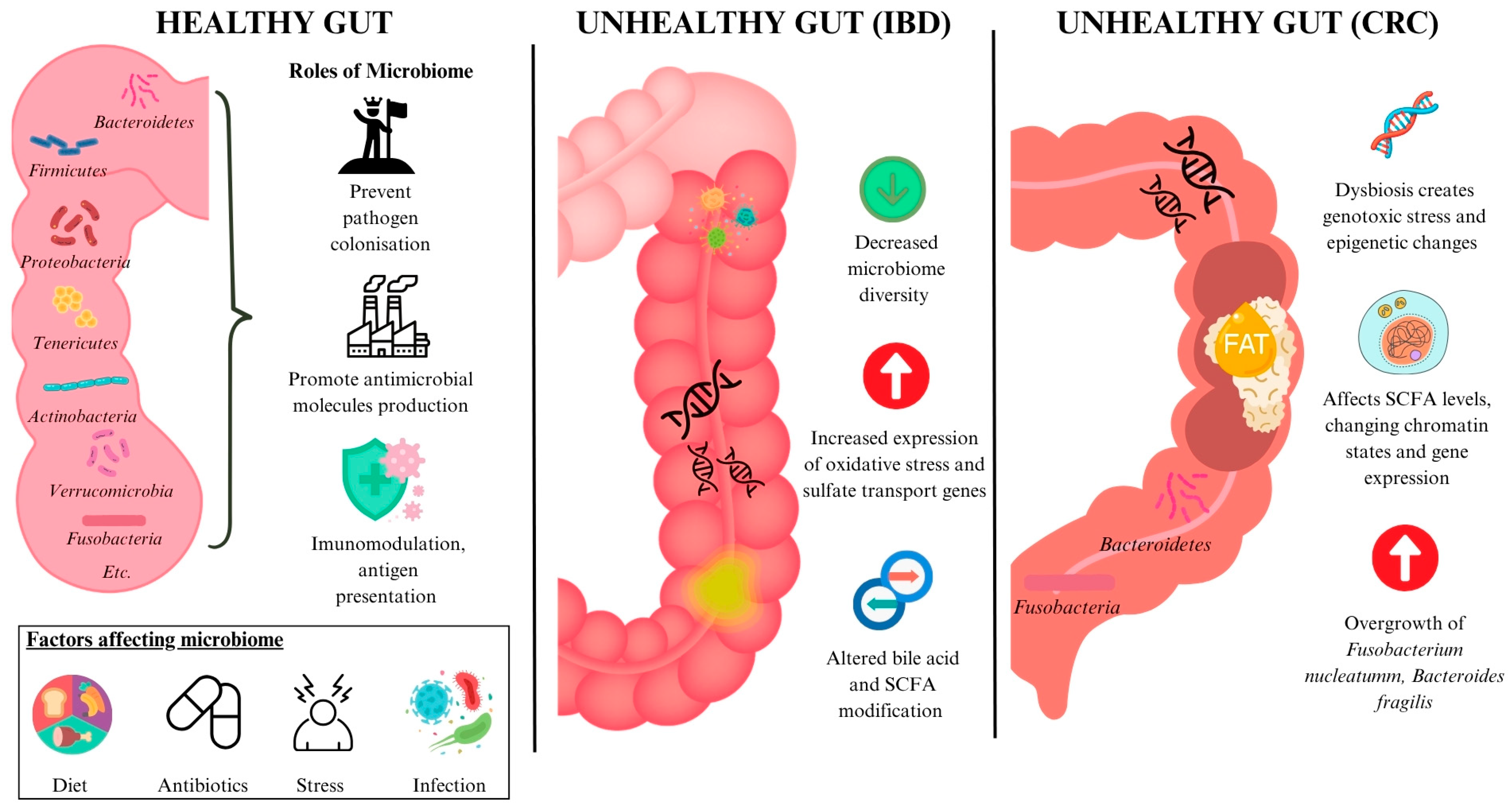
1.1. The Gut Microbiota
1.2. Gut Dysbiosis
2. Gut Dysbiosis in Pathogenesis of Diseases
2.1. Gut Dysbiosis in Inflammatory Bowel Disease (IBD)
2.2. Gut Dysbiosis in Colorectal Cancer (CRC)
3. Current Therapeutic Strategies
3.1. IBD Treatment
3.2. CRC Treatment
4. Nanomedicine
5. Diagnostic Nanomedicine
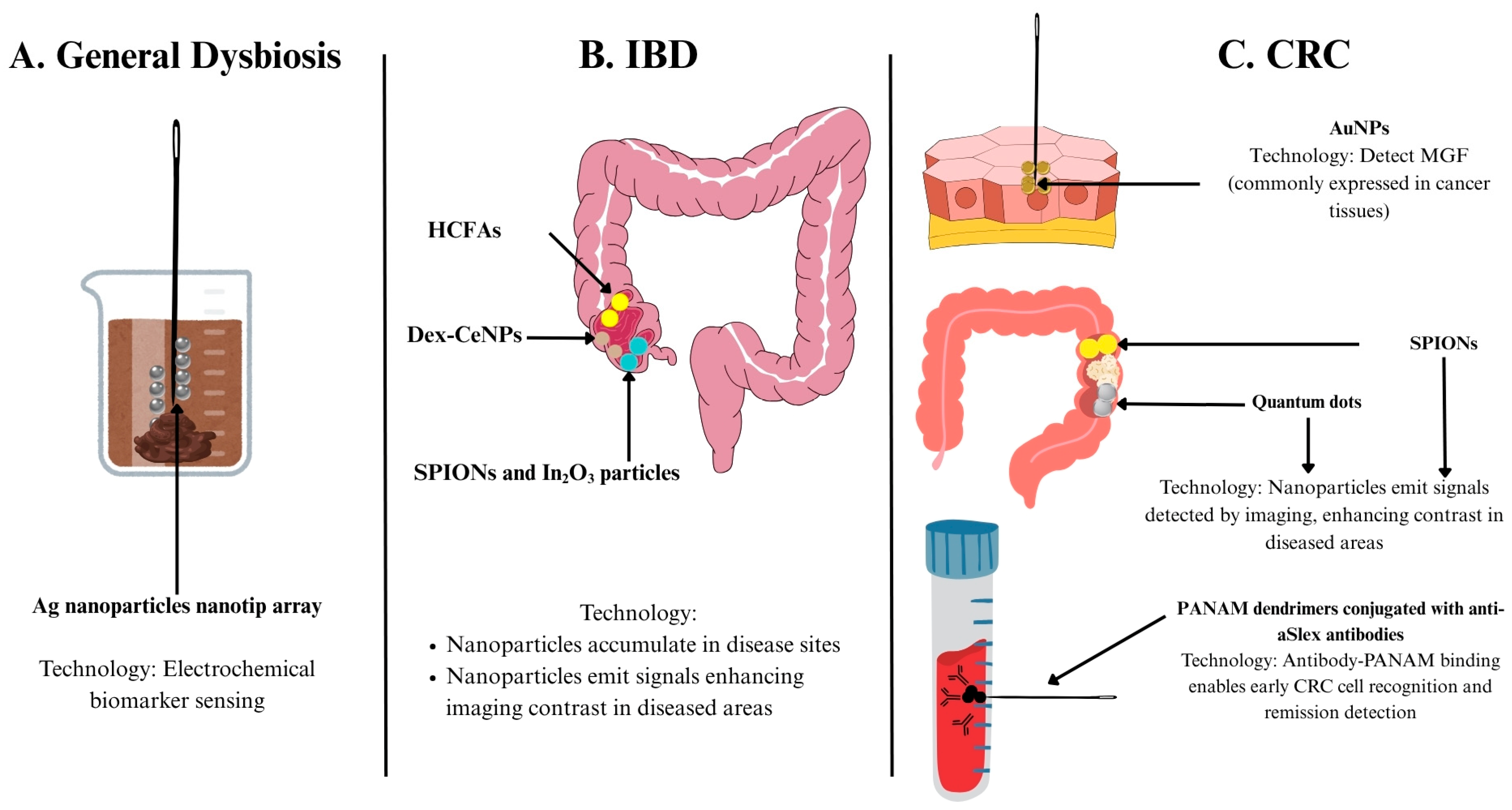
5.1. Nanomedicine for Measuring Diagnostic Biomarkers
5.1.1. Biomarkers of Gut Dysbiosis
| Application | Disease | Nanotechnology Used | Mechanism | Advantages | Stage | Reference |
|---|---|---|---|---|---|---|
| Biomarker Detection | General dysbiosis | Ag nanoparticles nanotip array | Electrochemical sensing | Low cost, simple preparation | Preclinical | [77] |
| CRC | Monolayer-capped AuNPs | Electrochemical sensing | Quick, simple, disturbance-resistant | [82] | ||
| Diagnostic Imaging | IBD | SPIONs and In2O3 particles | MRI | MRI enhancement, tracks disease activity | [83] | |
| Dex-CeNP | CT imaging | Targets disease, enhances CT contrast | [84] | |||
| HCFA | Hypoxia-activatable fluorescence probes | Detects hypoxia degrees for precision therapy | [85] | |||
| CRC | AuNPs | Detect MGF in CRC tissues | Identifies cancer; stronger, more stable emission | [86] | ||
| SPIONs | MRI contrast agent | MRI enhancement | [87] | |||
| Quantum dots | Contrast agent for fluorescence imaging | Size-modulated absorbance and emission, high photostability, longer excited state etc. | [88] | |||
| PANAM dendrimers conjugated with various aSlex antibodies | Detects circulating tumour cells | High capture, sensitive, noninvasive prognostic tool | [89] |
5.1.2. Biomarkers in CRC
5.2. Nanomedicine in Diagnostic Imaging
5.2.1. Diagnostic Imaging of IBD
5.2.2. Diagnostic Imaging of CRC
6. Therapeutic Nanomedicine
6.1. Nanomedicine in Drug Delivery
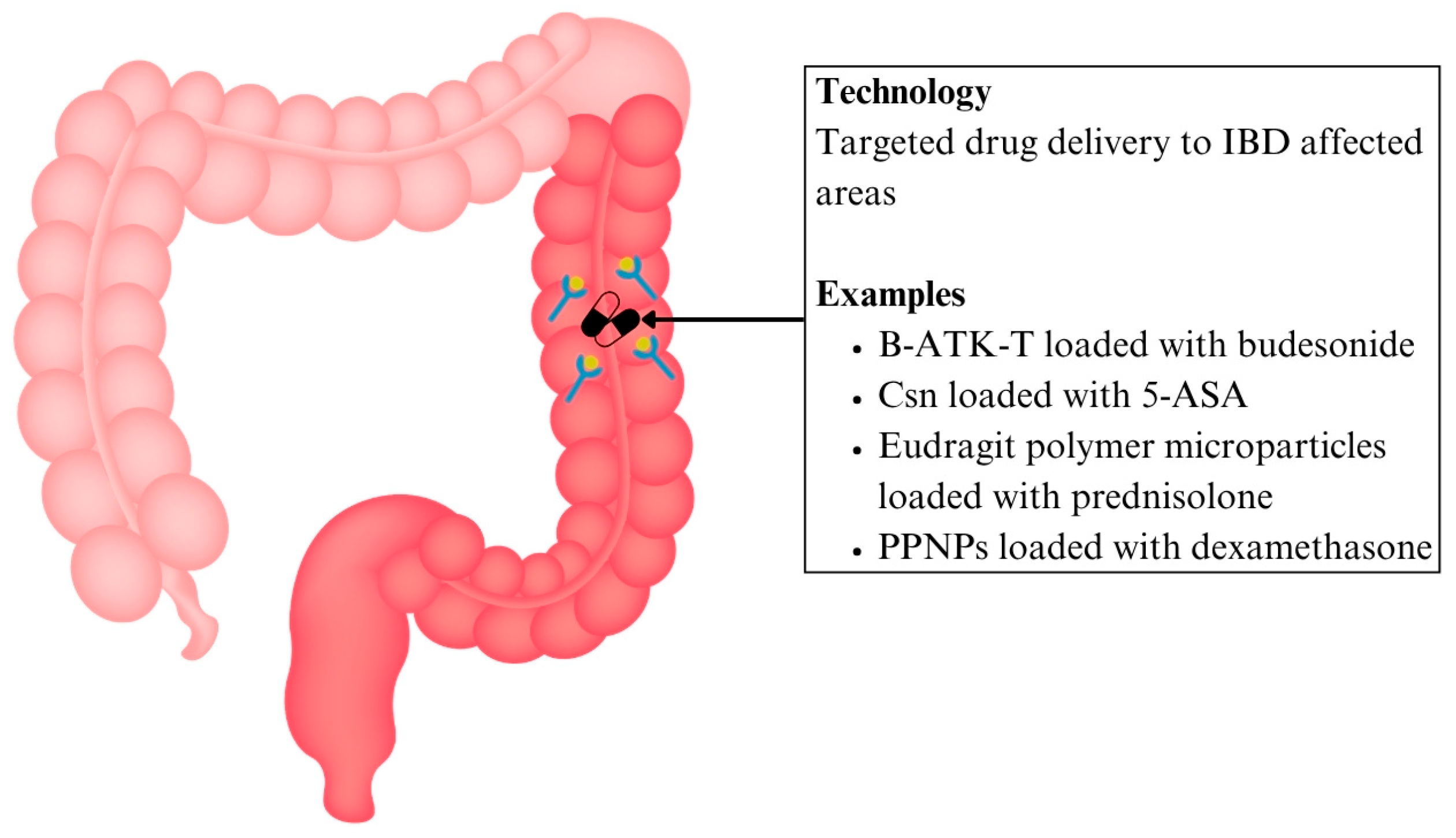
6.1.1. Drug Delivery for IBD
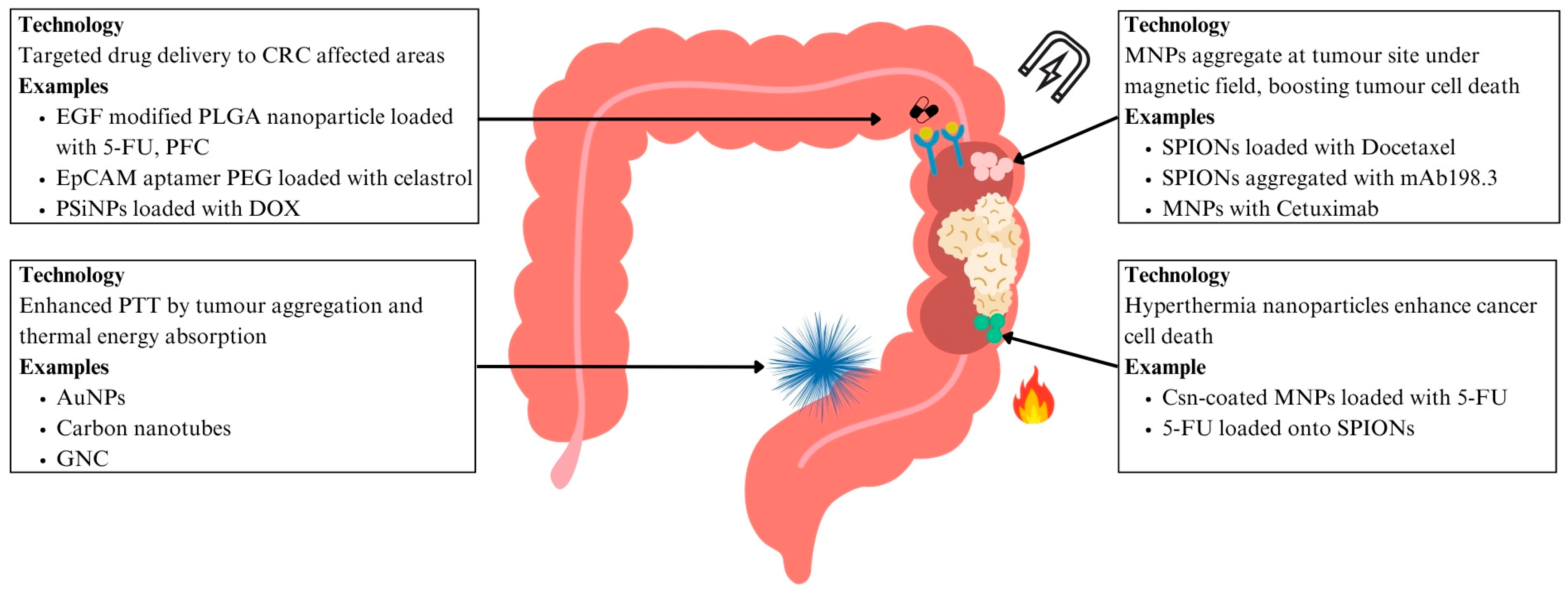
6.1.2. Drug Delivery for CRC
6.2. Nanomedicine in Targeted Therapies
6.2.1. Hyperthermia Treatment for CRC
6.2.2. Magnetic Drug Targeting for CRC
6.2.3. Photothermal Therapy (PTT) for CRC
6.3. Nanotechnology and the Gut Microbiota
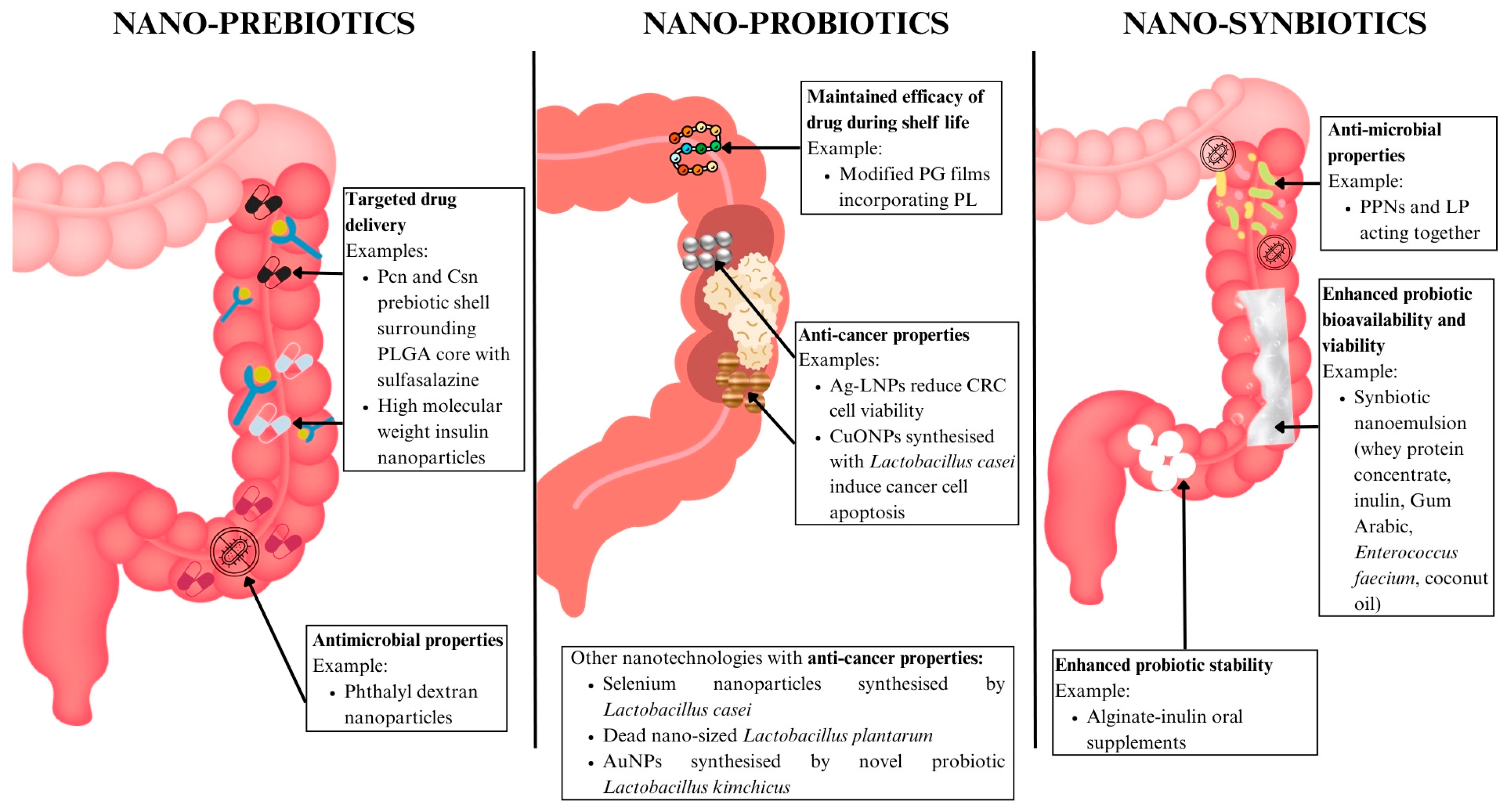
6.3.1. Nano-Prebiotics
6.3.2. Nano-Probiotics
6.3.3. Nano-Synbiotics
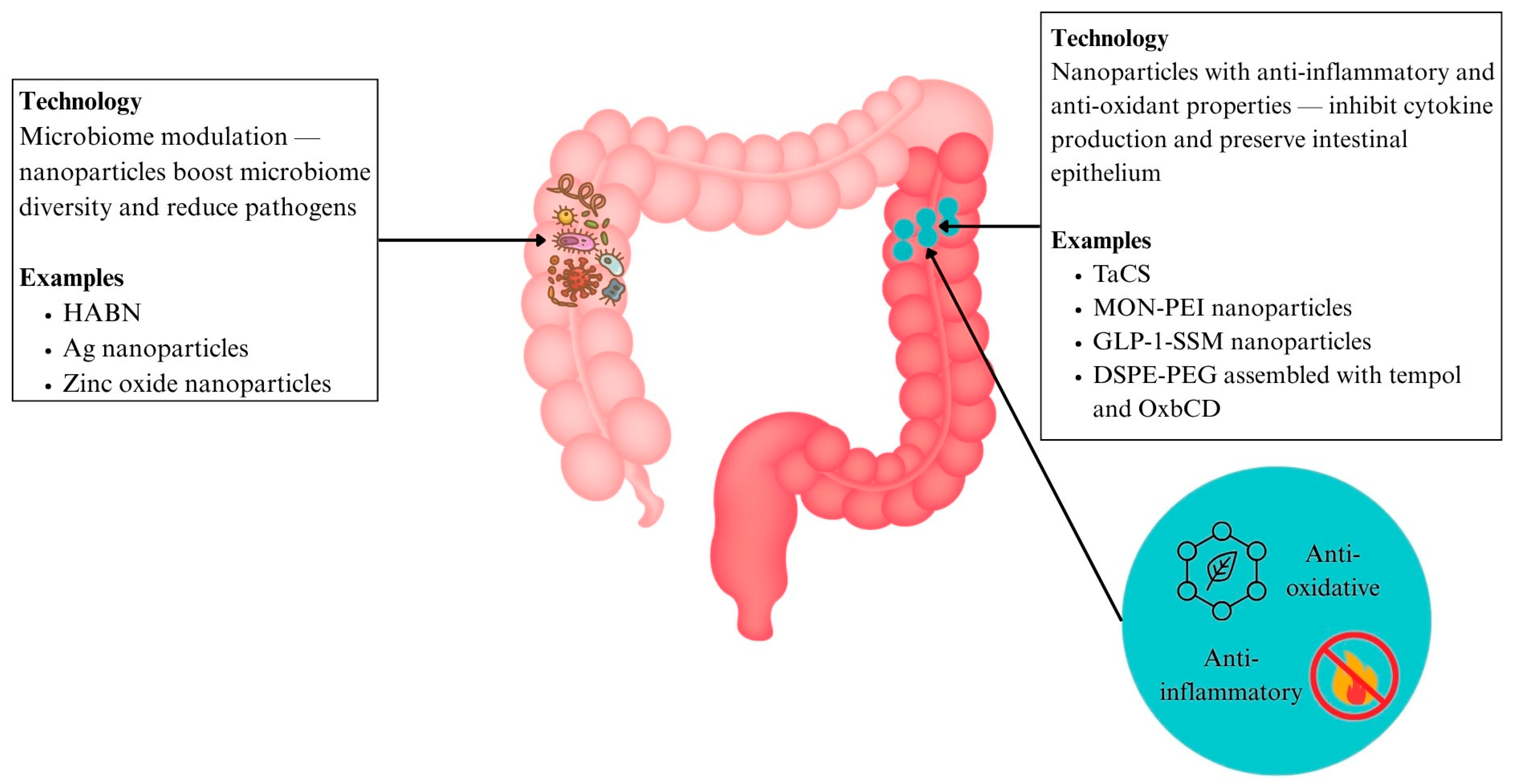
6.3.4. Nanoparticles with Antioxidant and Anti-Inflammatory Effects (IBD)
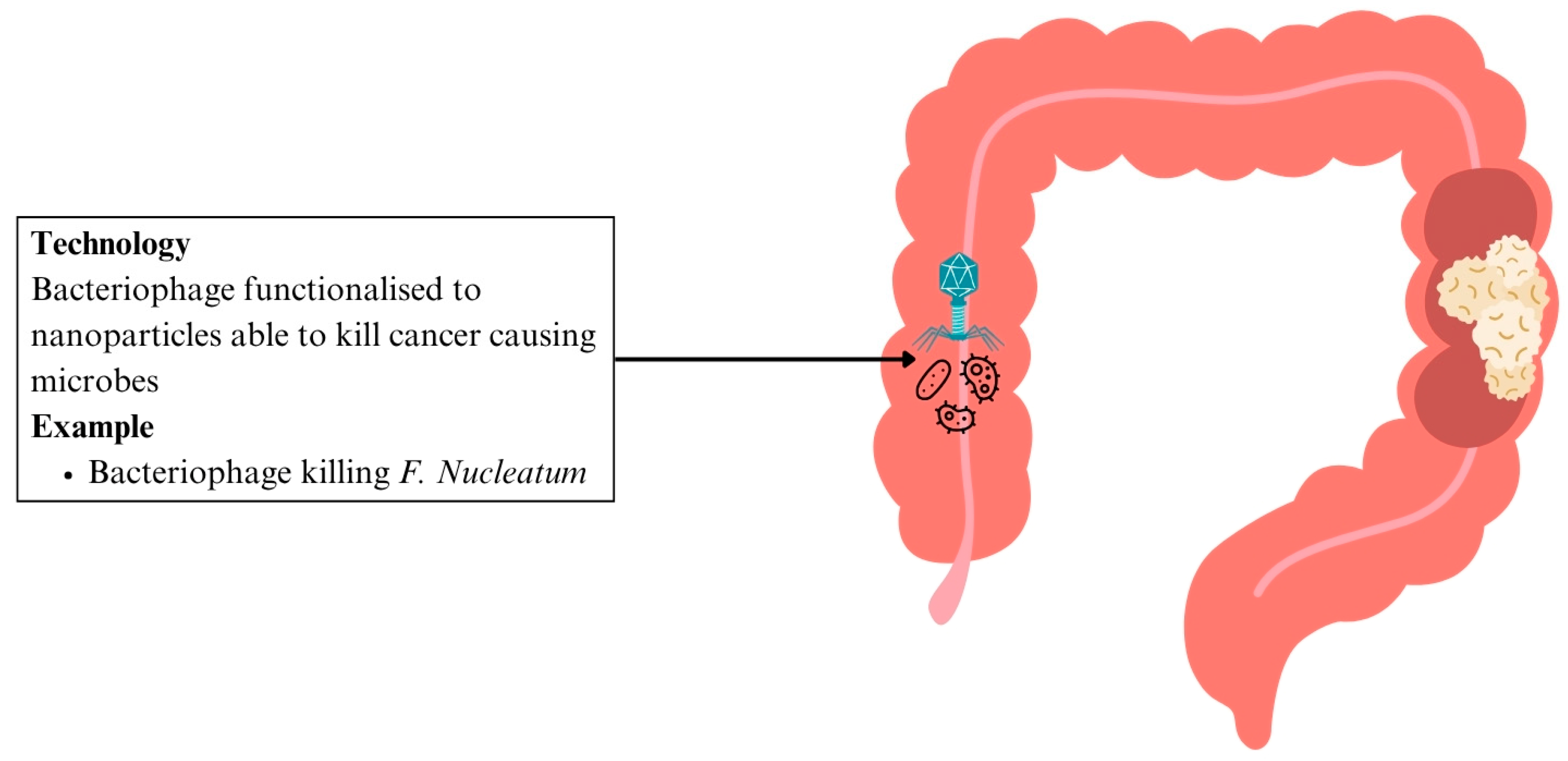
6.3.5. Nanomedicine for Microbiome Modulation
- In IBD
- In CRC
| Application | Disease | Nanotechnology Used | Mechanism | Advantages | Stage | Reference |
|---|---|---|---|---|---|---|
| Drug delivery | IBD | B-ATK-T nanoparticle prodrug linked with budesonide | Thioketal bonds between budesonide and temporal degrade in ROS-rich areas | Targeted high dose delivery, reducing systemic side effects | Preclinical | [92] |
| Csn bound ginger nanocarrier linked with 5-ASA | pH-sensitive drug carrier complex that facilitates colon-specific drug release | Site-specific delivery, lowering pill burden, and systemic exposure | [94] | |||
| Eudragit polymer microparticles containing prednisolone | pH-sensitive drug carrier complex that facilitates colon-specific drug release | Localised immunosuppression, minimising systemic effects | [95] | |||
| PPNP loaded with dexamethasone | Esterase-responsive systems trigger phenol hydrolysis and drug activation | Localised immunosuppression, minimising systemic effects | [96] | |||
| CRC | EGF modified PLGA nanoparticles loaded with 5-FU and PFC | EGFR-targeting nanoparticles enable direct interaction with CRC cells | Enhanced tumour suppression and apoptosis induction | [100] | ||
| PEG dendrimer nanoparticles with EpCAM aptamer loaded with Celastrol | EpCAM aptamer functionalised nanoparticles selectively bind to cancer cells | Improved drug precision with reduced local and systemic toxicity | [101] | |||
| PSiNPs loaded with DOX | Nanocarriers enhance tumour accumulation and penetration in CSCs | Improved chemotherapy efficacy | [103] | |||
| Hyperthermia treatment | CRC | Csn-coated MNPs with 5-FU | Hyperthermia with chemotherapy enhances tumour regression | Improved chemotherapy efficacy | [104] | |
| 5-FU loaded onto PLGA encapsulating iron oxide nanoparticles | Increased cytotoxic activity against colon cancer cells | Lower effective doses, reducing systemic toxicity | [107] | |||
| Magnetic drug targeting | CRC | Docetaxel encapsulated with oil core polymeric SPIONs | Magnetic field induced nanoparticle aggregation | Efficient cytotoxicity with precise delivery and minimal systemic side effects | [109] | |
| SPIONs aggregated with mAb198.3 | mAb198.3 stains CRC cells | Significant tumour growth reduction | [110] | |||
| MNPs combined with Cetuximab | MNPs induce oxidative stress, overcome Cetuximab resistance | Potential to overcome Cetuximab-resistant CRC | [111,112] | |||
| PTT | CRC | AuNPs functionalised with A33 antibody | A33-antibody functionalised PTAs absorb NIR light, targeting CRC tumour with effective accumulation and cancer cell death | Targeted PTT with no other organ toxicity | [117,118,119] | |
| Carbon nanotubes functionalised with nanocomposite POSS-PCU | Functionalised carbon nanotubes aggregate in CRC tumours, reducing cancer cell viability | Enhanced targeting of PTT through antibody functionalisation | [120,121] | |||
| GNC-Gal@CMaP nanocomposites loaded with galunisertib, surface-functionalised with anti-PD-L1 antibodies | Tumour-selective nanoparticle accumulation for enhanced PTT | Antibody-functionalised PTT eliminates primary tumours while inhibiting metastases | [122] | |||
| Multifunctional endoscope-based interventional system | Fluorescence mapping, radio frequency-based ablation, targeted photo/chemotherapy | Novel minimally invasive CRC treatment | [123] | |||
| Nano-prebiotics | IBD | Pcn and Csn prebiotic shell surrounding PLGA core loaded with sulfasalazine | pH-responsive prebiotic shell for drug protection and delivery | Improved drug concentration at target sites | [128] | |
| High molecular weight insulin nanoparticles | Improved drug delivery | No peripheral toxicity | [129] | |||
| Nano-probiotics | General dysbiosis | Modified PG films incorporating varying levels of PL | Active packaging sustaining probiotic GABA production with antimicrobial effects | Preserves probiotic viability and function throughout shelf life | [131] | |
| CRC | Ag-LNPs | ROS-induced CRC cell death | Reduced CRC cell viability | [132] | ||
| CuONPs synthesised with Lactobacillus casei | Anti-cancer, pro-oxidative, apoptotic, antimicrobial | CRC cytotoxicity | [133] | |||
| Nanosynbiotics | IBD | PPNs and LP | Improved gut barrier and microbiome balance | Antimicrobial and anti-dysbiosis effects | [138] | |
| Nanoemulsion incorporating whey protein concentrate, inulin, Gum Arabic, and Enterococcus faecium, coconut oil | Improved probiotic stability and absorption | Enhanced probiotic efficacy | [139] | |||
| Alginate beads incorporated with inulin to protect probiotic strains Pediococcus acidilactici, Lactobacillus reuteri, and Lactobacillus salivarius | Enhanced gut-targeted probiotic efficacy | Enhanced gut probiotic action | [140] | |||
| Antioxidant and anti-inflammatory nanoparticles | IBD | TACS | Anti-inflammatory and mitochondrial protection | Reduced IBD inflammation | [141] | |
| MON-PEI | Multitargeted anti-inflammatory properties with improved safety | Lower dosing and improved safety compared to mesalazine | [144] | |||
| GLP-1-SSM | Preserves intestinal architecture and reduces colonic inflammation | Alleviates colonic inflammation and diarrhoea | [145] | |||
| DSPE-PEG assembled with tempol and OxbCD | Targeted ROS-triggered anti-inflammatory action | Superior anti-inflammatory effect compared to free tempol | [92] | |||
| Microbiome Modulation | IBD | HABN | Targets gut inflammation, barrier repair, and dysbiosis | Restores gut microbiome and epithelial integrity | [145] | |
| Ag nanoparticles targeting Fusobacteriaceae | Precision targeting of pathogenic Fusobacteriaceae | Targets IBD-related dysbiosis | [146] | |||
| Zinc oxide nanoparticles | Modulates SCFA levels and microbiome implicated in IBD | Modulates IBD pathogenesis | [146] | |||
| CRC | Bacteriophage targeting F. nucleatum functionalised to nanoparticles | Selective elimination of oncogenic bacteria | Selective killing of oncogenic microbes | [147] |
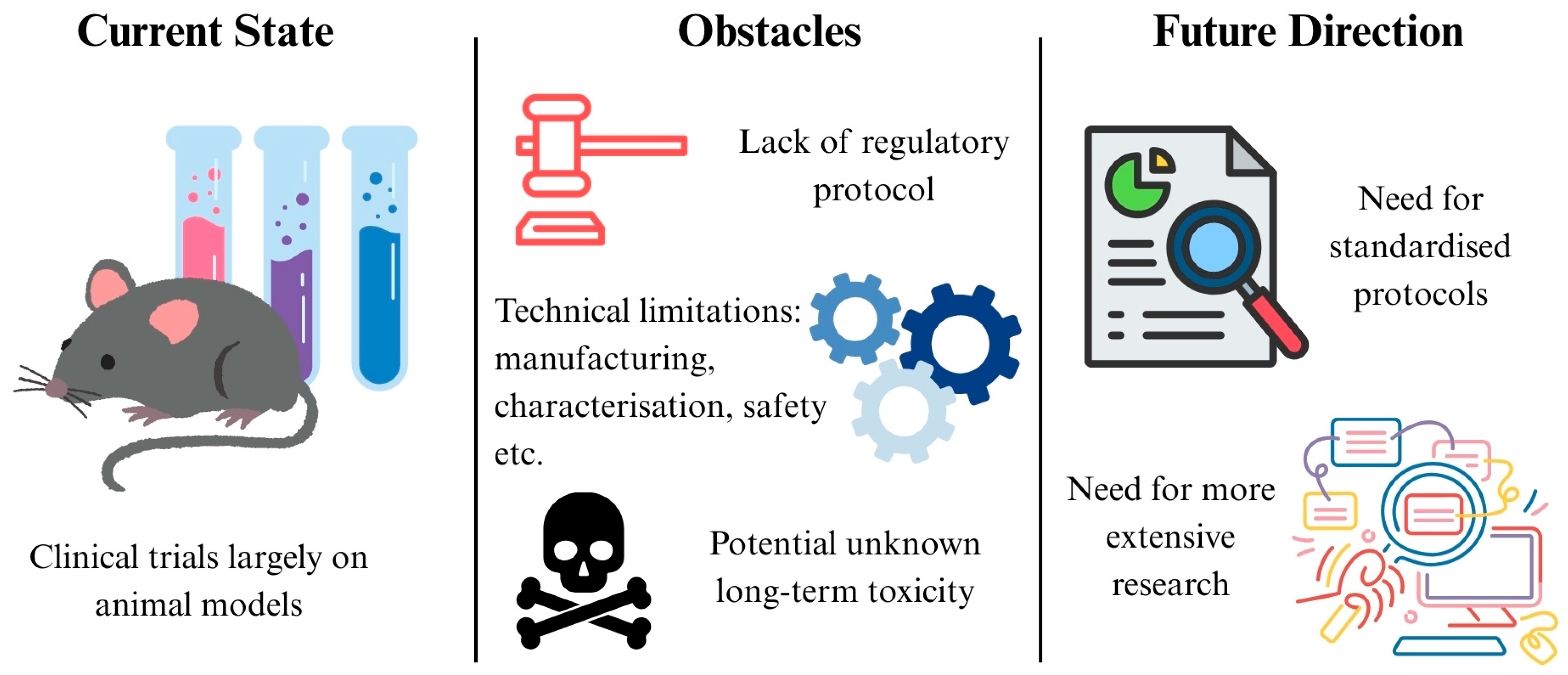
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ASA | 5-aminosalicylic acid |
| 5-FU | 5-fluorouracil |
| Ag | silver |
| Ag-LNP | silver/Lactobacillus rhamnosus GG nanoparticle |
| Anti-TNF-α | anti-tumour necrosis factor-alpha |
| aSlex | anti-Slex |
| AuNP | gold nanoparticle |
| B-ATK-T | Bud-ATK-Tem |
| CD | Crohn’s disease |
| cfDNA | cell-free DNA |
| CRC | colorectal cancer |
| CuONP | copper oxide nanoparticle |
| CSC | cancer stem cell |
| Csn | chitosan |
| DDS | drug delivery system |
| Dex-CeNP | dextran coated cerium oxide nanoparticle |
| DOX | doxorubicin |
| DPP-4 | dipeptidyl peptidase IV |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| EpCAM | epithelial cell adhesion molecule |
| EPR | enhanced permeability and retention |
| FDA | food and drug administration |
| FMT | faecal microbiota transplantation |
| GABA | gamma-aminobutyric acid |
| GC-MS | gas chromatography linked to the mass spectrometry technique |
| GIT | gastrointestinal tract |
| GLP-1 | glucagon-like peptide-1 |
| GLP-1-SSM | GLP-1 in sterically stabilised phospholipid micelles |
| GNC | gold nanocages |
| HABN | hyaluronic acid-bilirubin nanomedicine |
| HCFA | hypoxia-activatable and cytoplasmic protein-powered fluorescence cascade amplifier |
| HEK | human embryonic kidney |
| HPLC | high-performance liquid chromatography |
| IBD | inflammatory bowel disease |
| IC50 | inhibitory concentration 50% |
| IGF | insulin-like growth factor |
| IL | interleukin |
| In2O3 | indium (111) oxide |
| LP | Lactobacillus plantarum |
| MGF | mechano-growth factor |
| MNP | magnetic nanoparticle |
| MON | mesoporous organosilica nanoparticles |
| MON-PEI | polyethylenimine-mesoporous organosilica |
| MRI | magnetic resonance imaging |
| Nab | nanoparticle albumin bound |
| NF-κB | nuclear factor κB |
| NIR | near-infrared |
| OxbCD | β-cyclodextrin-derived material |
| PANAM | poly(amidoamine) |
| Pcn | pectin |
| PD-L1 | programmed cell death ligand-1 |
| PEG | polyethylene glycol |
| PG | poly(L-glutamic acid) |
| PL | poly(L-lysine) |
| PLGA | poly (lactic-co-glycolic acid) |
| PFC | perfluorocarbon |
| POSS-PCU | polyhedral oligomeric silsesquioxane poly (carbonate-urea) urethane |
| PPAR-γ | peroxisome proliferator-activated receptor-gamma |
| PPN | phthalyl pullulan nanoparticle |
| PPNP | polyphenols and polymers self-assembled nanoparticle |
| PSiNP | porous silicon nanoparticle |
| PTA | photothermal agent |
| PTT | photothermal therapy |
| ROS | reactive oxygen species |
| SCFA | short chain fatty acid |
| SPIO | superparamagnetic iron oxide |
| SPION | superparamagnetic iron oxide nanoparticle |
| Ta | Tantalum |
| TACS | Ta2C modified with chondroitin sulfate |
| TGF | transforming growth factor |
| TLR | toll-like receptor |
| TNF-α | tumour necrosis factor-alpha |
| UC | ulcerative colitis |
| VEGF | vascular endothelial growth factor |
| VOC | volatile organic compounds |
References
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatr. 2015, 3, 17. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The gut microbiome, aging, and longevity: A systematic review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Q. Factors affecting gut microbiome in daily diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- Kumbhare, S.V.; Patangia, D.V.V.; Patil, R.H.; Shouche, Y.S.; Patil, N.P. Factors influencing the gut microbiome in children: From infancy to childhood. J. Biosci. 2019, 44, 49. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef]
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G327–G333. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Cénit, M.C.; Matzaraki, V.; Tigchelaar, E.F.; Zhernakova, A. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim. Biophys. Acta 2014, 1842, 1981–1992. [Google Scholar] [CrossRef]
- Brusca, S.B.; Abramson, S.B.; Scher, J.U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 2014, 26, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Antibiotic-therapy-induced gut dysbiosis affecting gut microbiota-brain axis and cognition: Restoration by intake of probiotics and synbiotics. Int. J. Mol. Sci. 2023, 24, 3074. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Chandel, N.; Maile, A.; Shrivastava, S.; Verma, A.K.; Thakur, V. Establishment and perturbation of human gut microbiome: Common trends and variations between Indian and global populations. Gut Microbiome 2024, 5, e8. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Pareek, S.; Kurakawa, T.; Das, B.; Motooka, D.; Nakaya, S.; Rongsen-Chandola, T.; Goyal, N.; Kayama, H.; Dodd, D.; Okumura, R.; et al. Comparison of Japanese and Indian intestinal microbiota shows diet-dependent interaction between bacteria and fungi. NPJ Biofilms Microbiomes 2019, 5, 37. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; von Bergen, M.; et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef]
- Hernández, E.; Bargiela, R.; Diez, M.S.; Friedrichs, A.; Pérez-Cobas, A.E.; Gosalbes, M.J.; Knecht, H.; Martínez-Martínez, M.; Seifert, J.; von Bergen, M.; et al. Functional consequences of microbial shifts in the human gastrointestinal tract linked to antibiotic treatment and obesity. Gut Microbes 2013, 4, 306–315. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and gut dysbiosis. In Precision Medicine for Investigators, Practitioners and Providers; Faintuch, J., Faintuch, S., Eds.; Experientia Supplementum; Springer: Cham, Switzerland, 2018; pp. 459–476. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Hansen, J.; Gulati, A.; Sartor, R.B. The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr. Opin. Gastroenterol. 2010, 26, 564–571. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, L.A.; Jones, B.V. Dysbiosis modulates capacity for bile acid modification in the gut microbiomes of patients with inflammatory bowel disease: A mechanism and marker of disease? Gut 2012, 61, 1642–1643. [Google Scholar] [CrossRef] [PubMed]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G.; et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef]
- Yu, Y.N.; Fang, J.Y. Gut Microbiota and Colorectal Cancer. Gastrointest. Tumors 2015, 2, 26–32. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, W.C.; Kong, M.S.; Shi, H.N.; Walker, W.A.; Lin, C.Y.; Jung, S.M.; Lin, T.Y. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef]
- Gagnière, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- Belcheva, A.; Irrazabal, T.; Martin, A. Gut microbial metabolism and colon cancer: Can manipulations of the microbiota be useful in the management of gastrointestinal health? BioEssays 2015, 37, 403–412. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S.; et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C. Linking Gut Microbiota to Colorectal Cancer. J. Cancer 2017, 8, 3378–3395. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.L.; Garrett, W.S. Microbes, microbiota, and colon cancer. Cell Host Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Damião, A.O.M.C.; de Azevedo, M.F.C.; Carlos, A.S.; Wada, M.Y.; Silva, T.V.M.; Feitosa, F.C. Conventional therapy for moderate to severe inflammatory bowel disease: A systematic literature review. World J. Gastroenterol. 2019, 25, 1142–1157. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Baert, F. Medical therapy of inflammatory bowel disease. Med. Clin. N. Am. 1994, 78, 1413–1426. [Google Scholar] [CrossRef]
- Hvas, C.L.; Bendix, M.; Dige, A.; Dahlerup, J.F.; Agnholt, J. Current, experimental, and future treatments in inflammatory bowel disease: A clinical review. Immunopharmacol. Immunotoxicol. 2018, 40, 446–460. [Google Scholar] [CrossRef]
- Desreumaux, P.; Ghosh, S. Review article: Mode of action and delivery of 5-aminosalicylic acid—New evidence. Aliment. Pharmacol. Ther. 2006, 24 (Suppl. S1), 2–9. [Google Scholar] [CrossRef]
- Loftus EVJr Kane, S.V.; Bjorkman, D. Systematic review: Short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 19, 179–189. [Google Scholar] [CrossRef]
- Troncone, E.; Monteleone, G. The safety of non-biological treatments in Ulcerative Colitis. Expert. Opin. Drug Saf. 2017, 16, 779–789. [Google Scholar] [CrossRef]
- Toruner, M.; Loftus EVJr Harmsen, W.S.; Zinsmeister, A.R.; Orenstein, R.; Sandborn, W.J.; Colombel, J.F.; Egan, L.J. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008, 134, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.C.; May, G.R.; Fick, G.H.; Sutherland, L.R. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann. Intern. Med. 1995, 123, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Van den Brande, J.M.; Braat, H.; van den Brink, G.R.; Versteeg, H.H.; Bauer, C.A.; Hoedemaeker, I.; van Montfrans, C.; Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology 2003, 124, 1774–1785. [Google Scholar] [CrossRef]
- Lügering, A.; Schmidt, M.; Lügering, N.; Pauels, H.G.; Domschke, W.; Kucharzik, T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology 2001, 121, 1145–1157. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Pender, S.L.; Jackson, C.L.; Prothero, J.D.; Gordon, J.N.; Picariello, L.; Rovedatti, L.; Docena, G.; Monteleone, G.; Rampton, D.S.; et al. Functional modulation of Crohn’s disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology 2007, 133, 137–149. [Google Scholar] [CrossRef]
- Arijs, I.; De Hertogh, G.; Machiels, K.; Van Steen, K.; Lemaire, K.; Schraenen, A.; Van Lommel, L.; Quintens, R.; Van Assche, G.; Vermeire, S.; et al. Mucosal gene expression of cell adhesion molecules, chemokines, and chemokine receptors in patients with inflammatory bowel disease before and after infliximab treatment. Am. J. Gastroenterol. 2011, 106, 748–761. [Google Scholar] [CrossRef]
- Vos, A.C.; Wildenberg, M.E.; Arijs, I.; Duijvestein, M.; Verhaar, A.P.; de Hertogh, G.; Vermeire, S.; Rutgeerts, P.; van den Brink, G.R.; Hommes, D.W. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm. Bowel Dis. 2012, 18, 401–408. [Google Scholar] [CrossRef]
- Ainsworth, M.A.; Bendtzen, K.; Brynskov, J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am. J. Gastroenterol. 2008, 103, 944–948. [Google Scholar] [CrossRef]
- Nyboe Andersen, N.; Pasternak, B.; Friis-Møller, N.; Andersson, M.; Jess, T. Association between tumour necrosis factor-α inhibitors and risk of serious infections in people with inflammatory bowel disease: Nationwide Danish cohort study. BMJ 2015, 350, h2809. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Na, S.Y.; Moon, W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver 2019, 13, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent advances in the treatment of colorectal cancer: A review. J. Nippon. Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Poston, G.J.; Figueras, J.; Giuliante, F.; Nuzzo, G.; Sobrero, A.F.; Gigot, J.F.; Nordlinger, B.; Adam, R.; Gruenberger, T.; Choti, M.A.; et al. Urgent need for a new staging system in advanced colorectal cancer. J. Clin. Oncol. 2008, 26, 4828–4833. [Google Scholar] [CrossRef]
- Viswanath, B.; Kim, S.; Lee, K. Recent insights into nanotechnology development for detection and treatment of colorectal cancer. Int. J. Nanomed. 2016, 11, 2491–2504. [Google Scholar] [CrossRef]
- Yau, T.O. Precision treatment in colorectal cancer: Now and the future. JGH Open 2019, 3, 361–369. [Google Scholar] [CrossRef]
- Verma, M. Personalized medicine and cancer. J. Pers. Med. 2012, 2, 1–14. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Nel, A.E.; Brinker, C.J.; Chan, W.C.; Chen, C.; Chen, X.; Ho, D.; Hu, T.; Kataoka, K.; Kotov, N.; et al. What do we mean when we say nanomedicine? ACS Nano 2022, 16, 13257–13259. [Google Scholar] [CrossRef]
- Bansal, M.; Kumar, A.; Malinee, M.; Sharma, T.K. Nanomedicine: Diagnosis, treatment, and potential prospects. Nanosci. Med. 2020, 1, 297–331. [Google Scholar]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem. Int. Ed. Engl. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Brydges, C.R.; Fiehn, O.; Mayberg, H.S.; Schreiber, H.; Dehkordi, S.M.; Bhattacharyya, S.; Cha, J.; Choi, K.S.; Craighead, W.E.; Krishnan, R.R.; et al. Mood Disorders Precision Medicine Consortium. Indoxyl sulfate, a gut microbiome-derived uremic toxin, is associated with psychic anxiety and its functional magnetic resonance imaging-based neurologic signature. Sci. Rep. 2021, 11, 21011. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Maehara, S.; Tabuchi, N.; Okamura, N. Anthraquinone-containing compound in rhubarb prevents indole production via functional changes in gut microbiota. J. Nat. Med. 2021, 75, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Yang, C.Y.; Tarng, D.C. Diet, gut microbiome and indoxyl sulphate in chronic kidney disease patients. Nephrology 2018, 23 (Suppl. S4), 16–20. [Google Scholar] [CrossRef]
- Wang, X.; Shi, S.; Zhang, F.; Li, S.; Tan, J.; Su, B.; Cheng, Q.; Gou, Y.; Zhang, Y. Application of a nanotip array-based electrochemical sensing platform for detection of indole derivatives as key indicators of gut microbiota health. Alex. Eng. J. 2023, 85, 294–299. [Google Scholar] [CrossRef]
- Reiter, S.; Dunkel, A.; Metwaly, A.; Panes, J.; Salas, A.; Haller, D.; Hofmann, T. Development of a highly sensitive ultra-high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry quantitation method for fecal bile acids and application on Crohn’s disease studies. J. Agric. Food Chem. 2021, 69, 5238–5251. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Khalsa, A.S.; Bailey, M.T.; Kelleher, K.; Spees, C.; Zhu, J. Accurate and reliable quantitation of short chain fatty acids from human feces by ultra high-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS). J. Pharm. Biomed. Anal. 2021, 200, 114066. [Google Scholar] [CrossRef]
- Salihović, S.; Dickens, A.M.; Schoultz, I.; Fart, F.; Sinisalu, L.; Lindeman, T.; Halfvarson, J.; Orešič, M.; & Hyötyläinen, T. Simultaneous determination of perfluoroalkyl substances and bile acids in human serum using ultra-high-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 2251–2259. [Google Scholar] [CrossRef]
- Luo, Z.; Lv, T.; Zhu, K.; Li, Y.; Wang, L.; Gooding, J.J.; Liu, G.; Liu, B. Paper-based ratiometric fluorescence analytical devices towards point-of-care testing of human serum albumin. Angew. Chem. Int. Ed. Engl. 2020, 59, 3131–3136. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Briley-Saebo, K.; Xie, J.; Zhang, R.; Wang, Z.; He, C.; Tang, C.Y.; Tao, X. Inflammatory bowel disease: MR- and SPECT/CT-based macrophage imaging for monitoring and evaluating disease activity in experimental mouse model—Pilot study. Radiology 2014, 271, 400–407. [Google Scholar] [CrossRef]
- Naha, P.C.; Hsu, J.C.; Kim, J.; Shah, S.; Bouché, M.; Si-Mohamed, S.; Rosario-Berrios, D.N.; Douek, P.; Hajfathalian, M.; Yasini, P.; et al. Dextran-coated cerium oxide nanoparticles: A computed tomography contrast agent for imaging the gastrointestinal tract and inflammatory bowel disease. ACS Nano 2020, 14, 10187–10197. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, S.; Guo, J.; Dong, H.; Yin, K.; Huang, W.T.; Yang, R. In vivo imaging of hypoxia associated with inflammatory bowel disease by a cytoplasmic protein-powered fluorescence cascade amplifier. Anal. Chem. 2020, 92, 5787–5794. [Google Scholar] [CrossRef]
- Alagaratnam, S.; Yang, S.Y.; Loizidou, M.; Fuller, B.; Ramesh, B. Mechano-growth factor expression in colorectal cancer investigated with fluorescent gold nanoparticles. Anticancer Res. 2019, 39, 1705–1710. [Google Scholar] [CrossRef]
- Perumal, K.; Ahmad, S.; Mohd-Zahid, M.H.; Wan Hanaffi, W.N.; Alias, I.Z.; Six, J.L.; Ferji, K.; Jaafar, J.; Boer, J.C.; Plebanski, M.; et al. Nanoparticles and gut microbiota in colorectal cancer. Front. Nanotechnol. 2021, 3, 681760. [Google Scholar] [CrossRef]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.G.; Calaminus, S.D.J.; Stasiuk, G.J. NIR-quantum dots in biomedical imaging and their future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Chen, H.; Shen, W.; Sinko, P.J.; Dong, H.; Zhao, R.; Lu, Y.; Zhu, Y.; Jia, L. Multivalent conjugation of antibody to dendrimers for the enhanced capture and regulation on colon cancer cells. Sci. Rep. 2015, 5, 9445. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emerg. Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Sedghi, S.; Fields, J.Z.; Klamut, M.; Urban, G.; Durkin, M.; Winship, D.; Fretland, D.; Olyaee, M.; Keshavarzian, A. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut 1993, 34, 1191–1197. [Google Scholar] [CrossRef]
- Abed, O.A.; Attlassy, Y.; Xu, J.; Han, K.; Moon, J.J. Emerging Nanotechnologies and Microbiome Engineering for the Treatment of Inflammatory Bowel Disease. Mol. Pharm. 2022, 19, 4393–4410. [Google Scholar] [CrossRef] [PubMed]
- Sonu, I.; Lin, M.V.; Blonski, W.; Lichtenstein, G.R. Clinical pharmacology of 5-ASA compounds in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2010, 39, 559–599. [Google Scholar] [CrossRef] [PubMed]
- Markam, R.; Bajpai, A.K. Functionalization of ginger derived nanoparticles with chitosan to design drug delivery system for controlled release of 5-amino salicylic acid (5-ASA) in treatment of inflammatory bowel diseases: An in vitro study. React. Funct. Polym. 2020, 149, 104520. [Google Scholar] [CrossRef]
- Shams, T.; Illangakoon, U.E.; Parhizkar, M.; Harker, A.H.; Edirisinghe, S.; Orlu, M.; Edirisinghe, M. Electrosprayed microparticles for intestinal delivery of prednisolone. J. R. Soc. Interface 2018, 15, 20180491. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.J.; Wang, L.; Pan, D.; Yang, R.; Xu, Y.; Sheng, J.; Huang, Q.; Zhao, H.; Yang, M. Rational design of polyphenol-poloxamer nanovesicles for targeting inflammatory bowel disease therapy. Chem. Mater. 2018, 30, 4073–4080. [Google Scholar] [CrossRef]
- Linton, S.S.; Sherwood, S.G.; Drews, K.C.; Kester, M. Targeting cancer cells in the tumor microenvironment: Opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 208–222. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Tian, Z.; Wu, X.; Peng, L.; Yu, N.; Gou, G.; Zuo, W.; Yang, J. pH-responsive bufadienolides nanocrystals decorated by chitosan quaternary ammonium salt for treating colon cancer. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124819. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, Q.; Zhu, H.; Zhuang, Y.; Bao, J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-Fluorouracil and perfluorocarbon. BMC Cancer 2020, 20, 354. [Google Scholar] [CrossRef]
- Ge, P.; Niu, B.; Wu, Y.; Xu, W.; Li, M.; Sun, H.; Zhou, H.; Zhang, X.; Xie, J. Enhanced cancer therapy of celastrol in vitro and in vivo by smart dendrimers delivery with specificity and biosafety. Chem. Eng. J. 2020, 383, 123228. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. 2016, 11, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Rasa, S.M.M.; Cirri, E.; Ori, A.; Neri, F.; Quaas, R.; Hilger, I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress, and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics 2021, 13, 1625. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Mini, E.; Dombrowski, J.; Moroson, B.A.; Bertino, J.R. Cytotoxic effects of hyperthermia, 5-fluorouracil and their combination on a human leukemia T-lymphoblast cell line, CCRF-CEM. Eur. J. Cancer Clin. Oncol. 1986, 22, 927–934. [Google Scholar] [CrossRef]
- Eynali, S.; Khoei, S.; Khoee, S.; Esmaelbeygi, E. Evaluation of the cytotoxic effects of hyperthermia and 5-fluorouracil-loaded magnetic nanoparticles on human colon cancer cell line HT-29. Int. J. Hyperth. 2017, 33, 327–335. [Google Scholar] [CrossRef]
- Wang, N.; Chen, L.; Huang, W.; Gao, Z.; Jin, M. Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment. Nanomaterials 2024, 14, 557. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Bai, J.; Wang, J.T.; Protti, A.; Southern, P.; Bogart, L.; Heidari, H.; Li, X.; Cakebread, A.; Asker, D.; et al. Magnetic Drug Targeting: Preclinical in Vivo Studies, Mathematical Modeling, and Extrapolation to Humans. Nano Lett. 2016, 16, 5652–5660. [Google Scholar] [CrossRef]
- Grifantini, R.; Taranta, M.; Gherardini, L.; Naldi, I.; Parri, M.; Grandi, A.; Giannetti, A.; Tombelli, S.; Lucarini, G.; Ricotti, L.; et al. Magnetically driven drug delivery systems improving targeted immunotherapy for colon-rectal cancer. J. Control. Release 2018, 280, 76–86. [Google Scholar] [CrossRef]
- Yousefi, M.; Farzi-Khajeh, H.; Akbarzadeh-Khiavi, M.; Safary, A.; Adibkia, K. Improved Biological Impacts of anti-EGFR Monoclonal Antibody in KRAS-Mutant Colorectal Cancer Cells by Silica-Coated Magnetic Nanoparticle Conjugation. Pharm. Sci. 2024, 30, 444–455. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.A.; Ali, D.; Alarafi, S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109891. [Google Scholar] [CrossRef]
- Khot, M.I.; Andrew, H.; Svavarsdottir, H.S.; Armstrong, G.; Quyn, A.J.; Jayne, D.G. A Review on the Scope of Photothermal Therapy-Based Nanomedicines in Preclinical Models of Colorectal Cancer. Clin. Color. Cancer 2019, 18, e200–e209. [Google Scholar] [CrossRef]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef]
- Kirui, D.K.; Krishnan, S.; Strickland, A.D.; Batt, C.A. PAA-derived gold nanorods for cellular targeting and photothermal therapy. Macromol. Biosci. 2011, 11, 779–788. [Google Scholar] [CrossRef]
- Goodrich, G.P.; Bao, L.; Gill-Sharp, K.; Sang, K.L.; Wang, J.; Payne, J.D. Photothermal therapy in a murine colon cancer model using near-infrared absorbing gold nanorods. J. Biomed. Opt. 2010, 15, 018001. [Google Scholar] [CrossRef]
- Tan, A.; Madani, S.Y.; Rajadas, J.; Pastorin, G.; Seifalian, A.M. Synergistic photothermal ablative effects of functionalizing carbon nanotubes with a POSS-PCU nanocomposite polymer. J. Nanobiotechnol. 2012, 10, 34. [Google Scholar] [CrossRef]
- Graham, E.G.; MacNeill, C.M.; Levi-Polyachenko, N.H. Quantifying folic acid-functionalized multi-walled carbon nanotubes bound to colorectal cancer cells for improved photothermal ablation. J. Nanopart. Res. 2013, 15, 1649. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; Cao, K.; Zhang, L.; Fang, X.; Chen, F.; Feng, S.; Yan, F. Photothermal therapy mediated by gold nanocages composed of anti-PDL1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomater. 2021, 134, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.; Song, C.; Cho, H.R.; Ghaffari, R.; Choi, T.K.; Kim, K.H.; Lee, Y.B.; Ling, D.; Lee, H.; et al. An endoscope with integrated transparent bioelectronics and theranostic nanoparticles for colon cancer treatment. Nat. Commun. 2015, 6, 10059. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Atanasov, A.G.; Souto, E.B.; Novellino, E.; Capasso, R.; Santini, A. An Updated Overview on Nanonutraceuticals: Focus on Nanoprebiotics and Nanoprobiotics. Int. J. Mol. Sci. 2020, 21, 2285. [Google Scholar] [CrossRef] [PubMed]
- Kedir, W.M.; Deresa, E.M.; Diriba, T.F. Pharmaceutical and drug delivery applications of pectin and its modified nanocomposites. Heliyon 2022, 8, e10654. [Google Scholar] [CrossRef]
- Ładniak, A.; Jurak, M.; Palusińska-Szysz, M.; Wiącek, A.E. The Influence of Polysaccharides/TiO2 on the Model Membranes of Dipalmitoylphosphatidylglycerol and Bacterial Lipids. Molecules 2022, 27, 343. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems-A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Y.; Cui, L.; Yang, Z.; Wang, J.; Zhang, Z.; Chen, K.; Zhao, C.; He, N.; Li, S. Combining Gut Microbiota Modulation and Enzymatic-Triggered Colonic Delivery by Prebiotic Nanoparticles Improves Mouse Colitis Therapy. Biomater. Res. 2024, 28, 0062. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.; Pérez-Morales, R.; Goycoolea, F.M.; Mueller, M.; Praznik, W.; Loeppert, R.; Bermúdez-Morales, V.; Zavala-Padilla, G.; Ayala, M.; Olvera, C. Self-assembled high molecular weight inulin nanoparticles: Enzymatic synthesis, physicochemical and biological properties. Carbohydr. Polym. 2019, 215, 160–169. [Google Scholar] [CrossRef]
- Kim, W.S.; Han, G.G.; Hong, L.; Kang, S.K.; Shokouhimehr, M.; Choi, Y.J.; Cho, C.S. Novel production of natural bacteriocin via internalization of dextran nanoparticles into probiotics. Biomaterials 2019, 218, 119360. [Google Scholar] [CrossRef]
- Karimi, M.; Yazdi, F.T.; Mortazavi, S.A.; Shahabi-Ghahfarrokhi, I.; Chamani, J. Development of active antimicrobial poly (l-glutamic) acid-poly (l-lysine) packaging material to protect probiotic bacterium. Polym. Test. 2020, 83, 106338. [Google Scholar] [CrossRef]
- Aziz Mousavi, S.M.A.; Mirhosseini, S.A.; Rastegar Shariat Panahi, M.; Mahmoodzadeh Hosseini, H. Characterization of Biosynthesized Silver Nanoparticles Using Lactobacillus rhamnosus GG and its In Vitro Assessment Against Colorectal Cancer Cells. Probiotics Antimicrob. Proteins 2020, 12, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Kouhkan, M.; Ahangar, P.; Babaganjeh, L.A.; Allahyari-Devin, M. Biosynthesis of copper oxide nanoparticles using Lactobacillus casei subsp. Casei and its anticancer and antibacterial activities. Curr. Nanosci. 2020, 16, 101–111. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guo, Y.; Qiao, L.; Ma, L.; Cheng, Y.; Roman, A. Biogenic Synthesis of Novel Functionalized Selenium Nanoparticles by Lactobacillus casei ATCC 393 and Its Protective Effects on Intestinal Barrier Dysfunction Caused by Enterotoxigenic Escherichia coli K88. Front. Microbiol. 2018, 9, 1129. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, H.; Lee, K.W.; Park, K.Y. Dead Nano-Sized Lactobacillus plantarum Inhibits Azoxymethane/Dextran Sulfate Sodium-Induced Colon Cancer in Balb/c Mice. J. Med. Food 2015, 18, 1400–1405. [Google Scholar] [CrossRef]
- Markus, J.; Mathiyalagan, R.; Kim, Y.J.; Abbai, R.; Singh, P.; Ahn, S.; Perez, Z.E.J.; Hurh, J.; Yang, D.C. Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb. Technol. 2016, 95, 85–93. [Google Scholar] [CrossRef]
- Hong, L.; Lee, S.M.; Kim, W.S.; Choi, Y.J.; Oh, S.H.; Li, Y.L.; Choi, S.H.; Chung, D.H.; Jung, E.; Kang, S.K.; et al. Synbiotics Containing Nanoprebiotics: A Novel Therapeutic Strategy to Restore Gut Dysbiosis. Front. Microbiol. 2021, 12, 715241. [Google Scholar] [CrossRef]
- Krithika, B.; Preetha, R. Formulation of protein based inulin incorporated synbiotic nanoemulsion for enhanced stability of probiotic. Mater. Res. Express 2019, 6, 114003. [Google Scholar] [CrossRef]
- Atia, A.; Gomaa, A.; Fliss, I.; Beyssac, E.; Garrait, G.; Subirade, M. A prebiotic matrix for encapsulation of probiotics: Physicochemical and microbiological study. J. Microencapsul. 2016, 33, 89–101. [Google Scholar] [CrossRef]
- Song, X.; Huang, Q.; Yang, Y.; Ma, L.; Liu, W.; Ou, C.; Chen, Q.; Zhao, T.; Xiao, Z.; Wang, M.; et al. Efficient therapy of inflammatory bowel disease (IBD) with highly specific and durable targeted Ta2C modified with chondroitin sulfate (TACS). Adv. Mater. 2023, 35, e2301585. [Google Scholar] [CrossRef]
- Shi, C.; Dawulieti, J.; Shi, F.; Yang, C.; Qin, Q.; Shi, T.; Wang, L.; Hu, H.; Sun, M.; Ren, L.; et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci. Adv. 2022, 8, eabj2372. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Schoenemeyer, A.; Visintin, A.; Fitzgerald, K.A.; Monks, B.G.; Knetter, C.F.; Lien, E.; Nilsen, N.J.; Espevik, T.; Golenbock, D.T. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 2004, 5, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, A.N.; Thaqi, M.; Priyamvada, S.; Jayawardena, D.; Kumar, A.; Gujral, T.; Chatterjee, I.; Mugarza, E.; Saksena, S.; Onyuksel, H.; et al. GLP-1 nanomedicine alleviates gut inflammation. Nanomedicine 2017, 13, 659–665. [Google Scholar] [CrossRef]
- Lee, Y.; Sugihara, K.; Gillilland MG3rd Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef]
- Long, D.; Merlin, D. Micro- and nanotechnological delivery platforms for treatment of dysbiosis-related inflammatory bowel disease. Nanomedicine 2021, 16, 1741–1745. [Google Scholar] [CrossRef]
- Song, W.; Anselmo, A.C.; Huang, L. Nanotechnology intervention of the microbiome for cancer therapy. Nat. Nanotechnol. 2019, 14, 1093–1103. [Google Scholar] [CrossRef]
- El Deeb, B.A.; Faheem, G.G.; Bakhit, M.S. Biosynthesis of silver nanoparticles by Talaromyces funiculosus for therapeutic applications and safety evaluation. Sci. Rep. 2025, 15, 13750. [Google Scholar] [CrossRef]
- Chiang, C.F.; Hsu, Y.H.; Hsieh, W.Y.; Liao, T.H.; Chen, C.L.; Chen, Y.C.; Liang, P.C.; Wang, S.J. IOP injection, a novel superparamagnetic iron oxide particle MRI contrast agent for the detection of hepatocellular carcinoma: A phase II clinical trial. J. Magn. Reson. Imaging 2023, 58, 1177–1188. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; López-Estévez, A.M.; Cordeiro, A.S.; Dacoba, T.G.; Crecente-Campo, J.; Torres, D.; Alonso, M.J. Nanotechnologies for the delivery of biologicals: Historical perspective and current landscape. Adv. Drug Deliv. Rev. 2021, 176, 113899. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in nanomaterial-mediated photothermal cancer therapies: Toward clinical applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Eftekharifar, M.; Heidari, R.; Mohaghegh, N.; Najafabadi, A.H.; Heidari, H. Advances in photoactivated carbon-based nanostructured materials for targeted cancer therapy. Adv. Drug Deliv. Rev. 2025, 222, 115604. [Google Scholar] [CrossRef] [PubMed]
- Herrero de la Parte, B.; Rodrigo, I.; Gutiérrez-Basoa, J.; Iturrizaga Correcher, S.; Mar Medina, C.; Echevarría-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; García-Alonso, I. Proposal of new safety limits for in vivo experiments of magnetic hyperthermia antitumor therapy. Cancers 2022, 14, 3084. [Google Scholar] [CrossRef] [PubMed]
- Venturini, J.; Chakraborty, A.; Baysal, M.A.; Tsimberidou, A.M. Developments in nanotechnology approaches for the treatment of solid tumors. Exp. Hematol. Oncol. 2025, 14, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V. Facilitating the translation of nanomedicines to a clinical product: Challenges and opportunities. Drug Discov. Today 2018, 23, 974–991. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, B.; Yu, H.; Song, Z.; Xu, X.; Zheng, Z.; Zhao, K.; Zhao, J.; Zhao, Y. Therapeutic applications and potential biological barriers of nano-delivery systems in common gastrointestinal disorders: A comprehensive review. Adv. Compos. Hybrid. Mater. 2025, 8, 227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, A.X.X.; Ong, B.Y.C.; Dinesh, T.; Srinivasan, D.K. Nanomedicine Strategies in the Management of Inflammatory Bowel Disease and Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 6465. https://doi.org/10.3390/ijms26136465
Tan AXX, Ong BYC, Dinesh T, Srinivasan DK. Nanomedicine Strategies in the Management of Inflammatory Bowel Disease and Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(13):6465. https://doi.org/10.3390/ijms26136465
Chicago/Turabian StyleTan, Asia Xiao Xuan, Brandon Yen Chow Ong, Tarini Dinesh, and Dinesh Kumar Srinivasan. 2025. "Nanomedicine Strategies in the Management of Inflammatory Bowel Disease and Colorectal Cancer" International Journal of Molecular Sciences 26, no. 13: 6465. https://doi.org/10.3390/ijms26136465
APA StyleTan, A. X. X., Ong, B. Y. C., Dinesh, T., & Srinivasan, D. K. (2025). Nanomedicine Strategies in the Management of Inflammatory Bowel Disease and Colorectal Cancer. International Journal of Molecular Sciences, 26(13), 6465. https://doi.org/10.3390/ijms26136465







