Integrative Analysis of GATA3 Expression and Variants as Prognostic Biomarkers in Urothelial Cancer
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics and Risk Factors
2.2. Association of GATA3 SNPs with UC Risk
2.3. GATA3 Expression by rs1244159 Genotype
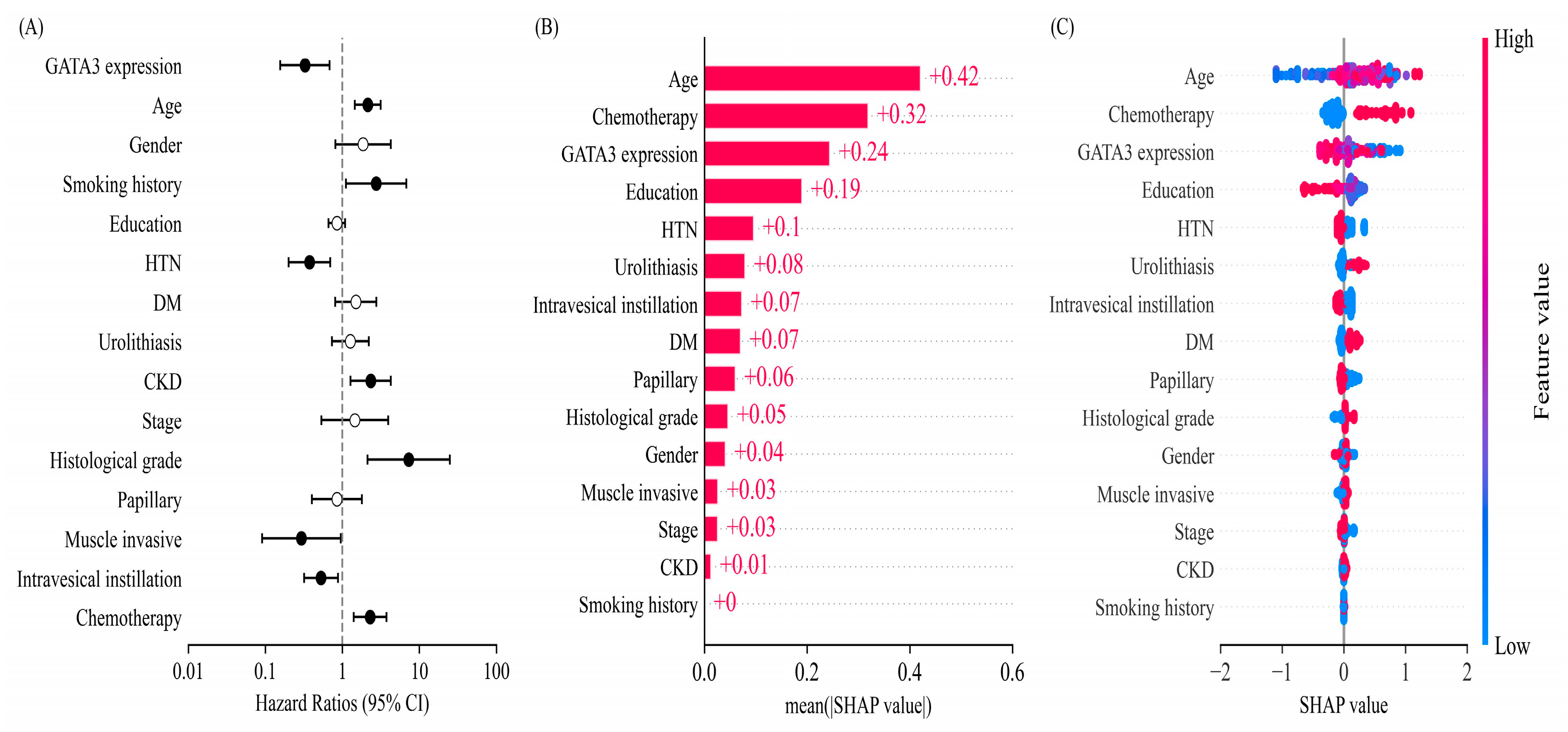
2.4. Multivariate Survival Analysis and Machine Learning-Based Feature Importance
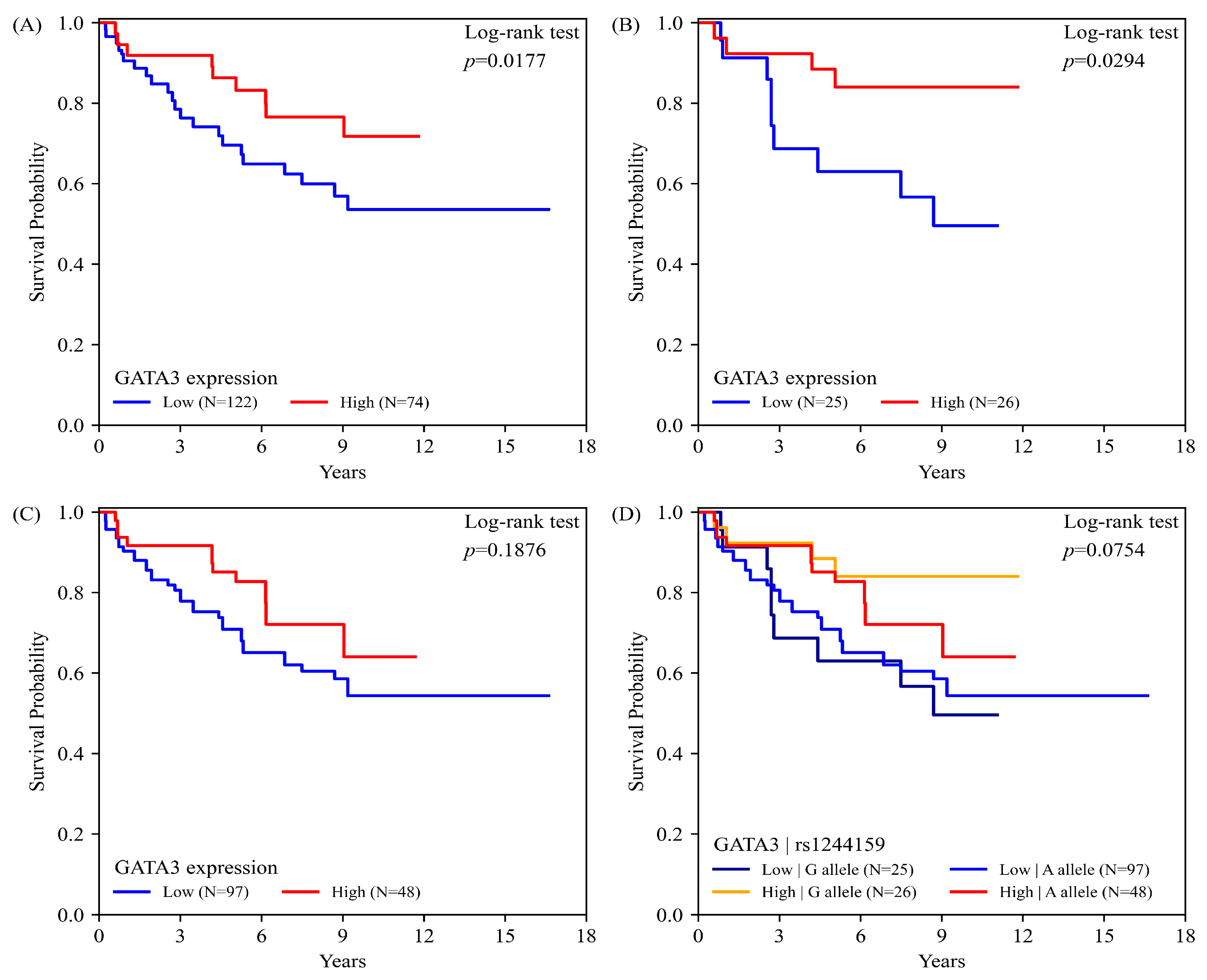
2.5. GATA3 Expression, rs1244159 Genotype, and UC Overall Survival
3. Discussion
4. Materials and Methods
4.1. Participant Recruitment, Questionnaires, and Biospecimen Collection
4.2. SNP Genotyping of GATA3 Locus
4.3. Tissues Specimen Collection and Clinicopathological Features
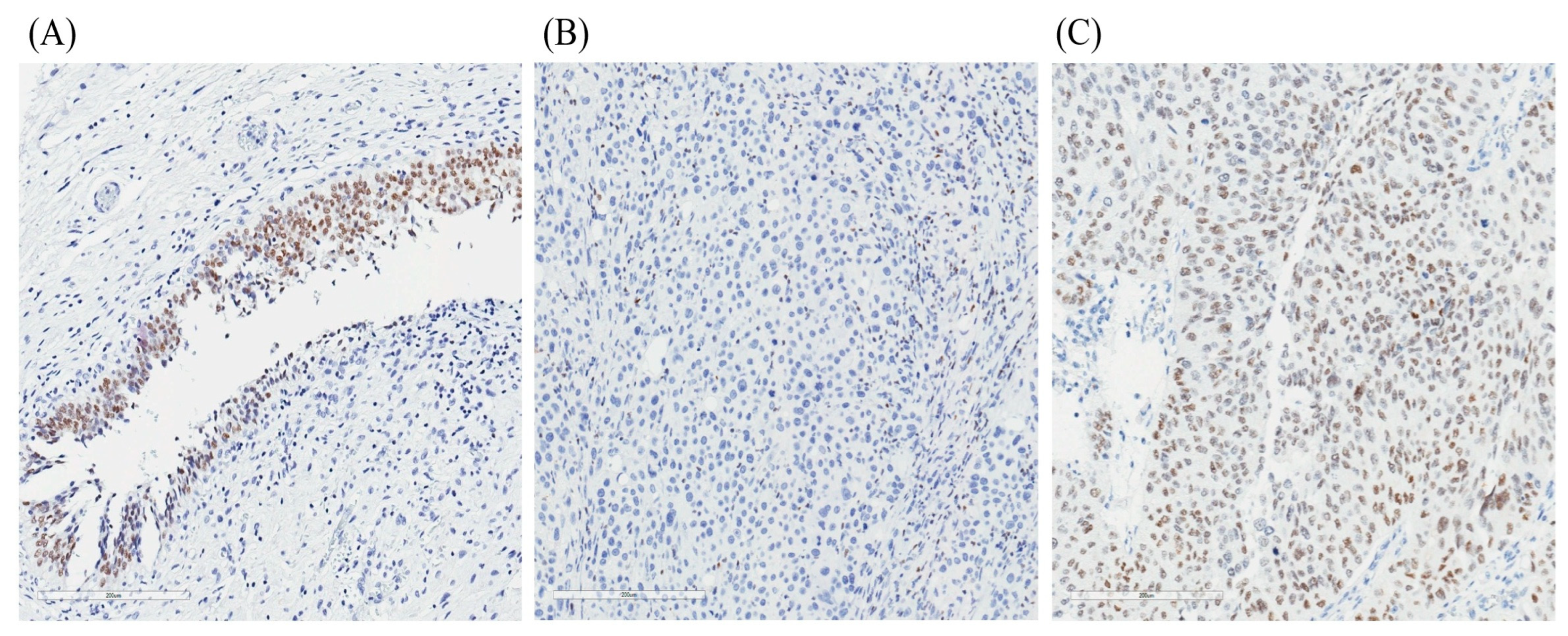
4.4. Tissue Microarray Construction and GATA3 Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Comperat, E.; Cowan, N.C.; De Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Van der Heijden, A.G.; Sherif, A.; European Association of, U. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Koutros, S.; Kiemeney, L.A.; Pal Choudhury, P.; Milne, R.L.; Lopez de Maturana, E.; Ye, Y.; Joseph, V.; Florez-Vargas, O.; Dyrskjot, L.; Figueroa, J.; et al. Genome-wide Association Study of Bladder Cancer Reveals New Biological and Translational Insights. Eur. Urol. 2023, 84, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Rothman, N.; Garcia-Closas, M.; Chatterjee, N.; Malats, N.; Wu, X.; Figueroa, J.D.; Real, F.X.; Van Den Berg, D.; Matullo, G.; Baris, D.; et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat. Genet. 2010, 42, 978–984. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Koutros, S.; Colt, J.S.; Kogevinas, M.; Garcia-Closas, M.; Real, F.X.; Friesen, M.C.; Baris, D.; Stewart, P.; Schwenn, M.; et al. Modification of Occupational Exposures on Bladder Cancer Risk by Common Genetic Polymorphisms. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, Q.; Chen, Z.; Tao, S.; Macnamara, L.; Kim, S.T.; Tian, L.; Xu, K.; Ding, Q.; Zheng, S.L.; et al. Systematic evaluation of bladder cancer risk-associated single-nucleotide polymorphisms in a Chinese population. Mol. Carcinog. 2013, 52, 916–921. [Google Scholar] [CrossRef]
- Murta-Nascimento, C.; Silverman, D.T.; Kogevinas, M.; Garcia-Closas, M.; Rothman, N.; Tardon, A.; Garcia-Closas, R.; Serra, C.; Carrato, A.; Villanueva, C.; et al. Risk of bladder cancer associated with family history of cancer: Do low-penetrance polymorphisms account for the increase in risk? Cancer Epidemiol. Biomark. Prev. 2007, 16, 1595–1600. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef]
- Plage, H.; Samtleben, H.; Hofbauer, S.; Kornienko, K.; Weinberger, S.; Bruch, P.G.; Elezkurtaj, S.; Rossner, F.; Schallenberg, S.; Kluth, M.; et al. GATA3 expression loss is linked to stage progression but is unrelated to prognosis in muscle-invasive urothelial carcinoma of the bladder. Hum. Pathol. 2022, 130, 10–17. [Google Scholar] [CrossRef]
- Debacker, C.; Catala, M.; Labastie, M.C. Embryonic expression of the human GATA-3 gene. Mech. Dev. 1999, 85, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Amin, A.; Gabrielson, E.; Illei, P.; Roden, R.B.; Sharma, R.; Epstein, J.I. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am. J. Surg. Pathol. 2012, 36, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Dadhania, V.; Zhang, M.; Zhang, L.; Bondaruk, J.; Majewski, T.; Siefker-Radtke, A.; Guo, C.C.; Dinney, C.; Cogdell, D.E.; Zhang, S.; et al. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine 2016, 12, 105–117. [Google Scholar] [CrossRef]
- So, J.S.; Epstein, J.I. GATA3 expression in paragangliomas: A pitfall potentially leading to misdiagnosis of urothelial carcinoma. Mod. Pathol. 2013, 26, 1365–1370. [Google Scholar] [CrossRef]
- Miyamoto, H.; Izumi, K.; Yao, J.L.; Li, Y.; Yang, Q.; McMahon, L.A.; Gonzalez-Roibon, N.; Hicks, D.G.; Tacha, D.; Netto, G.J. GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum. Pathol. 2012, 43, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Tsai, Y.C.; Jeng, Y.M. Biological significance of GATA3, cytokeratin 20, cytokeratin 5/6 and p53 expression in muscle-invasive bladder cancer. PLoS ONE 2019, 14, e0221785. [Google Scholar] [CrossRef]
- Inoue, S.; Mizushima, T.; Fujita, K.; Meliti, A.; Ide, H.; Yamaguchi, S.; Fushimi, H.; Netto, G.J.; Nonomura, N.; Miyamoto, H. GATA3 immunohistochemistry in urothelial carcinoma of the upper urinary tract as a urothelial marker and a prognosticator. Hum. Pathol. 2017, 64, 83–90. [Google Scholar] [CrossRef]
- Jangir, H.; Nambirajan, A.; Seth, A.; Sahoo, R.K.; Dinda, A.K.; Nayak, B.; Kaushal, S. Prognostic stratification of muscle invasive urothelial carcinomas using limited immunohistochemical panel of Gata3 and cytokeratins 5/6, 14 and 20. Ann. Diagn. Pathol. 2019, 43, 151397. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, Y.; Wang, S.; Zhang, Y.; Miao, Q.; Gao, F.; He, H. Expression status of GATA3 and mismatch repair proteins in upper tract urothelial carcinoma. Front. Med. 2019, 13, 730–740. [Google Scholar] [CrossRef]
- Liang, Y.; Heitzman, J.; Kamat, A.M.; Dinney, C.P.; Czerniak, B.; Guo, C.C. Differential expression of GATA-3 in urothelial carcinoma variants. Hum. Pathol. 2014, 45, 1466–1472. [Google Scholar] [CrossRef]
- Rodriguez Pena, M.D.C.; Chaux, A.; Eich, M.L.; Tregnago, A.C.; Taheri, D.; Borhan, W.; Sharma, R.; Rezaei, M.K.; Netto, G.J. Immunohistochemical assessment of basal and luminal markers in non-muscle invasive urothelial carcinoma of bladder. Virchows Arch. 2019, 475, 349–356. [Google Scholar] [CrossRef]
- Yuk, H.D.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Moon, K.C.; Ku, J.H. Clinical outcomes of muscle invasive bladder Cancer according to the BASQ classification. BMC Cancer 2019, 19, 897. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Jin, L.; Dai, G.; Yao, Q.; Xiang, H.; Zhang, Y.; Liu, X.; Xue, B. Biological and Clinical Significance of GATA3 Detected from TCGA Database and FFPE Sample in Bladder Cancer Patients. OncoTargets Ther. 2020, 13, 945–958. [Google Scholar] [CrossRef]
- Kiebach, J.; Beeren, I.; Aben, K.K.H.; Witjes, J.A.; van der Heijden, A.G.; Kiemeney, L.; Vrieling, A. Smoking behavior and the risks of tumor recurrence and progression in patients with non-muscle-invasive bladder cancer. Int. J. Cancer 2025, 156, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jian, Z.; Gao, X.; Yuan, C.; Jin, X.; Li, H.; Wang, K. Causal Associations Between Educational Attainment and 14 Urological and Reproductive Health Outcomes: A Mendelian Randomization Study. Front. Public Health 2021, 9, 742952. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.R.; Li, C.C.; Juan, Y.S.; Li, W.M.; Wu, W.J.; Chien, T.M. Risk Factors Predictive of Contralateral Recurrence of Upper Tract Urothelial Carcinoma Include Chronic Kidney Diseases and Postoperative Initiation of Dialysis. Cancers 2025, 17, 664. [Google Scholar] [CrossRef]
- Sung, H.L.; Hung, C.Y.; Tung, Y.C.; Lin, C.C.; Tsai, T.H.; Huang, K.H. Comparison between sodium-glucose cotransporter 2 inhibitors and dipeptidyl peptidase 4 inhibitors on the risk of incident cancer in patients with diabetes mellitus: A real-world evidence study. Diabetes/Metab. Res. Rev. 2024, 40, e3784. [Google Scholar] [CrossRef]
- van den Brandt, P.A. Diabetes and the risk of bladder cancer subtypes in men and women: Results from the Netherlands Cohort Study. Eur. J. Epidemiol. 2024, 39, 379–391. [Google Scholar] [CrossRef]
- Andrew, A.S.; Hu, T.; Gu, J.; Gui, J.; Ye, Y.; Marsit, C.J.; Kelsey, K.T.; Schned, A.R.; Tanyos, S.A.; Pendleton, E.M.; et al. HSD3B and gene-gene interactions in a pathway-based analysis of genetic susceptibility to bladder cancer. PLoS ONE 2012, 7, e51301. [Google Scholar] [CrossRef]
- Chen, Z.; Dazard, J.E.; Salerno, P.; Sirasapalli, S.K.; Makhlouf, M.H.; Rajagopalan, S.; Al-Kindi, S. Composite socio-environmental risk score for cardiovascular assessment: An explainable machine learning approach. Am. J. Prev. Cardiol. 2025, 22, 100964. [Google Scholar] [CrossRef]
- Wang, M.H.; Cordell, H.J.; Van Steen, K. Statistical methods for genome-wide association studies. Semin. Cancer Biol. 2019, 55, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. Neural Inf. Process. Syst. 2017, 30, 1–10. [Google Scholar]
| Variables | Control (n = 589) | UC Case (n = 461) | p-Value |
|---|---|---|---|
| Age; Mean (SD) | 66.70 (11.08) | 66.19 (11.51) | 0.4669 |
| age < 65 | 255 (43.29) | 199 (43.26) | 0.9672 |
| Age ≥ 65 | 334 (56.71) | 262 (56.74) | |
| Sex | |||
| Female | 256 (43.46) | 192 (41.65) | 0.5753 |
| Male | 333 (56.54) | 269 (58.35) | |
| Education | |||
| Elementary school or lower | 173 (29.62) | 220 (47.93) | <0.0001 |
| High school | 243 (41.61) | 160 (34.64) | |
| College or higher | 168 (28.77) | 80 (17.43) | |
| Smoking history | |||
| No | 432 (73.34) | 291 (62.4) | 0.0001 |
| Yes | 157 (26.66) | 170 (37.6) | |
| HTN | |||
| No | 368 (62.91) | 241 (53.67) | 0.0028 |
| Yes | 217 (37.09) | 208 (46.33) | |
| DM | |||
| No | 513 (87.4) | 361 (80.04) | 0.0021 |
| Yes | 76 (12.6) | 90 (19.96) | |
| CKD | |||
| No | 508 (86.25) | 213 (46.20) | <0.0001 |
| Yes | 81 (13.75) | 248 (53.80) | |
| Urinary stones | |||
| No | 497 (84.81) | 342 (75.83) | 0.0003 |
| Yes | 89 (15.19) | 109 (24.17) |
| SNP | Genotype | Control (%) | UC (%) | OR | pa | Adjusted OR | pb |
|---|---|---|---|---|---|---|---|
| rs12776126 | |||||||
| G/G | 460 (78.10) | 364 (78.96) | Ref | ||||
| G/T | 122 (20.71) | 96 (20.82) | 0.97 (0.72–1.32) | 0.1298 | 1.08 (0.76–1.52) | 0.5728 | |
| T/T | 7 (1.19) | 1 (0.22) | 5.07 (0.62–41.70) | 0.1281 | 2.18 (0.26–18.05) | 0.4917 | |
| T | 136 (11.54) | 98 (10.63) | 1.12 (0.83–1.53) | 0.4578 | 1.18 (0.86–1.63) | 0.3146 | |
| G c | 1042 (88.46) | 824 (89.37) | Ref allele | ||||
| rs1244159 | |||||||
| A/A | 321 (54.50) | 233 (50.65) | Ref | ||||
| A/G | 238 (40.41) | 194 (42.17) | 0.91 (0.70–1.17) | 0.3502 | 0.88 (0.66–1.18) | 0.2025 | |
| G/G | 30 (5.09) | 33 (7.17) | 0.60 (0.35–1.02) | 0.0836 | 0.48 (0.27–0.87) | 0.0231 | |
| G | 297 (25.26) | 260 (28.26) | 0.80 (0.64–0.99) | 0.0373 | 0.76 (0.61–0.95) | 0.0171 | |
| A c | 879 (74.74) | 660 (71.74) | Ref allele | ||||
| rs11255526 | |||||||
| G/G | 242 (41.30) | 172 (37.39) | Ref | ||||
| G/C | 274 (46.76) | 223 (48.48) | 0.92 (0.70–1.20) | 0.8366 | 0.95 (0.70–1.29) | 0.9084 | |
| C/C | 70 (11.95) | 65 (14.13) | 0.80 (0.54–1.18) | 0.3236 | 0.87 (0.56–1.36) | 0.5994 | |
| C | 413 (35.30) | 353 (38.37) | 0.98 (0.80–1.20) | 0.8599 | 0.94 (0.76–1.15) | 0.5488 | |
| G c | 757 (64.70) | 567 (61.63) | Ref allele | ||||
| rs11255528 | |||||||
| C/C | 304 (51.61) | 248 (54.03) | Ref | ||||
| C/T | 231 (39.22) | 170 (37.04) | 1.11 (0.85–1.44) | 0.6107 | 1.07 (0.79–1.44) | 0.8448 | |
| T/T | 54 (9.17) | 41 (8.93) | 1.05 (0.67–1.64) | 0.9920 | 1.06 (0.64–1.76) | 0.9118 | |
| T | 339 (28.83) | 252 (27.45) | 1.01 (0.81–1.24) | 0.9903 | 1.05 (0.84–1.31) | 0.6652 | |
| Cc | 837 (71.17) | 666 (72.55) | Ref allele |
| A Allele (n = 145) | G Allele (n= 51) | p Value | |
|---|---|---|---|
| Diagnosis age | 69.47 ± 12.37 | 65.05 ± 11.81 | 0.0180 |
| Gender | 0.9014 | ||
| Male | 64 (44.14) | 22 (43.14) | |
| Female | 81 (55.86) | 29 (56.86) | |
| Smoking history | 54 (37.24) | 14 (27.45) | 0.2064 |
| HTN | 75 (51.72) | 27 (55.10) | 0.6823 |
| DM | 45 (31.03) | 19 (38.78) | 0.3191 |
| CKD | 89 (61.38) | 31 (63.27) | 0.8142 |
| Urolithiasis | 33 (22.76) | 9 (18.37) | 0.5188 |
| TNM stage | 0.1462 | ||
| 0a to I | 74 (51.03) | 20 (39.22) | |
| II to IV | 71 (48.97) | 31 (60.78) | |
| Histological grade | 0.3459 | ||
| Low grade | 38 (26.21) | 10 (19.61) | |
| High grade | 107 (73.79) | 41 (80.39) | |
| Papillary | 0.4842 | ||
| No | 51 (35.66) | 21 (41.18) | |
| Yes | 92 (64.34) | 30 (58.82) | |
| Muscle invasive | 0.4233 | ||
| No | 45 (31.47) | 13 (25.49) | |
| Yes | 98 (68.53) | 38 (74.51) | |
| Intravesical instillation | 0.0789 | ||
| No | 89 (63.12) | 25 (49.02) | |
| Yes | 52 (36.88) | 26 (50.98) | |
| Chemotherapy | 0.5983 | ||
| No | 91 (64.54) | 35 (68.63) | |
| Yes | 50 (35.46) | 16 (31.37) | |
| GATA3 expression | 85. 41±45.17 | 96.82±49.61 | 0.0884 |
| Low | 97 (66.90) | 25 (49.02) | 0.0375 |
| High | 48 (33.10) | 26 (50.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, C.-M.; Chang, H.; Chang, C.-H.; Chang, Y.-H.; Hsiao, P.-J.; Lien, C.-S.; Chung, C.-J. Integrative Analysis of GATA3 Expression and Variants as Prognostic Biomarkers in Urothelial Cancer. Int. J. Mol. Sci. 2025, 26, 6378. https://doi.org/10.3390/ijms26136378
Chung C-M, Chang H, Chang C-H, Chang Y-H, Hsiao P-J, Lien C-S, Chung C-J. Integrative Analysis of GATA3 Expression and Variants as Prognostic Biomarkers in Urothelial Cancer. International Journal of Molecular Sciences. 2025; 26(13):6378. https://doi.org/10.3390/ijms26136378
Chicago/Turabian StyleChung, Chia-Min, Han Chang, Chao-Hsiang Chang, Yi-Huei Chang, Po-Jen Hsiao, Chi-Shun Lien, and Chi-Jung Chung. 2025. "Integrative Analysis of GATA3 Expression and Variants as Prognostic Biomarkers in Urothelial Cancer" International Journal of Molecular Sciences 26, no. 13: 6378. https://doi.org/10.3390/ijms26136378
APA StyleChung, C.-M., Chang, H., Chang, C.-H., Chang, Y.-H., Hsiao, P.-J., Lien, C.-S., & Chung, C.-J. (2025). Integrative Analysis of GATA3 Expression and Variants as Prognostic Biomarkers in Urothelial Cancer. International Journal of Molecular Sciences, 26(13), 6378. https://doi.org/10.3390/ijms26136378






