Epigenome Engineering Using dCas Systems for Biomedical Applications and Biotechnology: Current Achievements, Opportunities and Challenges
Abstract
1. Introduction
2. The Diversity of CRISPR/Cas-Based Tools for Epigenome Engineering
3. Perspectives of the Applications of dCas-Based Epigenomic Editors in Biomedicine
3.1. Hereditary Disorders: Haploinsufficiency (In Vivo Studies)
3.2. Hereditary Disorders: Imprinting-Related (In Vivo Studies)
3.3. Hereditary Disorders: X-Chromosome-Linked (In Vivo Studies and Clinical Trials)
3.4. Hereditary Disorders: Recessive Autosomal (In Vivo Studies)
3.5. Neurodegenerative Diseases (In Vitro and In Vivo Studies)
3.6. Psychoneurological Conditions and Addictions (In Vivo Studies)
3.7. Metabolic Diseases (In Vivo Studies)
3.8. Autoimmune Disorders (Only In Vitro Studies Yet)
3.9. Acute Organ Injury and Fibrosis (In Vivo Studies)
3.10. Normal and Accelerated Aging (In Vitro and In Vivo Studies)
3.11. Oncological Diseases (In Vitro Studies Only)
3.12. Viral Diseases (In Vitro, In Vivo Studies and Clinical Trials)
3.13. Regenerative Medicine and Cell-Based Therapies (In Vitro Studies)
4. Animal Biotechnology Applications
5. Applications of Epigenomic Editors in Agrobiotechnology
5.1. Peculiarities of Epigenetic Regulation and Engineering in Plants
5.2. Perspectives of Epigenomic Editing in Plants
5.3. Real-World Applications and Regulatory Considerations
6. Industrial Biotechnology Application
7. Challenges Associated with the Use of Epigenomic Editors and Ways to Overcome Them
7.1. Challenges in Genome Target Selection (Off-Target Effect) and Context-Dependent Effects
7.2. Potency of Epigenetic Editing and Persistence of Epigenetic States
7.3. Delivery Methods
7.4. Immunogenicity and Cytotoxicity of dCas-Based Epigenome Editors
7.5. Ethical and Regulatory Landscape for Introduction of dCas-Based Systems into Clinical Practice
8. Concluding Remarks, Outlook and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.Y.; Doudna, J.A. CRISPR Technology: A Decade of Genome Editing Is Only the Beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in Epigenetics Link Genetics to the Environment and Disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Allis, C.D.; Jenuwein, T. The Molecular Hallmarks of Epigenetic Control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal Structure of the Nucleosome Core Particle at 2.8 A Resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA Methylation: A Historical Perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Jurkowska, R.Z.; Jeltsch, A. Mechanisms and Biological Roles of DNA Methyltransferases and DNA Methylation: From Past Achievements to Future Challenges. In DNA Methyltransferases—Role and Function; Jeltsch, A., Jurkowska, R.Z., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2022; Volume 1389, pp. 1–19. ISBN 978-3-031-11453-3. [Google Scholar]
- Lyko, F. The DNA Methyltransferase Family: A Versatile Toolkit for Epigenetic Regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef]
- Davletgildeeva, A.T.; Kuznetsov, N.A. The Role of DNMT Methyltransferases and TET Dioxygenases in the Maintenance of the DNA Methylation Level. Biomolecules 2024, 14, 1117. [Google Scholar] [CrossRef]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone Post-Translational Modifications—Cause and Consequence of Genome Function. Nat. Rev. Genet. 2022. [Google Scholar] [CrossRef]
- Yang, X.-J.; Seto, E. HATs and HDACs: From Structure, Function and Regulation to Novel Strategies for Therapy and Prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef]
- Michalak, E.M.; Burr, M.L.; Bannister, A.J.; Dawson, M.A. The Roles of DNA, RNA and Histone Methylation in Ageing and Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 573–589. [Google Scholar] [CrossRef]
- Shin, Y. Histone Tail Cleavage as a Mechanism for Epigenetic Regulation. Int. J. Mol. Sci. 2024, 25, 10789. [Google Scholar] [CrossRef] [PubMed]
- Draizen, E.J.; Shaytan, A.K.; Mariño-Ramírez, L.; Talbert, P.B.; Landsman, D.; Panchenko, A.R. HistoneDB 2.0: A Histone Database with Variants—An Integrated Resource to Explore Histones and Their Variants. Database 2016, 2016, baw014. [Google Scholar] [CrossRef] [PubMed]
- Clapier, C.R.; Iwasa, J.; Cairns, B.R.; Peterson, C.L. Mechanisms of Action and Regulation of ATP-Dependent Chromatin-Remodelling Complexes. Nat. Rev. Mol. Cell Biol. 2017, 18, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Willcockson, M.A.; Healton, S.E.; Weiss, C.N.; Bartholdy, B.A.; Botbol, Y.; Mishra, L.N.; Sidhwani, D.S.; Wilson, T.J.; Pinto, H.B.; Maron, M.I.; et al. H1 Histones Control the Epigenetic Landscape by Local Chromatin Compaction. Nature 2021, 589, 293–298. [Google Scholar] [CrossRef]
- Sanulli, S.; Trnka, M.J.; Dharmarajan, V.; Tibble, R.W.; Pascal, B.D.; Burlingame, A.L.; Griffin, P.R.; Gross, J.D.; Narlikar, G.J. HP1 Reshapes Nucleosome Core to Promote Phase Separation of Heterochromatin. Nature 2019, 575, 390–394. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Razin, S.V.; Ulianov, S.V. Genome-Directed Cell Nucleus Assembly. Biology 2022, 11, 708. [Google Scholar] [CrossRef]
- Razin, S.V.; Gavrilov, A.A. The Role of Liquid–Liquid Phase Separation in the Compartmentalization of Cell Nucleus and Spatial Genome Organization. Biochem. Mosc. 2020, 85, 643–650. [Google Scholar] [CrossRef]
- Skvortsova, K.; Iovino, N.; Bogdanović, O. Functions and Mechanisms of Epigenetic Inheritance in Animals. Nat. Rev. Mol. Cell Biol. 2018, 19, 774–790. [Google Scholar] [CrossRef]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in Human Disease and Prospects for Epigenetic Therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef]
- Galkin, F.; Kovalchuk, O.; Koldasbayeva, D.; Zhavoronkov, A.; Bischof, E. Stress, Diet, Exercise: Common Environmental Factors and Their Impact on Epigenetic Age. Ageing Res. Rev. 2023, 88, 101956. [Google Scholar] [CrossRef] [PubMed]
- Beerli, R.R.; Segal, D.J.; Dreier, B.; Barbas, C.F. Toward Controlling Gene Expression at Will: Specific Regulation of the erbB-2/HER-2 Promoter by Using Polydactyl Zinc Finger Proteins Constructed from Modular Building Blocks. Proc. Natl. Acad. Sci. USA 1998, 95, 14628–14633. [Google Scholar] [CrossRef] [PubMed]
- Method of the Year 2011. Nat. Methods 2012, 9, 1. [CrossRef]
- Zhang, F.; Cong, L.; Lodato, S.; Kosuri, S.; Church, G.M.; Arlotta, P. Efficient Construction of Sequence-Specific TAL Effectors for Modulating Mammalian Transcription. Nat. Biotechnol. 2011, 29, 149–153. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef]
- Clark, T.; Waller, M.A.; Loo, L.; Moreno, C.L.; Denes, C.E.; Neely, G.G. CRISPR Activation Screens: Navigating Technologies and Applications. Trends Biotechnol. 2024, 42, 1017–1034. [Google Scholar] [CrossRef]
- McCutcheon, S.R.; Rohm, D.; Iglesias, N.; Gersbach, C.A. Epigenome Editing Technologies for Discovery and Medicine. Nat. Biotechnol. 2024, 42, 1199–1217. [Google Scholar] [CrossRef]
- Ueda, J.; Yamazaki, T.; Funakoshi, H. Toward the Development of Epigenome Editing-Based Therapeutics: Potentials and Challenges. Int. J. Mol. Sci. 2023, 24, 4778. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, A.; Rots, M.G. (Eds.) Epigenome Editing: Methods and Protocols; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2018; Volume 1767, ISBN 978-1-4939-7773-4. [Google Scholar]
- Roth, G.V.; Gengaro, I.R.; Qi, L.S. Precision Epigenetic Editing: Technological Advances, Enduring Challenges, and Therapeutic Applications. Cell Chem. Biol. 2024, 31, 1422–1446. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, K.M.; Ovchinnikova, V.Y.; Dashinimaev, E.B. Cell Reprogramming with CRISPR/Cas9 Based Transcriptional Regulation Systems. Front. Bioeng. Biotechnol. 2020, 8, 882. [Google Scholar] [CrossRef]
- Cofsky, J.C.; Soczek, K.M.; Knott, G.J.; Nogales, E.; Doudna, J.A. CRISPR–Cas9 Bends and Twists DNA to Read Its Sequence. Nat. Struct. Mol. Biol. 2022, 29, 395–402. [Google Scholar] [CrossRef]
- Butterfield, G.L.; Rohm, D.; Roberts, A.; Nethery, M.A.; Rizzo, A.J.; Morone, D.J.; Garnier, L.; Iglesias, N.; Barrangou, R.; Gersbach, C.A. Characterization of Diverse Cas9 Orthologs for Genome and Epigenome Editing. Proc. Natl. Acad. Sci. USA 2025, 122, e2417674122. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary Classification of CRISPR-Cas Systems: A Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Feng, B. The Rapidly Advancing Class 2 CRISPR-Cas Technologies: A Customizable Toolbox for Molecular Manipulations. J. Cell. Mol. Med. 2020, 24, 3256–3270. [Google Scholar] [CrossRef]
- Kovalev, M.A.; Davletshin, A.I.; Karpov, D.S. Engineering Cas9: Next Generation of Genomic Editors. Appl. Microbiol. Biotechnol. 2024, 108, 209. [Google Scholar] [CrossRef]
- Escobar, M.; Li, J.; Patel, A.; Liu, S.; Xu, Q.; Hilton, I.B. Quantification of Genome Editing and Transcriptional Control Capabilities Reveals Hierarchies among Diverse CRISPR/Cas Systems in Human Cells. ACS Synth. Biol. 2022, 11, 3239–3250. [Google Scholar] [CrossRef]
- Xu, X.; Chemparathy, A.; Zeng, L.; Kempton, H.R.; Shang, S.; Nakamura, M.; Qi, L.S. Engineered Miniature CRISPR-Cas System for Mammalian Genome Regulation and Editing. Mol. Cell 2021, 81, 4333–4345.e4. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.; Scheiman, J.; Vora, S.; Pruitt, B.W.; Tuttle, M.; Iyer, E.P.R.; Lin, S.; Kiani, S.; Guzman, C.D.; Wiegand, D.J.; et al. Highly Efficient Cas9-Mediated Transcriptional Programming. Nat. Methods 2015, 12, 326–328. [Google Scholar] [CrossRef]
- Nuñez, J.K.; Chen, J.; Pommier, G.C.; Cogan, J.Z.; Replogle, J.M.; Adriaens, C.; Ramadoss, G.N.; Shi, Q.; Hung, K.L.; Samelson, A.J.; et al. Genome-Wide Programmable Transcriptional Memory by CRISPR-Based Epigenome Editing. Cell 2021, 184, 2503–2519.e17. [Google Scholar] [CrossRef]
- Omachi, K.; Miner, J.H. Comparative Analysis of dCas9-VP64 Variants and Multiplexed Guide RNAs Mediating CRISPR Activation. PLoS ONE 2022, 17, e0270008. [Google Scholar] [CrossRef]
- Yeo, N.C.; Chavez, A.; Lance-Byrne, A.; Chan, Y.; Menn, D.; Milanova, D.; Kuo, C.-C.; Guo, X.; Sharma, S.; Tung, A.; et al. An Enhanced CRISPR Repressor for Targeted Mammalian Gene Regulation. Nat. Methods 2018, 15, 611–616. [Google Scholar] [CrossRef]
- Neumann, E.N.; Bertozzi, T.M.; Wu, E.; Serack, F.; Harvey, J.W.; Brauer, P.P.; Pirtle, C.P.; Coffey, A.; Howard, M.; Kamath, N.; et al. Brainwide Silencing of Prion Protein by AAV-Mediated Delivery of an Engineered Compact Epigenetic Editor. Science 2024, 384, ado7082. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, C.; Zhang, T.; Li, F.; Yang, W.; Kaminski, R.; Fagan, P.R.; Putatunda, R.; Young, W.-B.; Khalili, K.; et al. CRISPR/gRNA-Directed Synergistic Activation Mediator (SAM) Induces Specific, Persistent and Robust Reactivation of the HIV-1 Latent Reservoirs. Sci. Rep. 2015, 5, 16277. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Gilbert, L.A.; Qi, L.S.; Weissman, J.S.; Vale, R.D. A Protein-Tagging System for Signal Amplification in Gene Expression and Fluorescence Imaging. Cell 2014, 159, 635–646. [Google Scholar] [CrossRef]
- Wang, H.; Han, M.; Qi, L.S. Engineering 3D Genome Organization. Nat. Rev. Genet. 2021, 22, 343–360. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, R.; Jiang, Y.; Zheng, Q.; Yang, Y.; Liu, J.; Wu, G.; Zhao, W.; Li, Z.; Peng, C.; et al. Rationally Designed Campylobacter Jejuni Cas9 Enables Efficient Gene Activation and Base Editing. Mol. Ther. Nucleic Acids 2024, 35, 102366. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.; Cheng, J.; Xiao, R.; VanDusen, N.J.; Quintino, L.; Pu, W.T.; Vandenberghe, L.H.; Chavez, A.; Church, G. Rational Design of a Compact CRISPR-Cas9 Activator for AAV-Mediated Delivery. bioRxiv 2018. [Google Scholar] [CrossRef]
- Carosso, G.A.; Yeo, R.W.; Gainous, T.B.; Jawaid, M.Z.; Yang, X.; Kim, J.Y.S.; Jadhav, K.; Juan-Sing, N.; Boregowda, S.V.; Cutillas, V.; et al. Discovery of Hypercompact Epigenetic Modulators for Persistent CRISPR-Mediated Gene Activation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Swain, T.; Pflueger, C.; Freytag, S.; Poppe, D.; Pflueger, J.; Nguyen, T.V.; Li, J.K.; Lister, R. A Modular dCas9-Based Recruitment Platform for Combinatorial Epigenome Editing. Nucleic Acids Res. 2024, 52, 474–491. [Google Scholar] [CrossRef]
- Kieu Nguyen, N.T.; Tu, Y.; Lee, H.-S.; Truong, V.A.; Chang, Y.-H.; Pham, N.N.; Chang, C.-W.; Lin, Y.-H.; Lai, P.-L.; Chen, P.-H.; et al. Split dCas12a Activator for lncRNA H19 Activation to Enhance BMSC Differentiation and Promote Calvarial Bone Healing. Biomaterials 2023, 297, 122106. [Google Scholar] [CrossRef]
- Nguyen, N.T.K.; Lee, S.; Chen, P.; Chang, Y.; Pham, N.N.; Chang, C.; Pham, D.H.; Ngo, D.K.T.; Dang, Q.T.; Truong, V.A.; et al. Enhanced Calvarial Bone Repair Using ASCs Engineered with RNA-Guided Split dCas12a System That Co-Activates Sox 5, Sox6, and Long Non-Coding RNA H19. Small 2024, 20, 2306612. [Google Scholar] [CrossRef]
- Zhang, X.; Bhattacharya, A.; Pu, C.; Dai, Y.; Liu, J.; Rao, L.; Tian, C. A Programmable CRISPR/dCas9-Based Epigenetic Editing System Enabling Loci-Targeted Histone Citrullination and Precise Transcription Regulation. J. Genet. Genom. 2024, 51, 1485–1493. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, S.; Liang, G.; An, W. MMP-9-Dependent Proteolysis of the Histone H3 N-Terminal Tail: A Critical Epigenetic Step in Driving Oncogenic Transcription and Colon Tumorigenesis. Mol. Oncol. 2024, 18, 2001–2019. [Google Scholar] [CrossRef]
- Altinbay, M.; Wang, J.; Chen, J.; Schäfer, D.; Sprang, M.; Blagojevic, B.; Wölfl, S.; Andrade-Navarro, M.A.; Dikic, I.; Knapp, S.; et al. Chem-CRISPR/dCas9FCPF: A Platform for Chemically Induced Epigenome Editing. Nucleic Acids Res. 2024, 52, 11587–11601. [Google Scholar] [CrossRef]
- Chen, R.; Shi, X.; Yao, X.; Gao, T.; Huang, G.; Ning, D.; Cao, Z.; Xu, Y.; Liang, W.; Tian, S.Z.; et al. Specific Multivalent Molecules Boost CRISPR-Mediated Transcriptional Activation. Nat. Commun. 2024, 15, 7222. [Google Scholar] [CrossRef]
- Qin, G.; Liu, Z.; Yang, J.; Liao, X.; Zhao, C.; Ren, J.; Qu, X. Targeting Specific DNA G-Quadruplexes with CRISPR-Guided G-Quadruplex-Binding Proteins and Ligands. Nat. Cell Biol. 2024, 26, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.L.; Kodikara, S.G.; Hoque, M.E.; Shiekh, S.; Alfehaid, J.; Basu, S.; Balci, H. Combining CRISPR Activation and Interference Capabilities Using dCas9 and G-Quadruplex Structures. NAR Mol. Med. 2025, 2, ugaf001. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Wang, J.; Sun, W.; Huang, W.; Cai, Z.; Zhao, G.; Wang, J. Reprogrammable CRISPR/dCas9-Based Recruitment of DNMT1 for Site-Specific DNA Demethylation and Gene Regulation. Cell Discov. 2019, 5, 1–4. [Google Scholar] [CrossRef]
- Levy, S.; Somasundaram, L.; Raj, I.X.; Ic-Mex, D.; Phal, A.; Schmidt, S.; Ng, W.I.; Mar, D.; Decarreau, J.; Moss, N.; et al. dCas9 Fusion to Computer-Designed PRC2 Inhibitor Reveals Functional TATA Box in Distal Promoter Region. Cell Rep. 2022, 38, 110457. [Google Scholar] [CrossRef]
- Maddineni, A.; Liang, Z.; Jambardi, S.; Roy, S.; Tycko, J.; Patil, A.; Manzano, M.; Gottwein, E. Cytotoxicity of Activator Expression in CRISPR-Based Transcriptional Activation Systems. bioRxiv 2024. [Google Scholar] [CrossRef]
- Johnson, A.F.; Nguyen, H.T.; Veitia, R.A. Causes and Effects of Haploinsufficiency. Biol. Rev. 2019, 94, 1774–1785. [Google Scholar] [CrossRef]
- Colasante, G.; Lignani, G.; Brusco, S.; Di Berardino, C.; Carpenter, J.; Giannelli, S.; Valassina, N.; Bido, S.; Ricci, R.; Castoldi, V.; et al. dCas9-Based Scn1a Gene Activation Restores Inhibitory Interneuron Excitability and Attenuates Seizures in Dravet Syndrome Mice. Mol. Ther. 2020, 28, 235–253. [Google Scholar] [CrossRef]
- Yamagata, T.; Raveau, M.; Kobayashi, K.; Miyamoto, H.; Tatsukawa, T.; Ogiwara, I.; Itohara, S.; Hensch, T.K.; Yamakawa, K. CRISPR/dCas9-Based Scn1a Gene Activation in Inhibitory Neurons Ameliorates Epileptic and Behavioral Phenotypes of Dravet Syndrome Model Mice. Neurobiol. Dis. 2020, 141, 104954. [Google Scholar] [CrossRef]
- Peter, C.J.; Saito, A.; Hasegawa, Y.; Tanaka, Y.; Nagpal, M.; Perez, G.; Alway, E.; Espeso-Gil, S.; Fayyad, T.; Ratner, C.; et al. In Vivo Epigenetic Editing of Sema6a Promoter Reverses Transcallosal Dysconnectivity Caused by C11orf46/Arl14ep Risk Gene. Nat. Commun. 2019, 10, 4112. [Google Scholar] [CrossRef]
- Li, M.M.; Madara, J.C.; Steger, J.S.; Krashes, M.J.; Balthasar, N.; Campbell, J.N.; Resch, J.M.; Conley, N.J.; Garfield, A.S.; Lowell, B.B. The Paraventricular Hypothalamus Regulates Satiety and Prevents Obesity via Two Genetically Distinct Circuits. Neuron 2019, 102, 653–667.e6. [Google Scholar] [CrossRef]
- Matharu, N.; Rattanasopha, S.; Tamura, S.; Maliskova, L.; Wang, Y.; Bernard, A.; Hardin, A.; Eckalbar, W.L.; Vaisse, C.; Ahituv, N. CRISPR-Mediated Activation of a Promoter or Enhancer Rescues Obesity Caused by Haploinsufficiency. Science 2019, 363, eaau0629. [Google Scholar] [CrossRef] [PubMed]
- Syding, L.A.; Nickl, P.; Kasparek, P.; Sedlacek, R. CRISPR/Cas9 Epigenome Editing Potential for Rare Imprinting Diseases: A Review. Cells 2020, 9, 993. [Google Scholar] [CrossRef] [PubMed]
- O’Geen, H.; Beitnere, U.; Garcia, M.S.; Adhikari, A.; Cameron, D.L.; Fenton, T.A.; Copping, N.A.; Deng, P.; Lock, S.; Halmai, J.A.N.M.; et al. Transcriptional Reprogramming Restores UBE3A Brain-Wide and Rescues Behavioral Phenotypes in an Angelman Syndrome Mouse Model. Mol. Ther. 2023, 31, 1088–1105. [Google Scholar] [CrossRef]
- Liu, Y.; Lou, S.; Li, J.; Liu, Y.; Huang, S.; Wei, Y.; Liu, J.; Lv, R.; Tang, J.; Shen, Z.; et al. Epigenetic Editing Alleviates Angelman Syndrome Phenotype in Mice by Unsilencing Paternal Ube3a. Cell Discov. 2024, 10, 97. [Google Scholar] [CrossRef]
- Li, J.; Shen, Z.; Liu, Y.; Yan, Z.; Liu, Y.; Lin, X.; Tang, J.; Lv, R.; Geng, G.; Xiong, Z.-Q.; et al. A High-Fidelity RNA-Targeting Cas13 Restores Paternal Ube3a Expression and Improves Motor Functions in Angelman Syndrome Mice. Mol. Ther. 2023, 31, 2286–2295. [Google Scholar] [CrossRef]
- Rohm, D.; Black, J.B.; McCutcheon, S.R.; Barrera, A.; Morone, D.J.; Nuttle, X.; De Esch, C.E.; Tai, D.J.C.; Talkowski, M.E.; Iglesias, N.; et al. Activation of the Imprinted Prader-Willi Syndrome Locus by CRISPR-Based Epigenome Editing. Cell Genom. 2025, 5, 100770. [Google Scholar] [CrossRef]
- Horii, T.; Morita, S.; Hino, S.; Kimura, M.; Hino, Y.; Kogo, H.; Nakao, M.; Hatada, I. Successful Generation of Epigenetic Disease Model Mice by Targeted Demethylation of the Epigenome. Genome Biol. 2020, 21, 77. [Google Scholar] [CrossRef]
- Liao, H.-K.; Hatanaka, F.; Araoka, T.; Reddy, P.; Wu, M.-Z.; Sui, Y.; Yamauchi, T.; Sakurai, M.; O’Keefe, D.D.; Núñez-Delicado, E.; et al. In Vivo Target Gene Activation via CRISPR/Cas9-Mediated Trans-Epigenetic Modulation. Cell 2017, 171, 1495–1507.e15. [Google Scholar] [CrossRef]
- Wu, R.; Li, P.; Xiao, P.; Zhang, S.; Wang, X.; Liu, J.; Sun, W.; Chang, Y.; Ai, X.; Chen, L.; et al. Activation of Endogenous Full-Length Utrophin by MyoAAV-UA as a Therapeutic Approach for Duchenne Muscular Dystrophy. Nat. Commun. 2025, 16, 2398. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Krzisch, M.; Wu, X.; Graef, J.; Muffat, J.; Hnisz, D.; Li, C.H.; Yuan, B.; Xu, C.; et al. Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene. Cell 2018, 172, 979. [Google Scholar] [CrossRef]
- Berman, R.F.; Buijsen, R.A.; Usdin, K.; Pintado, E.; Kooy, F.; Pretto, D.; Pessah, I.N.; Nelson, D.L.; Zalewski, Z.; Charlet-Bergeurand, N.; et al. Mouse Models of the Fragile X Premutation and Fragile X-Associated Tremor/Ataxia Syndrome. J. Neurodev. Disord. 2014, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Sandweiss, A.J.; Brandt, V.L.; Zoghbi, H.Y. Advances in Understanding of Rett Syndrome and MECP2 Duplication Syndrome: Prospects for Future Therapies. Lancet Neurol. 2020, 19, 689–698. [Google Scholar] [CrossRef]
- Qian, J.; Guan, X.; Xie, B.; Xu, C.; Niu, J.; Tang, X.; Li, C.H.; Colecraft, H.M.; Jaenisch, R.; Liu, X.S. Multiplex Epigenome Editing of MECP2 to Rescue Rett Syndrome Neurons. Sci. Transl. Med. 2023, 15, eadd4666. [Google Scholar] [CrossRef]
- Hong, W.; Haviland, I.; Pestana-Knight, E.; Weisenberg, J.L.; Demarest, S.; Marsh, E.D.; Olson, H.E. CDKL5 Deficiency Disorder-Related Epilepsy: A Review of Current and Emerging Treatment. CNS Drugs 2022, 36, 591–604. [Google Scholar] [CrossRef]
- Halmai, J.A.N.M.; Deng, P.; Gonzalez, C.E.; Coggins, N.B.; Cameron, D.; Carter, J.L.; Buchanan, F.K.B.; Waldo, J.J.; Lock, S.R.; Anderson, J.D.; et al. Artificial Escape from XCI by DNA Methylation Editing of the CDKL5 Gene. Nucleic Acids Res. 2020, 48, 2372–2387. [Google Scholar] [CrossRef]
- Monfort, B.; Want, K.; Gervason, S.; D’Autréaux, B. Recent Advances in the Elucidation of Frataxin Biochemical Function Open Novel Perspectives for the Treatment of Friedreich’s Ataxia. Front. Neurosci. 2022, 16, 838335. [Google Scholar] [CrossRef]
- Beaudin, M.; Manto, M.; Schmahmann, J.D.; Pandolfo, M.; Dupre, N. Recessive Cerebellar and Afferent Ataxias—Clinical Challenges and Future Directions. Nat. Rev. Neurol. 2022, 18, 257–272. [Google Scholar] [CrossRef]
- Tremblay, J.P.; Chapdelaine, P.; Coulombe, Z.; Rousseau, J. Transcription Activator-like Effector Proteins Induce the Expression of the Frataxin Gene. Hum. Gene Ther. 2012, 23, 883–890. [Google Scholar] [CrossRef]
- Chapdelaine, P.; Coulombe, Z.; Chikh, A.; Gérard, C.; Tremblay, J.P. A Potential New Therapeutic Approach for Friedreich Ataxia: Induction of Frataxin Expression with TALE Proteins. Mol. Ther. Nucleic Acids 2013, 2, e119. [Google Scholar] [CrossRef]
- Chapdelaine, P.; Gérard, C.; Sanchez, N.; Cherif, K.; Rousseau, J.; Ouellet, D.L.; Jauvin, D.; Tremblay, J.P. Development of an AAV9 Coding for a 3XFLAG-TALEfrat#8-VP64 Able to Increase in Vivo the Human Frataxin in YG8R Mice. Gene Ther. 2016, 23, 606–614. [Google Scholar] [CrossRef]
- Erwin, G.S.; Grieshop, M.P.; Ali, A.; Qi, J.; Lawlor, M.; Kumar, D.; Ahmad, I.; McNally, A.; Teider, N.; Worringer, K.; et al. Synthetic Transcription Elongation Factors License Transcription across Repressive Chromatin. Science 2017, 358, 1617–1622. [Google Scholar] [CrossRef]
- Kemaladewi, D.U.; Bassi, P.S.; Erwood, S.; Al-Basha, D.; Gawlik, K.I.; Lindsay, K.; Hyatt, E.; Kember, R.; Place, K.M.; Marks, R.M.; et al. A Mutation-Independent Approach for Muscular Dystrophy via Upregulation of a Modifier Gene. Nature 2019, 572, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Gräslund, T.; Li, X.; Magnenat, L.; Popkov, M.; Barbas, C.F. Exploring Strategies for the Design of Artificial Transcription Factors. J. Biol. Chem. 2005, 280, 3707–3714. [Google Scholar] [CrossRef] [PubMed]
- Wilber, A.; Tschulena, U.; Hargrove, P.W.; Kim, Y.-S.; Persons, D.A.; Barbas, C.F.; Nienhuis, A.W. A Zinc-Finger Transcriptional Activator Designed to Interact with the Gamma-Globin Gene Promoters Enhances Fetal Hemoglobin Production in Primary Human Adult Erythroblasts. Blood 2010, 115, 3033–3041. [Google Scholar] [CrossRef] [PubMed]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; De La Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Garriga-Canut, M.; Agustín-Pavón, C.; Herrmann, F.; Sánchez, A.; Dierssen, M.; Fillat, C.; Isalan, M. Synthetic Zinc Finger Repressors Reduce Mutant Huntingtin Expression in the Brain of R6/2 Mice. Proc. Natl. Acad. Sci. USA 2012, 109, E3136–E3145. [Google Scholar] [CrossRef]
- Zeitler, B.; Froelich, S.; Marlen, K.; Shivak, D.A.; Yu, Q.; Li, D.; Pearl, J.R.; Miller, J.C.; Zhang, L.; Paschon, D.E.; et al. Allele-Selective Transcriptional Repression of Mutant HTT for the Treatment of Huntington’s Disease. Nat. Med. 2019, 25, 1131–1142. [Google Scholar] [CrossRef]
- Wegmann, S.; DeVos, S.L.; Zeitler, B.; Marlen, K.; Bennett, R.E.; Perez-Rando, M.; MacKenzie, D.; Yu, Q.; Commins, C.; Bannon, R.N.; et al. Persistent Repression of Tau in the Brain Using Engineered Zinc Finger Protein Transcription Factors. Sci. Adv. 2021, 7, eabe1611. [Google Scholar] [CrossRef]
- Duarte, F. Multiplexed CRISPR Activation of Neuroprotective Genes for Alzheimer’s Disease. Master’s Thesis, Universidade de Lund, Suécia, Lund, Sweden, 2018. Available online: https://hdl.handle.net/10316/86445%20 (accessed on 24 June 2025).
- Lee, J.-H.; Ahn, N.-H.; Choi, S.-B.; Kwon, Y.; Yang, S.-H. Natural Products Targeting Amyloid Beta in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2341. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.; Zhang, S.; Iyaswamy, A.; Sun, J.; Wang, J.; Yang, C. Novel Insight into Functions of Transcription Factor EB (TFEB) in Alzheimer’s Disease and Parkinson’s Disease. Aging Dis. 2023, 14, 652. [Google Scholar] [CrossRef]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling Pathways in Parkinson’s Disease: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef] [PubMed]
- Giehrl-Schwab, J.; Giesert, F.; Rauser, B.; Lao, C.L.; Hembach, S.; Lefort, S.; Ibarra, I.L.; Koupourtidou, C.; Luecken, M.D.; Truong, D.J.; et al. Parkinson’s Disease Motor Symptoms Rescue by CRISPRa-reprogramming Astrocytes into GABAergic Neurons. EMBO Mol. Med. 2022, 14, e14797. [Google Scholar] [CrossRef]
- Guzmán-Sastoque, P.; Sotelo, S.; Esmeral, N.P.; Albarracín, S.L.; Sutachan, J.-J.; Reyes, L.H.; Muñoz-Camargo, C.; Cruz, J.C.; Bloch, N.I. Assessment of CRISPRa-Mediated Gdnf Overexpression in an In Vitro Parkinson’s Disease Model. Front. Bioeng. Biotechnol. 2024, 12, 1420183. [Google Scholar] [CrossRef]
- Du, X.; Xie, X.; Liu, R. The Role of α-Synuclein Oligomers in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8645. [Google Scholar] [CrossRef]
- Udovin, L.; Quarracino, C.; Herrera, M.I.; Capani, F.; Otero-Losada, M.; Perez-Lloret, S. Role of Astrocytic Dysfunction in the Pathogenesis of Parkinson’s Disease Animal Models from a Molecular Signaling Perspective. Neural Plast. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Lau, C.-H.; Ho, J.W.-T.; Lo, P.K.; Tin, C. Targeted Transgene Activation in the Brain Tissue by Systemic Delivery of Engineered AAV1 Expressing CRISPRa. Mol. Ther. Nucleic Acids 2019, 16, 637–649. [Google Scholar] [CrossRef]
- Gerhardt, M.J.; Priglinger, S.G.; Biel, M.; Michalakis, S. Biology, Pathobiology and Gene Therapy of CNG Channel-Related Retinopathies. Biomedicines 2023, 11, 269. [Google Scholar] [CrossRef]
- Moreno, A.M.; Fu, X.; Zhu, J.; Katrekar, D.; Shih, Y.-R.V.; Marlett, J.; Cabotaje, J.; Tat, J.; Naughton, J.; Lisowski, L.; et al. In Situ Gene Therapy via AAV-CRISPR-Cas9-Mediated Targeted Gene Regulation. Mol. Ther. 2018, 26, 1818–1827. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Li, P.; Yao, K. Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies. Int. J. Mol. Sci. 2022, 23, 4883. [Google Scholar] [CrossRef]
- Newton, F.; Megaw, R. Mechanisms of Photoreceptor Death in Retinitis Pigmentosa. Genes 2020, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Splith, V.; Riedmayr, L.M.; Rötzer, R.D.; Gasparoni, G.; Nordström, K.J.V.; Wagner, J.E.; Hinrichsmeyer, K.S.; Walter, J.; Wahl-Schott, C.; et al. A Gene Therapy for Inherited Blindness Using dCas9-VPR-Mediated Transcriptional Activation. Sci. Adv. 2020, 6, eaba5614. [Google Scholar] [CrossRef]
- Moreno, A.M.; Alemán, F.; Catroli, G.F.; Hunt, M.; Hu, M.; Dailamy, A.; Pla, A.; Woller, S.A.; Palmer, N.; Parekh, U.; et al. Long-Lasting Analgesia via Targeted in Situ Repression of NaV1.7 in Mice. Sci. Transl. Med. 2021, 13, eaay9056. [Google Scholar] [CrossRef]
- Colasante, G.; Qiu, Y.; Massimino, L.; Di Berardino, C.; Cornford, J.H.; Snowball, A.; Weston, M.; Jones, S.P.; Giannelli, S.; Lieb, A.; et al. In Vivo CRISPRa Decreases Seizures and Rescues Cognitive Deficits in a Rodent Model of Epilepsy. Brain 2020, 143, 891–905. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and Regional Prevalence, Burden, and Risk Factors for Carotid Atherosclerosis: A Systematic Review, Meta-Analysis, and Modelling Study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (CVDs). World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 24 June 2025).
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In Vivo CRISPR Base Editing of PCSK9 Durably Lowers Cholesterol in Primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef]
- Thakore, P.I.; Kwon, J.B.; Nelson, C.E.; Rouse, D.C.; Gemberling, M.P.; Oliver, M.L.; Gersbach, C.A. RNA-Guided Transcriptional Silencing in Vivo with S. Aureus CRISPR-Cas9 Repressors. Nat. Commun. 2018, 9, 1674. [Google Scholar] [CrossRef]
- Schoger, E.; Carroll, K.J.; Iyer, L.M.; McAnally, J.R.; Tan, W.; Liu, N.; Noack, C.; Shomroni, O.; Salinas, G.; Groß, J.; et al. CRISPR-Mediated Activation of Endogenous Gene Expression in the Postnatal Heart. Circ. Res. 2020, 126, 6–24. [Google Scholar] [CrossRef]
- Alheib, O.; Da Silva, L.P.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Preclinical Research Studies for Treating Severe Muscular Injuries: Focus on Tissue-Engineered Strategies. Trends Biotechnol. 2023, 41, 632–652. [Google Scholar] [CrossRef]
- Karpov, D.S.; Sosnovtseva, A.O.; Pylina, S.V.; Bastrich, A.N.; Petrova, D.A.; Kovalev, M.A.; Shuvalova, A.I.; Eremkina, A.K.; Mokrysheva, N.G. Challenges of CRISPR/Cas-Based Cell Therapy for Type 1 Diabetes: How Not to Engineer a “Trojan Horse”. Int. J. Mol. Sci. 2023, 24, 17320. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Ishahak, M.; Millman, J.R. Developments in Stem Cell-Derived Islet Replacement Therapy for Treating Type 1 Diabetes. Cell Stem Cell 2023, 30, 530–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, Y.; Zhang, B.; Meng, G.; Liu, Z.; Liew, S.Y.; Liang, R.; Zhang, Z.; Cai, X.; Wu, S.; et al. Transplantation of Chemically Induced Pluripotent Stem-Cell-Derived Islets under Abdominal Anterior Rectus Sheath in a Type 1 Diabetes Patient. Cell 2024, 187, 6152–6164.e18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Y.; Yin, Z.; Ye, Z.; Qin, Y.; Cheng, Z.; Shen, Y.; Yin, Z.; Ma, J.; Tang, Y.; et al. Three-Dimensional Chromosomal Landscape Revealing miR-146a Dysfunctional Enhancer in Lupus and Establishing a CRISPR-Mediated Approach to Inhibit the Interferon Pathway. Arthritis Rheumatol. 2023, 76, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kanamori, M.; Someya, K.; Nakatsukasa, H.; Yoshimura, A. Stabilization of Foxp3 Expression by CRISPR-dCas9-Based Epigenome Editing in Mouse Primary T Cells. Epigenet. Chromatin 2017, 10, 24. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Ying, Y.; Wang, Y.; Arnold, P.R.; Wang, G.; Li, J.; Ghobrial, R.M.; Chen, W.; Xiao, X.; et al. Epigenetically Modifying the Foxp3 Locus for Generation of Stable Antigen-Specific Tregs as Cellular Therapeutics. Am. J. Transpl. 2020, 20, 2366–2379. [Google Scholar] [CrossRef]
- Sato, Y.; Passerini, L.; Piening, B.D.; Uyeda, M.J.; Goodwin, M.; Gregori, S.; Snyder, M.P.; Bertaina, A.; Roncarolo, M.; Bacchetta, R. Human-engineered Treg-like Cells Suppress FOXP3-deficient T Cells but Preserve Adaptive Immune Responses in Vivo. Clin. Transl. Immunol. 2020, 9, e1214. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From Mechanisms to Medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Xu, X.; Tan, X.; Tampe, B.; Wilhelmi, T.; Hulshoff, M.S.; Saito, S.; Moser, T.; Kalluri, R.; Hasenfuss, G.; Zeisberg, E.M.; et al. High-Fidelity CRISPR/Cas9- Based Gene-Specific Hydroxymethylation Rescues Gene Expression and Attenuates Renal Fibrosis. Nat. Commun. 2018, 9, 3509. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-Fidelity CRISPR–Cas9 Nucleases with No Detectable Genome-Wide off-Target Effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Aleksic, S.; Berger, D.M.; Sierra, F.; Kuchel, G.A.; Barzilai, N. Geroscience-guided Repurposing of FDA-approved Drugs to Target Aging: A Proposed Process and Prioritization. Aging Cell 2022, 21, e13596. [Google Scholar] [CrossRef]
- Gilmour, B.C.; Bergersen, L.H.; Fang, E.F. The Hallmarks of Aging. In Molecular, Cellular, and Metabolic Fundamentals of Human Aging; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–6. ISBN 978-0-323-91617-2. [Google Scholar]
- Aging Biomarker Consortium; Bao, H.; Cao, J.; Chen, M.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Y.; et al. Biomarkers of Aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar] [CrossRef] [PubMed]
- Liesenfelder, S.; Mabrouk, M.H.E.; Iliescu, J.; Baranda, M.V.; Mizi, A.; Wessiepe, M.; Papantonis, A.; Wagner, W. Epigenetic Editing at Individual Age-Associated CpGs Affects the Genome-Wide Epigenetic Aging Landscape. Nat. Aging. 2025, 5, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, Y.; Kim, S.; Chang, Y.; Kim, Y.; Kwon, Y.; Kim, J. Transcriptional Activation of Endogenous Oct4 via the CRISPR/dCas9 Activator Ameliorates Hutchinson-Gilford Progeria Syndrome in Mice. Aging Cell 2023, 22, e13825. [Google Scholar] [CrossRef]
- Chakravarti, R.; Lenka, S.K.; Gautam, A.; Singh, R.; Ravichandiran, V.; Roy, S.; Ghosh, D. A Review on CRISPR-Mediated Epigenome Editing: A Future Directive for Therapeutic Management of Cancer. Curr. Drug Targets 2022, 23, 836–853. Available online: http://www.eurekaselect.com (accessed on 24 June 2025).
- Garcia-Bloj, B.; Moses, C.; Sgro, A.; Plani-Lam, J.; Arooj, M.; Duffy, C.; Thiruvengadam, S.; Sorolla, A.; Rashwan, R.; Mancera, R.L.; et al. Waking up Dormant Tumor Suppressor Genes with Zinc Fingers, TALEs and the CRISPR/dCas9 System. Oncotarget 2016, 7, 60535–60554. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Shan, L.; Han, L.; Ma, S.; Zhang, Y.; Hao, B.; Lin, Y.; Rong, Z. Gene Activation in Human Cells Using CRISPR/Cpf1-P300 and CRISPR/Cpf1-SunTag Systems. Protein Cell 2018, 9, 380–383. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Cui, Y.; Lubecka, K.; Stefanska, B.; Irudayaraj, J. CRISPR-dCas9 Mediated TET1 Targeting for Selective DNA Demethylation at BRCA1 Promoter. Oncotarget 2016, 7, 46545–46556. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, L.; Wang, Y.; Deng, J.; Lin, Y.; Wang, Q.; Fang, C.; Ma, Z.; Wang, H.; Shi, G.; et al. Targeted Demethylation of the SARI Promotor Impairs Colon Tumour Growth. Cancer Lett. 2019, 448, 132–143. [Google Scholar] [CrossRef]
- Morita, S.; Noguchi, H.; Horii, T.; Nakabayashi, K.; Kimura, M.; Okamura, K.; Sakai, A.; Nakashima, H.; Hata, K.; Nakashima, K.; et al. Targeted DNA Demethylation in Vivo Using dCas9–Peptide Repeat and scFv–TET1 Catalytic Domain Fusions. Nat. Biotechnol. 2016, 34, 1060–1065. [Google Scholar] [CrossRef]
- Yoshida, M.; Yokota, E.; Sakuma, T.; Yamatsuji, T.; Takigawa, N.; Ushijima, T.; Yamamoto, T.; Fukazawa, T.; Naomoto, Y. Development of an Integrated CRISPRi Targeting ΔNp63 for Treatment of Squamous Cell Carcinoma. Oncotarget 2018, 9, 29220–29232. [Google Scholar] [CrossRef]
- Wang, H.; Guo, R.; Du, Z.; Bai, L.; Li, L.; Cui, J.; Li, W.; Hoffman, A.R.; Hu, J.-F. Epigenetic Targeting of Granulin in Hepatoma Cells by Synthetic CRISPR dCas9 Epi-Suppressors. Mol. Ther. Nucleic Acids 2018, 11, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.-F.; Chen, D.-S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.-L. Hepatitis B Virus Infection. Nat. Rev. Dis. Primers 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.-K.; Lo, Y.-R.; Pawlotsky, J.-M.; Yuen, M.-F. Chronic Hepatitis B Virus Infection. Lancet 2018, 392, 2313–2324. [Google Scholar] [CrossRef]
- Prescott, N.A.; Mansisidor, A.; Bram, Y.; Biaco, T.; Faulkner, S.C.; Lemmon, A.A.; Lim, C.; Koche, R.P.; Risca, V.I.; Schwartz, R.E.; et al. A Nucleosome Switch Primes Hepatitis B Virus Infection. bioRxiv 2024. [Google Scholar] [CrossRef]
- Luo, W.; Wang, J.; Xu, D.; Bai, H.; Zhang, Y.; Zhang, Y.; Li, X. Engineered Zinc-Finger Transcription Factors Inhibit the Replication and Transcription of HBV in Vitro and in Vivo. Int. J. Mol. Med. 2018, 41, 2169–2176. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Z.; Guo, J.; Huang, P.; Zhu, X.; Zhou, X.; Yang, Z.; Zhao, L.; Xu, L.; Xu, J.; et al. Creation of a Six-Fingered Artificial Transcription Factor That Represses the Hepatitis B Virus HBx Gene Integrated into a Human Hepatocellular Carcinoma Cell Line. J. Biomol. Screen. 2012, 17, 581–590. [Google Scholar] [CrossRef]
- Bloom, K.; Kaldine, H.; Cathomen, T.; Mussolino, C.; Ely, A.; Arbuthnot, P. Inhibition of Replication of Hepatitis B Virus Using Transcriptional Repressors That Target the Viral DNA. BMC Infect. Dis. 2019, 19, 802. [Google Scholar] [CrossRef]
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Ponomareva, N.; Bayurova, E.; Zakirova, N.; Kondrashova, A.; Goptar, I.; Nikiforova, A.; Sudina, A.; et al. Transient and Tunable CRISPRa Regulation of APOBEC/AID Genes for Targeting Hepatitis B Virus. Mol. Ther. Nucleic Acids 2023, 32, 478–493. [Google Scholar] [CrossRef]
- Espinosa-Medina, I.; Feliciano, D.; Belmonte-Mateos, C.; Linda Miyares, R.; Garcia-Marques, J.; Foster, B.; Lindo, S.; Pujades, C.; Koyama, M.; Lee, T. TEMPO Enables Sequential Genetic Labeling and Manipulation of Vertebrate Cell Lineages. Neuron 2023, 111, 345–361.e10. [Google Scholar] [CrossRef]
- Cosgrove, B. Epigenetic Editing for the Treatment of HBV. 2023. Available online: https://tunetx.com/wp-content/uploads/2023/12/HEPDART_Dec5_Web.pdf (accessed on 24 June 2025).
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Zerbato, J.M.; Purves, H.V.; Lewin, S.R.; Rasmussen, T.A. Between a Shock and a Hard Place: Challenges and Developments in HIV Latency Reversal. Curr. Opin. Virol. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bialek, J.K.; Dunay, G.A.; Voges, M.; Schäfer, C.; Spohn, M.; Stucka, R.; Hauber, J.; Lange, U.C. Targeted HIV-1 Latency Reversal Using CRISPR/Cas9-Derived Transcriptional Activator Systems. PLoS ONE 2016, 11, e0158294. [Google Scholar] [CrossRef]
- Ji, H.; Jiang, Z.; Lu, P.; Ma, L.; Li, C.; Pan, H.; Fu, Z.; Qu, X.; Wang, P.; Deng, J.; et al. Specific Reactivation of Latent HIV-1 by dCas9-SunTag-VP64-Mediated Guide RNA Targeting the HIV-1 Promoter. Mol. Ther. 2016, 24, 508–521. [Google Scholar] [CrossRef]
- Saayman, S.M.; Lazar, D.C.; Scott, T.A.; Hart, J.R.; Takahashi, M.; Burnett, J.C.; Planelles, V.; Morris, K.V.; Weinberg, M.S. Potent and Targeted Activation of Latent HIV-1 Using the CRISPR/dCas9 Activator Complex. Mol. Ther. 2016, 24, 488–498. [Google Scholar] [CrossRef]
- Keng, C.T.; Yogarajah, T.; Lee, R.C.H.; Muhammad, I.B.H.; Chia, B.S.; Vasandani, S.R.; Lim, D.S.; Guo, K.; Wong, Y.H.; Mok, C.K.; et al. AAV-CRISPR-Cas13 Eliminates Human Enterovirus and Prevents Death of Infected Mice. eBioMedicine 2023, 93, 104682. [Google Scholar] [CrossRef]
- Sokka, J.; Yoshihara, M.; Kvist, J.; Laiho, L.; Warren, A.; Stadelmann, C.; Jouhilahti, E.-M.; Kilpinen, H.; Balboa, D.; Katayama, S.; et al. CRISPR Activation Enables High-Fidelity Reprogramming into Human Pluripotent Stem Cells. Stem Cell Rep. 2022, 17, 413–426. [Google Scholar] [CrossRef]
- Xiong, K.; Zhou, Y.; Blichfeld, K.A.; Hyttel, P.; Bolund, L.; Freude, K.K.; Luo, Y. RNA-Guided Activation of Pluripotency Genes in Human Fibroblasts. Cell. Reprogram. 2017, 19, 189–198. [Google Scholar] [CrossRef]
- Balboa, D.; Weltner, J.; Eurola, S.; Trokovic, R.; Wartiovaara, K.; Otonkoski, T. Conditionally Stabilized dCas9 Activator for Controlling Gene Expression in Human Cell Reprogramming and Differentiation. Stem Cell Rep. 2015, 5, 448–459. [Google Scholar] [CrossRef]
- Weltner, J.; Balboa, D.; Katayama, S.; Bespalov, M.; Krjutškov, K.; Jouhilahti, E.-M.; Trokovic, R.; Kere, J.; Otonkoski, T. Human Pluripotent Reprogramming with CRISPR Activators. Nat. Commun. 2018, 9, 2643. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lin, C.-C.; Thomas, J.L.; Li, J.-A.; Lin, H.-Y. Cellular Reprogramming with Multigene Activation by the Delivery of CRISPR/dCas9 Ribonucleoproteins via Magnetic Peptide-Imprinted Chitosan Nanoparticles. Mater. Today Bio. 2021, 9, 100091. [Google Scholar] [CrossRef]
- Abujarour, R.; Dinella, J.; Pribadi, M.; Fong, L.K.; Denholtz, M.; Gutierrez, A.; Haynes, M.; Mahmood, E.; Lee, T.T.; Ding, S.; et al. A Chemical Approach Facilitates CRISPRa-Only Human iPSC Generation and Minimizes the Number of Targeted Loci Required. Future Sci. 2024, 10, FSO964. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Cabrera, D.; Hsiang-Chi Liou, R.; Lin, J.; Shi, Y.; Liu, K.; Hung, S.S.C.; Hewitt, A.W.; Wang, P.-Y.; Ching-Bong Wong, R. Combinatorial Approach of Binary Colloidal Crystals and CRISPR Activation to Improve Induced Pluripotent Stem Cell Differentiation into Neurons. ACS Appl. Mater. Interfaces 2022, 14, 8669–8679. [Google Scholar] [CrossRef] [PubMed]
- Black, J.B.; McCutcheon, S.R.; Dube, S.; Barrera, A.; Klann, T.S.; Rice, G.A.; Adkar, S.S.; Soderling, S.H.; Reddy, T.E.; Gersbach, C.A. Master Regulators and Cofactors of Human Neuronal Cell Fate Specification Identified by CRISPR Gene Activation Screens. Cell Rep. 2020, 33, 108460. [Google Scholar] [CrossRef]
- Schoger, E.; Zimmermann, W.-H.; Cyganek, L.; Zelarayán, L.C. Establishment of Two Homozygous CRISPR Interference (CRISPRi) Knock-in Human Induced Pluripotent Stem Cell (hiPSC) Lines for Titratable Endogenous Gene Repression. Stem Cell Res. 2021, 55, 102473. [Google Scholar] [CrossRef]
- Karbassi, E.; Padgett, R.; Bertero, A.; Reinecke, H.; Klaiman, J.M.; Yang, X.; Hauschka, S.D.; Murry, C.E. Targeted CRISPR Activation Is Functional in Engineered Human Pluripotent Stem Cells but Undergoes Silencing after Differentiation into Cardiomyocytes and Endothelium. Cell. Mol. Life Sci. 2024, 81, 95. [Google Scholar] [CrossRef]
- Qiao, A.; Wei, Y.; Liu, Y.; Kahn-Krell, A.; Ye, L.; Nguyen, T.; Zhang, J. Doxycycline-Mediated Control of Cyclin D2 Overexpression in Human-Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2024, 25, 8714. [Google Scholar] [CrossRef]
- Pfaff, N.; Lachmann, N.; Ackermann, M.; Kohlscheen, S.; Brendel, C.; Maetzig, T.; Niemann, H.; Antoniou, M.N.; Grez, M.; Schambach, A.; et al. A Ubiquitous Chromatin Opening Element Prevents Transgene Silencing in Pluripotent Stem Cells and Their Differentiated Progeny. Stem Cells 2013, 31, 488–499. [Google Scholar] [CrossRef]
- Guo, J.; Ma, D.; Huang, R.; Ming, J.; Ye, M.; Kee, K.; Xie, Z.; Na, J. An Inducible CRISPR-ON System for Controllable Gene Activation in Human Pluripotent Stem Cells. Protein Cell 2017, 8, 379–393. [Google Scholar] [CrossRef]
- Schoger, E.; Argyriou, L.; Zimmermann, W.-H.; Cyganek, L.; Zelarayán, L.C. Generation of Homozygous CRISPRa Human Induced Pluripotent Stem Cell (hiPSC) Lines for Sustained Endogenous Gene Activation. Stem Cell Res. 2020, 48, 101944. [Google Scholar] [CrossRef]
- Hazelbaker, D.Z.; Beccard, A.; Angelini, G.; Mazzucato, P.; Messana, A.; Lam, D.; Eggan, K.; Barrett, L.E. A Multiplexed gRNA piggyBac Transposon System Facilitates Efficient Induction of CRISPRi and CRISPRa in Human Pluripotent Stem Cells. Sci. Rep. 2020, 10, 635. [Google Scholar] [CrossRef]
- Tian, R.; Abarientos, A.; Hong, J.; Hashemi, S.H.; Yan, R.; Dräger, N.; Leng, K.; Nalls, M.A.; Singleton, A.B.; Xu, K.; et al. Genome-Wide CRISPRi/a Screens in Human Neurons Link Lysosomal Failure to Ferroptosis. Nat. Neurosci. 2021, 24, 1020–1034. [Google Scholar] [CrossRef] [PubMed]
- Furuhata, Y.; Nihongaki, Y.; Sato, M.; Yoshimoto, K. Control of Adipogenic Differentiation in Mesenchymal Stem Cells via Endogenous Gene Activation Using CRISPR-Cas9. ACS Synth. Biol. 2017, 6, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-N.; Liao, H.-T.; Truong, V.A.; Huang, K.-L.; Yu, F.-J.; Chen, H.-H.; Nguyen, T.K.N.; Makarevich, P.; Parfyonova, Y.; Hu, Y.-C. CRISPR-Based Activation of Endogenous Neurotrophic Genes in Adipose Stem Cell Sheets to Stimulate Peripheral Nerve Regeneration. Theranostics 2019, 9, 6099–6111. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xiao, J.; Huo, J.; Geng, Z.; Ma, K.; Sun, X.; Fu, X. Targeting Ectodysplasin Promotor by CRISPR/dCas9-Effector Effectively Induces the Reprogramming of Human Bone Marrow-Derived Mesenchymal Stem Cells into Sweat Gland-like Cells. Stem Cell Res. Ther. 2018, 9, 8. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; You, L.; Deng, T.; Pang, Q.; Meng, X.; Zhu, B. dCas9-Based PDGFR–β Activation ADSCs Accelerate Wound Healing in Diabetic Mice through Angiogenesis and ECM Remodeling. Int. J. Mol. Sci. 2023, 24, 5949. [Google Scholar] [CrossRef]
- Petazzi, P.; Torres-Ruiz, R.; Fidanza, A.; Roca-Ho, H.; Gutierrez-Agüera, F.; Castaño, J.; Rodriguez-Perales, S.; Díaz De La Guardia, R.; López-Millán, B.; Bigas, A.; et al. Robustness of Catalytically Dead Cas9 Activators in Human Pluripotent and Mesenchymal Stem Cells. Mol. Ther. Nucleic Acids 2020, 20, 196–204. [Google Scholar] [CrossRef]
- Shi, S.; Ge, Y.; Yan, Q.; Wan, S.; Li, M.; Li, M. Activating UCHL1 through the CRISPR Activation System Promotes Cartilage Differentiation Mediated by HIF-1α/SOX9. J. Cell. Mol. Med. 2024, 28, e70051. [Google Scholar] [CrossRef]
- Zhao, L.; Lai, Y.; Jiao, H.; Li, J.; Lu, K.; Huang, J. CRISPR-Mediated Sox9 Activation and RelA Inhibition Enhance Cell Therapy for Osteoarthritis. Mol. Ther. 2024, 32, 2549–2562. [Google Scholar] [CrossRef]
- Adisasmita, M.; Lee, H.K.; An, Y.; Kim, M.; Mamo, M.G.; Hur, J.K.; Choi, D.; Shin, J.H.; Jung, Y.K. Epigenetic Modulation Inhibits Epithelial-Mesenchymal Transition-Driven Fibrogenesis and Enhances Characteristics of Chemically-Derived Hepatic Progenitors. Ann. Surg. Treat. Res. 2024, 106, 274. [Google Scholar] [CrossRef]
- Perez-Garcia, V.; Lea, G.; Lopez-Jimenez, P.; Okkenhaug, H.; Burton, G.J.; Moffett, A.; Turco, M.Y.; Hemberger, M. BAP1/ASXL Complex Modulation Regulates Epithelial-Mesenchymal Transition during Trophoblast Differentiation and Invasion. eLife 2021, 10, e63254. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Shu, J.; Dadon, D.; Young, R.A.; Jaenisch, R. Editing DNA Methylation in the Mammalian Genome. Cell 2016, 167, 233–247.e17. [Google Scholar] [CrossRef] [PubMed]
- Black, J.B.; Adler, A.F.; Wang, H.-G.; D’Ippolito, A.M.; Hutchinson, H.A.; Reddy, T.E.; Pitt, G.S.; Leong, K.W.; Gersbach, C.A. Targeted Epigenetic Remodeling of Endogenous Loci by CRISPR/Cas9-Based Transcriptional Activators Directly Converts Fibroblasts to Neuronal Cells. Cell Stem Cell 2016, 19, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-K.; Fu, X.-B.; Sun, X.-Y. In-Vitro Construction of Engineered Sweat Gland Organoids. Jie Fang Jun Yi Xue Za Zhi 2020, 45, 384. [Google Scholar] [CrossRef]
- Alejandra, G.C.; Lucia, C.; Ho, H.S.; Luis, G.; Juan, R.P.; Federico, P.-B. Activation of Pancreatic β-Cell Genes by Multiplex Epigenetic CRISPR-Editing. bioRxiv 2020. [Google Scholar] [CrossRef]
- Alzhanuly, B.; Mukhatayev, Z.Y.; Botbayev, D.M.; Ashirbekov, Y.; Katkenov, N.D.; Dzhaynakbaev, N.T.; Sharipov, K.O. Modulation of Insulin Gene Expression with CRISPR/Cas9-Based Transcription Factors. Open Access Maced. J. Med. Sci. 2021, 9, 876–881. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Lin, C.-Y.; Li, Y.-C.E.; Lin, H.-Y. Nanoparticle-Mediated CRISPR/dCas9a Activation of Multiple Transcription Factors to Engineer Insulin-Producing Cells. J. Mater. Chem. B 2023, 11, 1866–1870. [Google Scholar] [CrossRef]
- Đorđević, M.; Stepper, P.; Feuerstein-Akgoz, C.; Gerhauser, C.; Paunović, V.; Tolić, A.; Rajić, J.; Dinić, S.; Uskoković, A.; Grdović, N.; et al. EpiCRISPR Targeted Methylation of Arx Gene Initiates Transient Switch of Mouse Pancreatic Alpha to Insulin-Producing Cells. Front. Endocrinol. 2023, 14, 1134478. [Google Scholar] [CrossRef]
- Roberston, M.J.; Raghunathan, S.; Potaman, V.N.; Zhang, F.; Stewart, M.D.; McConnell, B.K.; Schwartz, R.J. CRISPR-Cas9–Induced IGF1 Gene Activation as a Tool for Enhancing Muscle Differentiation via Multiple Isoform Expression. FASEB J. 2020, 34, 555–570. [Google Scholar] [CrossRef]
- Luo, N.; Li, J.; Chen, Y.; Xu, Y.; Wei, Y.; Lu, J.; Dong, R. Hepatic Stellate Cell Reprogramming via Exosome-Mediated CRISPR/dCas9-VP64 Delivery. Drug Deliv. 2021, 28, 10–18. [Google Scholar] [CrossRef]
- Joo, H.J.; Ma, D.J.; Hwang, J.S.; Shin, Y.J. SIRT1 Activation Using CRISPR/dCas9 Promotes Regeneration of Human Corneal Endothelial Cells through Inhibiting Senescence. Antioxidants 2020, 9, 1085. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Manoochehri, H.; Dama, P. Use of CAR T-Cell for Acute Lymphoblastic Leukemia (ALL) Treatment: A Review Study. Cancer Gene Ther. 2022, 29, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.; Riddell, S.; Maloney, D. CD19-Targeted Chimeric Antigen Receptor-modified T-cell Immunotherapy for B-cell Malignancies. Clin. Pharmacol. Ther. 2016, 100, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, M.; Mansilla-Soto, J.; Rivière, I.; Sadelain, M. Programming CAR T Cell Tumor Recognition: Tuned Antigen Sensing and Logic Gating. Cancer Discov. 2023, 13, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Fry, T.J. Mechanisms of Resistance to CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-Cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Watanabe, K.; Terakura, S.; Martens, A.C.; Van Meerten, T.; Uchiyama, S.; Imai, M.; Sakemura, R.; Goto, T.; Hanajiri, R.; Imahashi, N.; et al. Target Antigen Density Governs the Efficacy of Anti–CD20-CD28-CD3 ζ Chimeric Antigen Receptor–Modified Effector CD8+ T Cells. J. Immunol. 2015, 194, 911–920. [Google Scholar] [CrossRef]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Olive, D.; Kuchroo, V.; Zarour, H.M. CD8+ T Cells Specific for Tumor Antigens Can Be Rendered Dysfunctional by the Tumor Microenvironment through Upregulation of the Inhibitory Receptors BTLA and PD-1. Cancer Res. 2012, 72, 887–896. [Google Scholar] [CrossRef]
- Yang, Z.; Li, L.; Turkoz, A.; Chen, P.; Harari-Steinfeld, R.; Bobbin, M.; Stefanson, O.; Choi, H.; Pietrobon, V.; Alphson, B.; et al. Contextual Reprogramming of CAR-T Cells for Treatment of HER2+ Cancers. J. Transl. Med. 2021, 19, 459. [Google Scholar] [CrossRef]
- Yang, Z.; Pietrobon, V.; Bobbin, M.; Stefanson, O.; Yang, J.; Goswami, A.; Alphson, B.; Choi, H.; Magallanes, K.; Cai, Q.; et al. Nanoscale, Antigen Encounter-Dependent, IL-12 Delivery by CAR T Cells plus PD-L1 Blockade for Cancer Treatment. J. Transl. Med. 2023, 21, 158. [Google Scholar] [CrossRef]
- Smith, K.A. Interleukin-2: Inception, Impact, and Implications. Science 1988, 240, 1169–1176. [Google Scholar] [CrossRef]
- Lee, D.; Ahn, H.; Sim, H.-I.; Choi, E.; Choi, S.; Jo, Y.; Yun, B.; Song, H.K.; Oh, S.J.; Denda-Nagai, K.; et al. A CRISPR Activation Screen Identifies MUC-21 as Critical for Resistance to NK and T Cell-Mediated Cytotoxicity. J. Exp. Clin. Cancer Res. 2023, 42, 272. [Google Scholar] [CrossRef] [PubMed]
- Pacalin, N.M.; Steinhart, Z.; Shi, Q.; Belk, J.A.; Dorovskyi, D.; Kraft, K.; Parker, K.R.; Shy, B.R.; Marson, A.; Chang, H.Y. Bidirectional Epigenetic Editing Reveals Hierarchies in Gene Regulation. Nat. Biotechnol. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, P.; Abarientos, A.B.; Tian, R.; Seyler, M.; Leong, J.T.; Chen, M.; Choudhry, P.; Hechler, T.; Shah, N.; Wong, S.W.; et al. CRISPR-Based Screens Uncover Determinants of Immunotherapy Response in Multiple Myeloma. Blood Adv. 2020, 4, 2899–2911. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Nath Yogi, L. A Review on Recent Advances in Animal Biotechnology. Sci. Herit. J. 2023, 7, 79–82. [Google Scholar] [CrossRef]
- Gomez, J.A.; Beitnere, U.; Segal, D.J. Live-Animal Epigenome Editing: Convergence of Novel Techniques. Trends Genet. 2019, 35, 527–541. [Google Scholar] [CrossRef]
- Yosef, I.; Mahata, T.; Chen, Y.; Bar-Joseph, H.; Shalgi, R.; Munitz, A.; Gerlic, M.; Qimron, U. Engineering Mice for Female-Biased Progeny without Impacting Genetic Integrity and Litter Size. EMBO Rep. 2019, 20, e48269. [Google Scholar] [CrossRef]
- Yang, H.; Deng, M.; Lv, W.; Wei, Z.; Cai, Y.; Cheng, P.; Wang, F.; Zhang, Y. Overexpression of Bmp4, Dazl, Nanos3 and Sycp2 in Hu Sheep Leydig Cells Using CRISPR/Dcas9 System Promoted Male Germ Cell Related Gene Expression. Biology 2022, 11, 289. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Zhang, Y.; Ran, Y.; Qu, B.; Wang, C.; Zhao, F.; Zhang, L. dCas9-guided Demethylation of the AKT1 Promoter Improves Milk Protein Synthesis in a Bovine Mastitis Mammary Gland Epithelial Model Induced by Using Staphylococcus aureus. Cell Biol. Int. 2024, 48, 300–310. [Google Scholar] [CrossRef]
- Wang, K.; Ouyang, H.; Xie, Z.; Yao, C.; Guo, N.; Li, M.; Jiao, H.; Pang, D. Efficient Generation of Myostatin Mutations in Pigs Using the CRISPR/Cas9 System. Sci. Rep. 2015, 5, 16623. [Google Scholar] [CrossRef]
- You, W.; Li, M.; Qi, Y.; Wang, Y.; Chen, Y.; Liu, Y.; Li, L.; Ouyang, H.; Pang, D. CRISPR/Cas9-Mediated Specific Integration of Fat-1 and IGF-1 at the pRosa26 Locus. Genes 2021, 12, 1027. [Google Scholar] [CrossRef]
- Devlin, R.H.; Yesaki, T.Y.; Biagi, C.A.; Donaldson, E.M.; Swanson, P.; Chan, W.-K. Extraordinary Salmon Growth. Nature 1994, 371, 209–210. [Google Scholar] [CrossRef]
- Lai, L.; Kolber-Simonds, D.; Park, K.-W.; Cheong, H.-T.; Greenstein, J.L.; Im, G.-S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of α-1,3-Galactosyltransferase Knockout Pigs by Nuclear Transfer Cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Biotechnological Exploitation of Marine Animals. In Animal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 541–562. ISBN 978-0-12-416002-6. [Google Scholar]
- Masih, S.; Jain, P.; Baz, R.E.; Khan, Z.K. Transgenic Animals and Their Applications. In Animal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 407–423. ISBN 978-0-12-416002-6. [Google Scholar]
- Barel-Cohen, K.; Shore, L.S.; Shemesh, M.; Wenzel, A.; Mueller, J.; Kronfeld-Schor, N. Monitoring of Natural and Synthetic Hormones in a Polluted River. J. Environ. Manage. 2006, 78, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, E.; Cren-Olivé, C.; Grenier-Loustalot, M.-F. Occurrence of Pharmaceuticals and Hormones in Drinking Water Treated from Surface Waters. Environ. Chem. Lett. 2011, 9, 103–114. [Google Scholar] [CrossRef]
- Thiebaut, F.; Hemerly, A.S.; Ferreira, P.C.G. A Role for Epigenetic Regulation in the Adaptation and Stress Responses of Non-Model Plants. Front. Plant Sci. 2019, 10, 246. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.-K. Epigenetic Gene Regulation in Plants and Its Potential Applications in Crop Improvement. Nat. Rev. Mol. Cell Biol. 2024, 26, 51–67. [Google Scholar] [CrossRef]
- Miryeganeh, M.; Saze, H. Epigenetic Inheritance and Plant Evolution. Popul. Ecol. 2020, 62, 17–27. [Google Scholar] [CrossRef]
- Koornneef, M.; Hanhart, C.J.; van der Veen, J.H. A Genetic and Physiological Analysis of Late Flowering Mutants in Arabidopsis Thaliana. Mol. Gen. Genet. MGG 1991, 229, 57–66. [Google Scholar] [CrossRef]
- Chouard, P. Vernalization and Its Relations to Dormancy. Annu. Rev. Plant Biol. 1960, 11, 191–238. [Google Scholar] [CrossRef]
- Kim, D.-H.; Sung, S. Genetic and Epigenetic Mechanisms Underlying Vernalization. Arab. Book Am. Soc. Plant Biol. 2014, 12, e0171. [Google Scholar] [CrossRef]
- Nakamura, M.; Hennig, L. Inheritance of Vernalization Memory at FLOWERING LOCUS C during Plant Regeneration. J. Exp. Bot. 2017, 68, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, C.C.; Hills, M.J.; Lister, C.; Dean, C.; Dennis, E.S.; Peacock, W.J. Resetting of FLOWERING LOCUS C Expression after Epigenetic Repression by Vernalization. Proc. Natl. Acad. Sci. USA 2008, 105, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, E.J.; Genger, R.K.; Kovac, K.; Peacock, W.J.; Dennis, E.S. DNA Methylation and the Promotion of Flowering by Vernalization. Proc. Natl. Acad. Sci. USA 1998, 95, 5824–5829. [Google Scholar] [CrossRef] [PubMed]
- Pikaard, C.S.; Mittelsten Scheid, O. Epigenetic Regulation in Plants. Cold Spring Harb. Perspect. Biol. 2014, 6, a019315. [Google Scholar] [CrossRef]
- Debbarma, J.; Sarki, Y.N.; Saikia, B.; Boruah, H.P.D.; Singha, D.L.; Chikkaputtaiah, C. Ethylene Response Factor (ERF) Family Proteins in Abiotic Stresses and CRISPR–Cas9 Genome Editing of ERFs for Multiple Abiotic Stress Tolerance in Crop Plants: A Review. Mol. Biotechnol. 2019, 61, 153–172. [Google Scholar] [CrossRef]
- Karlson, C.K.S.; Mohd-Noor, S.N.; Nolte, N.; Tan, B.C. CRISPR/dCas9-Based Systems: Mechanisms and Applications in Plant Sciences. Plants 2021, 10, 2055. [Google Scholar] [CrossRef]
- Heyl, A.; Ramireddy, E.; Brenner, W.G.; Riefler, M.; Allemeersch, J.; Schmülling, T. The Transcriptional Repressor ARR1-SRDX Suppresses Pleiotropic Cytokinin Activities in Arabidopsis. Plant Physiol. 2008, 147, 1380–1395. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Belachew, A.; Ma, S.F.; Young, M.; Ade, J.; Shen, Y.; Marion, C.M.; Holtan, H.E.; Bailey, A.; Stone, J.K.; et al. The EDLL Motif: A Potent Plant Transcriptional Activation Domain from AP2/ERF Transcription Factors. Plant J. 2012, 70, 855–865. [Google Scholar] [CrossRef]
- Meyer, P. DNA Methylation Systems and Targets in Plants. FEBS Lett. 2011, 585, 2008–2015. [Google Scholar] [CrossRef]
- Morales-Ruiz, T.; Ortega-Galisteo, A.P.; Ponferrada-Marín, M.I.; Martínez-Macías, M.I.; Ariza, R.R.; Roldán-Arjona, T. DEMETER and REPRESSOR OF SILENCING 1 Encode 5-Methylcytosine DNA Glycosylases. Proc. Natl. Acad. Sci. USA 2006, 103, 6853–6858. [Google Scholar] [CrossRef]
- Ortega-Galisteo, A.P.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T. Arabidopsis DEMETER-LIKE Proteins DML2 and DML3 Are Required for Appropriate Distribution of DNA Methylation Marks. Plant Mol. Biol. 2008, 67, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Jacobsen, S.E. Epigenetic Modifications in Plants: An Evolutionary Perspective. Curr. Opin. Plant Biol. 2011, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Abdulraheem, M.I.; Xiong, Y.; Moshood, A.Y.; Cadenas-Pliego, G.; Zhang, H.; Hu, J. Mechanisms of Plant Epigenetic Regulation in Response to Plant Stress: Recent Discoveries and Implications. Plants 2024, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, H.; Liu, S.; Li, Z.; Yang, X.; Yan, J.; Li, J.; Tran, L.-S.P.; Qin, F. A Transposable Element in a NAC Gene Is Associated with Drought Tolerance in Maize Seedlings. Nat. Commun. 2015, 6, 8326. [Google Scholar] [CrossRef]
- Zhang, J.-B.; He, S.-P.; Luo, J.-W.; Wang, X.-P.; Li, D.-D.; Li, X.-B. A Histone Deacetylase, GhHDT4D, Is Positively Involved in Cotton Response to Drought Stress. Plant Mol. Biol. 2020, 104, 67–79. [Google Scholar] [CrossRef]
- Pathak, H.; Kumar, M.; Molla, K.A.; Chakraborty, K. Abiotic Stresses in Rice Production: Impacts and Management. ORYZA- Int. J. Rice 2021, 58, 103–125. [Google Scholar] [CrossRef]
- Crespo-Salvador, Ó.; Sánchez-Giménez, L.; López-Galiano, M.J.; Fernández-Crespo, E.; Scalschi, L.; García-Robles, I.; Rausell, C.; Real, M.D.; González-Bosch, C. The Histone Marks Signature in Exonic and Intronic Regions Is Relevant in Early Response of Tomato Genes to Botrytis Cinerea and in miRNA Regulation. Plants 2020, 9, 300. [Google Scholar] [CrossRef]
- Law, S.S.Y.; Liou, G.; Nagai, Y.; Giménez-Dejoz, J.; Tateishi, A.; Tsuchiya, K.; Kodama, Y.; Fujigaya, T.; Numata, K. Polymer-Coated Carbon Nanotube Hybrids with Functional Peptides for Gene Delivery into Plant Mitochondria. Nat. Commun. 2022, 13, 2417. [Google Scholar] [CrossRef]
- Mao, Y.; Botella, J.R.; Liu, Y.; Zhu, J.-K. Gene Editing in Plants: Progress and Challenges. Natl. Sci. Rev. 2019, 6, 421–437. [Google Scholar] [CrossRef]
- Okpe, A.O.; Nkaa, F.A. Comparative Review of Plant Transformation Techniques. J. Adv. Biol. Biotechnol. 2021, 1–18. [Google Scholar] [CrossRef]
- Charrier, A.; Vergne, E.; Dousset, N.; Richer, A.; Petiteau, A.; Chevreau, E. Efficient Targeted Mutagenesis in Apple and First Time Edition of Pear Using the CRISPR-Cas9 System. Front. Plant Sci. 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-H.; Yu, M.; Lai, E.-M. Agrobacterium-Mediated Plant Transformation: Biology and Applications. Arab. Book 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.A.; Naqvi, R.Z.; Rahman, S.U.; Amin, I.; Mansoor, S. Plant Virus-Derived Vectors for Plant Genome Engineering. Viruses 2023, 15, 531. [Google Scholar] [CrossRef]
- Jones, H.D.; Sparks, C.A. Stable Transformation of Plants. In Plant Genomics: Methods and Protocols; Gustafson, J.P., Langridge, P., Somers, D.J., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 111–130. ISBN 978-1-59745-427-8. [Google Scholar]
- Shohag, M.J.I.; Khan, F.Z.; Tang, L.; Wei, Y.; He, Z.; Yang, X. COVID-19 Crisis: How Can Plant Biotechnology Help? Plants 2021, 10, 352. [Google Scholar] [CrossRef]
- Prado, G.S.; Bamogo, P.K.A.; de Abreu, J.A.C.; Gillet, F.-X.; dos Santos, V.O.; Silva, M.C.M.; Brizard, J.-P.; Bemquerer, M.P.; Bangratz, M.; Brugidou, C.; et al. Nicotiana Benthamiana Is a Suitable Transient System for High-Level Expression of an Active Inhibitor of Cotton Boll Weevil α-Amylase. BMC Biotechnol. 2019, 19, 15. [Google Scholar] [CrossRef]
- Sheludko, Y.V.; Gerasymenko, I.M.; Warzecha, H. Transient Expression of Human Cytochrome P450s 2D6 and 3A4 in Nicotiana Benthamiana Provides a Possibility for Rapid Substrate Testing and Production of Novel Compounds. Biotechnol. J. 2018, 13, e1700696. [Google Scholar] [CrossRef]
- Canto, T. Transient Expression Systems in Plants: Potentialities and Constraints. In Advanced Technologies for Protein Complex Production and Characterization; Vega, M.C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 287–301. ISBN 978-3-319-27216-0. [Google Scholar]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-Free Genome Editing in Plants with Preassembled CRISPR-Cas9 Ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Haroon, M.; Wang, X.; Afzal, R.; Zafar, M.M.; Idrees, F.; Batool, M.; Khan, A.S.; Imran, M. Novel Plant Breeding Techniques Shake Hands with Cereals to Increase Production. Plants 2022, 11, 1052. [Google Scholar] [CrossRef]
- Piatek, A.; Ali, Z.; Baazim, H.; Li, L.; Abulfaraj, A.; Al-Shareef, S.; Aouida, M.; Mahfouz, M.M. RNA-guided Transcriptional Regulation in Planta via Synthetic dCas9-based Transcription Factors. Plant Biotechnol. J. 2015, 13, 578–589. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhang, D.; Baltes, N.J.; Paul, J.W., III; Tang, X.; Zheng, X.; Voytas, D.F.; Hsieh, T.-F.; Zhang, Y.; Qi, Y. A CRISPR/Cas9 Toolbox for Multiplexed Plant Genome Editing and Transcriptional Regulation. Plant Physiol. 2015, 169, 971–985. [Google Scholar] [CrossRef]
- Vazquez-Vilar, M.; Bernabé-Orts, J.M.; Fernandez-del-Carmen, A.; Ziarsolo, P.; Blanca, J.; Granell, A.; Orzaez, D. A Modular Toolbox for gRNA–Cas9 Genome Engineering in Plants Based on the GoldenBraid Standard. Plant Methods 2016, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, D.; Xiong, X.; Yan, B.; Xie, W.; Sheen, J.; Li, J.-F. A Potent Cas9-Derived Gene Activator for Plant and Mammalian Cells. Nat. Plants 2017, 3, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Roca Paixão, J.F.; Gillet, F.-X.; Ribeiro, T.P.; Bournaud, C.; Lourenço-Tessutti, I.T.; Noriega, D.D.; de Melo, B.P.; de Almeida-Engler, J.; Grossi-de-Sa, M.F. Improved Drought Stress Tolerance in Arabidopsis by CRISPR/dCas9 Fusion with a Histone AcetylTransferase. Sci. Rep. 2019, 9, 8080. [Google Scholar] [CrossRef] [PubMed]
- Zalatan, J.G.; Lee, M.E.; Almeida, R.; Gilbert, L.A.; Whitehead, E.H.; La Russa, M.; Tsai, J.C.; Weissman, J.S.; Dueber, J.E.; Qi, L.S.; et al. Engineering Complex Synthetic Transcriptional Programs with CRISPR RNA Scaffolds. Cell 2015, 160, 339–350. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhou, J.; Zhang, Y.; Malzahn, A.; Zhong, Z.; Hsieh, T.-F.; Voytas, D.F.; Zhang, Y.; Qi, Y. Robust Transcriptional Activation in Plants Using Multiplexed CRISPR-Act2.0 and mTALE-Act Systems. Mol. Plant 2018, 11, 245–256. [Google Scholar] [CrossRef]
- Pan, C.; Wu, X.; Markel, K.; Malzahn, A.A.; Kundagrami, N.; Sretenovic, S.; Zhang, Y.; Cheng, Y.; Shih, P.M.; Qi, Y. CRISPR–Act3.0 for Highly Efficient Multiplexed Gene Activation in Plants. Nat. Plants 2021, 7, 942–953. [Google Scholar] [CrossRef]
- Casas-Mollano, J.A.; Zinselmeier, M.H.; Sychla, A.; Smanski, M.J. Efficient Gene Activation in Plants by the MoonTag Programmable Transcriptional Activator. Nucleic Acids Res. 2023, 51, 7083–7093. [Google Scholar] [CrossRef]
- Zinselmeier, M.H.; Casas-Mollano, J.A.; Cors, J.; Sychla, A.; Heinsch, S.C.; Voytas, D.F.; Smanski, M.J. Optimized dCas9 Programmable Transcriptional Activators for Plants. Plant Biotechnol. J. 2024, 22, 3202. [Google Scholar] [CrossRef]
- Xu, L.; Sun, B.; Liu, S.; Gao, X.; Zhou, H.; Li, F.; Li, Y. The Evaluation of Active Transcriptional Repressor Domain for CRISPRi in Plants. Gene 2023, 851, 146967. [Google Scholar] [CrossRef]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q.; et al. A CRISPR–Cpf1 System for Efficient Genome Editing and Transcriptional Repression in Plants. Nat. Plants 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Ming, M.; Ren, Q.; Pan, C.; He, Y.; Zhang, Y.; Liu, S.; Zhong, Z.; Wang, J.; Malzahn, A.A.; Wu, J.; et al. CRISPR–Cas12b Enables Efficient Plant Genome Engineering. Nat. Plants 2020, 6, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sretenovic, S.; Fan, T.; Cheng, Y.; Li, G.; Qi, A.; Tang, X.; Xu, Y.; Guo, W.; Zhong, Z.; et al. Hypercompact CRISPR–Cas12j2 (CasΦ) Enables Genome Editing, Gene Activation, and Epigenome Editing in Plants. Plant Commun. 2022, 3, 100453. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Shen, R.; Shao, Y.; Tian, Y.; Han, P.; Zhang, X.; Zhu, J.-K.; Lu, Y. Efficient and Multiplex Gene Upregulation in Plants through CRISPR-Cas-Mediated Knockin of Enhancers. Mol. Plant 2024, 17, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Vázquez, L.A.; Méndez-García, A.; Chamu-García, V.; Rodríguez, A.L.; Bandyopadhyay, A.; Paul, S. The Applications of CRISPR/Cas-Mediated microRNA and lncRNA Editing in Plant Biology: Shaping the Future of Plant Non-Coding RNA Research. Planta 2023, 259, 32. [Google Scholar] [CrossRef]
- de Melo, B.P.; Lourenço-Tessutti, I.T.; Paixão, J.F.R.; Noriega, D.D.; Silva, M.C.M.; de Almeida-Engler, J.; Fontes, E.P.B.; Grossi-de-Sa, M.F. Transcriptional Modulation of AREB-1 by CRISPRa Improves Plant Physiological Performance under Severe Water Deficit. Sci. Rep. 2020, 10, 16231. [Google Scholar] [CrossRef]
- Park, J.-J.; Dempewolf, E.; Zhang, W.; Wang, Z.-Y. RNA-Guided Transcriptional Activation via CRISPR/dCas9 Mimics Overexpression Phenotypes in Arabidopsis. PLoS ONE 2017, 12, e0179410. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z.; Ding, X.; Alariqi, M.; Zhang, C.; Zhu, X.; Fan, S.; Zhu, L.; Zhang, X.; Jin, S. Developing an Efficient CRISPR–dCas9–TV-Derived Transcriptional Activation System to Create Three Novel Cotton Germplasm Materials. Plant Commun. 2023, 4, 100600. [Google Scholar] [CrossRef]
- Ren, C.; Li, H.; Liu, Y.; Li, S.; Liang, Z. Highly Efficient Activation of Endogenous Gene in Grape Using CRISPR/dCas9-Based Transcriptional Activators. Hortic. Res. 2022, 9, uhab037. [Google Scholar] [CrossRef]
- Valencia-Lozano, E.; Cabrera-Ponce, J.L.; Barraza, A.; López-Calleja, A.C.; García-Vázquez, E.; Rivera-Toro, D.M.; deFolter, S.; Alvarez-Venegas, R. Editing of SlWRKY29 by CRISPR-Activation Promotes Somatic Embryogenesis in Solanum Lycopersicum Cv. Micro-Tom. PLoS ONE 2024, 19, e0301169. [Google Scholar] [CrossRef]
- Karlson, C.K.S.; Mohd Noor, S.N.; Khalid, N.; Tan, B.C. CRISPRi-Mediated Down-Regulation of the Cinnamate-4-Hydroxylase (C4H) Gene Enhances the Flavonoid Biosynthesis in Nicotiana Tabacum. Biology 2022, 11, 1127. [Google Scholar] [CrossRef]
- He, J. Enhanced Expression of the Rice Gene OsCTF Using Epigenetic Regulation for Low Cadmium Planting in Rice. Sci. Technol. Eng. Chem. Environ. Prot. 2024, 1. [Google Scholar] [CrossRef]
- Bhatt, R.; Tiwari, B.S. CRISPRi/dCas9-KRAB Mediated Suppression of Solanidine Galactosyltransferase (Sgt1) in Solanum Tuberosum Leads to the Reduction in α-Solanine Level in Potato Tubers without Any Compensatory Effect in α-Chaconine. Biocatal. Agric. Biotechnol. 2024, 58, 103133. [Google Scholar] [CrossRef]
- Ghose, A.K.; Abdullah, S.N.A.; Md Hatta, M.A.; Megat Wahab, P.E. DNA Free CRISPR/DCAS9 Based Transcriptional Activation System for UGT76G1 Gene in Stevia Rebaudiana Bertoni Protoplasts. Plants 2022, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Bartolomé, J.; Gardiner, J.; Liu, W.; Papikian, A.; Ghoshal, B.; Kuo, H.Y.; Zhao, J.M.-C.; Segal, D.J.; Jacobsen, S.E. Targeted DNA Demethylation of the Arabidopsis Genome Using the Human TET1 Catalytic Domain. Proc. Natl. Acad. Sci. USA 2018, 115. [Google Scholar] [CrossRef]
- Ghoshal, B.; Vong, B.; Picard, C.L.; Feng, S.; Tam, J.M.; Jacobsen, S.E. A Viral Guide RNA Delivery System for CRISPR-Based Transcriptional Activation and Heritable Targeted DNA Demethylation in Arabidopsis Thaliana. PLoS Genet. 2020, 16, e1008983. [Google Scholar] [CrossRef]
- Papikian, A.; Liu, W.; Gallego-Bartolomé, J.; Jacobsen, S.E. Site-Specific Manipulation of Arabidopsis Loci Using CRISPR-Cas9 SunTag Systems. Nat. Commun. 2019, 10, 729. [Google Scholar] [CrossRef]
- Ghoshal, B.; Picard, C.L.; Vong, B.; Feng, S.; Jacobsen, S.E. CRISPR-Based Targeting of DNA Methylation in Arabidopsis Thaliana by a Bacterial CG-Specific DNA Methyltransferase. Proc. Natl. Acad. Sci. USA 2021, 118, e2125016118. [Google Scholar] [CrossRef]
- Lee, J.E.; Neumann, M.; Duro, D.I.; Schmid, M. CRISPR-Based Tools for Targeted Transcriptional and Epigenetic Regulation in Plants. PLoS ONE 2019, 14, e0222778. [Google Scholar] [CrossRef]
- Shin, H.; Choi, W.L.; Lim, J.Y.; Huh, J.H. Epigenome Editing: Targeted Manipulation of Epigenetic Modifications in Plants. Genes Genom. 2022, 44, 307–315. [Google Scholar] [CrossRef]
- Fal, K.; Masson, M.L.; Berr, A.; Carles, C.C. Manipulating Plant Development by Editing Histone Methylation with the dCas9 Tool: The CUC3 Boundary Gene as a Case Study. bioRxiv 2024. [Google Scholar] [CrossRef]
- Qi, X.; Gao, H.; Lv, R.; Mao, W.; Zhu, J.; Liu, C.; Mao, L.; Li, X.; Xie, C. CRISPR/dCas-Mediated Gene Activation Toolkit Development and Its Application for Parthenogenesis Induction in Maize. Plant Commun. 2023, 4, 100449. [Google Scholar] [CrossRef] [PubMed]
- García-Murillo, L.; Valencia-Lozano, E.; Priego-Ranero, N.A.; Cabrera-Ponce, J.L.; Duarte-Aké, F.P.; Vizuet-de-Rueda, J.C.; Rivera-Toro, D.M.; Herrera-Ubaldo, H.; de Folter, S.; Alvarez-Venegas, R. CRISPRa-Mediated Transcriptional Activation of the SlPR-1 Gene in Edited Tomato Plants. Plant Sci. 2023, 329, 111617. [Google Scholar] [CrossRef] [PubMed]
- Gentzel, I.N.; Park, C.H.; Bellizzi, M.; Xiao, G.; Gadhave, K.R.; Murphree, C.; Yang, Q.; LaMantia, J.; Redinbaugh, M.G.; Balint-Kurti, P.; et al. A CRISPR/dCas9 Toolkit for Functional Analysis of Maize Genes. Plant Methods 2020, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, L.; Li, F.; Li, Y. Transcriptional Regulation by CRISPR/dCas9 in Common Wheat. Gene 2022, 807, 145919. [Google Scholar] [CrossRef]
- Hooghvorst, I.; Altpeter, F. dCas9-3xSRDX-Mediated Transcriptional Repression in Sugarcane. Plant Cell Rep. 2023, 42, 1837–1840. [Google Scholar] [CrossRef]
- Guo, T.; Lu, Z.-Q.; Shan, J.-X.; Ye, W.-W.; Dong, N.-Q.; Lin, H.-X. ERECTA1 Acts Upstream of the OsMKKK10-OsMKK4-OsMPK6 Cascade to Control Spikelet Number by Regulating Cytokinin Metabolism in Rice. Plant Cell 2020, 32, 2763. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Yuan, D.; Liu, Y.; Che, R.; Hu, Y.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H.; et al. Expression of the Nitrate Transporter Gene OsNRT1.1A/OsNPF6.3 Confers High Yield and Early Maturation in Rice. Plant Cell 2018, 30, 638. [Google Scholar] [CrossRef]
- Tian, L. Recent Advances in Understanding Carotenoid-Derived Signaling Molecules in Regulating Plant Growth and Development. Front. Plant Sci. 2015, 6, 790. [Google Scholar] [CrossRef]
- Hudson, A.; Carpenter, R.; Doyle, S.; Coen, E.S. Olive: A Key Gene Required for Chlorophyll Biosynthesis in Antirrhinum Majus. EMBO J. 1993, 12, 3711. [Google Scholar] [CrossRef]
- Komninakas, I. Enhancing Tomato Fruit Quality: Application of CRISPR/dCas9-Mediated Methylation on the PG Gene Promoter Region. Holster Scholar Projects. 2023. Available online: https://digitalcommons.lib.uconn.edu/srhonors_holster/53/ (accessed on 24 June 2025).
- Damodharan, S.; Corem, S.; Gupta, S.K.; Arazi, T. Tuning of SlARF10A Dosage by Sly-miR160a Is Critical for Auxin-Mediated Compound Leaf and Flower Development. Plant J. Cell Mol. Biol. 2018, 96, 855–868. [Google Scholar] [CrossRef]
- Uranga, M.; Daròs, J.-A. Tools and Targets: The Dual Role of Plant Viruses in CRISPR-Cas Genome Editing. Plant Genome 2023, 16, e20220. [Google Scholar] [CrossRef] [PubMed]
- Fullwood, M.J.; Wei, C.-L.; Liu, E.T.; Ruan, Y. Next-Generation DNA Sequencing of Paired-End Tags (PET) for Transcriptome and Genome Analyses. Genome Res. 2009, 19, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Stalidzans, E.; Dace, E. Sustainable Metabolic Engineering for Sustainability Optimisation of Industrial Biotechnology. Comput. Struct. Biotechnol. J. 2021, 19, 4770–4776. [Google Scholar] [CrossRef]
- Goel, A.; Wortel, M.T.; Molenaar, D.; Teusink, B. Metabolic Shifts: A Fitness Perspective for Microbial Cell Factories. Biotechnol. Lett. 2012, 34, 2147–2160. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae Biorefinery: High Value Products Perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- de Souza, F.M.; Gupta, R.K. Bacteria for Bioplastics: Progress, Applications, and Challenges. ACS Omega 2024, 9, 8666–8686. [Google Scholar] [CrossRef]
- Koppolu, V.; Vasigala, V.K. Role of Escherichia coli in Biofuel Production. Microbiol. Insights 2016. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus Subtilis: A Universal Cell Factory for Industry, Agriculture, Biomaterials and Medicine. Microb. Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef]
- Cairns, T.C.; Nai, C.; Meyer, V. How a Fungus Shapes Biotechnology: 100 Years of Aspergillus Niger Research. Fungal Biol. Biotechnol. 2018, 5, 13. [Google Scholar] [CrossRef]
- Al-lwayzy, S.H.; Yusaf, T.; Al-Juboori, R.A. Biofuels from the Fresh Water Microalgae Chlorella Vulgaris (FWM-CV) for Diesel Engines. Energies 2014, 7, 1829–1851. [Google Scholar] [CrossRef]
- Kubik, S.; Bruzzone, M.J.; Shore, D. Establishing Nucleosome Architecture and Stability at Promoters: Roles of Pioneer Transcription Factors and the RSC Chromatin Remodeler. BioEssays 2017, 39, 1600237. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces Cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Donohoue, P.D.; Barrangou, R.; May, A.P. Advances in Industrial Biotechnology Using CRISPR-Cas Systems. Trends Biotechnol. 2018, 36, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Kang, J.W.; Kang, A.; Lee, T.S. Redirecting Metabolic Flux via Combinatorial Multiplex CRISPRi-Mediated Repression for Isopentenol Production in Escherichia coli. ACS Synth. Biol. 2019, 8, 391–402. [Google Scholar] [CrossRef]
- Kim, J.; Lee, T.S. Enhancing Isoprenol Production by Systematically Tuning Metabolic Pathways Using CRISPR Interference in E. coli. Front. Bioeng. Biotechnol. 2023, 11, 1296132. [Google Scholar] [CrossRef]
- Cleto, S.; Jensen, J.V.; Wendisch, V.F.; Lu, T.K. Corynebacterium Glutamicum Metabolic Engineering with CRISPR Interference (CRISPRi). ACS Synth. Biol. 2016, 5, 375–385. [Google Scholar] [CrossRef]
- Kaczmarzyk, D.; Cengic, I.; Yao, L.; Hudson, E.P. Diversion of the Long-Chain Acyl-ACP Pool in Synechocystis to Fatty Alcohols through CRISPRi Repression of the Essential Phosphate Acyltransferase PlsX. Metab. Eng. 2018, 45, 59–66. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, T.; Liu, Y.; Tian, R.; Lv, X.; Li, J.; Du, G.; Chen, J.; Ledesma-Amaro, R.; Liu, L. Design of a Programmable Biosensor-CRISPRi Genetic Circuits for Dynamic and Autonomous Dual-Control of Metabolic Flux in Bacillus Subtilis. Nucleic Acids Res. 2020, 48, 996–1009. [Google Scholar] [CrossRef]
- Peng, R.; Wang, Y.; Feng, W.; Yue, X.; Chen, J.; Hu, X.; Li, Z.; Sheng, D.; Zhang, Y.; Li, Y. CRISPR/dCas9-Mediated Transcriptional Improvement of the Biosynthetic Gene Cluster for the Epothilone Production in Myxococcus Xanthus. Microb. Cell Factories 2018, 17, 15. [Google Scholar] [CrossRef]
- Arber, W. DNA Modification and Restriction. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 1974; Volume 14, pp. 1–37. ISBN 978-0-12-540014-5. [Google Scholar]
- Vasu, K.; Nagaraja, V. Diverse Functions of Restriction-Modification Systems in Addition to Cellular Defense. Microbiol. Mol. Biol. Rev. 2013, 77, 53–72. [Google Scholar] [CrossRef]
- Beaulaurier, J.; Schadt, E.E.; Fang, G. Deciphering Bacterial Epigenomes Using Modern Sequencing Technologies. Nat. Rev. Genet. 2019, 20, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Marinus, M.G.; Løbner-Olesen, A. DNA Methylation. EcoSal. Plus 2014, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-L.; Gao, W.-L.; Xu, W.-F.; Lyu, Z.-Y.; Ma, L.; Luo, S.; Chen, X.-A.; Mao, X.-M.; Li, Y.-Q. m4C DNA Methylation Regulates Biosynthesis of Daptomycin in Streptomyces Roseosporus L30. Synth. Syst. Biotechnol. 2022, 7, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Won, C.; Yim, S.S. Emerging Methylation-Based Approaches in Microbiome Engineering. Biotechnol. Biofuels Bioprod. 2024, 17, 96. [Google Scholar] [CrossRef]
- Smith, J.D.; Suresh, S.; Schlecht, U.; Wu, M.; Wagih, O.; Peltz, G.; Davis, R.W.; Steinmetz, L.M.; Parts, L.; St.Onge, R.P. Quantitative CRISPR Interference Screens in Yeast Identify Chemical-Genetic Interactions and New Rules for Guide RNA Design. Genome Biol. 2016, 17, 45. [Google Scholar] [CrossRef]
- Ciurkot, K.; Gorochowski, T.E.; Roubos, J.A.; Verwaal, R. Efficient Multiplexed Gene Regulation in Saccharomyces cerevisiae Using dCas12a. Nucleic Acids Res. 2021, 49, 7775–7790. [Google Scholar] [CrossRef]
- Ferreira, R.; Skrekas, C.; Hedin, A.; Sánchez, B.J.; Siewers, V.; Nielsen, J.; David, F. Model-Assisted Fine-Tuning of Central Carbon Metabolism in Yeast through dCas9-Based Regulation. ACS Synth. Biol. 2019, 8, 2457–2463. [Google Scholar] [CrossRef]
- Zhai, H.; Cui, L.; Xiong, Z.; Qi, Q.; Hou, J. CRISPR-Mediated Protein-Tagging Signal Amplification Systems for Efficient Transcriptional Activation and Repression in Saccharomyces cerevisiae. Nucleic Acids Res. 2022, 50, 5988–6000. [Google Scholar] [CrossRef]
- Jensen, E.D.; Ferreira, R.; Jakočiūnas, T.; Arsovska, D.; Zhang, J.; Ding, L.; Smith, J.D.; David, F.; Nielsen, J.; Jensen, M.K.; et al. Transcriptional Reprogramming in Yeast Using dCas9 and Combinatorial gRNA Strategies. Microb. Cell Factories 2017, 16, 46. [Google Scholar] [CrossRef]
- Alland, L.; Muhle, R.; Hou, H.; Potes, J.; Chin, L.; Schreiber-Agus, N.; DePinho, R.A. Role for N-CoR and Histone Deacetylase in Sin3-Mediated Transcriptional Repression. Nature 1997, 387, 49–55. [Google Scholar] [CrossRef]
- Muñoz-Miranda, L.A.; Zepeda-Peña, A.C.; Casas-Godoy, L.; Pereira-Santana, A.; Méndez-Zamora, A.; Barrera-Martínez, I.; Rodríguez-Zapata, L.; Gschaedler-Mathis, A.C.; Figueroa-Yáñez, L.J. CRISPRi-Induced Transcriptional Regulation of IAH1 Gene and Its Influence on Volatile Compounds Profile in Kluyveromyces Marxianus DU3. World J. Microbiol. Biotechnol. 2024, 40, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, L.; Pan, L.; Wang, B.; Pan, L. CRISPR/dCas9-Mediated Epigenetic Modification Reveals Differential Regulation of Histone Acetylation on Aspergillus Niger Secondary Metabolite. Microbiol. Res. 2021, 245, 126694. [Google Scholar] [CrossRef] [PubMed]
- Shafreen, M.; Mukesh, K.; Saleena, L.M. Microbial Cell Factories for Melanin Production: Progress and Opportunities for Industrial Production. Biologia 2024, 79, 1461–1470. [Google Scholar] [CrossRef]
- Preciado, S.; Mendive-Tapia, L.; Torres-García, C.; Zamudio-Vázquez, R.; Soto-Cerrato, V.; Pérez-Tomás, R.; Albericio, F.; Nicolás, E.; Lavilla, R. Synthesis and Biological Evaluation of a Post-Synthetically Modified Trp-Based Diketopiperazine. MedChemComm 2013, 4, 1171. [Google Scholar] [CrossRef]
- Ben Ameur Mehdi, R.; Shaaban, K.A.; Rebai, I.K.; Smaoui, S.; Bejar, S.; Mellouli, L. Five Naturally Bioactive Molecules Including Two Rhamnopyranoside Derivatives Isolated from the Streptomyces Sp. Strain TN58. Nat. Prod. Res. 2009, 23, 1095–1107. [Google Scholar] [CrossRef]
- Roux, I.; Woodcraft, C.; Hu, J.; Wolters, R.; Gilchrist, C.L.M.; Chooi, Y.-H. CRISPR-Mediated Activation of Biosynthetic Gene Clusters for Bioactive Molecule Discovery in Filamentous Fungi. ACS Synth. Biol. 2020, 9, 1843–1854. [Google Scholar] [CrossRef]
- Kao, P.-H.; Ng, I.-S. CRISPRi Mediated Phosphoenolpyruvate Carboxylase Regulation to Enhance the Production of Lipid in Chlamydomonas Reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef]
- Lin, J.; Lin, W.; Ng, I. CRISPRa/i with Adaptive Single Guide Assisted Regulation DNA (ASGARD) Mediated Control of Chlorella sorokiniana to Enhance Lipid and Protein Production. Biotechnol. J. 2022, 17, 2100514. [Google Scholar] [CrossRef]
- Shaytan, A.K.; Novikov, R.V.; Vinnikov, R.S.; Gribkova, A.K.; Glukhov, G.S. From DNA-Protein Interactions to the Genetic Circuit Design Using CRISPR-dCas Systems. Front. Mol. Biosci. 2022, 9, 1070526. [Google Scholar] [CrossRef]
- Modell, A.E.; Lim, D.; Nguyen, T.M.; Sreekanth, V.; Choudhary, A. CRISPR-Based Therapeutics: Current Challenges and Future Applications. Trends Pharmacol. Sci. 2022, 43, 151–161. [Google Scholar] [CrossRef]
- Policarpi, C.; Munafò, M.; Tsagkris, S.; Carlini, V.; Hackett, J.A. Systematic Epigenome Editing Captures the Context-Dependent Instructive Function of Chromatin Modifications. Nat. Genet. 2024, 56, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Hofacker, D.; Broche, J.; Laistner, L.; Adam, S.; Bashtrykov, P.; Jeltsch, A. Engineering of Effector Domains for Targeted DNA Methylation with Reduced Off-Target Effects. Int. J. Mol. Sci. 2020, 21, 502. [Google Scholar] [CrossRef] [PubMed]
- Labuhn, M.; Adams, F.F.; Ng, M.; Knoess, S.; Schambach, A.; Charpentier, E.M.; Schwarzer, A.; Mateo, J.L.; Klusmann, J.-H.; Heckl, D. Refined sgRNA Efficacy Prediction Improves Large- and Small-Scale CRISPR–Cas9 Applications. Nucleic Acids Res. 2018, 46, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, M.; Thumberger, T.; Del Sol Keyer, M.; Wittbrodt, J.; Mateo, J.L. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS ONE 2015, 10, e0124633. [Google Scholar] [CrossRef]
- Xu, X.; Gao, J.; Dai, W.; Wang, D.; Wu, J.; Wang, J. Gene Activation by a CRISPR-Assisted Trans Enhancer. eLife 2019, 8, e45973. [Google Scholar] [CrossRef]
- Li, K.; Liu, Y.; Cao, H.; Zhang, Y.; Gu, Z.; Liu, X.; Yu, A.; Kaphle, P.; Dickerson, K.E.; Ni, M.; et al. Interrogation of Enhancer Function by Enhancer-Targeting CRISPR Epigenetic Editing. Nat. Commun. 2020, 11, 485. [Google Scholar] [CrossRef]
- O’Geen, H.; Ren, C.; Nicolet, C.M.; Perez, A.A.; Halmai, J.; Le, V.M.; Mackay, J.P.; Farnham, P.J.; Segal, D.J. dCas9-Based Epigenome Editing Suggests Acquisition of Histone Methylation Is Not Sufficient for Target Gene Repression. Nucleic Acids Res. 2017, 45, 9901–9916. [Google Scholar] [CrossRef]
- Sanson, K.R.; Hanna, R.E.; Hegde, M.; Donovan, K.F.; Strand, C.; Sullender, M.E.; Vaimberg, E.W.; Goodale, A.; Root, D.E.; Piccioni, F.; et al. Optimized Libraries for CRISPR-Cas9 Genetic Screens with Multiple Modalities. Nat. Commun. 2018, 9, 5416. [Google Scholar] [CrossRef]
- Savell, K.E.; Bach, S.V.; Zipperly, M.E.; Revanna, J.S.; Goska, N.A.; Tuscher, J.J.; Duke, C.G.; Sultan, F.A.; Burke, J.N.; Williams, D.; et al. A Neuron-Optimized CRISPR/dCas9 Activation System for Robust and Specific Gene Regulation. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Mu, W.; Luo, T.; Barrera, A.; Bounds, L.R.; Klann, T.S.; Weele, M.T.; Bryois, J.; Crawford, G.E.; Sullivan, P.F.; Gersbach, C.A.; et al. Machine Learning Methods for Predicting Guide RNA Effects in CRISPR Epigenome Editing Experiments. bioRxiv 2024. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, L.; Meng, J.; Ma, L.; Zuo, E.; Sun, Y. EpiCas-DL: Predicting sgRNA Activity for CRISPR-Mediated Epigenome Editing by Deep Learning. Comput. Struct. Biotechnol. J. 2023, 21, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, S.; Zhu, R.; Lu, C.; Xu, X.; Li, C.; Huang, X.; Zhao, X.; Mao, F.; Li, K. CRISPRepi: A Multi-Omic Atlas for CRISPR-Based Epigenome Editing. Nucleic Acids Res. 2025, 53, D901–D913. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.S.; Cabrera, A.; Spence, J.P.; Goell, J.; Anand, S.S.; Hilton, I.B.; Song, Y.S. Predicting the Effect of CRISPR-Cas9-Based Epigenome Editing. bioRxiv 2023. [Google Scholar] [CrossRef]
- Beyersdorf, J.P.; Bawage, S.; Iglesias, N.; Peck, H.E.; Hobbs, R.A.; Wroe, J.A.; Zurla, C.; Gersbach, C.A.; Santangelo, P.J. Robust, Durable Gene Activation In Vivo via mRNA-Encoded Activators. ACS Nano 2022, 16, 5660–5671. [Google Scholar] [CrossRef]
- Cappelluti, M.A.; Mollica Poeta, V.; Valsoni, S.; Quarato, P.; Merlin, S.; Merelli, I.; Lombardo, A. Durable and Efficient Gene Silencing in Vivo by Hit-and-Run Epigenome Editing. Nature 2024, 627, 416–423. [Google Scholar] [CrossRef]
- Tremblay, F.; Xiong, Q.; Shah, S.S.; Ko, C.-W.; Kelly, K.; Morrison, M.S.; Giancarlo, C.; Ramirez, R.N.; Hildebrand, E.M.; Voytek, S.B.; et al. A Potent Epigenetic Editor Targeting Human PCSK9 for Durable Reduction of Low-Density Lipoprotein Cholesterol Levels. Nat. Med. 2025, 31, 1329–1338. [Google Scholar] [CrossRef]
- Du, Y.; Liu, Y.; Hu, J.; Peng, X.; Liu, Z. CRISPR/Cas9 Systems: Delivery Technologies and Biomedical Applications. Asian J. Pharm. Sci. 2023, 18, 100854. [Google Scholar] [CrossRef]
- Sari-Ak, D.; Alomari, O.; Shomali, R.; Lim, J.; Thimiri Govinda Raj, D. Advances in CRISPR-Cas9 for the Baculovirus Vector System: A Systematic Review. Viruses 2022, 15, 54. [Google Scholar] [CrossRef]
- Duan, D.; Sharma, P.; Yang, J.; Yue, Y.; Dudus, L.; Zhang, Y.; Fisher, K.J.; Engelhardt, J.F. Circular Intermediates of Recombinant Adeno-Associated Virus Have Defined Structural Characteristics Responsible for Long-Term Episomal Persistence in Muscle Tissue. J. Virol. 1998, 72, 8568–8577. [Google Scholar] [CrossRef]
- Chandler, R.J.; Sands, M.S.; Venditti, C.P. Recombinant Adeno-Associated Viral Integration and Genotoxicity: Insights from Animal Models. Hum. Gene Ther. 2017, 28, 314–322. [Google Scholar] [CrossRef]
- Song, R.; Pekrun, K.; Khan, T.A.; Zhang, F.; Paşca, S.P.; Kay, M.A. Selection of rAAV Vectors That Cross the Human Blood-Brain Barrier and Target the Central Nervous System Using a Transwell Model. Mol. Ther. Methods Clin. Dev. 2022, 27, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Calcedo, R.; Vandenberghe, L.H.; Gao, G.; Lin, J.; Wilson, J.M. Worldwide Epidemiology of Neutralizing Antibodies to Adeno-Associated Viruses. J. Infect. Dis. 2009, 199, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Lu, C.-H.; Luo, W.-Y.; Chang, Y.-H.; Sung, L.-Y.; Chiu, H.-Y.; Hu, Y.-C. Baculovirus as a Gene Delivery Vector for Cartilage and Bone Tissue Engineering. Curr. Gene Ther. 2010, 10, 242–254. [Google Scholar] [CrossRef]
- Covo-Vergara, Á.; Salaberry, L.; Silva-Pilipich, N.; Hervas-Stubbs, S.; Smerdou, C. Cell-Specific Delivery of CRISPR-Cas9 with Pseudotyped Lentiviral Particles: Just Change the Envelope. Mol. Ther. Nucleic Acids 2024, 35, 102395. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, J.; Li, D.; Cheng, Y. Strategies in the Delivery of Cas9 Ribonucleoprotein for CRISPR/Cas9 Genome Editing. Theranostics 2021, 11, 614–648. [Google Scholar] [CrossRef]
- Chen, K.; Han, H.; Zhao, S.; Xu, B.; Yin, B.; Lawanprasert, A.; Trinidad, M.; Burgstone, B.W.; Murthy, N.; Doudna, J.A. Lung and Liver Editing by Lipid Nanoparticle Delivery of a Stable CRISPR–Cas9 Ribonucleoprotein. Nat. Biotechnol. 2024, 1–13. [Google Scholar] [CrossRef]
- George, C.A.; Sahu, S.U.; De Oñate, L.; Souza, B.S.D.F.; Wilson, R.C. Genome Editing Therapy for the Blood: Ex Vivo Success and In Vivo Prospects. CRISPR J. 2024, 7, 231–248. [Google Scholar] [CrossRef]
- Schmidt, T.; Wiesbeck, M.; Egert, L.; Truong, T.-T.; Danese, A.; Voshagen, L.; Imhof, S.; Iraci Borgia, M.; Deeksha; Neuner, A.M.; et al. Efficient DNA- and Virus-Free Engineering of Cellular Transcriptomic States Using dCas9 Ribonucleoprotein (dRNP) Complexes. Nucleic Acids Res. 2025, 53, gkaf235. [Google Scholar] [CrossRef]
- Ewaisha, R.; Anderson, K.S. Immunogenicity of CRISPR Therapeutics—Critical Considerations for Clinical Translation. Front. Bioeng. Biotechnol. 2023, 11, 1138596. [Google Scholar] [CrossRef]
- Hakim, C.H.; Kumar, S.R.P.; Pérez-López, D.O.; Wasala, N.B.; Zhang, D.; Yue, Y.; Teixeira, J.; Pan, X.; Zhang, K.; Million, E.D.; et al. Cas9-Specific Immune Responses Compromise Local and Systemic AAV CRISPR Therapy in Multiple Dystrophic Canine Models. Nat. Commun. 2021, 12, 6769. [Google Scholar] [CrossRef]
- Lek, A.; Wong, B.; Keeler, A.; Blackwood, M.; Ma, K.; Huang, S.; Sylvia, K.; Batista, A.R.; Artinian, R.; Kokoski, D.; et al. Death after High-Dose rAAV9 Gene Therapy in a Patient with Duchenne’s Muscular Dystrophy. N. Engl. J. Med. 2023, 389, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Duan, D. Lethal Immunotoxicity in High-Dose Systemic AAV Therapy. Mol. Ther. 2023, 31, 3123–3126. [Google Scholar] [CrossRef] [PubMed]
- Goell, J.H.; Li, J.; Mahata, B.; Ma, A.J.; Kim, S.; Shah, S.; Shah, S.; Contreras, M.; Misra, S.; Reed, D.; et al. Tailoring a CRISPR/Cas-Based Epigenome Editor for Programmable Chromatin Acylation and Decreased Cytotoxicity. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zeps, N.; Lysaght, T.; Chadwick, R.; Erler, A.; Foo, R.; Giordano, S.; Lai, P.S.; Schaefer, G.O.; Xafis, V.; Chew, W.L.; et al. Ethics and Regulatory Considerations for the Clinical Translation of Somatic Cell Human Epigenetic Editing. Stem Cell Rep. 2021, 16, 1652–1655. [Google Scholar] [CrossRef]
- Huerne, K.; Palmour, N.; Wu, A.R.; Beck, S.; Berner, A.; Siebert, R.; Joly, Y. Auditing the Editor: A Review of Key Translational Issues in Epigenetic Editing. CRISPR J. 2022, 5, 203–212. [Google Scholar] [CrossRef]
- Alex, K.; Winkler, E.C. Comparative Ethical Evaluation of Epigenome Editing and Genome Editing in Medicine: First Steps and Future Directions. J. Med. Ethics 2024, 50, 398–406. [Google Scholar] [CrossRef]
- Perez, M.F.; Lehner, B. Intergenerational and Transgenerational Epigenetic Inheritance in Animals. Nat. Cell Biol. 2019, 21, 143–151. [Google Scholar] [CrossRef]
- Center for Biologics Evaluation and Research. Preclinical Assessment of Investigational Cellular and Gene Therapy Products. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/preclinical-assessment-investigational-cellular-and-gene-therapy-products (accessed on 23 June 2025).
- Center for Biologics Evaluation and Research. Long Term Follow-Up After Administration of Human Gene Therapy Products. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/long-term-follow-after-administration-human-gene-therapy-products (accessed on 23 June 2025).
- Center for Biologics Evaluation and Research. Human Gene Therapy Products Incorporating Human Genome Editing. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-gene-therapy-products-incorporating-human-genome-editing (accessed on 23 June 2025).
- Cure Rare Disease’s Drug Development Framework|Cure Rare Disease. Available online: https://www.cureraredisease.org/blog-posts/cure-rare-diseases-drug-development-framework (accessed on 23 June 2025).
- Morgan, S.L.; Mariano, N.C.; Bermudez, A.; Arruda, N.L.; Wu, F.; Luo, Y.; Shankar, G.; Jia, L.; Chen, H.; Hu, J.-F.; et al. Manipulation of Nuclear Architecture through CRISPR-Mediated Chromosomal Looping. Nat. Commun. 2017, 8, 15993. [Google Scholar] [CrossRef]
- Kim, J.H.; Rege, M.; Valeri, J.; Dunagin, M.C.; Metzger, A.; Titus, K.R.; Gilgenast, T.G.; Gong, W.; Beagan, J.A.; Raj, A.; et al. LADL: Light-Activated Dynamic Looping for Endogenous Gene Expression Control. Nat. Methods 2019, 16, 633–639. [Google Scholar] [CrossRef]
- Qin, G.; Yang, J.; Zhao, C.; Ren, J.; Qu, X. Manipulating Complex Chromatin Folding via CRISPR-Guided Bioorthogonal Chemistry. Proc. Natl. Acad. Sci. USA 2022, 119, e2204725119. [Google Scholar] [CrossRef]
- Kubo, N.; Ishii, H.; Xiong, X.; Bianco, S.; Meitinger, F.; Hu, R.; Hocker, J.D.; Conte, M.; Gorkin, D.; Yu, M.; et al. Promoter-Proximal CTCF Binding Promotes Distal Enhancer-Dependent Gene Activation. Nat. Struct. Mol. Biol. 2021, 28, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Tarjan, D.R.; Flavahan, W.A.; Bernstein, B.E. Epigenome Editing Strategies for the Functional Annotation of CTCF Insulators. Nat. Commun. 2019, 10, 4258. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Datlinger, P.; Chardon, F.; Coelho, M.A.; Dong, M.B.; Lawson, K.A.; Lu, T.; Maroc, L.; Norman, T.M.; Song, B.; et al. High-Content CRISPR Screening. Nat. Rev. Methods Primers 2022, 2, 1–23. [Google Scholar] [CrossRef]
- Schmidt, R.; Steinhart, Z.; Layeghi, M.; Freimer, J.W.; Bueno, R.; Nguyen, V.Q.; Blaeschke, F.; Ye, C.J.; Marson, A. CRISPR Activation and Interference Screens Decode Stimulation Responses in Primary Human T Cells. Science 2022, 375, eabj4008. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Daley, T.P.; Wang, F.; Cao, W.S.; Bhate, S.; Lin, X.; Still, C.; Liu, H.; Zhao, D.; et al. CRISPR Activation Screens Systematically Identify Factors That Drive Neuronal Fate and Reprogramming. Cell Stem Cell 2018, 23, 758–771.e8. [Google Scholar] [CrossRef]
- Yin, J.; Wan, H.; Kong, D.; Liu, X.; Guan, Y.; Wu, J.; Zhou, Y.; Ma, X.; Lou, C.; Ye, H.; et al. A Digital CRISPR-dCas9-Based Gene Remodeling Biocomputer Programmed by Dietary Compounds in Mammals. Cell Syst. 2024, 15, 941–955.e5. [Google Scholar] [CrossRef]
- Agapov, A.; Panteleev, V.; Kropocheva, E.; Kanevskaya, A.; Esyunina, D.; Kulbachinskiy, A. Prokaryotic Argonaute Nuclease Cooperates with Co-Encoded RNase to Acquire Guide RNAs and Target Invader DNA. Nucleic Acids Res. 2024, 52, 5895–5911. [Google Scholar] [CrossRef]
- Faure, G.; Saito, M.; Wilkinson, M.E.; Quinones-Olvera, N.; Xu, P.; Flam-Shepherd, D.; Kim, S.; Reddy, N.; Zhu, S.; Evgeniou, L.; et al. TIGR-Tas: A Family of Modular RNA-Guided DNA-Targeting Systems in Prokaryotes and Their Viruses. Science 2025, 388, eadv9789. [Google Scholar] [CrossRef]
- Ruffolo, J.A.; Nayfach, S.; Gallagher, J.; Bhatnagar, A.; Beazer, J.; Hussain, R.; Russ, J.; Yip, J.; Hill, E.; Pacesa, M.; et al. Design of Highly Functional Genome Editors by Modeling the Universe of CRISPR-Cas Sequences. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ichikawa, D.M.; Abdin, O.; Alerasool, N.; Kogenaru, M.; Mueller, A.L.; Wen, H.; Giganti, D.O.; Goldberg, G.W.; Adams, S.; Spencer, J.M.; et al. A Universal Deep-Learning Model for Zinc Finger Design Enables Transcription Factor Reprogramming. Nat. Biotechnol. 2023, 41, 1117–1129. [Google Scholar] [CrossRef]
- Hayes, T.; Rao, R.; Akin, H.; Sofroniew, N.J.; Oktay, D.; Lin, Z.; Verkuil, R.; Tran, V.Q.; Deaton, J.; Wiggert, M.; et al. Simulating 500 Million Years of Evolution with a Language Model. Science 2025, 387, 850–858. [Google Scholar] [CrossRef] [PubMed]
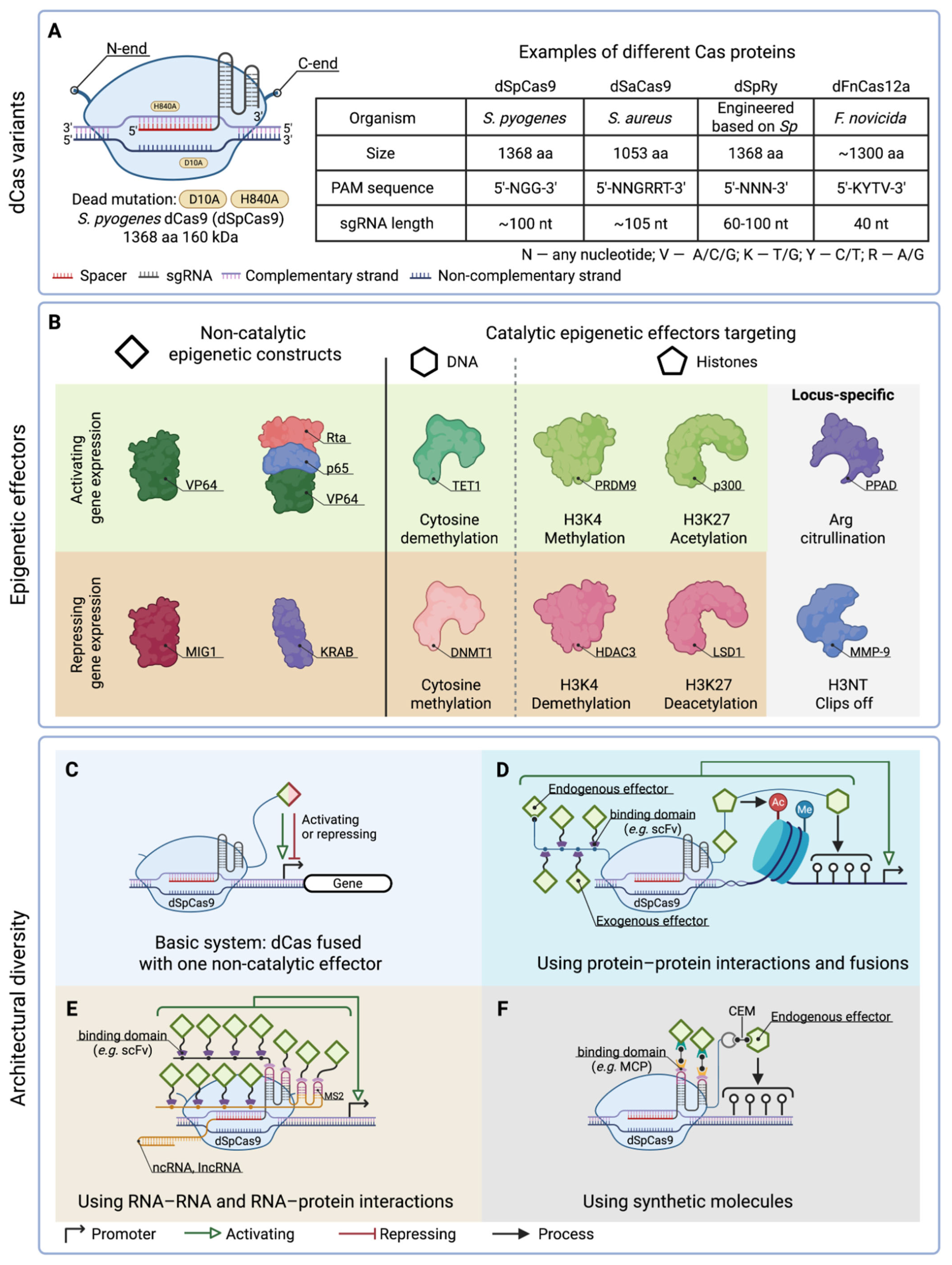
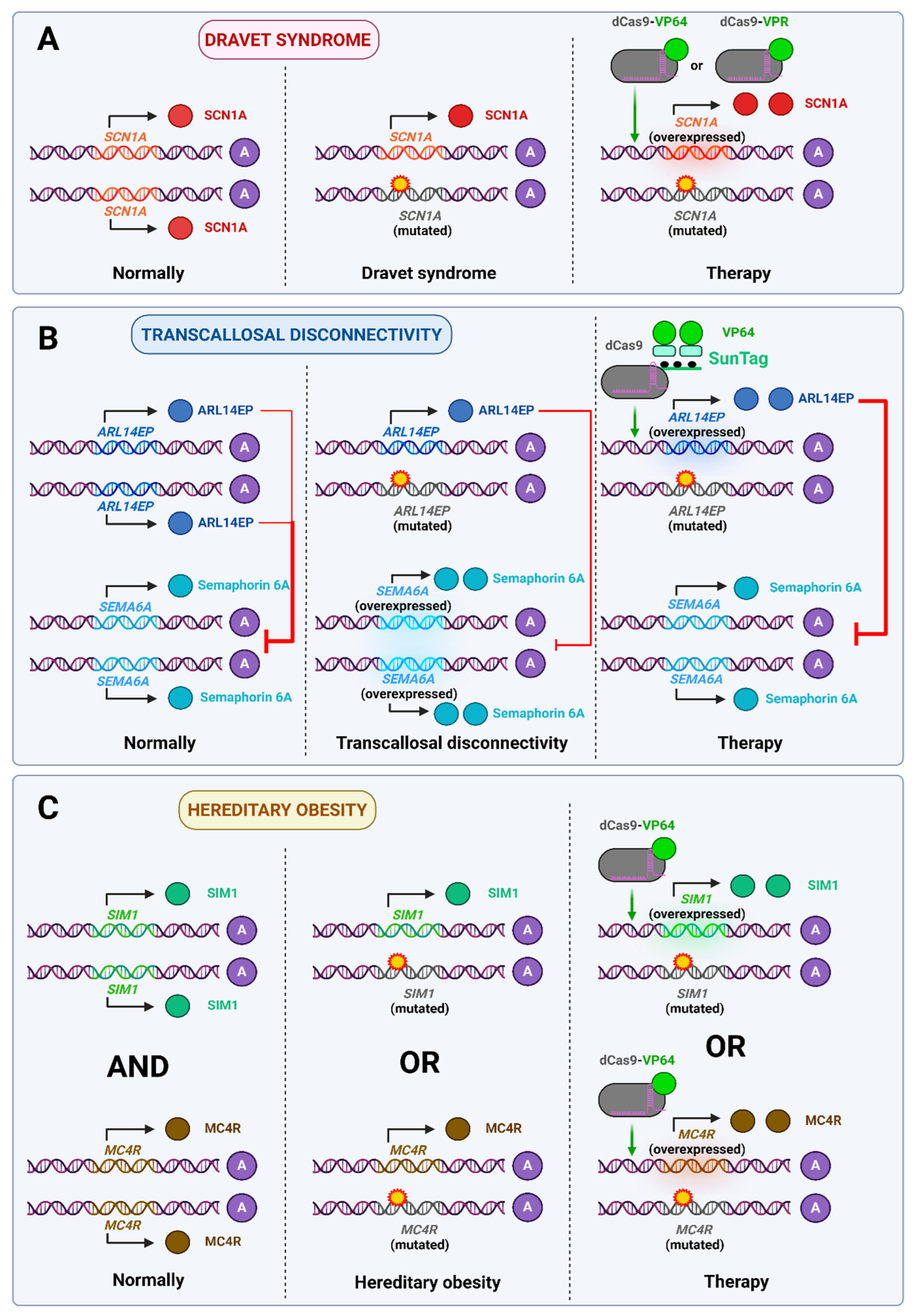
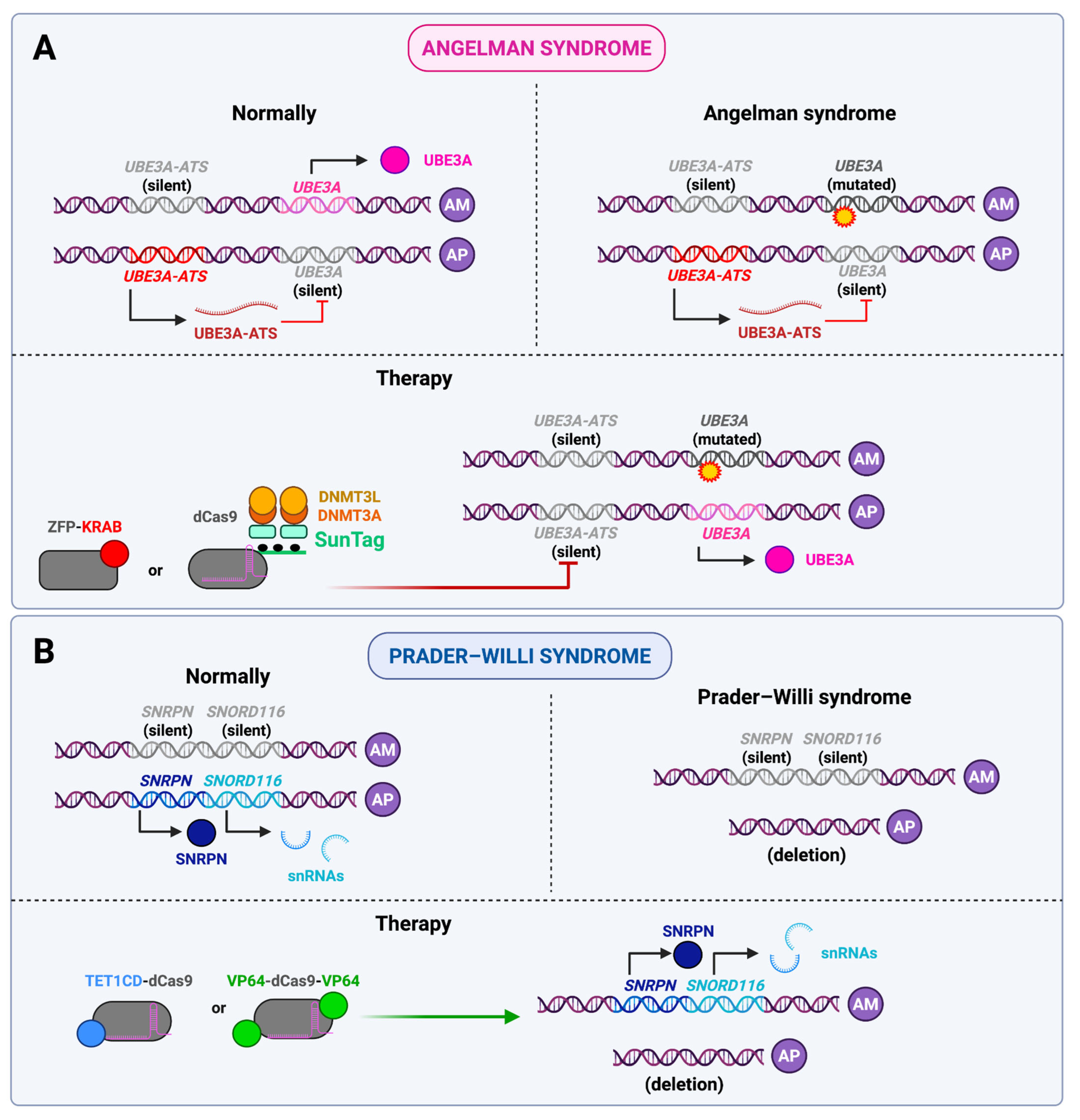
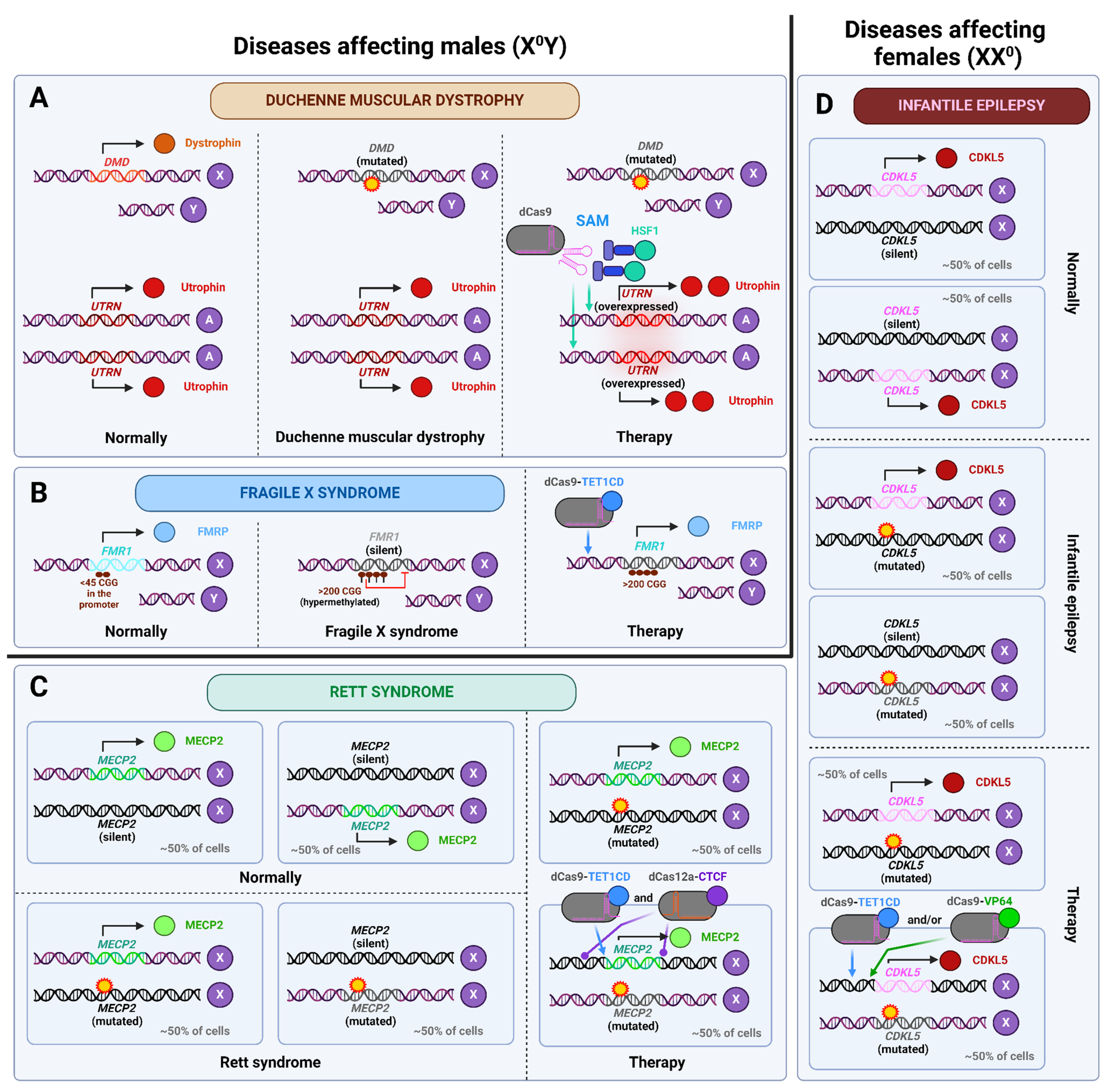
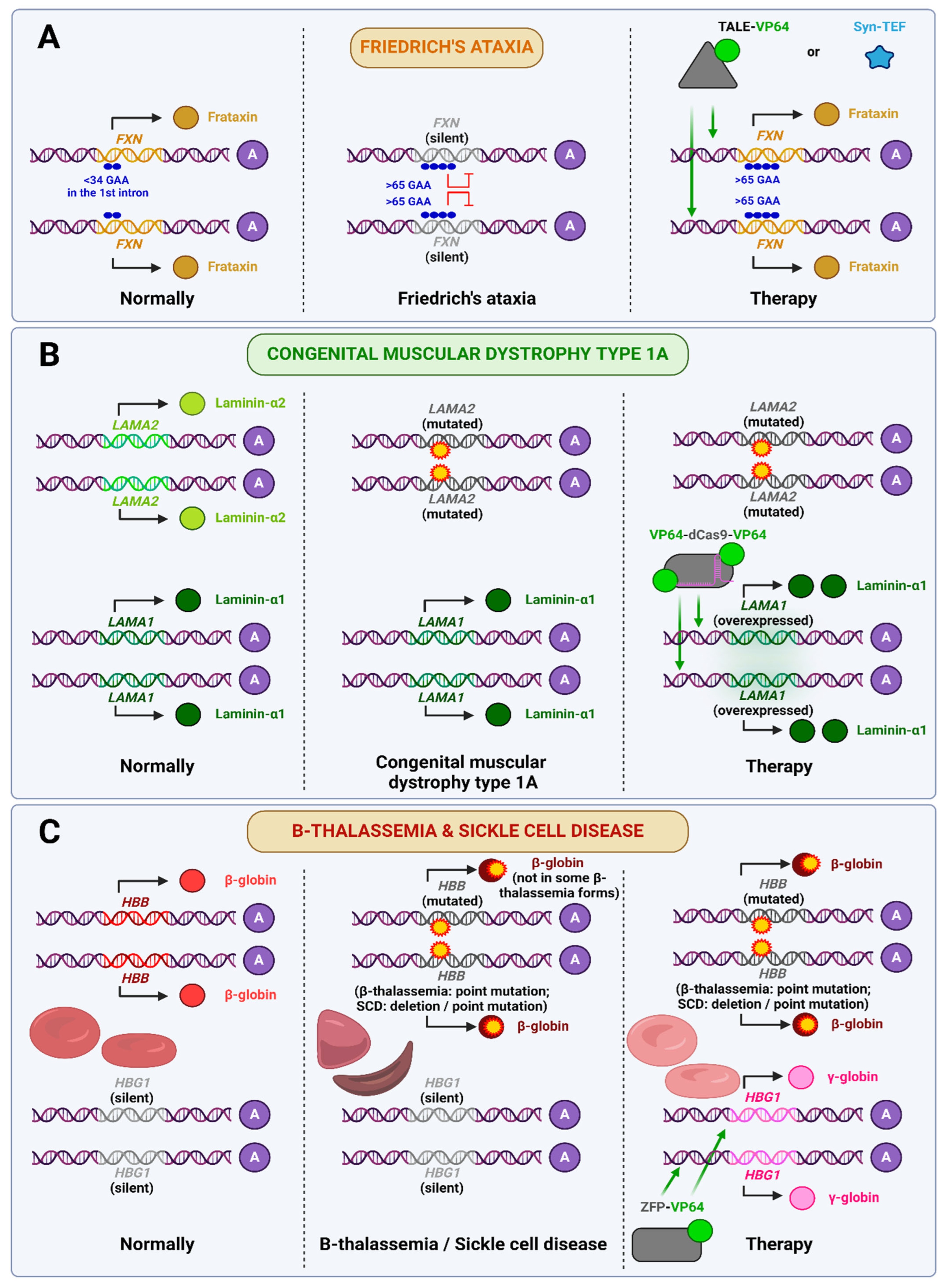
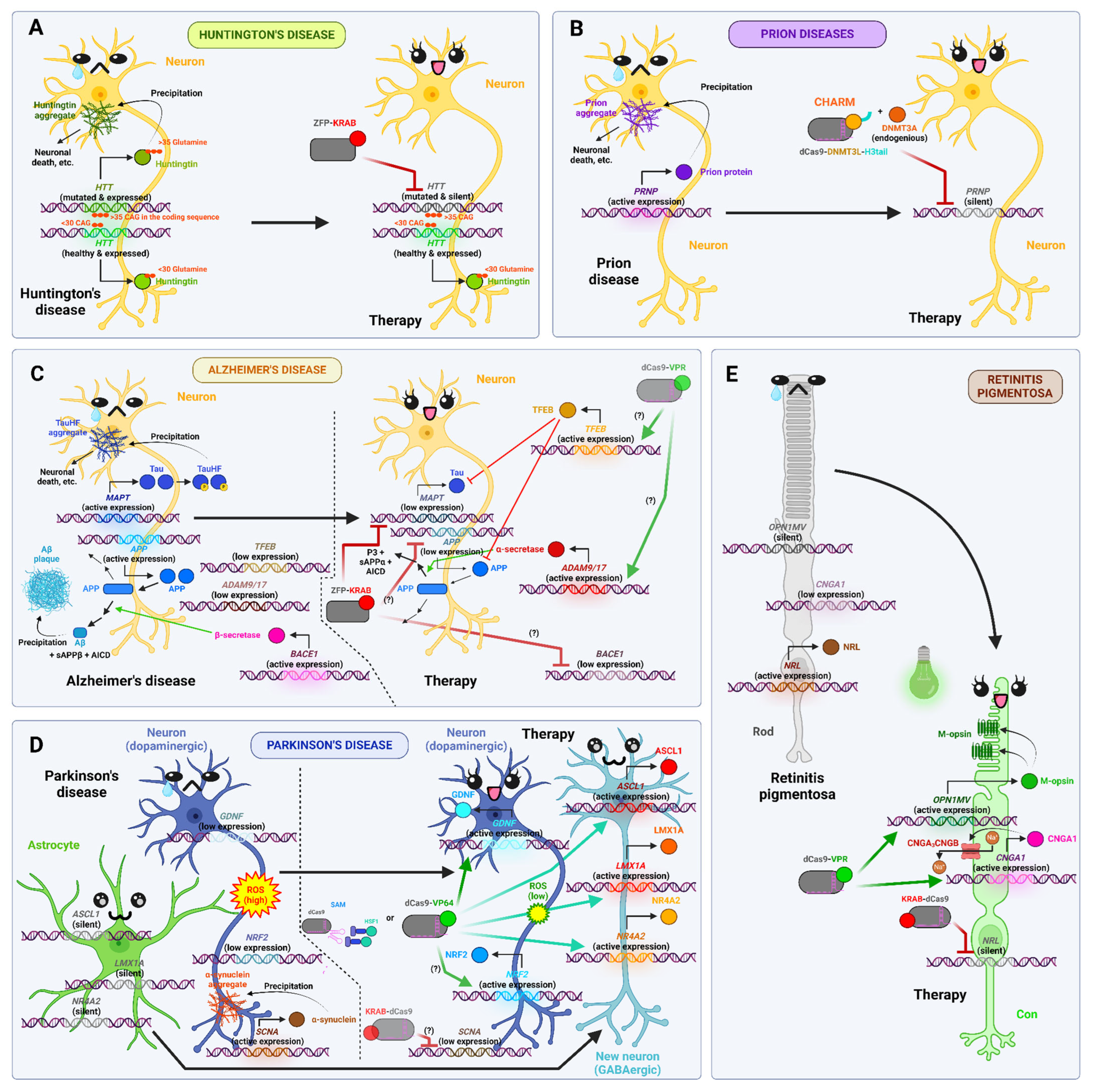
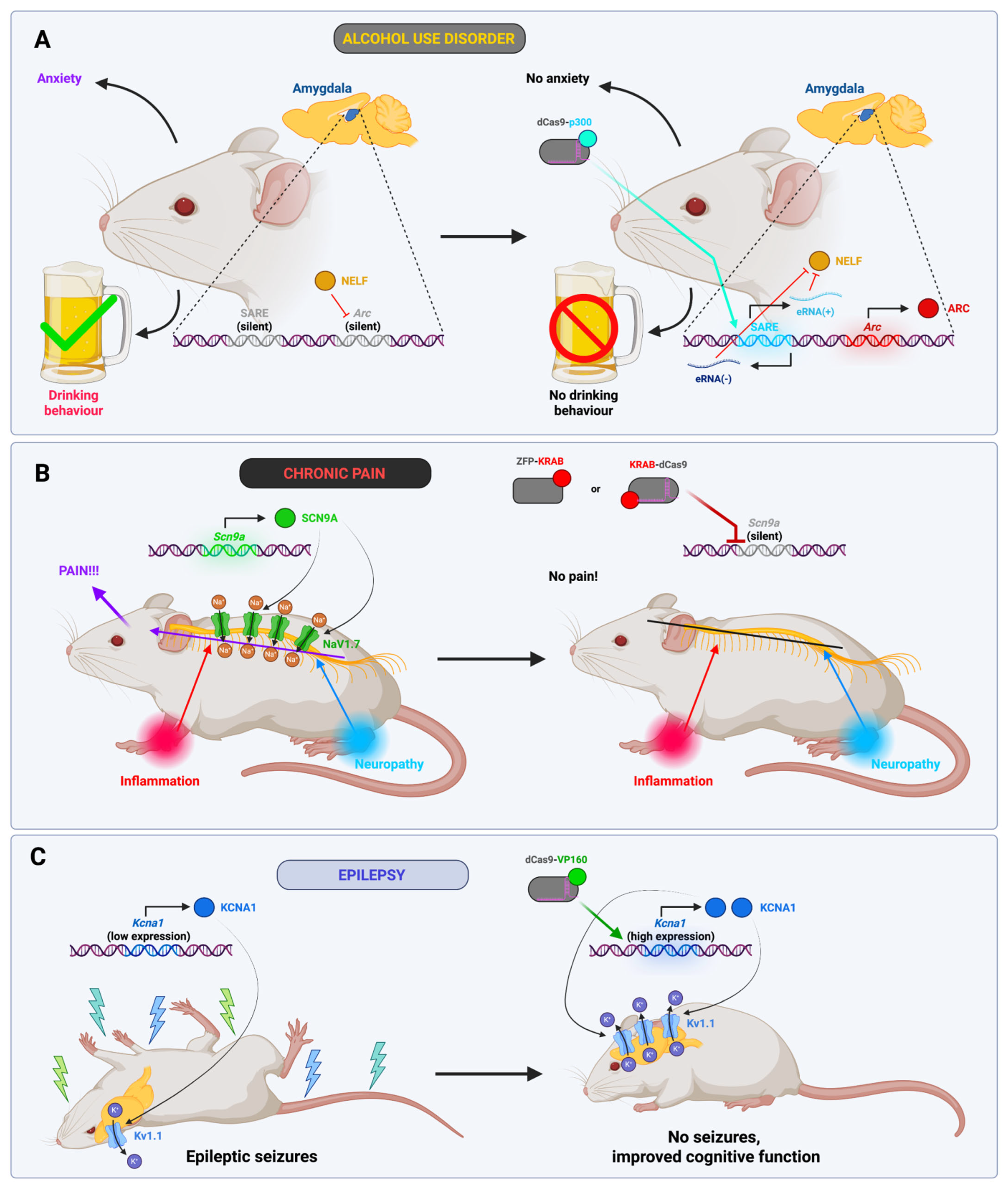
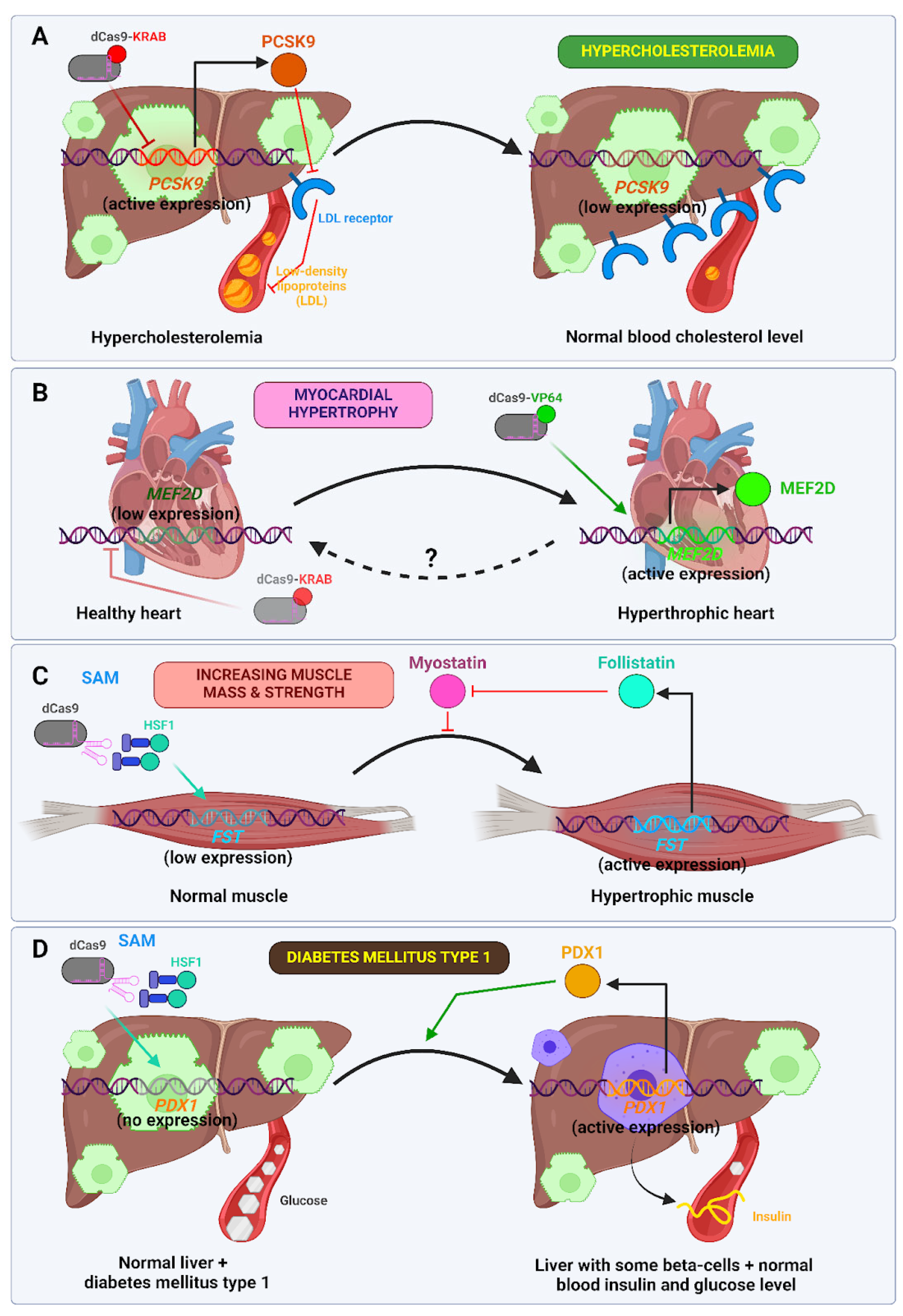

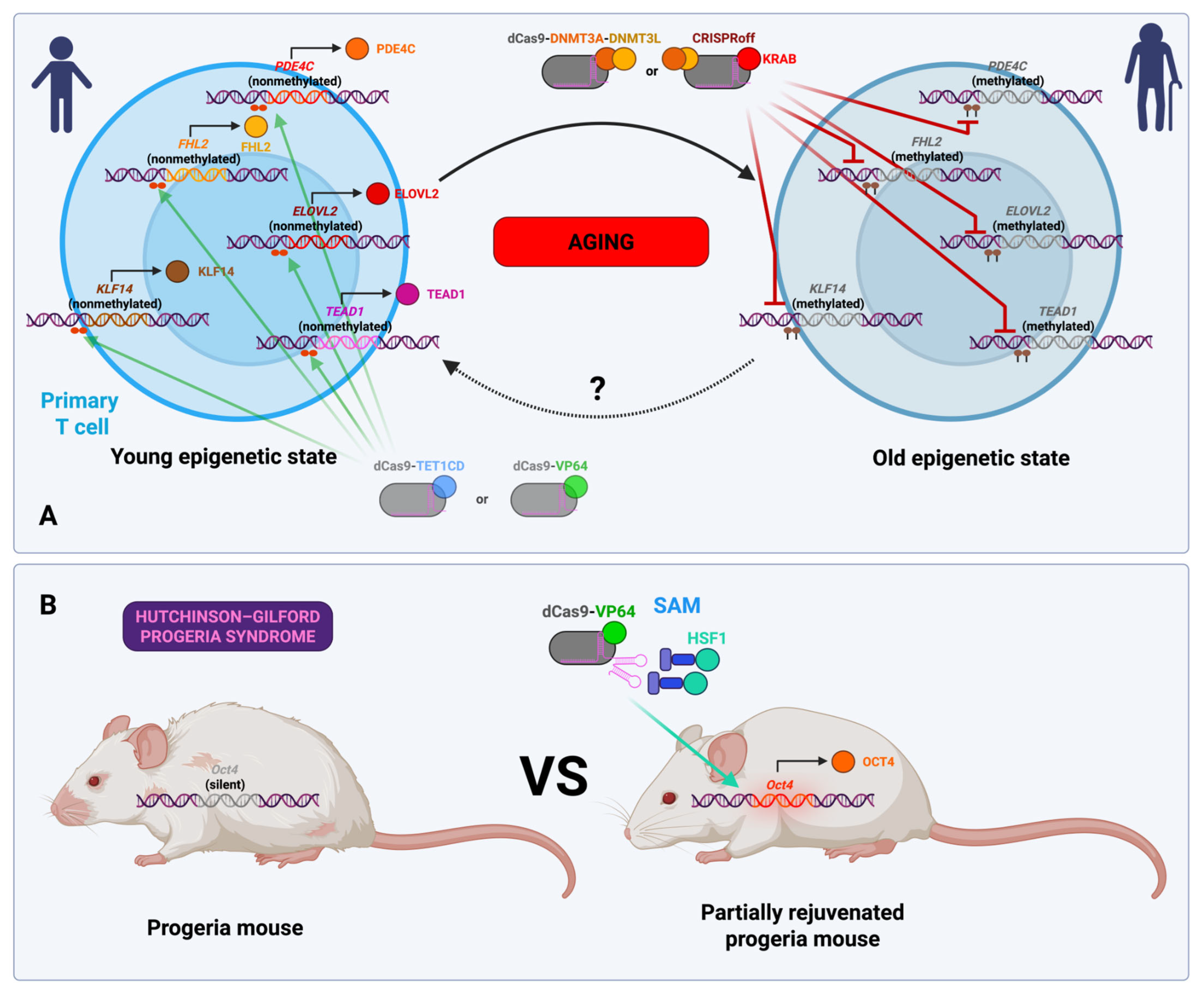
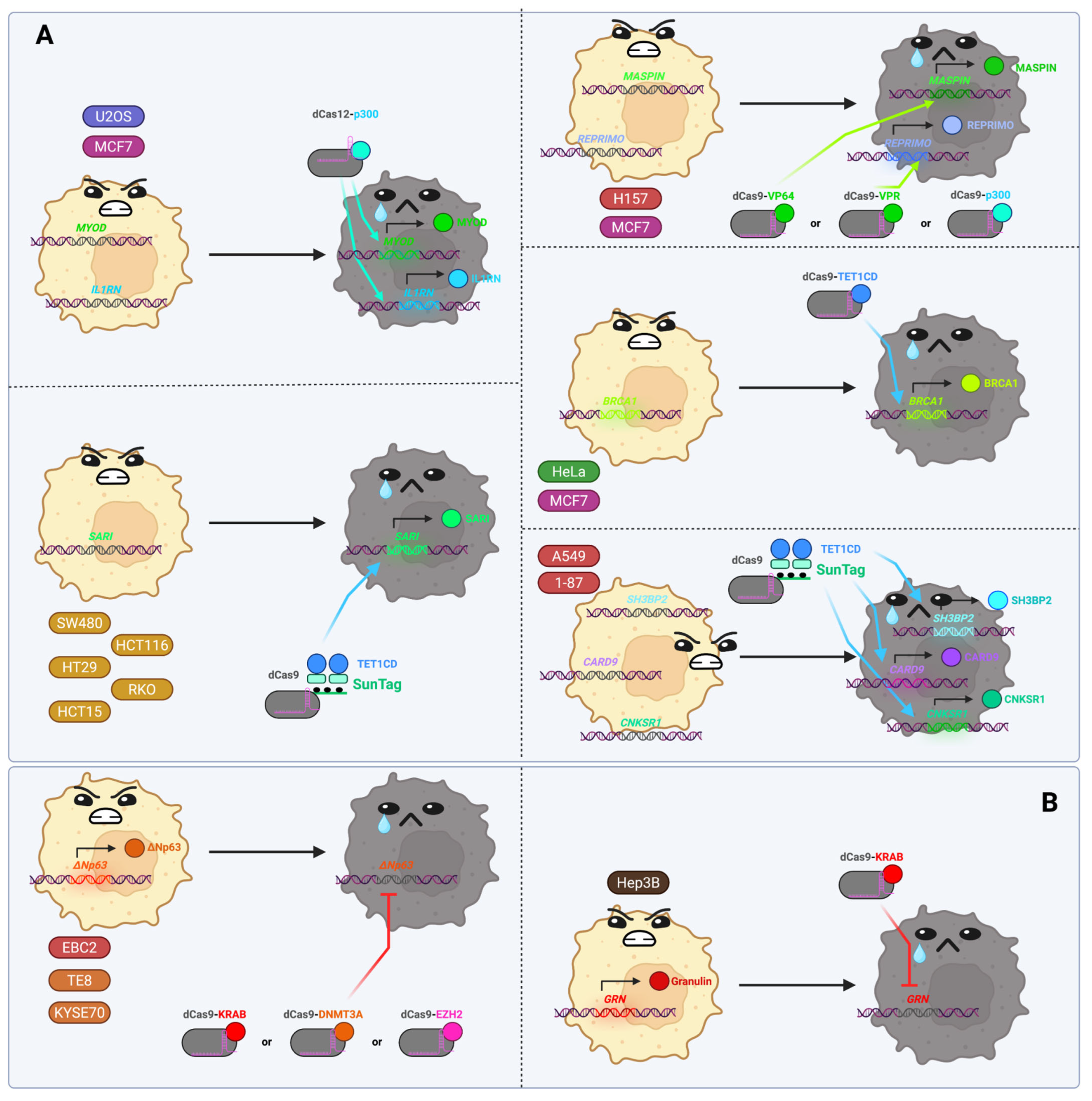
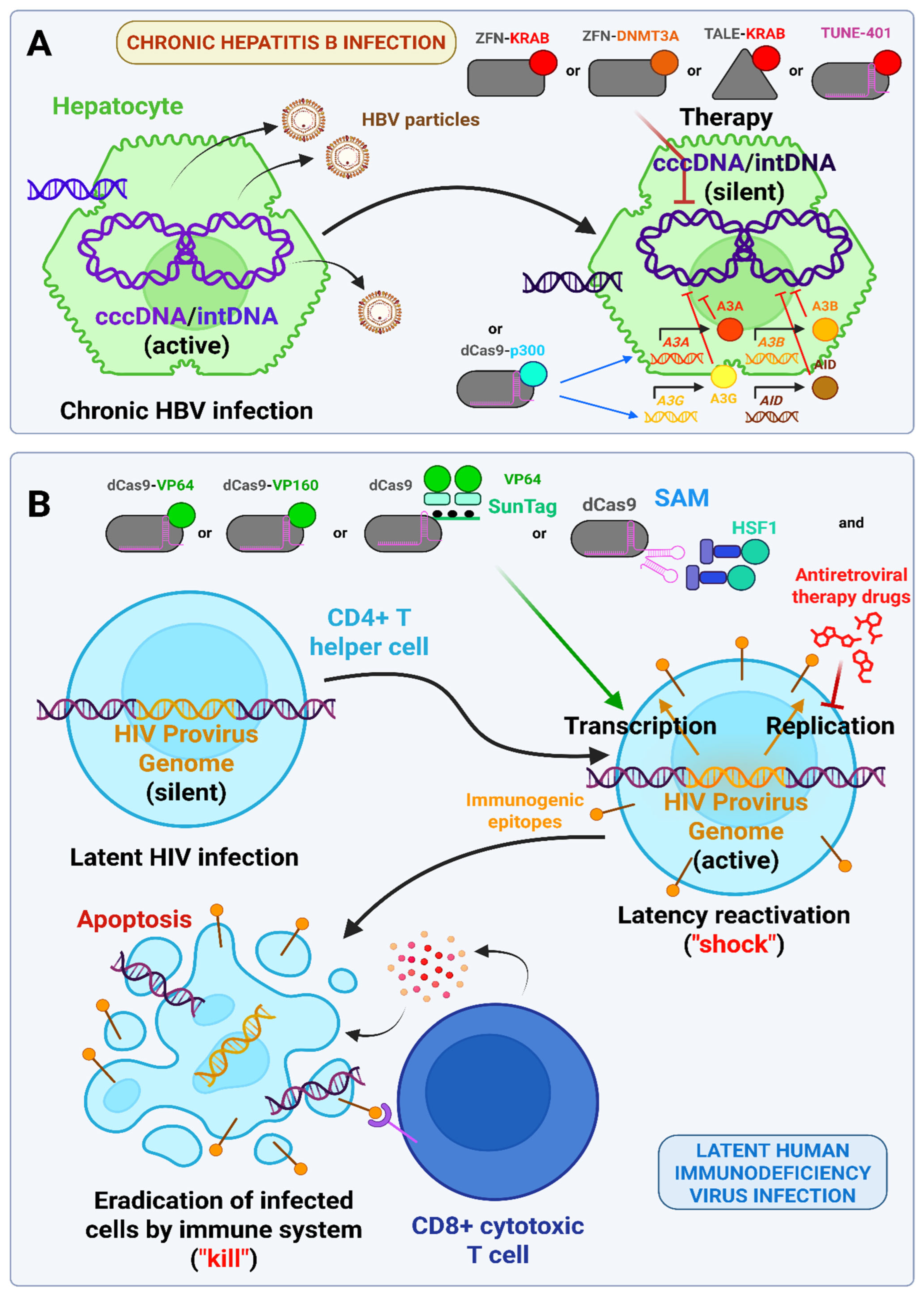
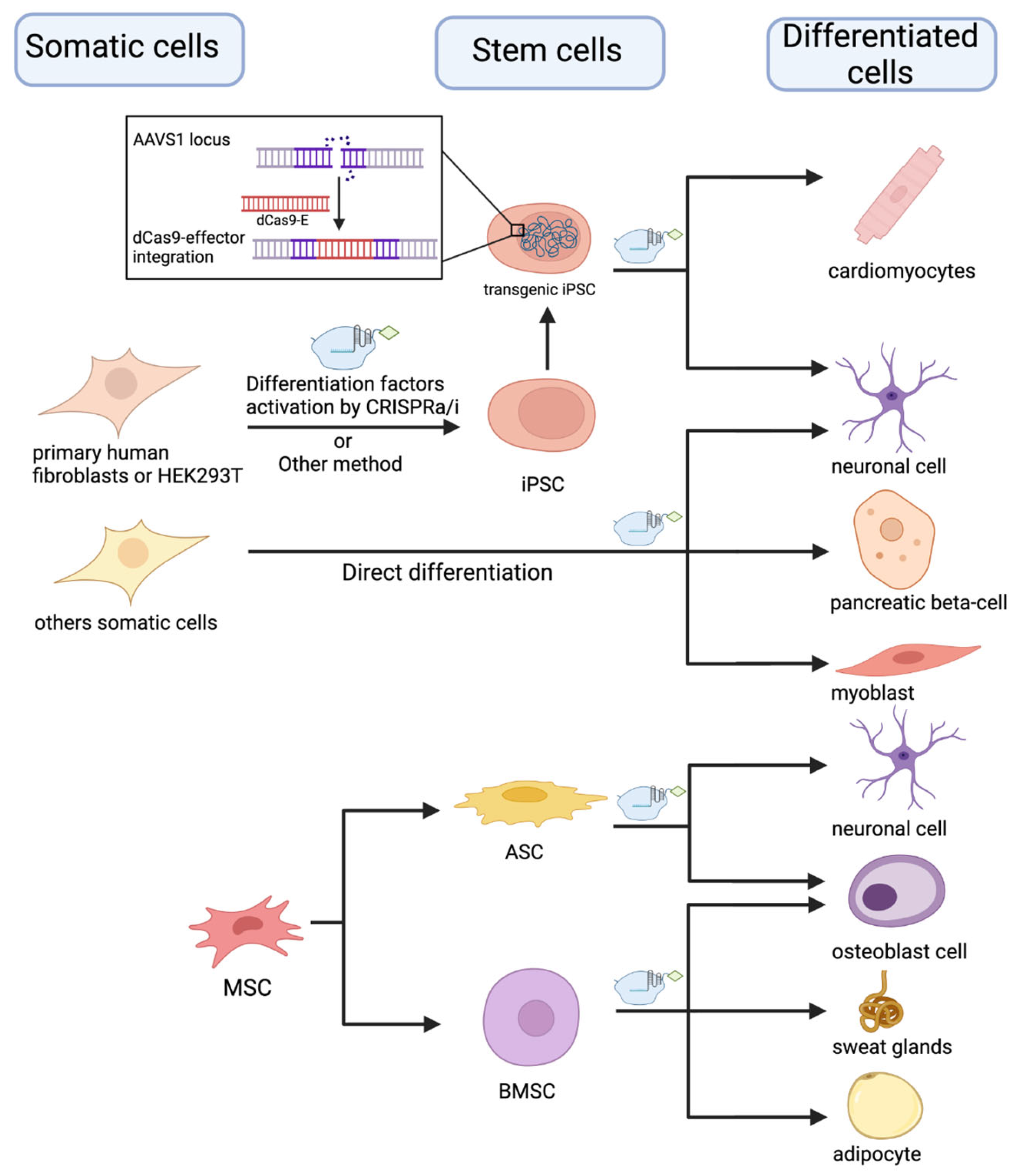
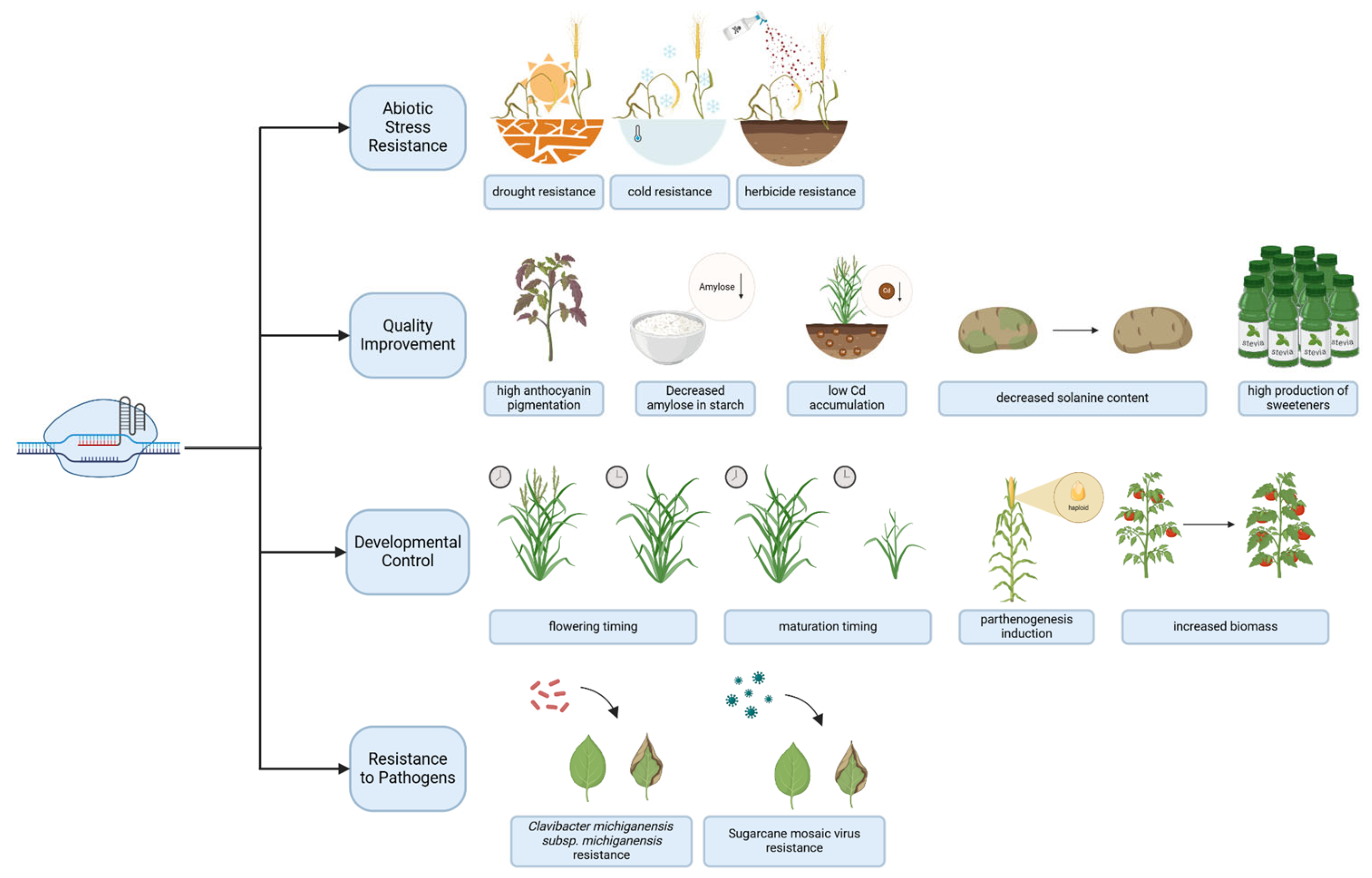
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalev, M.A.; Mamaeva, N.Y.; Kristovskiy, N.V.; Feskin, P.G.; Vinnikov, R.S.; Oleinikov, P.D.; Sosnovtseva, A.O.; Yakovlev, V.A.; Glukhov, G.S.; Shaytan, A.K. Epigenome Engineering Using dCas Systems for Biomedical Applications and Biotechnology: Current Achievements, Opportunities and Challenges. Int. J. Mol. Sci. 2025, 26, 6371. https://doi.org/10.3390/ijms26136371
Kovalev MA, Mamaeva NY, Kristovskiy NV, Feskin PG, Vinnikov RS, Oleinikov PD, Sosnovtseva AO, Yakovlev VA, Glukhov GS, Shaytan AK. Epigenome Engineering Using dCas Systems for Biomedical Applications and Biotechnology: Current Achievements, Opportunities and Challenges. International Journal of Molecular Sciences. 2025; 26(13):6371. https://doi.org/10.3390/ijms26136371
Chicago/Turabian StyleKovalev, Maxim A., Naida Yu. Mamaeva, Nikolay V. Kristovskiy, Pavel G. Feskin, Renat S. Vinnikov, Pavel D. Oleinikov, Anastasiia O. Sosnovtseva, Valeriy A. Yakovlev, Grigory S. Glukhov, and Alexey K. Shaytan. 2025. "Epigenome Engineering Using dCas Systems for Biomedical Applications and Biotechnology: Current Achievements, Opportunities and Challenges" International Journal of Molecular Sciences 26, no. 13: 6371. https://doi.org/10.3390/ijms26136371
APA StyleKovalev, M. A., Mamaeva, N. Y., Kristovskiy, N. V., Feskin, P. G., Vinnikov, R. S., Oleinikov, P. D., Sosnovtseva, A. O., Yakovlev, V. A., Glukhov, G. S., & Shaytan, A. K. (2025). Epigenome Engineering Using dCas Systems for Biomedical Applications and Biotechnology: Current Achievements, Opportunities and Challenges. International Journal of Molecular Sciences, 26(13), 6371. https://doi.org/10.3390/ijms26136371





