Metabolomic Signatures Predict Seven-Year Mortality in Clinically Stable COPD Patients
Abstract
1. Introduction
2. Results
2.1. Characterization of COPD Patients
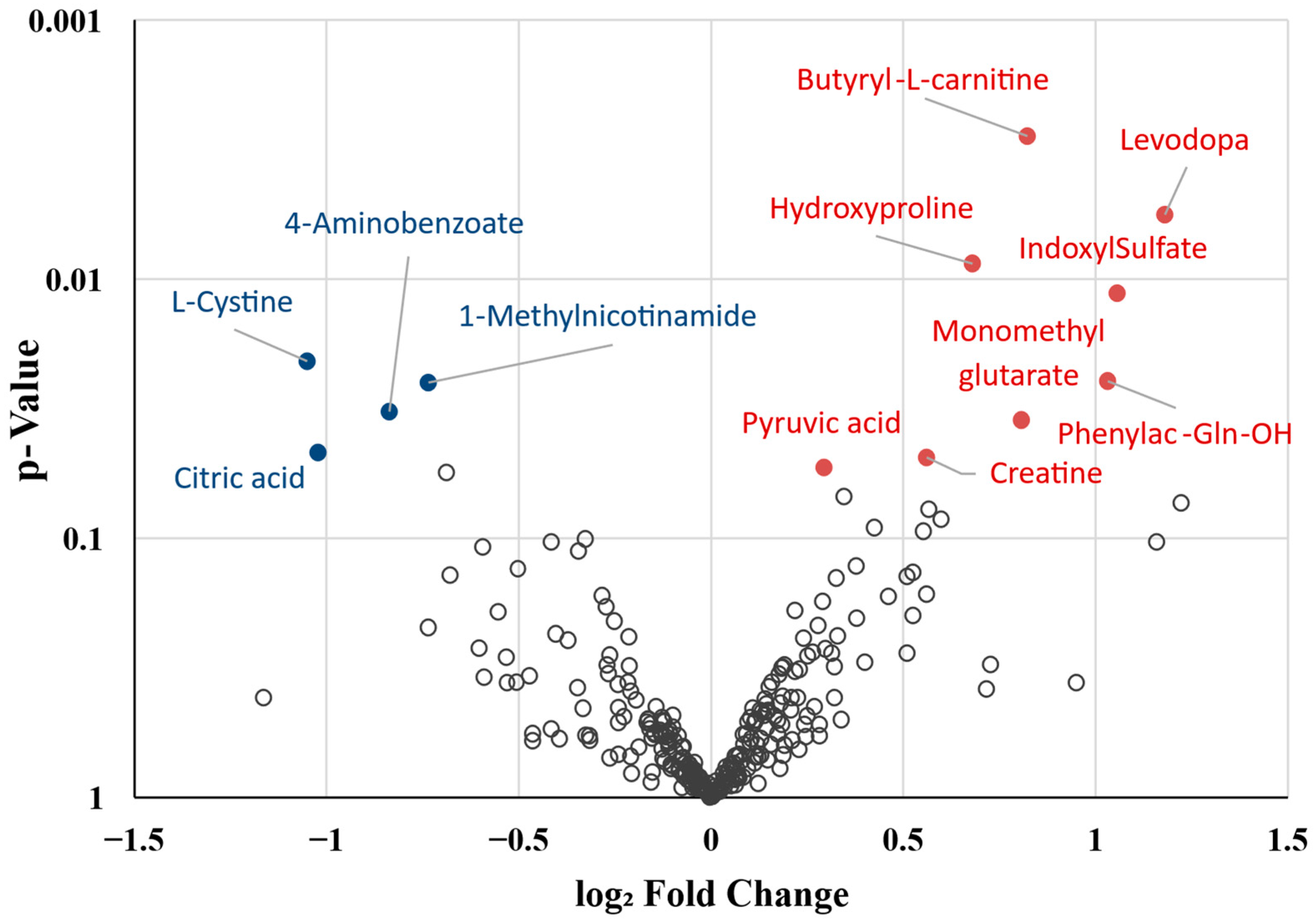
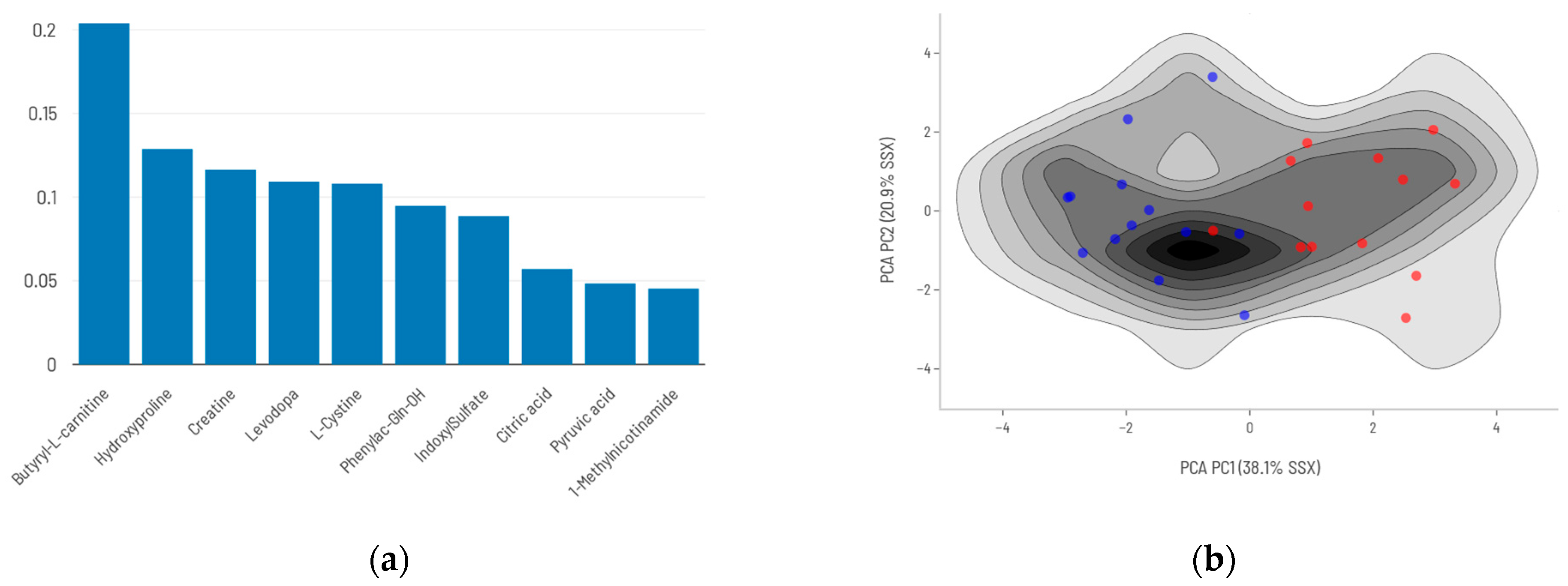
2.2. Differential Metabolite Profile of Deceased Patients
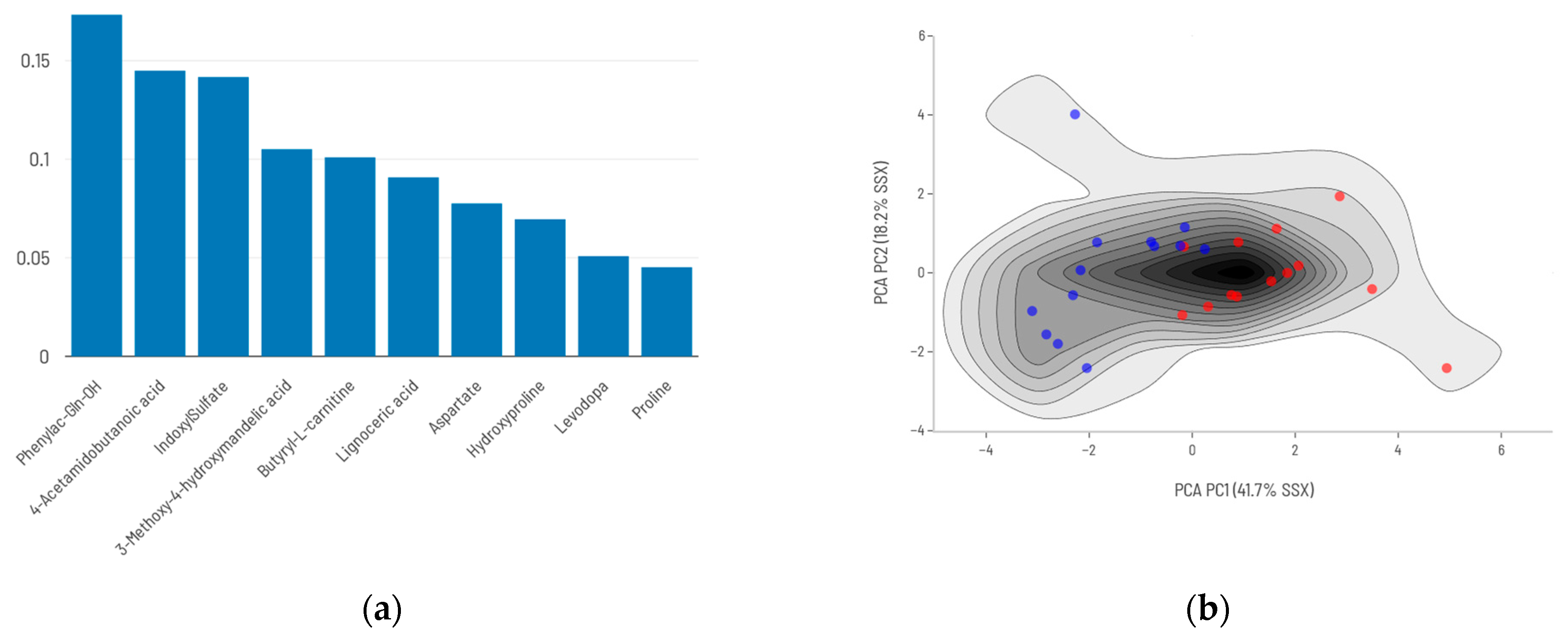
2.3. Biological Function Analysis
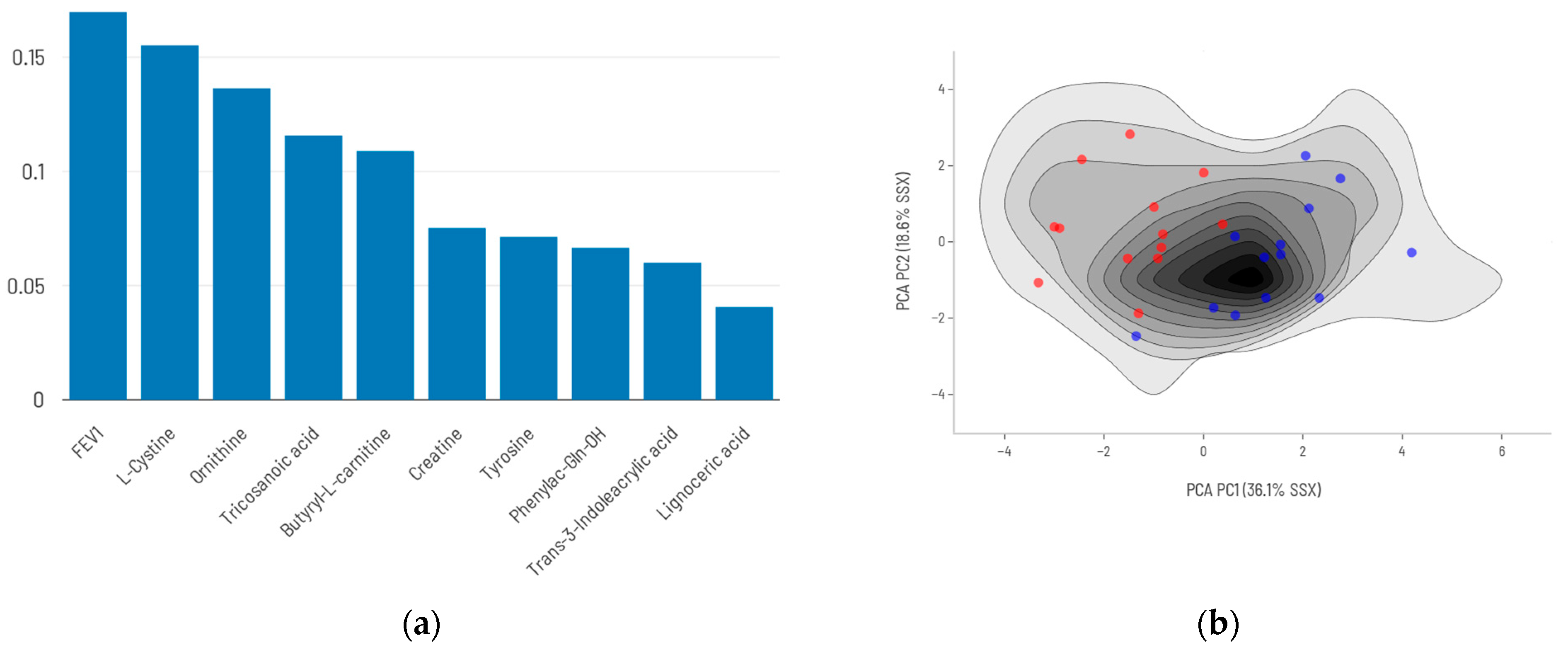
2.4. AI Modeling
2.5. Integration of FEV1 into the Predictive Model
3. Discussion
3.1. Dysregulation of Energy and Mitochondrial Metabolism
3.2. Alterations in Amino Acid and Nitrogen Metabolism
3.3. Redox Imbalance and Neuroendocrine Dysregulation
3.4. Microbiota-Derived Metabolites and Host–Microbiota Interactions
3.5. Metabolic Signature of Poor Prognosis
3.6. Strengths and Potential Limitations
4. Materials and Methods
4.1. Participants and Ethics
4.2. Blood Sample Collection
4.3. Metabolomics Workflow
4.3.1. Metabolite Collection and LC-MS Parameters
4.3.2. Metabolite Identification and Quantification
4.3.3. Metabolomic Outlier Identification and Quality Control
4.4. Data Analysis
4.4.1. Sample Size Calculation
4.4.2. Clinical Data Analysis
4.4.3. Metabolomic Data Preprocessing and Filtering
4.4.4. Covariate Adjustment and Differential Analysis
4.4.5. Data Export for Machine Learning
4.5. Generation of Predictive Models
4.5.1. Model Development
4.5.2. Initial Model Fitting
4.5.3. Internal Validation
4.5.4. Model Evaluation
4.5.5. Biological Function Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | Aggregated Conformal Predictors |
| AI | Artificial Intelligence |
| BD | Bronchodilator |
| BMI | Body Mass Index |
| BCAA | Branched-Chain Amino Acids |
| COPD | Chronic Obstructive Pulmonary Disease |
| DAMs | Differentially Abundant Metabolites |
| DLco | Diffusing Capacity for Carbon Monoxide |
| FDR | False Discovery Rate |
| FEV1 | Forced Expiratory Volume in 1 Second |
| FVC | Forced Vital Capacity |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| HPLC | High Performance Liquid Chromatography |
| HMDB | Human Metabolome Database |
| IQR | Interquartile Range |
| KNN | K-Nearest Neighbors |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| MCC | Matthews Correlation Coefficient |
| MNA | Methylnicotinamide |
| MS | Mass Spectrometry |
| NPV | Negative Predictive Value |
| PAGIn | N-Phenylacetylglutamine |
| PCA | Principal Component Analysis |
| PPV | Positive Predictive Value |
| QC | Quality Control |
| RF | Random Forest |
| SVA | Surrogate Variable Analysis |
| SMPDB | Small Molecule Pathway Database |
| TCA | Tricarboxylic Acid (Cycle) |
| UPLC | Ultra-Performance Liquid Chromatography |
Appendix A
Appendix B
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2025 Report; Global Initiative for Chronic Obstructive Lung Disease: Deer Park, IL, USA, 2025. [Google Scholar]
- American Lung Association COPD Trends Brief—Mortality. Available online: https://www.lung.org/research/trends-in-lung-disease/copd-trends-brief/copd-mortality (accessed on 27 May 2025).
- Fermont, J.M.; Masconi, K.L.; Jensen, M.T.; Ferrari, R.; Di Lorenzo, V.A.P.; Marott, J.M.; Schuetz, P.; Watz, H.; Waschki, B.; Müllerova, H.; et al. Biomarkers and Clinical Outcomes in COPD: A Systematic Review and Meta-Analysis. Thorax 2019, 74, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.I.; Hillas, G.; Loukides, S.; Vassilakopoulos, T. Mortality Prevention as the Centre of COPD Management. ERJ Open Res. 2024, 10, 00850–02023. [Google Scholar] [CrossRef]
- Enríquez-Rodríguez, C.J.; Pascual-Guardia, S.; Casadevall, C.; Caguana-Vélez, O.A.; Rodríguez-Chiaradia, D.; Barreiro, E.; Gea, J. Proteomic Blood Profiles Obtained by Totally Blind Biological Clustering in Stable and Exacerbated COPD Patients. Cells 2024, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Rodríguez, C.J.; Casadevall, C.; Faner, R.; Pascual-Guardia, S.; Castro-Acosta, A.; López-Campos, J.L.; Peces-Barba, G.; Seijo, L.; Caguana-Vélez, O.A.; Monsó, E.; et al. A Pilot Study on Proteomic Predictors of Mortality in Stable COPD. Cells 2024, 13, 1351. [Google Scholar] [CrossRef]
- Gea, J.; Enríquez-Rodríguez, C.J.; Pascual-Guardia, S. Metabolomics in COPD. Arch. Bronconeumol. 2023, 59, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Godbole, S.; Bowler, R.P. Metabolome Features of COPD: A Scoping Review. Metabolites 2022, 12, 621. [Google Scholar] [CrossRef]
- Enríquez-Rodríguez, C.J.; Casadevall, C.; Faner, R.; Castro-Costa, A.; Pascual-Guàrdia, S.; Seijó, L.; López-Campos, J.L.; Peces-Barba, G.; Monsó, E.; Barreiro, E.; et al. COPD: Systemic Proteomic Profiles in Frequent and Infrequent Exacerbators. ERJ Open Res. 2024, 10, 00004–02024. [Google Scholar] [CrossRef]
- Gea, J.; Casadevall, C.; Nebot, P.; Enríquez Rodríguez, C.J.; Faner, M.R.; Cosio, B.G.; Haro, N.; Pascual-Guardia, S.; Peces-Barba, G.; Monsó, E.; et al. Aging and Metabolic Changes in COPD Patients. In Proceedings of the B70. COPD in the Spotlight: Insights Into Disease Pathogenesis, San Diego, CA, USA, 6 May 2024; p. A4314. [Google Scholar]
- Solanes, I.; Casan, P.; Sangenís, M.; Calaf, N.; Giraldo, B.; Güell, R. Factores de riesgo de mortalidad en la EPOC. Arch. Bronconeumol. 2007, 43, 445–449. [Google Scholar] [CrossRef]
- García Castillo, E.; Alonso Pérez, T.; Ancochea, J.; Pastor Sanz, M.T.; Almagro, P.; Martínez-Camblor, P.; Miravitlles, M.; Rodríguez-Carballeira, M.; Navarro, A.; Lamprecht, B.; et al. Mortality Prediction in Chronic Obstructive Pulmonary Disease Comparing the GOLD 2015 and GOLD 2019 Staging: A Pooled Analysis of Individual Patient Data. ERJ Open Res. 2020, 6, 00253–02020. [Google Scholar] [CrossRef]
- Marín, J.M. Viejos y nuevos criterios para clasificar la EPOC. Arch. Bronconeumol. 2004, 40, 9–15. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines Activate Proinflammatory Signaling Pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, C.; Agranovich, B.; Enríquez-Rodríguez, C.J.; Faner, R.; Pascual-Guàrdia, S.; Castro-Acosta, A.; Camps-Ubach, R.; Garcia-Aymerich, J.; Barreiro, E.; Monsó, E.; et al. Metabolomic Plasma Profile of Chronic Obstructive Pulmonary Disease Patients. Int. J. Mol. Sci. 2025, 26, 4526. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, M.; Liu, Q.; Weng, J.; Wei, L.; Chung, K.F.; Adcock, I.M.; Chang, Q.; Li, M.; Huang, Y.; et al. Changes in Targeted Metabolomics in Lung Tissue of Chronic Obstructive Pulmonary Disease. J. Thorac. Dis. 2023, 15, 2544–2558. [Google Scholar] [CrossRef]
- Novotna, B.; Abdel-Hamid, M.; Koblizek, V.; Svoboda, M.; Hejduk, K.; Rehacek, V.; Bis, J.; Salajka, F. A Pilot Data Analysis of a Metabolomic HPLC-MS/MS Study of Patients with COPD. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2018, 27, 531–539. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Q.; Liu, C.; Pang, R.; Yin, Y. Plasma Metabolomics and Lipidomics Reveal Perturbed Metabolites in Different Disease Stages of Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.E.; Mota, C.; Strowitzki, M.J.; Shigemura, M.; Sznajder, J.I.; Crowe, L.; Masterson, J.C.; Hayes, S.E.; Reddan, B.; Yin, X.; et al. Hypercapnia Alters Mitochondrial Gene Expression and Acylcarnitine Production in Monocytes. Immunol. Cell Biol. 2023, 101, 556–577. [Google Scholar] [CrossRef]
- Barreiro, E.; Gea, J. Muscle Fatigability of Patients With Severe COPD and Chronic Respiratory Failure: The Contribution of Respiratory Factors. Arch. Bronconeumol. 2024, 60, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Sancho-Muñoz, A.; Chalela, R. Nutritional Status and Muscle Dysfunction in Chronic Respiratory Diseases: Stable Phase versus Acute Exacerbations. J. Thorac. Dis. 2018, 10, S1332–S1354. [Google Scholar] [CrossRef] [PubMed]
- Swallow, E.B.; Reyes, D.; Hopkinson, N.S.; Man, W.D.-C.; Porcher, R.; Cetti, E.J.; Moore, A.J.; Moxham, J.; Polkey, M.I. Quadriceps Strength Predicts Mortality in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Thorax 2007, 62, 115–120. [Google Scholar] [CrossRef]
- Afzal, A.B.; Khalid, S.; Baksi, S. Association Between Low Serum Creatinine and Mortality in Patients With Severe Chronic Obstructive Pulmonary Disease. Cureus 2022, 14, e29376. [Google Scholar] [CrossRef]
- Kao, C.C.; Hsu, J.W.-C.; Bandi, V.; Hanania, N.A.; Kheradmand, F.; Jahoor, F. Glucose and Pyruvate Metabolism in Severe Chronic Obstructive Pulmonary Disease. J. Appl. Physiol. Bethesda Md. 1985 2012, 112, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, Y.; Liu, S.; Mao, S.; Ling, Y.; Liu, D.; He, X.; Wang, X. Metabonomic Profiling of Serum and Urine by 1H NMR-Based Spectroscopy Discriminates Patients with Chronic Obstructive Pulmonary Disease and Healthy Individuals. PLoS ONE 2013, 8, e65675. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.R.; Kadam, S.; Brahme, A.; Agrawal, M.; Apte, K.; Narke, G.; Kekan, K.; Madas, S.; Salvi, S. Systemic Immuno-Metabolic Alterations in Chronic Obstructive Pulmonary Disease (COPD). Respir. Res. 2019, 20, 171. [Google Scholar] [CrossRef]
- Choi, B.H.; Hyun, S.; Koo, S.-H. The Role of BCAA Metabolism in Metabolic Health and Disease. Exp. Mol. Med. 2024, 56, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank-Quinn, C.I.; Jacobson, S.; Hughes, G.; Powell, R.L.; Petrache, I.; Kechris, K.; Bowler, R.; Reisdorph, N. Metabolomics and Transcriptomics Pathway Approach Reveals Outcome-Specific Perturbations in COPD. Sci. Rep. 2018, 8, 17132. [Google Scholar] [CrossRef]
- Ubhi, B.K.; Riley, J.H.; Shaw, P.A.; Lomas, D.A.; Tal-Singer, R.; MacNee, W.; Griffin, J.L.; Connor, S.C. Metabolic Profiling Detects Biomarkers of Protein Degradation in COPD Patients. Eur. Respir. J. 2012, 40, 345–355. [Google Scholar] [CrossRef]
- Rahman, I.; MacNee, W. Lung Glutathione and Oxidative Stress: Implications in Cigarette Smoke-Induced Airway Disease. Am. J. Physiol. 1999, 277, L1067–L1088. [Google Scholar] [CrossRef]
- Norheim, K.L.; Ben Ezra, M.; Heckenbach, I.; Andreasson, L.M.; Eriksen, L.L.; Dyhre-Petersen, N.; Damgaard, M.V.; Berglind, M.; Pricolo, L.; Sampson, D.; et al. Effect of Nicotinamide Riboside on Airway Inflammation in COPD: A Randomized, Placebo-Controlled Trial. Nat. Aging 2024, 4, 1772–1781. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, S.; Wu, T.; Sun, F.; Hu, S.; Shi, L. Potential of Gut Microbiota Metabolites in Treating COPD: Network Pharmacology and Mendelian Randomization Approaches. Front. Microbiol. 2024, 15, 1416651. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-Dependent Regulation of Collagen Metabolism. Cell. Mol. Life Sci. CMLS 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Phang, J.M.; Pandhare, J.; Liu, Y. The Metabolism of Proline as Microenvironmental Stress Substrate. J. Nutr. 2008, 138, 2008S–2015S. [Google Scholar] [CrossRef] [PubMed]
- Martin-Mosquero, C.; Peces-Barba, G.; Rubio, M.L.; Ortega, M.; Rodriguez-Nieto, M.J.; Martinez Galan, L.; Gonzalez-Mangado, N. Increased Collagen Deposition Correlated with Lung Destruction in Human Emphysema. Histol. Histopathol. 2006, 21, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Xing, D.; Viera, L.; Burkes, R.M.; Wu, Y.; Bhatt, S.P.; Dransfield, M.T.; Couper, D.J.; O’Neal, W.; Hoffman, E.A.; et al. The Matrikine Acetyl-Proline-Glycine-Proline and Clinical Features of COPD: Findings from SPIROMICS. Respir. Res. 2019, 20, 254. [Google Scholar] [CrossRef]
- Ding, H.-Z.; Wang, H.; Wu, D.; Zhou, F.-C.; Zhu, J.; Tong, J.-B.; Gao, Y.-T.; Li, Z.-G. Serum Metabolomics Analysis of Patients with Chronic Obstructive Pulmonary Disease and “Frequent Exacerbator” Phenotype. Mol. Med. Rep. 2024, 30, 1–14. [Google Scholar] [CrossRef]
- Uc, E.Y.; Dienel, G.A.; Cruz, N.F.; Harik, S.I. β-Adrenergics Enhance Brain Extraction of Levodopa. Mov. Disord. 2002, 17, 54–59. [Google Scholar] [CrossRef]
- Imazu, M.; Fukuda, H.; Kanzaki, H.; Amaki, M.; Hasegawa, T.; Takahama, H.; Hitsumoto, T.; Tsukamoto, O.; Morita, T.; Ito, S.; et al. Plasma Indoxyl Sulfate Levels Predict Cardiovascular Events in Patients with Mild Chronic Heart Failure. Sci. Rep. 2020, 10, 16528. [Google Scholar] [CrossRef]
- Menezes, A.M.B.; Pérez-Padilla, R.; Wehrmeister, F.C.; Lopez-Varela, M.V.; Muiño, A.; Valdivia, G.; Lisboa, C.; Jardim, J.R.B.; de Oca, M.M.; Talamo, C.; et al. FEV1 Is a Better Predictor of Mortality than FVC: The PLATINO Cohort Study. PLoS ONE 2014, 9, e109732. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Pascual, S.; Castro-Acosta, A.; Hernández-Carcereny, C.; Castelo, R.; Márquez-Martín, E.; Montón, C.; Palou, A.; Faner, R.; Furlong, L.I.; et al. Miembros del grupo BIOMEPOC The BIOMEPOC Project: Personalized Biomarkers and Clinical Profiles in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2019, 55, 93–99. [Google Scholar] [CrossRef]
- Pastor, M.; Gómez-Tamayo, J.C.; Sanz, F. Flame: An Open Source Framework for Model Development, Hosting, and Usage in Production Environments. J. Cheminformatics 2021, 13, 31. [Google Scholar] [CrossRef]
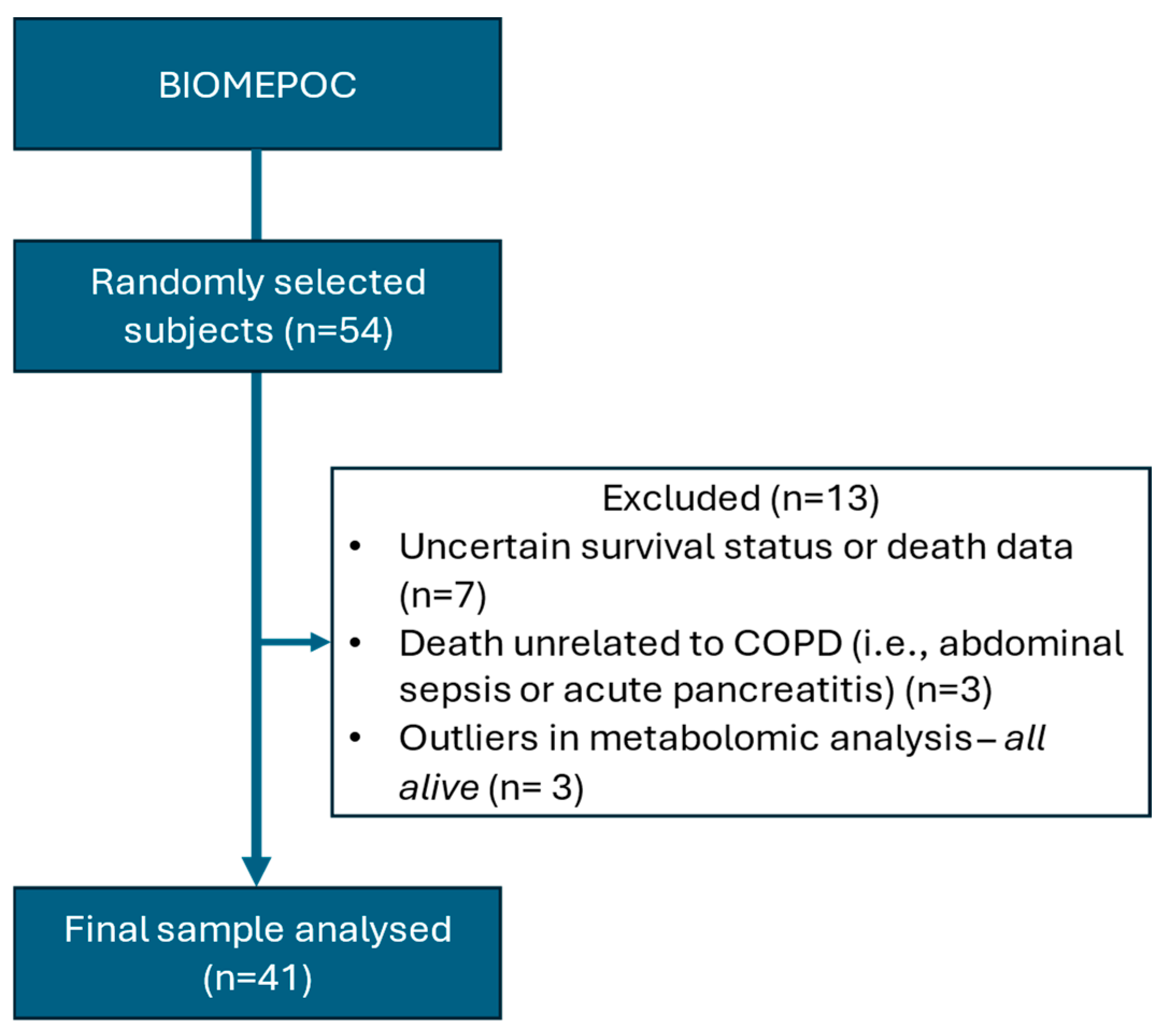


| Surviving (n = 28, 68%) | Deceased (n = 13, 32%) | p-Value | |
|---|---|---|---|
| Age, yrs. | 66.2 ± 9.0 | 73.4 ± 5.2 | 0.01 |
| Sex | 0.12 | ||
| -Male, n (%) | 15 (58) | 11(42) | |
| -Female, n (%) | 13 (87) | 2 (13) | |
| Smoking status | 0.92 | ||
| -Active smoker, n (%) | 13 (65) | 7 (35) | |
| -Ex-smoker, n (%) | 15 (71) | 6 (29) | |
| Pack Years | 56 ± 24 | 58 ± 29 | 0.83 |
| BMI, kg/m2 | 26.3 ± 5.9 | 24.4 ± 4.0 | 0.32 |
| Post-BD FEV1, % pred. | 48.5 [39.0–67.0] | 33.0 [23.0–37.0] | <0.01 |
| Post-BD FEV1/FVC, % pred. | 48.6 [38.5–59.0] | 39.0 [34.0–56.0] | 0.26 |
| DLco, % pred | 52.3 ± 18.5 | 38.2 ± 19.8 | 0.06 |
| GOLD I–IV, n (%) | 0.15 | ||
| -I (10%) | 4 (100) | 0 (0) | |
| -II (27%) | 9 (82) | 2 (18) | |
| -III (41%) | 11 65) | 6 (35) | |
| -IV (22%) | 4 (44) | 5 (56) | |
| GOLD A-D, n (%) | 0.21 | ||
| -A (20%) | 7 (88) | 1 (12) | |
| -B (20%) | 7 (88) | 1 (12) | |
| -E (60%) | 14 (56) | 11 (44) | |
| Charlson score | 3.0 [2.5–4.0] | 3.0 [2.8–4.3] | 0.67 |
| Metabolite | log2FC | %Δ | p-Value | Predominant Source | Taxonomy | Function/Action |
|---|---|---|---|---|---|---|
| ↑ Butyryl-L-carnitine | 0.8233 | 76.95 | <0.01 | Endogen | Fatty acid ester (short chain acylcarnitine) | Lipid β-oxidation, energy production, amino acid catabolism |
| ↑ L-DOPA | 1.1807 | 126.69 | 0.01 | Endogen | Amino acids, peptides and analogues | Catecholamine intermediate, amino acid metabolism |
| ↑ Hydroxyproline | 0.6793 | 60.14 | 0.01 | Endogen | Amino acids, peptides and analogues | Peptide structure of collagen, tissue remodeling, amino acid metabolism |
| ↑ Indoxyl sulfate | 1.0562 | 107.94 | 0.01 | Microbiota | Sulfated aromatic compound | Cardiotoxin and uremic toxin, Endothelial damage |
| ↓ L-Cystine | −1.0506 | −51.72 | 0.02 | Endogen | Amino acids, peptides and analogues | Amino acid metabolism, redox homeostasis |
| ↑ N-Phenylacetylglutamine (PAGIn) | 1.0313 | 104.38 | 0.02 | Mixed Source | Amino acids, peptides and analogues | Amino acid metabolism, Nitrogenous excretion, ↑ Platelet aggregation and thrombosis |
| ↑ Methylnicotinamide (MNA) | −0.7350 | −39.92 | 0.03 | Endogen | Nicotinamide derivative | Nicotinate and nicotinamide metabolism, energy production, redox balance |
| ↓ 4-Aminobenzoate | −0.8362 | −43.99 | 0.03 | Microbiota | Benzoic acids and derivatives | Folic acid synthesis |
| ↑ Monomethylglutarate | 0.8077 | 75.05 | 0.03 | Endogen | Fatty Acid ester | Lipid β-oxidation, energy production, amino acid metabolism, redox balance |
| ↓ Citric acid | −1.0226 | −50.78 | 0.05 | Endogen | Tricarboxylic acids and derivatives | TCA cycle, energy production, amino acid metabolism |
| ↑ Creatine | 0.5618 | 47.61 | 0.05 | Endogen | Amino acids, peptides and analogues | Energy reserve, amino acid metabolism |
| ↑ Pyruvic acid | 0.2938 | 22.58 | 0.05 | Endogen | α-Keto acids and derivatives | Glycolysis end-product, TCA cycle, urea cycle, energy production, amino acid metabolism |
| ≈↓ 2-Hydroxyglutarate | −0.6872 | −37.89 | 0.06 | Endogen | Short-chain hydroxy acids and derivatives | Butanoate and amino acid metabolism, epigenetic regulator |
| ≈↑ Proline | 0.3449 | 27.01 | 0.07 | Endogen | Amino acids, peptides and analogues | Peptide structure of collagen, tissue remodeling, amino acid metabolism |
| ≈↑ Phenol | 1.2227 | 133.39 | 0.08 | Microbiota | Benzenoids, phenolic compound | Sulfate/sulfite metabolism, dysbiosis |
| ≈↑ 3-Methyl-2-Oxovaleric acid | 0.5672 | 48.17 | 0.08 | Endogen | Short-chain keto acids and derivatives | Amino acid metabolism, energy production |
| ≈↑ 2-Phosphoglyceric acid (2-PG) | 0.5983 | 51.39 | 0.09 | Endogen | Carbohydrates and their conjugates | Glycolysis, gluconeogenesis, energy production |
| ≈↑ Aspartate | 0.4243 | 34.19 | 0.09 | Endogen | Amino acids, peptides and analogues | Energy metabolism and urea cycle, amino acid metabolism, nicotinate and nicotinamide metabolism |
| ≈↑ Threitol | 0.5532 | 46.73 | 0.09 | Mixed source | Carbohydrates and their conjugates | Redox balance, dysbiosis |
| Conventional | AI Free Choice | |
|---|---|---|
| Fitting | ||
| Sensitivity–Specificity–MCC | 1.00 | 1.00 |
| Coverage | 0.96 | 1.00 |
| Prediction | ||
| Sensitivity | 0.92 | 0.75 |
| Specificity | 0.91 | 0.90 |
| MCC | 0.83 | 0.65 |
| Coverage | 0.92 | 0.85 |
| PPV (rep/our) | 0.90/0.83 | 0.87/0.78 |
| NPV (rep/our) | 0.93/0.96 | 0.81/0.89 |
| Accuracy (rep/our) | 0.92/0.91 | 0.83/0.85 |
| Conventional + FEV1 | AI Free Choice + FEV1 | |
|---|---|---|
| Fitting | ||
| Sensitivity–Specificity–MCC | 1.00 | 1.00 |
| Coverage | 1.00 | 1.00 |
| Prediction | ||
| Sensitivity | 0.83 | 1.00 |
| Specificity | 1.00 | 0.92 |
| MCC | 0.83 | 0.92 |
| Coverage | 0.85 | 0.96 |
| PPV (rep/our) | 1.00/1.00 | 0.92/0.86 |
| NPV (rep/our) | 0.87/0.93 | 1.00/1.00 |
| Accuracy (rep/our) | 0.92/0.95 | 0.96/0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enríquez-Rodríguez, C.J.; Agranovich, B.; Pascual-Guàrdia, S.; Faner, R.; Camps-Ubach, R.; Castro-Acosta, A.; López-Campos, J.L.; Peces-Barba, G.; Seijo, L.; Caguana-Vélez, O.A.; et al. Metabolomic Signatures Predict Seven-Year Mortality in Clinically Stable COPD Patients. Int. J. Mol. Sci. 2025, 26, 6373. https://doi.org/10.3390/ijms26136373
Enríquez-Rodríguez CJ, Agranovich B, Pascual-Guàrdia S, Faner R, Camps-Ubach R, Castro-Acosta A, López-Campos JL, Peces-Barba G, Seijo L, Caguana-Vélez OA, et al. Metabolomic Signatures Predict Seven-Year Mortality in Clinically Stable COPD Patients. International Journal of Molecular Sciences. 2025; 26(13):6373. https://doi.org/10.3390/ijms26136373
Chicago/Turabian StyleEnríquez-Rodríguez, César Jessé, Bella Agranovich, Sergi Pascual-Guàrdia, Rosa Faner, Ramon Camps-Ubach, Ady Castro-Acosta, José Luis López-Campos, Germán Peces-Barba, Luis Seijo, Oswaldo Antonio Caguana-Vélez, and et al. 2025. "Metabolomic Signatures Predict Seven-Year Mortality in Clinically Stable COPD Patients" International Journal of Molecular Sciences 26, no. 13: 6373. https://doi.org/10.3390/ijms26136373
APA StyleEnríquez-Rodríguez, C. J., Agranovich, B., Pascual-Guàrdia, S., Faner, R., Camps-Ubach, R., Castro-Acosta, A., López-Campos, J. L., Peces-Barba, G., Seijo, L., Caguana-Vélez, O. A., Rodríguez-Chiaradia, D., Barreiro, E., Monsó, E., Cosío, B. G., Abramovich, I., Agustí, A., Casadevall, C., Gea, J., & on behalf of the BIOMEPOC Group. (2025). Metabolomic Signatures Predict Seven-Year Mortality in Clinically Stable COPD Patients. International Journal of Molecular Sciences, 26(13), 6373. https://doi.org/10.3390/ijms26136373










