Transcription Factors and Methods for the Pharmacological Correction of Their Activity
Abstract
1. Introduction
2. Main Classes of Transcription Factors
2.1. Classification of Transcription Factors by Structural Complexity
2.1.1. Class I Simple Transcription Factors (DBD + Effector Domain)
2.1.2. Class II: Intermediate/Modular Transcription Factors
2.1.3. Class III: Complex Signal-Regulated TFs
2.1.4. Class IV: Nuclear Receptors
2.2. Classification of Transcription Factors by Functional Activity
3. NF-κB
Pharmacological Modulation
4. p53
Pharmacological Modulation
5. HIF-1α
Pharmacological Modulation
6. STAT (STAT1/3/5)
Pharmacological Modulation
7. AP-1
Pharmacological Modulation
8. Nrf2
Pharmacological Modulation
9. Nuclear Receptor Transcription Factors
Pharmacological Modulation
10. Pharmacological Approaches to Modulation of Transcription Factor Activity
10.1. Direct and Indirect Small Molecule TF Inhibitors
10.2. Activators and Restorers of TF Function
10.3. PROTAC and Molecular “Glues” (Targeted Degradation)
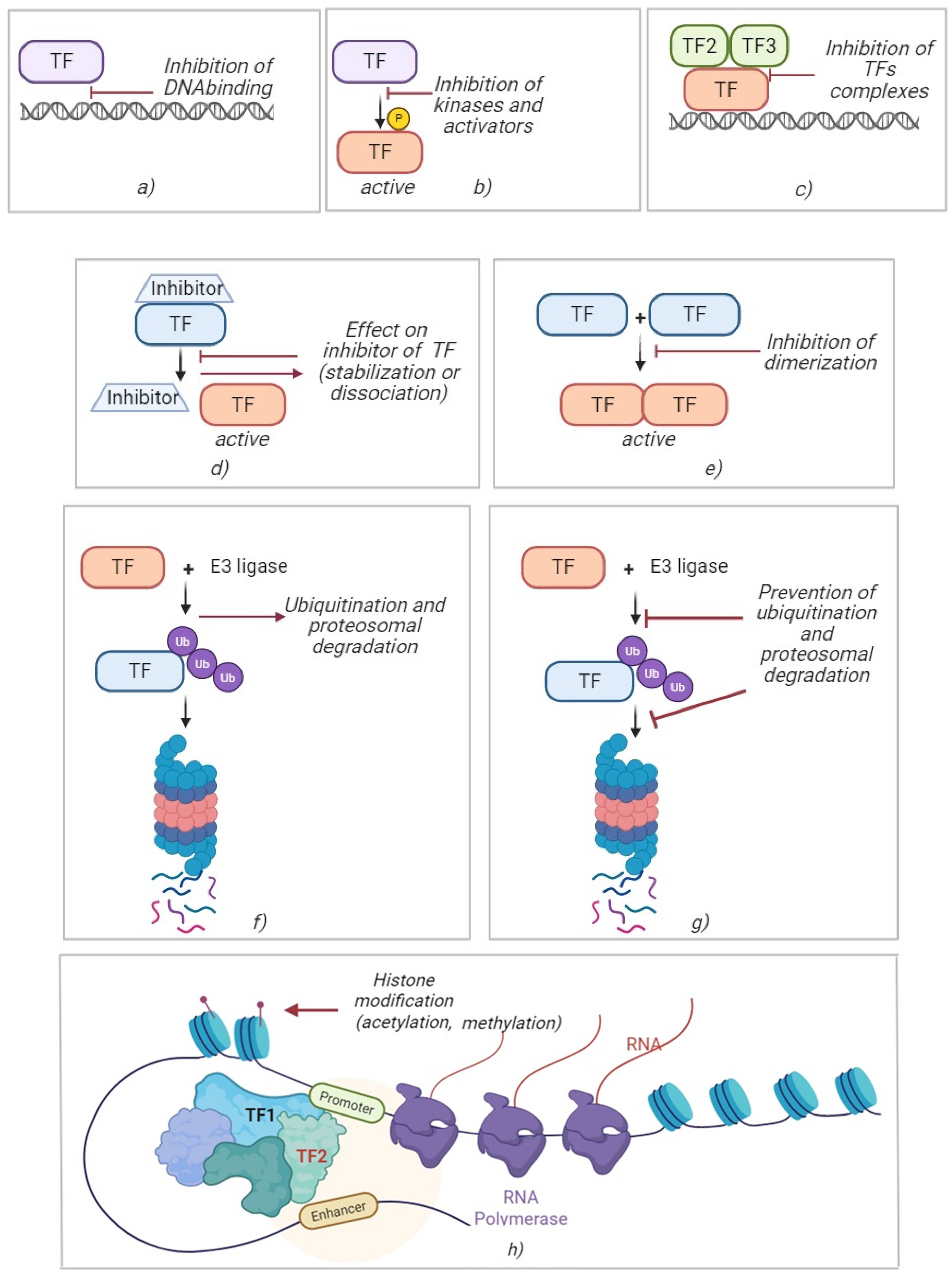
10.4. Epigenetic Modulators
11. Challenges, Limitations, and Solutions in Developing TF-Targeted Drugs
12. Conclusions
Funding
Conflicts of Interest
References
- Shen, C.H. Diagnostic Molecular Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2023; 472p. [Google Scholar]
- Spitz, F.; Furlong, E.E.M. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Preikšaitienė, E.; Kučinskas, V. Genomic control process: Development and evolution. Eur. J. Hum. Genet. 2016, 24, 1093. [Google Scholar] [CrossRef][Green Version]
- Rothenberg, E.V. Transcription factors specifically control change. Genes Dev. 2022, 36, 1097–1099. [Google Scholar] [CrossRef]
- Biggin, M.D. Animal transcription networks as highly connected, quantitative continua. Dev. Cell 2011, 21, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Revilla-i-Domingo, R.; Bilic, I.; Vilagos, B.; Tagoh, H.; Ebert, A.; Tamir, I.M.; Smeenk, L.; Trupke, J.; Sommer, A.; Jaritz, M.; et al. The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis. EMBO J. 2012, 31, 3130–3146. [Google Scholar] [CrossRef]
- Lo, J.H.-H.; Edwards, M.; Langerman, J.; Sridharan, R.; Plath, K.; Smale, S.T. Sox2 binding is essential for establishing but not maintaining active and silent states of dynamically regulated genes in pluripotent cells. Genes Dev. 2022, 36, 1079–1095. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Stilgenbauer, M.; Koehler, A.N. Small-Molecule Approaches to Target Transcription Factors. Annu. Rev. Cancer Biol. 2024, 8, 395–415. [Google Scholar] [CrossRef]
- Pan, T.; Coleman, J.E. GAL4 transcription factor is not a “zinc finger” but forms a Zn(II)2Cys6 binuclear cluster. Proc. Natl. Acad. Sci. USA 1990, 87, 2077–2081. [Google Scholar] [CrossRef]
- Resendes, K.K.; Rosmarin, A.G. Sp1 control of gene expression in myeloid cells. Crit. Rev. Eukaryot. Gene Expr. 2004, 14, 171–181. [Google Scholar] [CrossRef]
- Carnesecchi, J.; Pinto, P.B.; Lohmann, I. Hox transcription factors: An overview of multi-step regulators of gene expression. Int. J. Dev. Biol. 2018, 62, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef]
- Jindrich, K.; Degnan, B.M. The diversification of the basic leucine zipper family in eukaryotes correlates with the evolution of multicellularity. BMC Evol. Biol. 2016, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Bogoyevitch, M.A.; Kobe, B. Uses for JNK: The many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 1061–1095. [Google Scholar] [CrossRef] [PubMed]
- Kamada, R.; Nomura, T.; Anderson, C.W.; Sakaguchi, K. Cancer-associated p53 tetramerization domain mutants: Quantitative analysis reveals a low threshold for tumor suppressor inactivation. J. Biol. Chem. 2011, 286, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Joerger, A.C.; Fersht, A.R. The tumor suppressor p53: From structures to drug discovery. Cold Spring Harb. Perspect. Biol. 2010, 2, a000919. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, J.; Huang, H.; Li, J.; Yu, Y.; Jin, H.; Li, Y.; Deng, X.; Gao, J.; Zhao, Q.; et al. Crucial role of c-Jun phosphorylation at Ser63/73 mediated by PHLPP protein degradation in the cheliensisin a inhibition of cell transformation. Cancer Prev. Res. 2014, 7, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Wan, F.; Lenardo, M.J. The nuclear signaling of NF-κB: Current knowledge, new insights, and future perspectives. Cell Res. 2010, 20, 24–33. [Google Scholar] [CrossRef]
- Liongue, C.; Sobah, M.L.; Ward, A.C. Signal Transducer and Activator of Transcription Proteins at the Nexus of Immunodeficiency, Autoimmunity and Cancer. Biomedicines 2024, 12, 45. [Google Scholar] [CrossRef]
- Chia, Z.J.; Kumarapperuma, H.; Zhang, R.; Little, P.J.; Kamato, D. Smad transcription factors as mediators of 7 transmembrane G protein-coupled receptor signalling. Acta Pharmacol. Sin. 2025, 46, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kim, L.K.; Choi, J.M. Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases. Front. Immunol. 2018, 9, 2747. [Google Scholar] [CrossRef]
- Rastinejad, F.; Huang, P.; Chandra, V.; Khorasanizadeh, S. Understanding nuclear receptor form and function using structural biology. J. Mol. Endocrinol. 2013, 51, T1–T21. [Google Scholar] [CrossRef] [PubMed]
- Rastinejad, F. Allosteric communications between domains of nuclear receptors. Steroids 2025, 214, 109551. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Claessens, F.; Joniau, S.; Helsen, C. Comparing the rules of engagement of androgen and glucocorticoid receptors. Cell. Mol. Life Sci. 2017, 74, 2217–2228. [Google Scholar] [CrossRef]
- Kocanova, S.; Mazaheri, M.; Caze-Subra, S.; Bystricky, K. Ligands specify estrogen receptor alpha nuclear localization and degradation. BMC Cell Biol. 2010, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Koenig, R.J. Thyroid hormone receptor coactivators and corepressors. Thyroid 1998, 8, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, M.; Huderson, B.; Murphy, L.; Rowan, B.G. Post-translational modifications of nuclear receptors and human disease. Nucl. Recept. Signal. 2012, 10, e001. [Google Scholar] [CrossRef]
- Awasthi, N.; Liongue, C.; Ward, A.C. STAT proteins: A kaleidoscope of canonical and non-canonical functions in immunity and cancer. J. Hematol. Oncol. 2021, 14, 198. [Google Scholar] [CrossRef]
- Meng, X.; Grötsch, B.; Luo, Y.; Knaup, K.X.; Wiesener, M.S.; Chen, X.X.; Jantsch, J.; Fillatreau, S.; Schett, G.; Bozec, A. Hypoxia-Inducible Factor-1α Is a Critical Transcription Factor for IL-10-Producing B Cells in Autoimmune Disease. Nat. Commun. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Nicoll, M.; Ingham, R.J. AP-1 Family Transcription Factors: A Diverse Family of Proteins That Regulate Varied Cellular Activities in Classical Hodgkin Lymphoma and ALK+ ALCL. Exp. Hematol. Oncol. 2021, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Baltimore, D. Multiple Nuclear Factors Interact with the Immunoglobulin Enhancer Sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Singh, H.; Sen, R.; Baltimore, D.; Sharp, P.A. A Nuclear Factor That Binds to a Conserved Sequence Motif in Transcriptional Control Elements of Immunoglobulin Genes. Nature 1986, 319, 154–158. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes. Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Williams, L.M.; Gilmore, T.D. Looking Down on NF-κB. Mol. Cell. Biol. 2020, 40, e00104-20. [Google Scholar] [CrossRef]
- Putnam, N.H.; Srivastava, M.; Hellsten, U.; Dirks, B.; Chapman, J.; Salamov, A.; Terry, A.; Shapiro, H.; Lindquist, E.; Kapitonov, V.V.; et al. Sea Anemone Genome Reveals Ancestral Eumetazoan Gene Repertoire and Genomic Organization. Science 2007, 317, 86–94. [Google Scholar] [CrossRef]
- Sun, X.F.; Zhang, H. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol. Histopathol. 2007, 22, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.C.; Kalaitzidis, D.; Gilmore, T.D.; Finnerty, J.R. Rel Homology Domain-Containing Transcription Factors in the Cnidarian Nematostella vectensis. Dev. Genes Evol. 2007, 217, 63–72. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Ovchinnikova, T.V. Innate Immunity Mechanisms in Marine Multicellular Organisms. Mar. Drugs 2022, 20, 549. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, B.; Zhang, X.; Li, J.; Zeng, D.; Huang, T.; Wu, J. NF-κB affected the serum levels of TNF-α and IL-1β via activation of the MAPK/NF-κB signaling pathway in rat model of acute pulmonary microthromboembolism. Pulm. Circ. 2024, 14, e12357. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Li, L.; Sun, Y.; Yang, H.; Ye, Z.; Zhao, J. Effects of the TLR4/Myd88/NF-κB Signaling Pathway on NLRP3 Inflammasome in Coronary Microembolization-Induced Myocardial Injury. Cell. Physiol. Biochem. 2018, 47, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Elinav, E. NF-κB Regulation by NLRs: T Cells Join the Club. Immunity 2015, 42, 595–597. [Google Scholar] [CrossRef][Green Version]
- Son, M.; Wang, A.G.; Keisham, B.; Tay, S. Processing stimulus dynamics by the NF-κB network in single cells. Exp. Mol. Med. 2023, 55, 2531–2540. [Google Scholar] [CrossRef]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Ghosh, S.; Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 1995, 376, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J.D.; Basak, S.; Werner, S.L.; Huang, C.S.; Hoffmann, A. IκBε Provides Negative Feedback to Control NF-κB Oscillations, Signaling Dynamics, and Inflammatory Gene Expression. J. Cell Biol. 2006, 173, 659–664. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Karin, M. Nuclear Factor-κB in Cancer Development and Progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Zhou, H.; Coveney, A.P.; Wu, M.; Huang, J.; Blankson, S.; Zhao, H.; O’Leary, D.P.; Bai, Z.; Li, Y.; Redmond, H.P.; et al. Activation of Both TLR and NOD Signaling Confers Host Innate Immunity-Mediated Protection Against Microbial Infection. Front. Immunol. 2019, 10, 3082. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Núñez, G. Intracellular Sensors of Microbes and Danger. Immunol. Rev. 2011, 243, 5–8. [Google Scholar] [CrossRef]
- Gorshkova, R.P.; Isakov, V.V.; Nazarenko, E.L.; Ovodov, Y.S.; Guryanova, S.V.; Dmitriev, B.A. Structure of the O-specific polysaccharide of the lipopolysaccharide from Yersinia kristensenii O:25.35. Carbohydr. Res. 1993, 241, 201–208. [Google Scholar] [CrossRef] [PubMed]
- L’vov, V.L.; Gur’yanova, S.V.; Rodionov, A.V.; Gorshkova, R.P. Structure of the repeating unit of the O-specific polysaccharide of the lipopolysaccharide of Yersinia kristensenii strain 490 (O:12,25). Carbohydr. Res. 1992, 228, 415–422. [Google Scholar] [CrossRef]
- L’vov, V.L.; Gur’ianova, S.V.; Rodionov, A.V.; Dmitriev, B.A.; Shashkov, A.S.; Ignatenko, A.V.; Gorshkova, R.P.; Ovodov, I.S. The structure of a repetitive unit of the glycerolphosphate- containing O-specific polysaccharide chain from Yersinia kristensenii strain 103 (0:12,26) lipopolysaccharide. Bioorg. Khim. 1990, 16, 379–389. [Google Scholar] [PubMed]
- Meshcheryakova, E.; Makarov, E.; Philpott, D.; Andronova, T.; Ivanov, V. Evidence for correlation between the intensities of adjuvant effects and NOD2 activation by monomeric, dimeric and lipophylic derivatives of N-acetylglucosaminyl-N-acetylmuramyl peptides. Vaccine 2007, 25, 4515–4520. [Google Scholar] [CrossRef]
- Meshcheriakova, E.A.; Gur’ianova, S.V.; Makarov, E.A.; Andronova, T.M.; Ivanov, V.T. Structure-functional study of glycosaminylmuramoyl peptides. The effect of chemical modification of N-acetylglucosaminyl-N-acetylmuramoyldipeptide on its immunomodulating properties in vivo and in vitro. Bioorg. Chem. 1991, 17, 1157–1165. [Google Scholar] [PubMed]
- Bartek, J.; Lukas, J. Cell Biology. The Stress of Finding NEMO. Science 2006, 311, 1110–1111. [Google Scholar] [CrossRef] [PubMed]
- McCool, K.W.; Miyamoto, S. DNA Damage-Dependent NF-κB Activation: NEMO Turns Nuclear Signaling Inside Out. Immunol. Rev. 2012, 246, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-κB, Inflammation, Immunity and Cancer: Coming of Age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Ghislat, G.; Cheema, A.S.; Baudoin, E.; Verthuy, C.; Ballester, P.J.; Crozat, K.; Attaf, N.; Dong, C.; Milpied, P.; Malissen, B.; et al. NF-κB-Dependent IRF1 Activation Programs cDC1 Dendritic Cells to Drive Antitumor Immunity. Sci. Immunol. 2021, 6, eabg3570. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Gigani, O.B.; Gudima, G.O.; Kataeva, A.M.; Kolesnikova, N.V. Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life 2022, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Rechkina, E.A.; Denisova, G.F.; Masalova, O.V.; Lideman, L.F.; Denisov, D.A.; Lesnova, E.I.; Ataullakhanov, R.I.; Gur’ianova, S.V.; Kushch, A. Epitope mapping of antigenic determinants of hepatitis C virus proteins by phage display. Mol. Biol. 2006, 40, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V. Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin. Microorganisms 2022, 10, 1526. [Google Scholar] [CrossRef]
- Kolesnikova, N.V.; Kozlov, I.G.; Guryanova, S.V.; Kokov, E.A.; Andronova, T.M. Clinical and immunological efficiency of muramyl dipeptide in the treatment of atopic diseases. Med. Immunol. 2016, 1, 15–20. [Google Scholar] [CrossRef]
- Guryanova, S.; Khaitov, R. Glucosaminylmuramyldipeptide—GMDP: Effect on mucosal immunity (on the issue of immunotherapy and immunoprophylaxis). Immunologiya 2020, 41, 174–183. [Google Scholar] [CrossRef]
- Shih, V.F.; Kearns, J.D.; Basak, S.; Savinova, O.V.; Ghosh, G.; Hoffmann, A. Kinetic control of negative feedback regulators of NF-κB/RelA determines their pathogen- and cytokine-receptor signaling specificity. Proc. Natl. Acad. Sci. USA 2009, 106, 9619–9624. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, M.M.; Clark, H.R.; Hughey, J.J.; Maayan, I.; Kudo, T.; Gutschow, M.V.; Covert, M.W.; Regot, S. NF-κB signaling dynamics is controlled by a dose-sensing autoregulatory loop. Sci. Signal. 2019, 12, eaau3568. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-κB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Cildir, G.; Low, K.C.; Tergaonkar, V. Noncanonical NF-κB Signaling in Health and Disease. Trends Mol. Med. 2016, 22, 414–429. [Google Scholar] [CrossRef]
- Sun, S.-C. The Non-Canonical NF-κB Pathway in Immunity and Inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Xiao, G.; Harhaj, E.W.; Sun, S.-C. NF-κB-Inducing Kinase Regulates the Processing of NF-κB2 p100. Mol. Cell 2001, 7, 401–409. [Google Scholar] [CrossRef]
- Liao, G.; Sun, S.-C. Regulation of NF-κB2/p100 Processing by Its Nuclear Shuttling. Oncogene 2003, 22, 4868–4874. [Google Scholar] [CrossRef][Green Version]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, S.; Shinkura, R.; Kamata, T.; Nogaki, F.; Ikuta, K.; Tashiro, K.; Honjo, T. Alymphoplasia (aly)-Type Nuclear Factor κB-Inducing Kinase (NIK) Causes Defects in Secondary Lymphoid Tissue Chemokine Receptor Signaling and Homing of Peritoneal Cells to the Gut-Associated Lymphatic Tissue System. J. Exp. Med. 2000, 191, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Kurashima, Y.; Gohda, M.; Higuchi, M.; Ishikawa, I.; Miura, F.; Ogahara, I.; Kiyono, H. Sphingosine 1-Phosphate-Dependent Trafficking of Peritoneal B Cells Requires Functional NF-κB-Inducing Kinase in Stromal Cells. Blood 2008, 111, 4646–4652. [Google Scholar] [CrossRef]
- Yamada, T.; Mitani, T.; Yorita, K.; Uchida, D.; Matsushima, A.; Iwamasa, K.; Fujita, S.; Matsumoto, M. Abnormal Immune Function of Hemopoietic Cells from Alymphoplasia (aly) Mice, a Natural Strain with Mutant NF-κB-Inducing Kinase. J. Immunol. 2000, 165, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Brightbill, H.D.; Suto, E.; Blaquiere, N.; Ramamoorthi, N.; Sujatha-Bhaskar, S.; Gogol, E.B.; Castanedo, G.M.; Jackson, B.T.; Kwon, Y.C.; Haller, S.; et al. Conditional Deletion of NF-κB-Inducing Kinase (NIK) in Adult Mice Disrupts Mature B Cell Survival and Activation. J. Immunol. 2015, 195, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Koike, R.; Saito, H.; Matsuki, N. The Splenic Marginal Zone is Absent in Alymphoplastic Aly Mutant Mice. Eur. J. Immunol. 1996, 26, 669–675. [Google Scholar] [CrossRef]
- Weih, D.S.; Yilmaz, Z.B.; Weih, F. Essential Role of RelB in Germinal Center and Marginal Zone Formation and Proper Expression of Homing Chemokines. J. Immunol. 2001, 167, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Hu, H.; Jin, J.; Puebla-Osorio, N.; Xiao, Y.; Gilbert, B.E.; Brink, R.; Ullrich, S.E.; Sun, S.C. TRAF3 Regulates the Effector Function of Regulatory T Cells and Humoral Immune Responses. J. Exp. Med. 2014, 211, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.J.; Van Wier, S.; Tiedemann, R.; Shi, C.X.; Sebag, M.; et al. Promiscuous Mutations Activate the Noncanonical NF-κB Pathway in Multiple Myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef]
- Demchenko, Y.N.; Glebov, O.K.; Zingone, A.; Keats, J.J.; Bergsagel, P.L.; Kuehl, W.M. Classical and/or Alternative NF-κB Pathway Activation in Multiple Myeloma. Blood 2010, 115, 3541–3552. [Google Scholar] [CrossRef]
- Zhang, B.; Calado, D.P.; Wang, Z.; Fröhler, S.; Köchert, K.; Qian, Y.; Koralov, S.B.; Schmidt-Supprian, M.; Sasaki, Y.; Unitt, C.; et al. An Oncogenic Role for Alternative NF-κB Signaling in DLBCL Revealed Upon Deregulated BCL6 Expression. Cell Rep. 2015, 11, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Deaglio, S.; Dominguez-Sola, D.; Rasi, S.; Vaisitti, T.; Agostinelli, C.; Spina, V.; Bruscaggin, A.; Monti, S.; Cerri, M.; et al. Alteration of BIRC3 and Multiple Other NF-κB Pathway Genes in Splenic Marginal Zone Lymphoma. Blood 2011, 118, 4930–4934. [Google Scholar] [CrossRef]
- Demchenko, Y.N.; Kuehl, W.M. A Critical Role for the NFκB Pathway in Multiple Myeloma. Oncotarget 2010, 1, 59–68. [Google Scholar] [CrossRef]
- Thakur, S.; Lin, H.C.; Tseng, W.T.; Kumar, S.; Bravo, R.; Foss, F.; Gélinas, C.; Rabson, A.B. Rearrangement and Altered Expression of the NFKB-2 Gene in Human Cutaneous T-Lymphoma Cells. Oncogene 1994, 9, 2335–2344. [Google Scholar] [PubMed]
- Lanzillotta, A.; Porrini, V.; Bellucci, A.; Benarese, M.; Branca, C.; Parrella, E.; Spano, P.F.; Pizzi, M. NF-κB in Innate Neuroprotection and Age-Related Neurodegenerative Diseases. Front. Neurol. 2015, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Meffert, M.K. Roles for NF-κB in Nerve Cell Survival, Plasticity, and Disease. Cell Death Differ. 2006, 13, 852–860. [Google Scholar] [CrossRef]
- Inta, I.; Paxian, S.; Maegele, I.; Zhang, W.; Pizzi, M.; Spano, P.; Sarnico, I.; Muhammad, S.; Herrmann, O.; Inta, D.; et al. Bim and Noxa are Candidates to Mediate the Deleterious Effect of the NF-κB Subunit RelA in Cerebral Ischemia. J. Neurosci. 2006, 26, 12896–12903. [Google Scholar] [CrossRef] [PubMed]
- Sarnico, I.; Lanzillotta, A.; Boroni, F.; Benarese, M.; Alghisi, M.; Schwaninger, M.; Inta, I.; Battistin, L.; Spano, P.; Pizzi, M. NF-κB p50/RelA and c-Rel-Containing Dimers: Opposite Regulators of Neuron Vulnerability to Ischaemia. J. Neurochem. 2009, 108, 475–485. [Google Scholar] [CrossRef]
- Pizzi, M.; Sarnico, I.; Boroni, F.; Benetti, A.; Benarese, M.; Spano, P.F. Inhibition of IκBα Phosphorylation Prevents Glutamate-Induced NF-κB Activation and Neuronal Cell Death. Acta Neurochir. Suppl. 2005, 93, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.; Sarnico, I.; Boroni, F.; Benarese, M.; Steimberg, N.; Mazzoleni, G.; Dietz, G.P.; Bähr, M.; Liou, H.C.; Spano, P.F. NF-κB Factor c-Rel Mediates Neuroprotection Elicited by mGlu5 Receptor Agonists Against Amyloid β-Peptide Toxicity. Cell Death Differ. 2005, 12, 761–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanzillotta, A.; Sarnico, I.; Ingrassia, R.; Boroni, F.; Branca, C.; Benarese, M.; Faraco, G.; Blasi, F.; Chiarugi, A.; Spano, P.; et al. The Acetylation of RelA in Lys310 Dictates the NF-κB-Dependent Response in Post-Ischemic Injury. Cell Death Dis. 2010, 1, e96. [Google Scholar] [CrossRef]
- Baiguera, C.; Alghisi, M.; Pinna, A.; Bellucci, A.; De Luca, M.A.; Frau, L.; Morelli, M.; Ingrassia, R.; Benarese, M.; Porrini, V.; et al. Late-Onset Parkinsonism in NF-κB/c-Rel-Deficient Mice. Brain 2012, 135, 2750–2765. [Google Scholar] [CrossRef]
- Hunot, S.; Brugg, B.; Ricard, D.; Michel, P.P.; Muriel, M.P.; Ruberg, M.; Faucheux, B.A.; Agid, Y.; Hirsch, E.C. Nuclear Translocation of NF-κB is Increased in Dopaminergic Neurons of Patients with Parkinson Disease. Proc. Natl. Acad. Sci. USA 1997, 94, 7531–7536. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-κB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Vuscan, P.; Kischkel, B.; Joosten, L.A.B.; Netea, M.G. Trained immunity: General and emerging concepts. Immunol. Rev. 2024, 323, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V. Bacteria and Allergic Diseases. Int. J. Mol. Sci. 2024, 25, 10298. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Kataeva, A. Inflammation Regulation by Bacterial Molecular Patterns. Biomedicines 2023, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V. Influence of muramyl peptides on the production of chemokines, growth factors, pro-inflammatory and anti-inflammatory cytokines. RUDN J. Med. 2024, 28, 365–376. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NF-κB-Signaling Pathway in Cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Spiller, S.E.; Logsdon, N.J.; Deckard, L.A.; Sontheimer, H. Inhibition of Nuclear Factor Kappa-B Signaling Reduces Growth in Medulloblastoma In Vivo. BMC Cancer 2011, 11, 136. [Google Scholar] [CrossRef]
- Wong, A.H.; Shin, E.M.; Tergaonkar, V.; Chng, W.J. Targeting NF-κB Signaling for Multiple Myeloma. Cancers 2020, 12, 2203. [Google Scholar] [CrossRef]
- Ito, S. Proteasome Inhibitors for the Treatment of Multiple Myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef]
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.; Dou, Q.P. Bortezomib as the First Proteasome Inhibitor Anticancer Drug: Current Status and Future Perspectives. Curr. Cancer Drug Targets 2011, 11, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Offidani, M.; Corvatta, L.; Bringhen, S.; Gentili, S.; Gay, F.; Maracci, L.; Boccadoro, M.; Leoni, P.; Palumbo, A. Infection Complications in 476 Patients with Newly Diagnosed Multiple Myeloma Treated with Lenalidomide or Bortezomib Combinations. Blood 2015, 126, 5365. [Google Scholar] [CrossRef]
- Teh, B.W.; Harrison, S.J.; Worth, L.J.; Thursky, K.A.; Slavin, M.A. Infection risk with immunomodulatory and proteasome inhibitor–based therapies across treatment phases for multiple myeloma: A systematic review and meta-analysis. Eur. J. Cancer 2016, 67, 21–37. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Huang, B.; Zheng, D.; Chen, M.; Zhou, Z. Drug-Induced Modulation of T Lymphocytes as a Potential Mechanism of Susceptibility to Infections in Patients with Multiple Myeloma During Bortezomib Therapy. Cell Biochem. Biophys. 2014, 71, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Is NF-κB a Good Target for Cancer Therapy? Hopes and Pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- 30 Feng, R.; Anderson, G.; Xiao, G.; Elliott, G.; Leoni, L.; Mapara, M.Y.; Roodman, G.D.; Lentzsch, S. SDX-308, a Nonsteroidal Anti-Inflammatory Agent, Inhibits NF-κB Activity, Resulting in Strong Inhibition of Osteoclast Formation/Activity and Multiple Myeloma Cell Growth. Blood 2007, 109, 2130–2138. [Google Scholar] [CrossRef]
- Hideshima, T.; Neri, P.; Tassone, P.; Yasui, H.; Ishitsuka, K.; Raje, N.; Chauhan, D.; Podar, K.; Mitsiades, C.; Dang, L.; et al. MLN120B, a Novel IκB Kinase β Inhibitor, Blocks Multiple Myeloma Cell Growth In Vitro and In Vivo. Clin. Cancer Res. 2006, 12, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Sanda, T.; Iida, S.; Ogura, H.; Asamitsu, K.; Murata, T.; Bacon, K.B.; Ueda, R.; Okamoto, T. Growth Inhibition of Multiple Myeloma Cells by a Novel IκB Kinase Inhibitor. Clin. Cancer Res. 2005, 11, 1974–1982. [Google Scholar] [CrossRef]
- Dai, Y.; Pei, X.Y.; Rahmani, M.; Conrad, D.H.; Dent, P.; Grant, S. Interruption of the NF-κB Pathway by Bay 11-7082 Promotes UCN-01-Mediated Mitochondrial Dysfunction and Apoptosis in Human Multiple Myeloma Cells. Blood 2004, 103, 2761–2770. [Google Scholar] [CrossRef]
- Aghai, Z.H.; Kumar, S.; Farhath, S.; Kumar, M.A.; Saslow, J.; Nakhla, T.; Eydelman, R.; Strande, L.; Stahl, G.; Hewitt, C.; et al. Dexamethasone Suppresses Expression of Nuclear Factor-κB in the Cells of Tracheobronchial Lavage Fluid in Premature Neonates with Respiratory Distress. Pediatr. Res. 2006, 59, 811–815. [Google Scholar] [CrossRef]
- Rosenberg, A.S. From Mechanism to Resistance—Changes in the Use of Dexamethasone in the Treatment of Multiple Myeloma. Leuk. Lymphoma 2023, 64, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M. NF-κB Signalling as a Pharmacological Target in COVID-19: Potential Roles for IKKβ Inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Mahjoubin-Tehran, M.; Rezaei, S.; Butler, A.E.; Sahebkar, A. Decoy Oligonucleotides Targeting NF-κB: A Promising Therapeutic Approach for Inflammatory Diseases. Inflamm. Res. 2025, 74, 47. [Google Scholar] [CrossRef] [PubMed]
- Guldenpfennig, C.; Teixeiro, E.; Daniels, M. NF-κB’s Contribution to B Cell Fate Decisions. Front. Immunol. 2023, 14, 1214095. [Google Scholar] [CrossRef]
- Fakharzadeh, S.S.; Trusko, S.P.; George, D.L. Tumorigenic Potential Associated with Enhanced Expression of a Gene that is Amplified in a Mouse Tumor Cell Line. EMBO J. 1991, 10, 1565–1569. [Google Scholar] [CrossRef]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer. 2020, 20, 471–480. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, H.; Ji, Y.; Zhou, Q.; Du, Z.; Tian, L.; Jiang, Y.; Yao, K.; Zhou, Z. Targeting p53-MDM2 interaction by small-molecule inhibitors: Learning from MDM2 inhibitors in clinical trials. J. Hematol. Oncol. 2022, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer. 2013, 13, 83–96. [Google Scholar] [CrossRef]
- Wang, W.; Albadari, N.; Du, Y.; Fowler, J.F.; Sang, H.T.; Xian, W.; McKeon, F.; Li, W.; Zhou, J.; Zhang, R. MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future. Pharmacol. Rev. 2024, 76, 414–453. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Arya, A.K.; El-Fert, A.; Devling, T.; Eccles, R.M.; Aslam, M.A.; Rubbi, C.P.; Vlatković, N.; Fenwick, J.; Lloyd, B.H.; Sibson, D.R.; et al. Nutlin-3, the small-molecule inhibitor of MDM2, promotes senescence and radiosensitises laryngeal carcinoma cells harbouring wild-type p53. Br. J. Cancer 2010, 103, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.; Wovkulich, P.; Pizzolato, G.; Lovey, A.; Ding, Q.; Jiang, N.; Liu, J.J.; Zhao, C.; Glenn, K.; Wen, Y.; et al. Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med. Chem. Lett. 2013, 4, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Dutta, A.; Mukhopadhyay, R. TP53 mutations and MDM2 polymorphisms in breast and ovarian cancers: Amelioration by drugs and natural compounds. Clin. Transl. Oncol. 2025, 27, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Espadinha, M.; Raimundo, L.; Ramos, H.; Gomes, A.S.; Gomes, S.; Loureiro, J.B.; Inga, A.; Reis, F.; Gomes, C.; et al. DIMP53-1: A novel small-molecule dual inhibitor of p53-MDM2/X interactions with multifunctional p53-dependent anticancer properties. Mol. Oncol. 2017, 11, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, C.; Aptullahoglu, E.; Woodhouse, L.; Lin, W.Y.; Wallis, J.P.; Marr, H.; Marshall, S.; Bown, N.; Willmore, E.; Lunec, J. Non-genotoxic MDM2 inhibition selectively induces a pro-apoptotic p53 gene signature in chronic lymphocytic leukemia cells. Haematologica 2019, 104, 2429–2442. [Google Scholar] [CrossRef]
- Zanjirband, M.; Edmondson, R.J.; Lunec, J. Pre-clinical efficacy and synergistic potential of the MDM2-p53 antagonists, Nutlin-3 and RG7388, as single agents and in combined treatment with cisplatin in ovarian cancer. Oncotarget 2016, 7, 40115–40134. [Google Scholar] [CrossRef]
- Abed, A.; Greene, M.K.; Alsa’d, A.A.; Lees, A.; Hindley, A.; Longley, D.B.; McDade, S.S.; Scott, C.J. Nanoencapsulation of MDM2 Inhibitor RG7388 and Class-I HDAC Inhibitor Entinostat Enhances their Therapeutic Potential Through Synergistic Antitumor Effects and Reduction of Systemic Toxicity. Mol. Pharm. 2024, 21, 1246–1255. [Google Scholar] [CrossRef]
- Konopleva, M.; Martinelli, G.; Daver, N.; Papayannidis, C.; Wei, A.; Higgins, B.; Ott, M.; Mascarenhas, J.; Andreeff, M. MDM2 inhibition: An important step forward in cancer therapy. Leukemia 2020, 34, 2858–2874. [Google Scholar] [CrossRef]
- Zhang, Q.; Bykov, V.J.N.; Wiman, K.G.; Zawacka-Pankau, J. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 2018, 9, 439. [Google Scholar] [CrossRef]
- Mlakar, V.; Jurkovic Mlakar, S.; Lesne, L.; Marino, D.; Rathi, K.S.; Maris, J.M.; Ansari, M.; Gumy-Pause, F. PRIMA-1MET-induced neuroblastoma cell death is modulated by p53 and mycn through glutathione level. J. Exp. Clin. Cancer Res. 2019, 38, 69. [Google Scholar] [CrossRef]
- Pellot Ortiz, K.I.; Rechberger, J.S.; Nonnenbroich, L.F.; Daniels, D.J.; Sarkaria, J.N. MDM2 Inhibition in the Treatment of Glioblastoma: From Concept to Clinical Investigation. Biomedicines 2023, 11, 1879. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L.; Falandry, C.; List, A. A Proactive Approach to Prevent Hematopoietic Exhaustion During Cancer Chemotherapy in Older Patients: Temporary Cell-Cycle Arrest. Drugs Aging 2023, 40, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L.; Falandry, C.; List, A. New Advances in Supportive Care: Chemoprotective Agents as Novel Opportunities in Geriatric Oncology. Curr. Oncol. Rep. 2022, 24, 1695–1703. [Google Scholar] [CrossRef]
- Falandry, C.; List, A.; Balducci, L. Cell cycle arrest: A breakthrough in the supportive care of older cancer patients. J. Am. Geriatr. Soc. 2023, 71, 2297–2307. [Google Scholar] [CrossRef]
- Twarda-Clapa, A. An update patent review of MDM2-p53 interaction inhibitors (2019-2023). Expert. Opin. Ther. Pat. 2024, 34, 1177–1198. [Google Scholar] [CrossRef] [PubMed]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.; Guryanova, A. sbv IMPROVER: Modern Approach to Systems Biology. Methods Mol. Biol. 2017, 1613, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, A.A.; Morales, A.F.; Lacave, Á.M.; Tallam, A.; Simovic, B.; Alfaro, D.G.; Bobbili, D.R.; Martin, F.; Androsova, G.; Shvydchenko, I.; et al. Community-Reviewed Biological Network Models for Toxicology and Drug Discovery Applications. Gene Regul. Syst. Biol. 2016, 10, 51–66. [Google Scholar] [CrossRef]
- Bhatia, K.; Sandhu, V.; Wong, M.H.; Iyer, P.; Bhatt, S. Therapeutic biomarkers in acute myeloid leukemia: Functional and genomic approaches. Front. Oncol. 2024, 14, 1275251. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, Z.F.; Wang, S.P.; Vong, C.T.; Yu, B.; Wang, Y.T. HIF-1: Structure, biology and natural modulators. Chin. J. Nat. Med. 2021, 19, 521–527. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef]
- Merelli, A.; Rodríguez, J.C.G.; Folch, J.; Regueiro, M.R.; Camins, A.; Lazarowski, A. Understanding the role of hypoxia inducible factor during neurodegeneration for new therapeutics opportunities. Curr. Neuropharmacol. 2018, 16, 1484–1498. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Li, Q.; Han, H. HIF-1α in cerebral ischemia (Review). Mol. Med. Rep. 2022, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Chow, C.C.T.; Kambe, G.; Suwa, T.; Kobayashi, M.; Takahashi, I.; Harada, H.; Nam, J.M. An overview of the recent development of anticancer agents targeting the hif-1 transcription factor. Cancers 2021, 13, 2813. [Google Scholar] [CrossRef]
- Martí-Díaz, R.; Montenegro, M.F.; Cabezas-Herrera, J.; Goding, C.R.; Rodríguez-López, J.N.; Sánchez-Del-campo, L. Acriflavine, a potent inhibitor of HIF-1α, disturbs glucose metabolism and suppresses ATF4-protective pathways in melanoma under non-hypoxic conditions. Cancers 2021, 13, 102. [Google Scholar] [CrossRef]
- Montigaud, Y.; Ucakar, B.; Krishnamachary, B.; Bhujwalla, Z.M.; Feron, O.; Préat, V.; Danhier, F.; Gallez, B.; Danhier, P. Optimized acriflavine-loaded lipid nanocapsules as a safe and effective delivery system to treat breast cancer. Int. J. Pharm. 2018, 551, 322–328. [Google Scholar] [CrossRef]
- Khdair, A.; Hamad, I.; Alkhatib, H.; Bustanji, Y.; Mohammad, M.; Tayem, R.; Aiedeh, K. Modified-chitosan nanoparticles: Novel drug delivery systems improve oral bioavailability of doxorubicin. Eur. J. Pharm. Sci. 2016, 93, 38–44. [Google Scholar] [CrossRef]
- Hamadneh, L.; Abu-Irmaileh, B.; Al-Majawleh, M.; Bustanji, Y.; Jarrar, Y.; Al-Qirim, T. Doxorubicin–paclitaxel sequential treatment: Insights of DNA methylation and gene expression changes of luminal A and triple negative breast cancer cell lines. Mol. Cell. Biochem. 2021, 476, 3647–3654. [Google Scholar] [CrossRef]
- Yu, W.; Denu, R.A.; Krautkramer, K.A.; Grindle, K.M.; Yang, D.T.; Asimakopoulos, F.; Hematti, P.; Denu, J.M. Loss of SIRT3 provides growth advantage for B cell malignancies. J. Biol. Chem. 2016, 291, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Viziteu, E.; Grandmougin, C.; Goldschmidt, H.; Seckinger, A.; Hose, D.; Klein, B.; Moreaux, J. Chetomin, targeting HIF-1α/p300 complex, exhibits antitumour activity in multiple myeloma. Br. J. Cancer 2016, 114, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; El-Shahawy, M.A.; Pecoits-Filho, R.; Van, B.P.; Houser, M.T.; Frison, L.; Little, D.J.; Guzman, N.J.; Pergola, P.E. Roxadustat for Treating Anemia in Patients with CKD Not on Dialysis: Results from a Randomized Phase 3 Study. J. Am. Soc. Nephrol. 2021, 32, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Pollock, C.A.; El-Shahawy, M.; Escudero, E.T.; Rastogi, A.; Van, B.P.; Frison, L.; Houser, M.; Pola, M.; Little, D.J.; et al. Roxadustat Versus Epoetin Alfa for Treating Anemia in Patients with Chronic Kidney Disease on Dialysis: Results from the Randomized Phase 3 ROCKIES Study. J. Am. Soc. Nephrol. 2022, 33, 850–866. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, L.; Wei, X.; Long, M.; Du, Y. Roxadustat: Not just for anemia. Front. Pharmacol. 2022, 13, 971795. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef]
- Klampfer, L. Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs. Curr. Cancer Drug Targets 2006, 6, 107–121. [Google Scholar] [CrossRef]
- Balendran, T.; Lim, K.; Hamilton, J.A.; Achuthan, A.A. Targeting transcription factors for therapeutic benefit in rheumatoid arthritis. Front. Immunol. 2023, 14, 1196931. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhang, C.; Martincuks, A.; Herrmann, A.; Yu, H. STAT proteins in cancer: Orchestration of metabolism. Nat. Rev. Cancer 2023, 23, 115–134. [Google Scholar] [CrossRef]
- Wong, G.L.; Manore, S.G.; Doheny, D.L.; Lo, H.W. STAT family of transcription factors in breast cancer: Pathogenesis and therapeutic opportunities and challenges. Semin. Cancer Biol. 2022, 86 Pt 3, 84–106. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Cao, X.; Rashid, K.; Liu, T. The STAT family: Key transcription factors mediating crosstalk between cancer stem cells and tumor immune microenvironment. Semin. Cancer Biol. 2023, 88, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Meli, M.; Grimaudo, S. STAT5 and STAT5 Inhibitors in Hematological Malignancies. Anticancer Agents Med. Chem. 2019, 19, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, L.A. STAT5 facts. Nat. Immunol. 2023, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, Z.; Tomc, J.; Debeljak, N.; Solár, P. STAT5 as a Key Protein of Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7109. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xu, F.; Pang, X.; Xiao, Q.; Wei, Q.; Lei, B.; Li, X.; Fan, X.; Tan, G. STAT3-Dependent Gene TRIM5γ Interacts with HBx Through a Zinc Binding Site on the BBox Domain. Front. Microbiol. 2021, 12, 663534. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Yokosawa, N.; Yokota, S.; Fujii, N. C terminal CYS-RICH region of mumps virus structural V protein correlates with block of interferon alpha and gamma signal transduction pathway through decrease of STAT 1-alpha. Biochem. Biophys. Res. Commun. 2001, 283, 255–259. [Google Scholar] [CrossRef]
- Schust, J.; Sperl, B.; Hollis, A.; Mayer, T.U.; Berg, T. Stattic: A small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 2006, 13, 1235–1242. [Google Scholar] [CrossRef]
- Imbaby, S.; Hattori, Y. Stattic ameliorates the cecal ligation and puncture-induced cardiac injury in septic mice via IL-6-gp130-STAT3 signaling pathway. Life Sci. 2023, 330, 122008. [Google Scholar] [CrossRef]
- Hosseini, M.; Ezzeddini, R.; Hashemi, S.M.; Soudi, S.; Salek Farrokhi, A. Enhanced anti-tumor efficacy of S3I-201 in breast cancer mouse model through Wharton jelly- exosome. Cancer Cell Int. 2024, 24, 318. [Google Scholar] [CrossRef]
- Busker, S.; Page, B.; Arnér, E.S.J. To inhibit TrxR1 is to inactivate STAT3-Inhibition of TrxR1 enzymatic function by STAT3 small molecule inhibitors. Redox Biol. 2020, 36, 101646. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Farran, B.; Farren, M.; Chalikonda, G.; Wu, C.; Lesinski, G.B.; El-Rayes, B.F. Napabucasin (BBI 608), a potent chemoradiosensitizer in rectal cancer. Cancer 2020, 126, 3360–3371. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, S.; Li, Z.; Liao, A.; Li, Y.; Yang, L.; Zhou, X.; Gao, Y.; Liu, X.; Zou, Y.; et al. Napabucasin deactivates STAT3 and promotes mitoxantrone-mediated cGAS-STING activation for hepatocellular carcinoma chemo-immunotherapy. Biomaterials 2025, 313, 122766. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Hu, X. The JAK/STAT pathway in rheumatoid arthritis: Pathogenic or protective? Arthritis Rheum. 2003, 48, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.A.; Fonseca, J.E. JAK inhibitors and modulation of b cell immune responses in rheumatoid arthritis. Front. Med. 2020, 7, 607725. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Dubowy, R.L.; Maltzman, J.D.; Cervantes, F.; Gotlib, J. SIMPLIFY-1: A Phase III Randomized Trial of Momelotinib Versus Ruxolitinib in Janus Kinase Inhibitor-Naïve Patients with Myelofibrosis. J. Clin. Oncol. 2017, 35, 3844–3850. [Google Scholar] [CrossRef]
- Wu, W.; Fu, J.; Gu, Y.; Wei, Y.; Ma, P.; Wu, J. JAK2/STAT3 regulates estrogen-related senescence of bone marrow stem cells. J. Endocrinol. 2020, 245, 141–153. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef]
- Zhou, H.; Bai, L.; Xu, R.; Zhao, Y.; Chen, J.; McEachern, D.; Chinnaswamy, K.; Wen, B.; Dai, L.; Kumar, P.; et al. Structure-Based Discovery of SD-36 as a Potent, Selective, and Efficacious PROTAC Degrader of STAT3 Protein. J. Med. Chem. 2019, 62, 11280–11300. [Google Scholar] [CrossRef]
- Heppler, L.N.; Frank, D.A. Inhibit versus Destroy: Are PROTAC Degraders the Solution to Targeting STAT3? Cancer Cell 2019, 36, 459–461. [Google Scholar] [CrossRef]
- Kaneshige, A.; Bai, L.; Wang, M.; McEachern, D.; Meagher, J.L.; Xu, R.; Wang, Y.; Jiang, W.; Metwally, H.; Kirchhoff, P.D.; et al. A selective small-molecule STAT5 PROTAC degrader capable of achieving tumor regression in vivo. Nat. Chem. Biol. 2023, 19, 703–711. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, H.; Xu, R.; Zhao, Y.; Chinnaswamy, K.; McEachern, D.; Chen, J.; Yang, C.Y.; Liu, Z.; Wang, M.; et al. A Potent and Selective Small-Molecule Degrader of STAT3 Achieves Complete Tumor Regression In Vivo. Cancer Cell 2019, 36, 498–511.e17. [Google Scholar] [CrossRef]
- Karin, M.; Liu, Z.G.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Bejjani, F.; Evanno, E.; Zibara, K.; Piechaczyk, M.; Jariel-Encontre, I. The AP-1 transcriptional complex: Local switch or remote command? Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Lian, Y.; Zhang, L. The potential of activator protein 1 (AP-1) in cancer targeted therapy. Front. Immunol. 2023, 14, 1224892. [Google Scholar] [CrossRef]
- Tang, X.; McMullen, T.P.W.; Brindley, D.N. Increasing the low lipid phosphate phosphatase 1 activity in breast cancer cells decreases transcription by AP-1 and expressions of matrix metalloproteinases and cyclin D1/D3. Theranostics 2019, 9, 6129–6142. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Seki, S.; Yahara, Y.; Shiozawa, S.; Aikawa, Y.; Motomura, H.; Nogami, M.; Watanabe, K.; Sainoh, T.; Ito, H.; et al. A selective inhibition of c-Fos/activator protein-1 as a potential therapeutic target for intervertebral disc degeneration and associated pain. Sci. Rep. 2017, 7, 16983. [Google Scholar] [CrossRef]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small molecule inhibitors targeting activator protein 1 (AP-1). J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Guo, S.; Yang, Z.; Han, L.; Du, J.; Chen, J.; Dun, X.; Wang, G. Roflumilast reduced the IL-18-Induced inflammatory response in fibroblast-like synoviocytes (FLS). ACS Omega 2021, 6, 2149–2155. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Aran, K.R. Unraveling the role of Nrf2 in dopaminergic neurons: A review of oxidative stress and mitochondrial dysfunction in Parkinson’s disease. Metab. Brain Dis. 2025, 40, 123. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Satoh, H.; Moriguchi, T.; Takai, J.; Ebina, M.; Yamamoto, M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013, 73, 4158–4168. [Google Scholar] [CrossRef]
- Manai, F.; Zanoletti, L.; Arfini, D.; Micco, S.G.D.; Gjyzeli, A.; Comincini, S.; Amadio, M. Dimethyl Fumarate and Intestine: From Main Suspect to Potential Ally against Gut Disorders. Int. J. Mol. Sci. 2023, 24, 9912. [Google Scholar] [CrossRef]
- Brück, J.; Dringen, R.; Amasuno, A.; Pau-Charles, I.; Ghoreschi, K. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp. Dermatol. 2018, 27, 611–624. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.X.; Zhu, J.Q.; Dou, Y.X.; Xian, Y.F.; Lin, Z.X. Natural Nrf2 Inhibitors: A Review of Their Potential for Cancer Treatment. Int. J. Biol. Sci. 2023, 19, 3029–3041. [Google Scholar] [CrossRef]
- Hammad, A.; Namani, A.; Elshaer, M.; Wang, X.J.; Tang, X. “NRF2 addiction” in lung cancer cells and its impact on cancer therapy. Cancer Lett. 2019, 467, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Motohashi, H. NRF2 addiction in cancer cells. Cancer Sci. 2018, 109, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chen, J.; Liu, X.M.; Zhao, R.; Zhe, H. Nrf2-Mediated Metabolic Reprogramming in Cancer. Oxid. Med. Cell. Longev. 2018, 2018, 9304091. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Nishi, S.; Sakai, M. Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis. Biochem. J. 2004, 380 Pt 2, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.K.C.; Le, H.; Surh, Y.J. Nrf2, A Target for Precision Oncology in Cancer Prognosis and Treatment. J. Cancer Prev. 2023, 28, 131–142. [Google Scholar] [CrossRef]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Marciano, D.P.; Chang, M.R.; Corzo, C.A.; Goswami, D.; Lam, V.Q.; Pascal, B.D.; Griffin, P.R. The therapeutic potential of nuclear receptor modulators for metabolic disorders. Cell Metab. 2014, 19, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.G. Nuclear receptors as drug targets for metabolic disease. Adv. Drug Deliv. Rev. 2010, 62, 1307–1315. [Google Scholar] [CrossRef]
- Taubenheim, J.; Kortmann, C.; Fraune, S. Function and Evolution of Nuclear Receptors in Environmental-Dependent Postembryonic Development. Front. Cell Dev. Biol. 2021, 9, 653792. [Google Scholar] [CrossRef]
- Nettles, K.W.; Greene, G.L. Ligand control of coregulator recruitment to nuclear receptors. Annu. Rev. Physiol. 2005, 67, 309–333. [Google Scholar] [CrossRef]
- Upadhyay, S.; Hailemariam, A.E.; Mariyam, F.; Hafiz, Z.; Martin, G.; Kothari, J.; Farkas, E.; Sivaram, G.; Bell, L.; Tjalkens, R.; et al. Bis-Indole Derivatives as Dual Nuclear Receptor 4A1 (NR4A1) and NR4A2 Ligands. Biomolecules 2024, 14, 284. [Google Scholar] [CrossRef] [PubMed]
- Frigo, D.E.; Bondesson, M.; Williams, C. Nuclear receptors: From molecular mechanisms to therapeutics. Essays Biochem. 2021, 65, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, S.; Gustafsson, J.Å. Nuclear Receptors: Recent Drug Discovery for Cancer Therapies. Endocr. Rev. 2019, 40, 1207–1249. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; Jeong, Y.; Downes, M.; Yu, R.T.; Evans, R.M.; Mangelsdorf, D.J. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 2006, 126, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, J.; Pei, L.; Evans, R.M. Nuclear receptors: Decoding metabolic disease. FEBS Lett. 2008, 582, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Ma, G.; He, J.; Yu, Y.; Xu, Y.; Yu, X.; Martinez, J.; Lonard, D.M.; Xu, J. Tamoxifen inhibits ER-negative breast cancer cell invasion and metastasis by accelerating Twist1 degradation. Int. J. Biol. Sci. 2015, 11, 618–628. [Google Scholar] [CrossRef]
- Guerrero, J.; Alfaro, I.E.; Gómez, F.; Protter, A.A.; Bernales, S. Enzalutamide, an androgen receptor signaling inhibitor, induces tumor regression in a mouse model of castration-resistant prostate cancer. Prostate 2013, 73, 1291–1305. [Google Scholar] [CrossRef]
- Sugii, S.; Olson, P.; Sears, D.D.; Saberi, M.; Atkins, A.R.; Barish, G.D.; Hong, S.H.; Castro, G.L.; Yin, Y.Q.; Nelson, M.C.; et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. USA 2009, 106, 22504–22509. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef]
- Ding, L.; Tian, Y.; Yu, L.; Dang, N.; Pang, S. Coordinated actions of FXR and LXR in metabolism: From pathogenesis to pharmacological targets for type 2 diabetes. J. Diabetes Res. 2014, 2014, 751859. [Google Scholar] [CrossRef] [PubMed]
- Van Moortel, L.; Gevaert, K.; De Bosscher, K. Improved glucocorticoid receptor ligands: Fantastic beasts, but how to find them? Front. Endocrinol. 2020, 11, 559673. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Ueki, M.; Morishita, J.; Ueno, M.; Shiozawa, S.; Maekawa, N. T-5224, a selective inhibitor of c-Fos/activator protein-1, improves survival by inhibiting serum high mobility group box-1 in lethal lipopolysaccharide-induced acute kidney injury model. J. Intensive Care 2015, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Yuan, X.; Shen, Y.H.; Wang, J.; Azeem, W.; Yang, S.; Gade, A.; Lellahi, S.M.; Øyan, A.M.; Ke, X.; et al. Novel STAT3 Inhibitors Targeting STAT3 Dimerization by Binding to the STAT3 SH2 Domain. Front. Pharmacol. 2022, 13, 836724. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Abrams, S.L.; Steelman, L.S.; Cocco, L.; Ratti, S.; Martelli, A.M.; Lombardi, P.; Gizak, A.; Duda, P. APR-246—The Mutant TP53 Reactivator—Increases the Effectiveness of Berberine and Modified Berberines to Inhibit the Proliferation of Pancreatic Cancer Cells. Biomolecules 2022, 12, 276. [Google Scholar] [CrossRef]
- Schoenbeck, K.L.; Wildes, T.M. Updated Perspectives on the Management of Multiple Myeloma in Older Patients: Focus on Lenalidomide. Clin. Interv. Aging 2020, 15, 619–633. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Geng, H.; Fraser, E.J.; Formaker, P.; Chen, L.; Sharma, J.; Killea, P.; Choi, K.; Ventura, J.; Kurhanewicz, J.; et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018, 2, 1595–1607. [Google Scholar] [CrossRef]
- Brioli, A.; Gengenbach, L.; Mancuso, K.; Binder, M.; Ernst, T.; Heidel, F.H.; Stauch, T.; Za-magni, E.; Hilgendorf, I.; Hochhaus, A.; et al. Pomalidomide combinations are a safe and effective option after daratumumab failure. J. Cancer Res. Clin. Oncol. 2023, 149, 6569–6574. [Google Scholar] [CrossRef]
- Sperling, A.S.; Burgess, M.; Keshishian, H.; Gasser, J.A.; Bhatt, S.; Jan, M.; Słabicki, M.; Sellar, R.S.; Fink, E.C.; Miller, P.G.; et al. Patterns of substrate affinity, competition, and degradation kinetics underlie biological activity of thalidomide analogs. Blood 2019, 134, 160–170. [Google Scholar] [CrossRef]
- Licht, J.D.; Shortt, J.; Johnstone, R. From anecdote to targeted therapy: The curious case of thalidomide in multiple myeloma. Cancer Cell. 2014, 25, 9–11. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Oriol, A.; Beksac, M.; Liberati, A.M.; Galli, M.; Schjesvold, F.; Lindsay, J.; Weisel, K.; White, D.; Facon, T.; et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): A randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Bai, G.; Zhao, J.; Wei, X.; Li, R.; Li, J.; Hu, S.; Peng, L.; Liu, P.; Mao, H. The BRD4 inhibitor JQ1 suppresses tumor growth by reducing c-Myc expression in endometrial cancer. J. Transl. Med. 2022, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Zhan, Z.; Gan, L.; Bai, O. Mechanisms of HDACs in cancer development. Front. Immunol. 2025, 16, 1529239. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Elhrech, H.; Aanniz, T.; Balahbib, A.; Lee, L.H.; Al Abdulmonem, W.; Bouyahya, A. Clinical efficacy and mechanistic insights of FDA-approved HDAC inhibitors in the treatment of lymphoma. Eur. J. Pharm. Sci. 2025, 208, 107057. [Google Scholar] [CrossRef]
- Mao, H.; Zhao, X.; Sun, S.C. NF-κB in inflammation and cancer. Cell. Mol. Immunol. 2025. [Google Scholar] [CrossRef]
- Yang, R.; Rincon, M. Mitochondrial Stat3, the Need for Design Thinking. Int. J. Biol. Sci. 2016, 12, 532–544. [Google Scholar] [CrossRef]
- Su, Y.; Huang, X.; Huang, Z.; Huang, T.; Xu, Y.; Yi, C. STAT3 Localizes in Mitochondria-Associated ER Membranes Instead of in Mitochondria. Front. Cell Dev. Biol. 2020, 8, 274. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef]
- Schapira, M.; Calabrese, M.F.; Bullock, A.N.; Crews, C.M. Targeted protein degradation: Expanding the toolbox. Nat. Rev. Drug Discov. 2019, 18, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Burris, H.A., III; Vuky, J.; Dreicer, R.; Sartor, A.O.; Sternberg, C.N.; Percent, I.J.; Hussain, M.H.A.; Kalebasty, A.R.; Shen, J. Phase 1/2 study of ARV-110, an androgen receptor (AR) PROTAC degrader, in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40, 17. [Google Scholar] [CrossRef]
- Garralda, E.; Beaulieu, M.E.; Moreno, V.; Casacuberta-Serra, S.; Martínez-Martín, S.; Foradada, L.; Alonso, G.; Massó-Vallés, D.; López-Estévez, S.; Jauset, T.; et al. MYC targeting by OMO-103 in solid tumors: A phase 1 trial. Nat. Med. 2024, 30, 762–771. [Google Scholar] [CrossRef]
- Markowski, M.C.; De Marzo, A.M.; Antonarakis, E.S. BET inhibitors in metastatic prostate cancer: Therapeutic implications and rational drug combinations. Expert Opin. Investig. Drugs 2017, 26, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Hahn, A.W.; Shore, N.; Agarwal, N.; Sieber, P.; Smith, M.R.; Dorff, T.; Monk, P.; Rettig, M.; Patel, R.; et al. Phase I Study of ORIC-101, a Glucocorticoid Receptor Antagonist, in Combination with Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer Progressing on Enzalutamide. Clin. Cancer Res. 2024, 30, 1111–1120. [Google Scholar] [CrossRef]
- Alvarez, M.J.; Shen, Y.; Giorgi, F.M.; Lachmann, A.; Ding, B.B.; Ye, B.H.; Califano, A. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat. Genet. 2016, 48, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Protein degraders push into novel target space. Nat. Rev. Drug Discov. 2024, 23, 799–802. [Google Scholar] [CrossRef]
- Soucek, L.; Whitfield, J.; Martins, C.P.; Finch, A.J.; Murphy, D.J.; Sodir, N.M.; Karnezis, A.N.; Swigart, L.B.; Nasi, S.; Evan, G.I. Modelling Myc inhibition as a cancer therapy. Nature 2008, 455, 679–683. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, Z.C.; Wu, Y.Y.; Pi, Y.N.; Lou, S.H.; Liu, T.B.; Lou, G.; Yang, C. Bromodomain and extraterminal (BET) proteins: Biological functions, diseases, and targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 420. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 2019, 52, 2435–2444. [Google Scholar] [CrossRef]
- Kalita, T.; Dezfouli, S.A.; Pandey, L.M.; Uludag, H. siRNA Functionalized Lipid Nanoparticles (LNPs) in Management of Diseases. Pharmaceutics 2022, 14, 2520. [Google Scholar] [CrossRef] [PubMed]
- Terada, C.; Oh, K.; Tsubaki, R.; Chan, B.; Aibara, N.; Ohyama, K.; Shibata, M.A.; Wada, T.; Harada-Shiba, M.; Yamayoshi, A.; et al. Dynamic and static control of the off-target interactions of antisense oligonucleotides using toehold chemistry. Nat. Commun. 2023, 14, 7972. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z. Current status of gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Hum. Gene Ther. 2005, 16, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, M.E.; Jauset, T.; Massó-Vallés, D.; Martínez-Martín, S.; Rahl, P.; Maltais, L.; Zacarias-Fluck, M.F.; Casacuberta-Serra, S.; Serrano Del Pozo, E.; Fiore, C.; et al. Intrinsic cell-penetrating activity propels Omomyc from proof of concept to viable anti-MYC therapy. Sci. Transl. Med. 2019, 11, eaar5012. [Google Scholar] [CrossRef]
| TF Class/Family | Representatives (Examples) | Key Functions | Associated Diseases | References |
|---|---|---|---|---|
| NF-κB (Rel) | p65 (RelA), p50, RelB, etc. | Regulation of inflammation and immune response | Autoimmunity, inflammation, cancer | [19,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124] |
| p53-like | p53, p63, p73 | Tumor suppressors, cell cycle and apoptosis control | Cancer (TP53 mutations), degeneration | [125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149] |
| STAT (JAK/STAT) | STAT1, STAT3, STAT5, etc. | Cytokine signaling, immune regulation | Inflammation, immune disorders, cancer | [150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165] |
| HIF (hypoxia factors) | HIF-1α, HIF-2α (in complex with ARNT) | Adaptation to hypoxia, angiogenesis | Cancer (hypoxia in tumors), ischemia | [166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192] |
| AP-1 (bZIP) | c-Fos, c-Jun, ATF | Stress response, differentiation, proliferation | Inflammation, cancer (stimulation of oncogenes) | [193,194,195,196,197,198,199] |
| Nrf2 (CNC-bZIP) | Nrf2 (NFE2L2) | Antioxidant response, detoxification | Neurodegeneration, chronic inflammation | [200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216] |
| Nuclear receptors | ER (estrogen), AR (androgen), PPAR, GR, etc. | Regulation of development and metabolism under the influence of ligands (hormones, lipids) | Cancer (hormone-dependent), metabolic diseases | [217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233] |
| Drug (Mechanism) | Target (TF or Pathway) | Mechanism of Action | Stage of Application |
|---|---|---|---|
| Bortezomib (proteasome inhibitor) | NF-κB (via stabilization of IκB) | Blocks proteasomal degradation of IκBα, preventing NF-κB from entering the nucleus. Indirectly suppresses expression of NF-κB-dependent genes [106]. | Approved (multiple myeloma); side effects: immunosuppression [107]. |
| Dexamethasone (glucocorticoid) | NF-κB (indirectly) | NF-κB (indirectly). Induces expression of NF-κB inhibitors (IκBα), inhibits translocation of NF-κB into the nucleus [117]. | Widely used as an anti-inflammatory; immunosuppressant, included in the standard treatment for multiple myeloma [118]. |
| Nutlin-3a (small molecule) | p53 (via MDM2) | Binds to MDM2, disrupting the MDM2-p53 interaction, stabilizes p53, and activates its target genes [129,144]. | Clinical trials in cancer, including reducing systemic toxicity [129,144,145,146]. |
| APR-246 (PRIMA-1^Met) | p53 (mutant forms) | Metabolized to an electrophilic compound that covalently binds mutant p53, restoring its wild-type conformation [139,140]. | Clinical trials (cancer with TP53 mutation) [139]. |
| Acriflavine (small molecule) | HIF-1α | Binds to HIF-1α/2α (PAS-B domain), preventing dimerization with HIF-1β and thereby inhibiting transcription of hypoxia-inducible genes [155]. | Preclinical studies; requires toxicity reduction [157]. |
| Roxadustat (PHD prolyl hydroxylase inhibitor) | HIF-1α (stabilization) | Inhibits HIF prolyl hydroxylases, preventing hydroxylation and degradation of HIF-1α; mimics hypoxia by enhancing erythropoietin synthesis [163]. | Approved for anemia of chronic kidney disease [163]. |
| Stattic (small molecule) | STAT3 | Allosterically binds to the SH2 domain of STAT3, blocks dimerization of STAT3 monomers and their phosphorylation, suppressing STAT3 activity [177,178,179]. | Preclinical tool; not used clinically [177,178,179]. |
| Ruxolitinib (JAK inhibitor) | STAT (JAK/STAT pathway) | Competitively inhibits JAK1/2 (ATP node), preventing phosphorylation of STAT1-3. Reduces production of proinflammatory cytokines [185,186,187]. | Approved for myelofibrosis, polycythemia, osteoporosis [185,186,187]. |
| SD-36 (PROTAC) | STAT3 | Hybrid: contains a ligand to STAT3 and Cereblon; causes ubiquitination and proteasomal degradation of STAT3. Completely eliminates STAT3 from cells [189,190,191]. | Preclinical (leukemia and lymphoma models); high efficacy [189,190,191]. |
| T-5224 (small molecule) | AP-1 (c-Fos/c-Jun) | Binds to the AP-1 complex, specifically inhibiting its DNA-binding activity. Reduces expression of cytokines and MMPs [197,198]. | Phase II clinical trials (rheumatoid arthritis); not approved [198]. |
| Dimethyl fumarate (electrophile) | Nrf2 (via Keap1) | Covalently modifies Keap1, leading to dissociation of Keap1-Nrf2 and activation of transcription of antioxidant genes [208,209,210,211]. | Approved (multiple sclerosis, psoriasis); main effects—immunomodulation [208,209]. |
| Lenalidomide (molecular glue) | IKZF1/3 (IRF family factors, B cells) | Binds to the E3 ligase CRBN, redirecting it against IKZF1/3 factors, causing their selective degradation. Indirectly suppresses Myc, IRF4 gene expression in myeloma [212,213]. | Approved for multiple myeloma, lymphoma; also for immunomodulation [212,213]. |
| Modality | Challenge | Established Solutions |
|---|---|---|
| Lack of ligandable pockets and “undruggable” interfaces | Many TFs are intrinsically disordered or have large, flat protein surfaces without the deep binding pockets that conventional small-molecule drugs require. As a result, although hundreds of disease-associated TFs are known, only a very small number have been successfully drugged with traditional inhibitors. Example: The oncogenic TF c-Myc exemplifies an “undruggable” target: it lacks a stable binding pocket and continuously changes conformation, foiling standard small-molecule design approaches [251]. | 1. Proteolysis-Targeting Chimeras (PROTACs). These bifunctional molecules recruit an E3 ubiquitin ligase to tag the TF for degradation, bypassing the need for a functional binding pocket. Even TFs that rely on protein–protein or DNA interactions can be eliminated via PROTACs. PROTAC degraders against historically “undruggable” TFs have entered the clinic; for example, TFs in hormone-driven cancers ARV-110 and ARV-471 degrade the androgen and estrogen receptors, and have shown efficacy in trials. PROTACs for TFs involved in cancer and immune diseases are developing [252,253]. 2. Peptide and Protein Inhibitors. Larger biomolecules can be engineered to disrupt TF function by mimicking key interfaces. Omomyc is a 91-amino-acid “mini-protein” designed to mimic Myc’s partner MAX, thereby sequestering Myc in an inactive complex. Omomyc overcomes Myc’s lack of pockets by using a large interaction surface to bind Myc. The purified Omomyc protein was found to inherently penetrate cancer cells, enabling it to be used as a drug. Omomyc (OMO-103) became the first direct Myc inhibitor to reach clinical trials where it showed a favorable safety profile and evidence of target engagement [254]. 3. Epigenetic Cofactor Modulators. An indirect strategy is to target the coactivators or chromatin regulators that TFs depend on. Bromodomain and extraterminal (BET) proteins like BRD4 are “epigenetic readers” that facilitate transcription of oncogenic genes (including MYC). Small-molecule BET inhibitors do not bind Myc itself but block BRD4’s function at super-enhancers, thereby downregulating MYC transcription and other tumor drivers. Several BET inhibitors have entered clinical testing in cancer, demonstrating proof-of-concept that modulating a TF’s epigenetic machinery can achieve a therapeutic effect when the TF itself is intractable. Combination approaches with BET inhibitors are also explored to enhance efficacy, given their limited success as monotherapy [244,255]. |
| Redundancy and Compensatory Pathways | TF often operate in complex, overlapping networks, so cells can compensate for the loss of a single factor. Redundancy in TF families and parallel pathways means that inhibiting one TF may trigger alternative regulators to take over its function. In cancer, for instance, blocking one oncogenic TF can lead tumor cells to upregulate a different TF or signaling route to maintain survival. Similar TF “crosstalk” and backup mechanisms occur in immune diseases, where multiple inflammatory TFs can induce overlapping gene programs and genetic disorders. | 1. Combination Therapies: A proven way to address network redundancy is to co-target multiple factors or pathways simultaneously. By inhibiting both the primary TF and its compensatory partner, one can prevent the escape mechanism. For example, preclinical and clinical studies suggest that inhibiting GR in addition to AR can overcome resistance in castration-resistant prostate cancer. GR antagonist (ORIC-101) has been tested in combination with AR pathway inhibitors to block the GR-mediated survival route. More broadly, combination regimens are commonly used in oncology and immunology to shut down parallel pathways. Using multiple targeted drugs or a targeted agent plus chemotherapy/immunotherapy reducing the chance that an alternative transcriptional program can sustain the disease. This strategy has been validated by improved outcomes in cases where single-agent TF targeting failed due to compensation [256]. 2. Broad-Spectrum or Sequential Targeting: In some scenarios, drugs that impact a broader transcriptional program can be employed to avoid narrow targeting of one TF. For instance, inhibiting a critical upstream signaling kinase might simultaneously dampen several TF outputs. While this comes with higher toxicity risk, it can be useful in short bursts or sequentially. Another approach is adaptive therapy: monitoring for pathway changes and then targeting the newly active TF once compensation is detected [257]. |
| Off-Target Effects and Specificity Issues | Achieving high specificity is difficult when targeting transcription factors. Small molecules intended to disrupt a TF’s protein–protein or DNA-binding interactions may unintentionally bind to other proteins or DNA regions, causing off-target effects. Likewise, therapies like DNA decoys or antisense oligonucleotides can have promiscuous actions; for example, a decoy sequence might sequester related TF family members, or exogenous DNA/RNA might activate innate immune sensors. These off-target activities can lead to unintended gene expression changes and toxicities. Ensuring that only the desired TF is affected is a major hurdle. Early TF inhibitors often suffered from poor selectivity, limiting their clinical utility. | 1. Targeted Delivery for Specificity minimizes off-target exposure elsewhere. Antibody-drug conjugates (ADCs) carry TF inhibitors or degraders as payloads. By attaching a TF-directed molecule to an antibody against an antigen on tumor or immune cells, the therapy is delivered predominantly to those cells. This approach has been used with a PROTAC whereby an anti-CD33 antibody was conjugated to a PROTAC molecule (BMS-986497) to deliver it specifically into CD33-positive leukemia cells. The result is highly selective TF modulation; the payload (which degrades a transcriptional regulator) is released inside target cells, sparing other tissues. Another delivery-based tactic is using tissue-localized administration. For instance, an oligonucleotide decoy against STAT3 was injected directly into head and neck tumors in a phase 0 trial, which abrogated STAT3’s activity in the tumor with negligible systemic exposure. By localizing treatment to disease sites off-target effects on other organs are greatly reduced [258]. 2. Enhanced Molecular Specificity. Allosteric modulators are designed to bind unique regulatory sites on a TF or its cofactor, distinct from those on other proteins. For example, drugs reactivating mutant p53 (a tumor-suppressor TF) are engineered to bind the mutant conformation of p53, which normal p53 in healthy cells does not adopt, providing a selective effect in p53-mutant tumors. PROTACs themselves can offer a specificity advantage: since a PROTAC must bind both the target TF and a specific E3 ligase, the probability of off-target degradation is lowered. Finally, the use of conditional activation, for example, drugs activated by light or by tumor-specific enzymes, has shown robust preclinical success in restricting a TF inhibitor’s action to particular contexts, thereby reducing off-target impact [191]. |
| Toxicity in Normal Cells and Tissues | On-target effects in normal cells lead to dose-limiting toxicity. Pan-inhibitors of BET bromodomain proteins caused significant hematological toxicity in trials; the most common serious side effect was thrombocytopenia, likely because normal megakaryocytes require BET function for platelet production. This on-target adverse effect has in some cases led to bleeding risks. Other TF-targeted interventions can similarly affect normal proliferative tissues; a broad inhibitor of c-Myc would be expected to impact intestinal crypts and bone marrow, and global NF-κB blockade could suppress needed immune responses. A key difficulty is finding a therapeutic window—a dose that hits the TF in diseased cells but spares enough activity in normal cells to avoid toxicity. | 1. Exploiting Differential Dependency. Often, cancer or diseased cells are more reliant on a given TF than normal cells, and this can be leveraged to widen the safety margin. In preclinical models, Myc inhibition via Omomyc had an “extraordinary therapeutic window”: it dramatically halted tumor growth while only slowing the proliferation of normal cells. Tumor cells, which are “addicted” to Myc, underwent apoptosis, whereas normal tissue tolerated partial Myc suppression with reversible effects. This differential dependency allows lower doses or partial inhibition to effectively kill diseased cells. Clinically, careful titration of dose and intermittent dosing schedules are used to maintain efficacy while giving normal tissues time to recover—an approach taken with some BET inhibitors and MYC-targeting agents [259]. 2. Mitigating Toxicity with Combinations. Combining therapies can also mitigate toxicity by enabling lower doses of each agent. A synergistic drug combination can achieve the desired anti-disease effect with each drug at a sub-maximal dose, reducing side effects. For example, preclinical studies showed that pairing BET inhibitors with other treatments allowed dose reductions that minimized overlapping toxicities. Combination regimens are often used, targeting different pathways or adding a less toxic baseline therapy, so that no single drug has to be pushed to a dose that causes intolerable harm. Additionally, selective targeting strategies directly contribute to reduced systemic toxicity by concentrating the drug in diseased tissue. In the case of the PROTAC-ADC example, the toxic effects of the degrader payload on normal cells were diminished by restricting its action to antigen-positive tumor cells. Finally, modern TF-based therapies are engineered to be reversible or tunable; for instance, some gene therapies include safety switches to turn off an introduced TF if needed [260]. |
| Drug Delivery and Bioavailability Challenges | Many TF-targeted therapeutics are large or biologically fragile molecules that struggle to reach their intracellular nuclear targets. Peptides, proteins, siRNAs, and antisense oligonucleotides (ASOs) face delivery barriers: they may be rapidly degraded in the bloodstream, unable to cross cell membranes, or poorly distributed to the relevant tissue. Thus, delivering a TF-directed agent to the right place in the body and into the cell nucleus at therapeutic levels is a major hurdle. Likewise, early protein-based TF inhibitors were thought impractical because of an assumption that they could not penetrate cells. | 1. Nanoparticles and Nucleic Acid Chemistry. Advances in drug delivery systems have largely solved the problem of getting nucleic-acid therapies inside cells. Lipid nanoparticles (LNPs) encapsulate siRNA or ASOs and ferry them across cell membranes. Such nanoparticles protect the cargo from degradation and can be targeted to specific organs by modifying their surface. TFs can be inhibited via RNA interference or antisense in vivo, provided an appropriate delivery carrier is used [261,262,263]. 2. Viral Vector Gene Therapy. Instead of delivering a drug to modulate a TF, another approach is to deliver the gene encoding a TF or a corrective version of it directly into cells. Gene therapy vectors can introduce DNA that either expresses a functional TF protein or a regulatory RNA to alter TF activity. A landmark example is Gendicine, a recombinant adenovirus carrying the wild-type TP53 gene. Rather than trying to drug the mutant p53 protein, Gendicine supplies a fresh copy of the p53 transcription factor gene to tumor cells, restoring its tumor-suppressor function [264]. 3. Direct and Targeted Delivery. The therapeutic can be injected or applied locally to maximize concentration at the target for diseases confined to certain sites. Another strategy is to equip therapeutics with cell-penetrating peptides (CPPs) or other delivery tags. In the case of the Myc inhibitor protein Omomyc, it was discovered to have intrinsic CPP-like properties that allowed it to cross cell membranes on its own. This finding enabled systemic administration of a mini-protein that homes to nuclei and blocks Myc. There are also antibody-based delivery systems and exosome-based delivery vehicles under investigation [265]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guryanova, S.V.; Maksimova, T.V.; Azova, M.M. Transcription Factors and Methods for the Pharmacological Correction of Their Activity. Int. J. Mol. Sci. 2025, 26, 6394. https://doi.org/10.3390/ijms26136394
Guryanova SV, Maksimova TV, Azova MM. Transcription Factors and Methods for the Pharmacological Correction of Their Activity. International Journal of Molecular Sciences. 2025; 26(13):6394. https://doi.org/10.3390/ijms26136394
Chicago/Turabian StyleGuryanova, Svetlana V., Tatiana V. Maksimova, and Madina M. Azova. 2025. "Transcription Factors and Methods for the Pharmacological Correction of Their Activity" International Journal of Molecular Sciences 26, no. 13: 6394. https://doi.org/10.3390/ijms26136394
APA StyleGuryanova, S. V., Maksimova, T. V., & Azova, M. M. (2025). Transcription Factors and Methods for the Pharmacological Correction of Their Activity. International Journal of Molecular Sciences, 26(13), 6394. https://doi.org/10.3390/ijms26136394






