Shifting Perspectives on the Role of Tocotrienol vs. Tocopherol in Brain Health: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. PICO Framework and Research Question
2.2. Protocol
2.3. Eligibility Criteria
2.4. Sources Database, Search Strategy and Selection of Evidence
2.5. Data Extraction, Charting and Data Item
2.6. Synthesis of Results
3. Results
3.1. Sources of Evidence
3.2. General Characteristics of the Included Studies
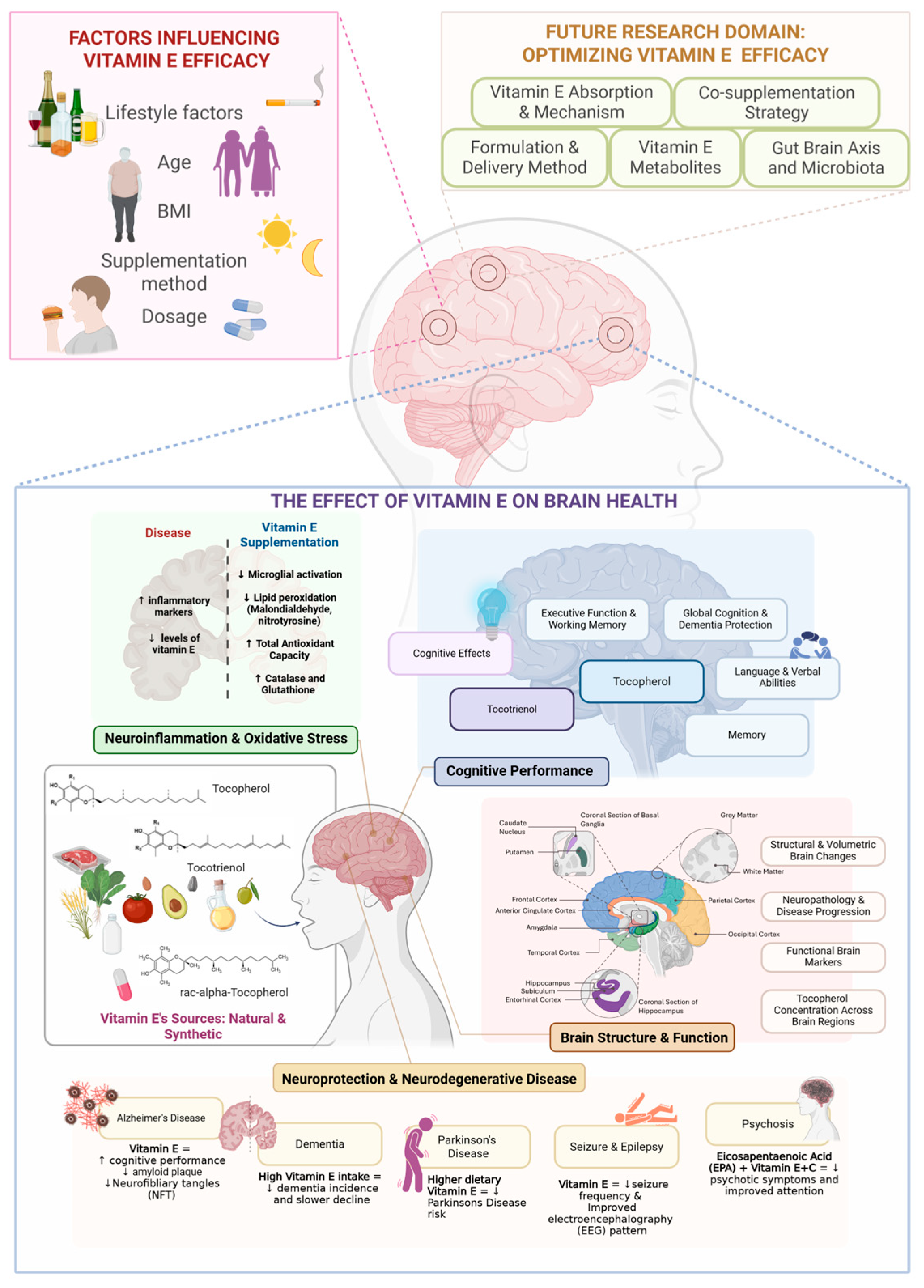
3.3. Overview of Vitamin E and Brain-Health Research for the Past 10 Years
3.4. Neuroinflammation and Oxidative Stress
3.5. Brain Structure and Function
3.6. Cognitive Performance
3.6.1. Level of Vitamin E
3.6.2. Intervention and Dietary Assessment
3.7. Neurodegenerative Diseases and Neuroprotection
3.7.1. Alzheimer’s Disease
3.7.2. Dementia
3.7.3. Parkinson’s Disease
3.7.4. Other Neuro-Related Diseases
4. Discussion
4.1. The Effects of Tocotrienol or Tocopherol Supplementation on Brain Health
4.1.1. Vitamin E Influences Neuroinflammation and Oxidative Stress
4.1.2. Vitamin E’s Ability to Protect the Brain Structure and Function
4.1.3. Vitamin E Delays Cognitive Decline
4.1.4. Vitamin E Protect Against Neurodegenerative Diseases
- 1.
- Alzheimer’s Disease
- 2.
- Dementia
- 3.
- Parkinson’s disease
- 4.
- Other Neurological Disorders
4.2. Other Possible Factors and Confounding Factors
4.3. How Do Tocotrienols and Tocopherols Compare in the Maintenance of Brain Health?
4.4. Future Directions: Optimizing Vitamin E Delivery and Efficacy for Brain Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ADCS-ADL | Alzheimer’s Disease Cooperative Study Activities of Daily Living |
| ADL | Activities of Daily Living |
| AE/SAE | Adverse Event/Serious Adverse Event |
| AF/AFT | Animal Fluency Test |
| APOE | Apolipoprotein E |
| BDNF | Brain-Derived Neurotrophic Factor |
| BPSD | Behavioral and Psychological Symptoms of Dementia |
| BVRT | Benton Visual Retention Test |
| CAS | Caregiver Activity Survey |
| CDR | Clinical Dementia Rating |
| CERAD | Consortium to Establish a Registry for Alzheimer’s Disease |
| CES-D | Center for Epidemiologic Studies Depression Scale |
| CLOX-1 | Clock Drawing Executive Function Test |
| CRP | C-reactive protein |
| DHA | Docosahexaenoic Acid |
| DRS | Dementia Rating Scale |
| DS-B | Digit Span Backward |
| DS-F | Digit Span Forward |
| DSRS | Dementia Severity Rating Scale |
| DSST | Digit Symbol Substitution Test |
| FORD | Free Oxygen Radical Defense |
| FORT | Free Oxygen Radical Test |
| GAF | Global Assessment of Functioning |
| GDS | Geriatric Depression Scale |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| GSHt/GSHr/GSSG | Total, Reduced, and Oxidized Glutathione |
| Hp | Haptoglobin |
| ICD | International Classification of Diseases |
| IL | Interleukin |
| INF-α2/IFN-γ | Interferon-alpha2/Interferon-gamma |
| LOTCA | Loewenstein Occupational Therapy Cognitive Assessment |
| LTL | Leukocyte Telomere Length |
| MCI | Mild Cognitive Impairment |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| NFT | Neurofibrillary Tangles |
| NPI | Neuropsychiatric Inventory |
| PANSS | Positive and Negative Syndrome Scale |
| PASAT | Paced Auditory Serial Addition Test |
| PHQ-9 | Patient Health Questionnaire-9 |
| PRISMA-ScR | Preferred Reporting Items for Systematic reviews and Meta-Analyses Scoping Review |
| PUFA | Polyunsaturated Fatty Acid |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Status |
| RNS/ROS | Reactive Nitrogen Species/Reactive Oxygen Species |
| SNARE | Soluble N-ethylmaleimide-sensitive factor Attachment protein Receptor |
| SOD | Superoxide Dismutase |
| STROOP | Stroop Color and Word Test |
| TAC | Total Antioxidant Capacity |
| TICS-m | Modified Telephone Interview for Cognitive Status |
| TMT | Trail Making Test |
| TNF-α/TNF-β | Tumor Necrosis Factor alpha/beta |
| UPDRS III | Unified Parkinson’s Disease Rating Scale Part III |
| VEGF | Vascular Endothelial Growth Factor |
| VLDL | Very Low-Density Lipoprotein |
| WMHs | White Matter Hyperintensities |
| WMLs | White Matter Lesions |
References
- de Leeuw, F.A.; Schneider, J.A.; Agrawal, S.; Leurgans, S.E.; Morris, M.C. Brain Tocopherol Levels Are Associated with Lower Activated Microglia Density in Elderly Human Cortex. Alzheimer’s Dement. Transl. Res. Clin. Interv. (TRCI) 2020, 6, e12021. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Power, R.; Howard, A.N.; Bergin, P.; Roche, W.; Prado-Cabrero, A.; Pope, G.; Cooke, J.; Power, T.; Mulcahy, R. Supplementation with Carotenoids, Omega-3 Fatty Acids, and Vitamin E Has a Positive Effect on the Symptoms and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, Y.; Shuaib, I.L.; Magosso, E.; Ansari, M.A.; Abu Bakar, M.R.; Wong, J.W.; Khan, N.A.K.; Liong, W.C.; Sundram, K.; Ng, B.H.; et al. Clinical Investigation of the Protective Effects of Palm Vitamin E Tocotrienols on Brain White Matter. Stroke 2014, 45, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Mehvari, J.; Motlagh, F.; Ghazvini, M.A.; Naeini, A.; Zare, M. Effects of Vitamin E on Seizure Frequency, Electroencephalogram Findings, and Oxidative Stress Status of Refractory Epileptic Patients. Adv. Biomed. Res. 2016, 5, 36. [Google Scholar] [CrossRef]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef]
- de Leeuw, F.A.; Honer, W.G.; Schneider, J.A.; Morris, M.C. Brain γ-Tocopherol Levels Are Associated with Presynaptic Protein Levels in Elderly Human Midfrontal Cortex. J. Alzheimer’s Dis. 2020, 77, 619–627. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Man, Y.; Li, N.; Zhou, Y.U. Effects of Vitamins E and C Combined with β-Carotene on Cognitive Function in the Elderly. Exp. Ther. Med. 2015, 9, 1489–1493. [Google Scholar] [CrossRef]
- Morris, M.C.; Schneider, J.A.; Li, H.; Tangney, C.C.; Nag, S.; Bennett, D.A.; Honer, W.G.; Barnes, L.L. Brain Tocopherols Related to Alzheimer’s Disease Neuropathology in Humans. Alzheimer’s Dement. 2014, 11, 32–39. [Google Scholar] [CrossRef]
- Paganini-Hill, A.; Bukhari, S.; Montine, T.J.; Corrada, M.M.; Kawas, C.H. Alzheimer’s Disease Neuropathologic Change and Vitamin Supplement Use Decades Earlier: The 90+ Study. Alzheimer Dis. Assoc. Disord. 2023, 37, 1–6. [Google Scholar] [CrossRef]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Di Lazzaro, G.; Franco, D.; Colona, V.L.; Alwardat, M.; Sinibaldi Salimei, P.; Mercuri, N.B.; Pierantozzi, M.; et al. Dietary Vitamin E as a Protective Factor for Parkinson’s Disease: Clinical and Experimental Evidence. Front. Neurol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Scott, T.M.; Johnson, M.A.; Poon, L.W.; Nelson, P.T.; Davey, A.; Woodard, J.L.; Vishwanathan, R.; Barbey, A.K.; Barger, K.; et al. Brain A-Tocopherol Concentration Is Inversely Associated with Neurofibrillary Tangle Counts in Brain Regions Affected in Earlier Braak Stages: A Cross-Sectional Finding in the Oldest Old. JAR Life 2021, 10, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Poli, G.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Febo, M.; Mazzon, E.; Bruscoli, S.; Brancorsini, S.; Mecocci, P. miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population. Nutrients 2023, 15, 634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Monahan, F.J.; McNulty, B.A.; Gibney, M.J.; Gibney, E.R. Effect of vitamin E intake from food and supplement sources on plasma α- and γ-tocopherol concentrations in a healthy Irish adult population. Br. J. Nutr. 2014, 112, 1575–1585. [Google Scholar] [CrossRef]
- Colombo, M.L. An Update on Vitamin E, Tocopherol and Tocotrienol—Perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, Y.M.; Choi, B.Y.; Kim, M.K.; Roh, S.; Kim, K.; Yang, Y.J. Associations of Serum Levels of Vitamins A, C, and E with the Risk of Cognitive Impairment Among Elderly Koreans. Nutr. Res. Pract. 2018, 12, 160. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of Antioxidant Supplement Use and Dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol. 2017, 74, 567. [Google Scholar] [CrossRef]
- Chan, J.P.; Tanprasertsuk, J.; Johnson, E.J.; Dey, P.; Bruno, R.S.; Johnson, M.A.; Poon, L.W.; Davey, A.; Woodard, J.L.; Kuchan, M.J. Associations between Brain Alpha-Tocopherol Stereoisomer Profile and Hallmarks of Brain Aging in Centenarians. Antioxidants 2024, 13, 997. [Google Scholar] [CrossRef]
- Kuchan, M.J.; Jensen, S.K.; Johnson, E.J.; Lieblein-Boff, J.C. The Naturally Occurring α-Tocopherol Stereoisomer RRR-α-tocopherol Is Predominant in the Human Infant Brain. Br. J. Nutr. 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Anwar, K.; Iqbal, J.; Hussain, M.M. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J. Lipid Res. 2007, 48, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Schmidt, K.; Péter, S.; Richards, J.; Winklhofer-Roob, B.; Hahn, A.; Obermüller-Jevic, U. Vitamin E: Not only a single stereoisomer. Free Radic. Biol. Med. 2024, 215, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Metabolism of natural forms of vitamin E and biological actions of vitamin E metabolites. Free Radic. Biol. Med. 2022, 179, 375–387. [Google Scholar] [CrossRef]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef]
- Goon, J.A.; Azman, N.H.E.N.; Ghani, S.M.A.; Hamid, Z.; Ngah, W.Z.W. Comparing palm oil tocotrienol rich fraction with α-tocopherol supplementation on oxidative stress in healthy older adults. Clin. Nutr. ESPEN 2017, 21, 1–12. [Google Scholar] [CrossRef]
- Nor Azman, N.H.E.; Goon, J.A.; Abdul Ghani, S.M.; Hamid, Z.; Wan Ngah, W.Z. Comparing palm oil, tocotrienol-rich fraction and α-tocopherol supplementation on the antioxidant levels of older adults. Antioxidants 2018, 7, 74. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef]
- Liu, S.; Luo, J.; Xiao, Z.; Wu, W.; Liang, X.; Ding, S.; Zhao, Q.; Zhao, X.; Wang, Y.; Ding, D. Low Dietary Vitamin E Intake Is Associated with High Risk of Incident Dementia Among Older Adults: The Shanghai Aging Study. Front. Nutr. 2022, 9, 1036795. [Google Scholar] [CrossRef]
- Aan, G.J.; Zainudin, M.S.A.; Karim, N.A.; Ngah, W.Z.W. Effect of the tocotrienol-rich fraction on the lifespan and oxidative biomarkers in Caenorhabditis elegans under oxidative stress. Clinics 2013, 68, 599–604. [Google Scholar] [CrossRef]
- Panagabko, C.; Morley, S.; Hernandez, M.; Cassolato, P.; Gordon, H.; Parsons, R.; Manor, D.; Atkinson, J. Ligand Specificity in the CRAL-TRIO Protein Family. Biochemistry 2003, 42, 6467–6474. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Rink, C.; Gordillo, G.M.; Khanna, S.; Gnyawali, U.; Roy, S.; Shneker, B.; Ganesh, K.; Phillips, G.; More, J.L.; et al. Oral Tocotrienols Are Transported to Human Tissues and Delay the Progression of the Model for End-Stage Liver Disease Score in Patients. J. Nutr. 2012, 142, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Che, H.-L.; Tan, D.M.-Y.; Teng, K.T. Bioavailability of Tocotrienols: Evidence in Human Studies. Nutr. Metab. 2014, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; François, R.; Yamauchi, T.; Pérez, M.; Sebti, S.d.M.; Malafa, M.P. Vitamin E δ-Tocotrienol Augments the Antitumor Activity of Gemcitabine and Suppresses Constitutive NF-κB Activation in Pancreatic Cancer. Mol. Cancer Ther. 2011, 10, 2363–2372. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Regner, L.; Mett, J.; Stahlmann, C.P.; Schorr, P.; Nelke, C.; Streidenberger, O.; Stoetzel, H.; Winkler, J.; Zaidan, S.; et al. Tocotrienol Affects Oxidative Stress, Cholesterol Homeostasis and the Amyloidogenic Pathway in Neuroblastoma Cells: Consequences for Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, 1809. [Google Scholar] [CrossRef]
- Shen, J.; Yang, T.; Xu, Y.; Luo, Y.; Zhong, X.; Shi, L.; Hu, T.; Guo, T.; Nie, Y.; Luo, F.; et al. δ-Tocotrienol, Isolated From Rice Bran, Exerts an Anti-Inflammatory Effect via MAPKs and PPARs Signaling Pathways in Lipopolysaccharide-Stimulated Macrophages. Int. J. Mol. Sci. 2018, 19, 3022. [Google Scholar] [CrossRef]
- Kumareswaran, A.; Ekeuku, S.O.; Mohamed, N.; Muhammad, N.; Hanafiah, A.; Pang, K.L.; Wong, S.K.; Chew, D.C.H.; Chin, K.Y. The Effects of Tocotrienol on Gut Microbiota: A Scoping Review. Life 2023, 13, 1882. [Google Scholar] [CrossRef]
- Sekikawa, T.; Kizawa, Y.; Li, Y.; Takara, T. Cognitive Function Improvement with Astaxanthin and Tocotrienol Intake: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Biochem. Nutr. 2020, 67, 307–316. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef]
- Latib, F.; Zafendi, M.A.I.; Mohd Lazaldin, M.A. The use of vitamin E in ocular health: Bridging omics approaches with Tocopherol and Tocotrienol in the management of glaucoma. Food Chem. Mol. Sci. 2024, 9, 100224. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Boon, M.H.; Thomson, H. The effect direction plot revisited: Application of the 2019 Cochrane Handbook guidance on alternative synthesis methods. Res. Synth. Methods 2021, 12, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, H.; Li, Q.; Gao, D.; Wang, X.; Wu, C.; Wang, Q.; Zhu, M. Dietary Vitamin E Intake and Risk of Parkinson’s Disease: A Cross-Sectional Study. Front. Nutr. 2024, 10, 1289238. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Zhang, L.; Li, J.; Liu, Y.; Chen, Z.; Wang, S.; Gao, C.; Sun, X. The Role of Periodontitis in the Link Between Alpha-Tocopherol Intake and Cognitive Performance: A Mediation Analysis in Older Adults. Front. Aging Neurosci. 2023, 15, 1129095. [Google Scholar] [CrossRef]
- Liu, X.; Finno, C.J.; Beck, T.; Dhana, K.; Tangney, C.; Desai, P.; Krueger, K.; Evans, D.A.; Rajan, K.B. Association of Vitamin E and Cognitive Decline in Older Adults with and Without the APOE Ɛ4 Allele: A Biracial Population-Based Community Study. J. Alzheimer’s Dis. 2023, 96, 1129–1138. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Zhuang, W.; Shang, Y.; Yan, G.; Lu, J.; Chen, Z.; Lyu, J. Non-Linear Relationship Between Dietary Vitamin E Intake and Cognitive Performance in Older Adults. Public Health 2023, 219, 10–17. [Google Scholar] [CrossRef]
- Dorey, C.K.; Gierhart, D.; Fitch, K.A.; Crandell, I.; Craft, N.E. Low Xanthophylls, Retinol, Lycopene, and Tocopherols in Grey and White Matter of Brains with Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 94, 1–17. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Gu, Z.; Hou, Z.; Yu, X.; Gao, M.; Cai, T.; Gao, Y.; Xie, J.; Gu, F.; et al. Association Between Dietary Vitamin E Intake and Cognitive Decline Among Old American: National Health and Nutrition Examination Survey. Eur. Geriatr. Med. 2023, 14, 1027–1036. [Google Scholar] [CrossRef]
- Power, R.; Nolan, J.M.; Prado-Cabrero, A.; Roche, W.; Coen, R.; Power, T.; Mulcahy, R. Omega-3 Fatty Acid, Carotenoid and Vitamin E Supplementation Improves Working Memory in Older Adults: A Randomised Clinical Trial. Clin. Nutr. 2022, 41, 405–414. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Gabr, S.A.; Anwer, S.; Li, H. Associations Between Vitamin E, Oxidative Stress Markers, Total Homocysteine Levels, and Physical Activity or Cognitive Capacity in Older Adults. Sci. Rep. 2021, 11, 12867. [Google Scholar] [CrossRef] [PubMed]
- Pantzaris, M.; Loukaides, G.; Paraskevis, D.; Kostaki, E.-G.; Patrikios, I. Neuroaspis PLP10™, a Nutritional Formula Rich in Omega-3 and Omega-6 Fatty Acids with Antioxidant Vitamins Including Gamma-Tocopherol in Early Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Neurol. Neurosurg. 2021, 210, 106954. [Google Scholar] [CrossRef] [PubMed]
- Stavrinou, P.S.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; Patrikios, I.S.; Giannaki, C.D. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 2020, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Livny, A.; Schnaider Beeri, M.; Heymann, A.; Moshier, E.; Berman, Y.; Mamistalov, M.; Shahar, D.-R.; Tsarfaty, G.; Leroith, D.; Preiss, R.; et al. Vitamin E Intake Is Associated with Lower Brain Volume in Haptoglobin 1-1 Elderly with Type 2 Diabetes. J. Alzheimer’s Dis. 2020, 74, 649–658. [Google Scholar] [CrossRef]
- Casati, M.; Boccardi, V.; Ferri, E.; Bertagnoli, L.; Bastiani, P.; Ciccone, S.; Mansi, M.; Scamosci, M.; Rossi, P.D.; Mecocci, P.; et al. Vitamin E and Alzheimer’s Disease: The Mediating Role of Cellular Aging. Aging Clin. Exp. Res. 2019, 32, 459–464. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Canas, J.A.; Fanelli-Kuczmarski, M.T.; Maldonado, A.I.; Shaked, D.; Kivimaki, M.; Evans, M.K.; Zonderman, A.B. Association of Antioxidant Vitamins A, C, E and Carotenoids with Cognitive Performance Over Time: A Cohort Study of Middle-Aged Adults. Nutrients 2020, 12, 3558. [Google Scholar] [CrossRef]
- Tanprasertsuk, J.; Mohn, E.S.; Matthan, N.R.; Lichtenstein, A.H.; Barger, K.; Vishwanathan, R.; Johnson, M.A.; Poon, L.W.; Johnson, E.J. Serum Carotenoids, Tocopherols, Total n-3 Polyunsaturated Fatty Acids, and n-6/n-3 Polyunsaturated Fatty Acid Ratio Reflect Brain Concentrations in a Cohort of Centenarians. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 74, 306–314. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, H.; Zhen, J.; Dong, S.; Guo, Y.; Van Halm-Lutterodt, N.; Yuan, L. Diminished Circulating Retinol and Elevated α-TOH/Retinol Ratio Predict an Increased Risk of Cognitive Decline in Aging Chinese Adults, Especially in Subjects with ApoE2 or ApoE4 Genotype. Aging 2018, 10, 4066–4083. [Google Scholar] [CrossRef]
- Bentsen, H.; Landrø, N.I. Neurocognitive effects of an omega-3 fatty acid and vitamins E+C in schizophrenia: A randomised controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 57–66. [Google Scholar] [CrossRef]
- Basambombo, L.L.; Carmichael, P.-H.; Côté, S.; Laurin, D. Use of Vitamin E and C Supplements for the Prevention of Cognitive Decline. Ann. Pharmacother. 2016, 51, 118–124. [Google Scholar] [CrossRef]
- Sano, M.; Aisen, P.S.; Andrews, H.F.; Tsai, W.-Y.; Lai, F.; Dalton, A.J.; None, N.; None, N.; McCallion, P.; Zendell, A.; et al. Vitamin E in Aging Persons with Down Syndrome. Neurology 2016, 86, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Kryscio, R.J.; Turner, J.; Jicha, G.A.; Cooper, G.; Caban-Holt, A.; Schmitt, F.A.; Abner, E.L. Self-Reported Sleep Apnea and Dementia Risk: Findings From the Prevention of Alzheimer’s Disease with Vitamin E and Selenium Trial. J. Am. Geriatr. Soc. 2016, 64, 2472–2478. [Google Scholar] [CrossRef]
- Smesny, S.; Milleit, B.; Schaefer, M.R.; Hipler, U.-C.; Milleit, C.; Wiegand, C.; Hesse, J.; Klier, C.M.; Holub, M.; Holzer, I.; et al. Effects of Omega-3 PUFA on the Vitamin E and Glutathione Antioxidant Defense System in Individuals at Ultra-High Risk of Psychosis. Prostaglandins Leukot. Essent. Fat. Acids 2015, 101, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Lortie, J.J.; Hoffmann, H.; Page, R.; Morrell, C.; Shea, T.B. A Nutritional Formulation for Cognitive Performance in Mild Cognitive Impairment: A Placebo-Controlled Trial with an Open-Label Extension. J. Alzheimer’s Dis. 2015, 48, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Doshanjh, L.; Fishman, P.; Luo, Y.; Smyers, K.; Page, R.; Morrell, C.; et al. A Phase II Randomized Clinical Trial of a Nutritional Formulation for Cognition and Mood in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 395–405. [Google Scholar] [CrossRef]
- Muss, C.; Mosgoeller, W.; Endler, T. Neuroprotective impact of a vitamin trace element composition—A randomized, double blind, placebo controlled clinical trial with healthy volunteers. Neuro Endocrinol. Lett. 2015, 36, 31–40. [Google Scholar]
- Polidori, M.C.; Ruggiero, C.; Croce, M.F.; Raichi, T.; Mangialasche, F.; Cecchetti, R.; Pelini, L.; Paolacci, L.; Ercolani, S.; Mecocci, P. Association of Increased Carotid Intima–media Thickness and Lower Plasma Levels of Vitamin C and Vitamin E in Old Age Subjects: Implications for Alzheimer’s Disease. J. Neural Transm. 2015, 122, 523–530. [Google Scholar] [CrossRef]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of Vitamin E and Memantine on Functional Decline in Alzheimer Disease. JAMA 2014, 311, 33. [Google Scholar] [CrossRef]
- Tan, J.K.; Then, S.M.; Mazlan, M.; Jamal, R.; Ngah, W.Z. Vitamin E, γ-tocotrienol, Protects Against Buthionine Sulfoximine-Induced Cell Death by Scavenging Free Radicals in SH-SY5Y Neuroblastoma Cells. Nutr. Cancer 2016, 68, 507–517. [Google Scholar] [CrossRef]
- Azzi, A.; Gysin, R.; Kempná, P.; Munteanu, A.; Negis, Y.; Villacorta, L.; Visarius, T.; Zingg, J.M. Vitamin E mediates cell signaling and regulation of gene expression. Ann. N. Y. Acad. Sci. 2004, 1031, 86–95. [Google Scholar] [CrossRef]
- Rimbach, G.; Moehring, J.; Huebbe, P.; Lodge, J.K. Gene-Regulatory Activity of α-Tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Han, S.N. Vitamin E: Regulatory role on gene and protein expression and metabolomics profiles. IUBMB Life 2019, 71, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Ghani, S.M.A.; Goon, J.A.; Azman, N.H.E.N.; Zakaria, S.N.A.; Hamid, Z.; Ngah, W.Z.W. Comparing the effects of vitamin E tocotrienol-rich fraction supplementation and α-tocopherol supplementation on gene expression in healthy older adults. Clinics 2019, 74, e688. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Fasciolo, G.; Di Meo, S.; Venditti, P. Vitamin E Supplementation and Mitochondria in Experimental and Functional Hyperthyroidism: A Mini-Review. Nutrients 2019, 11, 2900. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Ding, Y.; Luan, W.; Shen, X.; Wang, Z.; Cao, Y. LncRNA BDNF-AS as ceRNA regulates the miR-9-5p/BACE1 pathway affecting neurotoxicity in Alzheimer’s disease. Arch. Gerontol. Geriatr. 2022, 99, 104614. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Y.; Zhou, Y.; Liu, L.; Liu, Y.; Wang, D.; Zhang, S.; Yang, M. MiR-9 Regulates the Expression of BACE1 in Dementia Induced by Chronic Brain Hypoperfusion in Rats. Cell. Physiol. Biochem. 2017, 42, 1213–1226. [Google Scholar] [CrossRef]
- Chen, M.L.; Hong, C.G.; Yue, T.; Li, H.M.; Duan, R.; Hu, W.B.; Cao, J.; Wang, Z.X.; Chen, C.Y.; Hu, X.K.; et al. Inhibition of miR-331-3p and miR-9-5p ameliorates Alzheimer’s disease by enhancing autophagy. Theranostics 2021, 11, 2395–2409. [Google Scholar] [CrossRef]
- Yao, H.; Ma, R.; Yang, L.; Hu, G.; Chen, X.; Duan, M.; Kook, Y.; Niu, F.; Liao, K.; Fu, M.; et al. MiR-9 promotes microglial activation by targeting MCPIP1. Nat. Commun. 2014, 5, 4386. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.; Zheng, Q.; Hao, S.; Yang, L.; Xia, Q.; Qi, C.; Ge, J. MicroRNAs in Alzheimer’s disease: Potential diagnostic markers and therapeutic targets. Biomed. Pharmacother. 2022, 148, 112681. [Google Scholar] [CrossRef]
- Lee, P.; Ulatowski, L.M. Vitamin E: Mechanism of transport and regulation in the CNS. IUBMB Life 2019, 71, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Goldenstein, H.; Levy, N.S.; Ward, J.A.; Costacou, T.; Levy, A.P. Haptoglobin Genotype Is a Determinant of Hemoglobin Adducts and Vitamin E Content in HDL. J. Diabetes Res. 2018, 2018, 6125420. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.F.; Yanagisawa, D.; Durani, L.W.; Hamezah, H.S.; Damanhuri, H.A.; Wan Ngah, W.Z.; Tsuji, M.; Kiuchi, Y.; Ono, K.; Tooyama, I. Tocotrienol-rich fraction modulates amyloid pathology and improves cognitive function in AβPP/PS1 mice. J. Alzheimer’s Dis. 2016, 55, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.Z.W.; Ahmad, H.F.; Ankasha, S.J.; Makpol, S.; Tooyama, I. Dietary Strategies to Mitigate Alzheimer’s Disease: Insights into Antioxidant Vitamin Intake and Supplementation with Microbiota–Gut–Brain Axis Cross-Talk. Antioxidants 2024, 13, 1504. [Google Scholar] [CrossRef]
- Tooyama, I.; Kato, T.; Taguchi, H.; Kageyama, Y.; Irie, K.; Hirahara, Y.; Yanagisawa, D. Visualization of Amyloid Oligomers in the Brain of Patients with Alzheimer’s Disease. Acta Histochem. Cytochem. 2023, 56, 87–94. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Schmölz, L.; Birringer, M.; Lorkowski, S.; Wallert, M. Complexity of vitamin E metabolism. World J. Biol. Chem. 2016, 7, 14–43. [Google Scholar] [CrossRef]
- Carroll, M.F.; Schade, D.S. Timing of Antioxidant Vitamin Ingestion Alters Postprandial Proatherogenic Serum Markers. Circulation 2003, 108, 24–31. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the re-evaluation of tocopherol-rich extract (E 306), α-tocopherol (E 307), γ-tocopherol (E 308) and δ-tocopherol (E 309) as food additives. EFSA J. 2015, 13, 4247. [Google Scholar] [CrossRef]
- Ismail, M.; Alsalahi, A.; Imam, M.U.; Ooi, D.J.; Khaza’ai, H.; Aljaberi, M.A.; Shamsudin, M.N.; Zulkifli, I. Safety and Neuroprotective Efficacy of Palm Oil and Tocotrienol-Rich Fraction From Palm Oil: A Systematic Review. Nutrients 2020, 12, 521. [Google Scholar] [CrossRef]
- Ramli, F.F.; Ali, A.; Ibrahim, N.I. Protective Effects of Tocotrienols in Cerebral and Myocardial Ischemia-Reperfusion Injury: A Systematic Review. Appl. Sci. 2021, 11, 7994. [Google Scholar] [CrossRef]
- Sen, C.K.; Rink, C.; Khanna, S. Palm Oil–Derived Natural Vitamin E A-Tocotrienol in Brain Health and Disease. J. Am. Coll. Nutr. 2010, 29, 314S–323S. [Google Scholar] [CrossRef]
- Hamezah, H.S.; Durani, L.W.; Yanagisawa, D.; Ibrahim, N.F.; Aizat, W.M.; Makpol, S.; Wan Ngah, W.Z.; Damanhuri, H.A.; Tooyama, I. Modulation of proteome profile in AβPP/PS1 mice hippocampus, medial prefrontal cortex, and striatum by palm oil derived tocotrienol-rich fraction. J. Alzheimer’s Dis. 2019, 72, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, F.; Abdullah, A.; Makpol, S. Cellular Uptake and Bioavailability of Tocotrienol-Rich Fraction in SIRT1-Inhibited Human Diploid Fibroblasts. Sci. Rep. 2018, 8, 10471. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Khanna, S.; Roy, S.; Ezziddin, O.; Sen, C.K. Natural vitamin E α-tocotrienol: Retention in vital organs in response to long-term oral supplementation and withdrawal. Free Radic. Res. 2006, 40, 763–771. [Google Scholar] [CrossRef]
- Khanna, S.; Patel, V.; Rink, C.; Roy, S.; Sen, C.K. Delivery of orally supplemented α-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic. Biol. Med. 2005, 39, 1310–1319. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Enhanced solubility and oral bioavailability of γ-tocotrienol using a self-emulsifying drug delivery system (SEDDS). Lipids 2014, 49, 819–829. [Google Scholar] [CrossRef]
- Mohamad, N.V. Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System. Pharmaceuticals 2023, 16, 1403. [Google Scholar] [CrossRef]
- Lee, Y.Z.; Seow, E.K.; Lim, S.C.; Yuen, K.H.; Khan, N.A.K. Formulation of oily tocotrienols as a solid self-emulsifying dosage form for improved oral bioavailability in human subjects. J. Drug Deliv. Sci. Technol. 2022, 76, 103752. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Tejo, B.A.; May, C.Y.; Hassan, H.A.; Subramaniam, K.; Hung, L.C.; Basri, M.; Ismail, R. Nanostructured Lipid Carriers (NLC) for Efficient Delivery of Palm Phytonutrient. J. Oil Palm Res. 2011, 26, 232–239. [Google Scholar]
- Fu, J.-Y.; Meganathan, P.; Gunasegaran, N.; Tan, D.M.Y. Effect of nano-delivery systems on the bioavailability and tissue biodistribution of vitamin E tocotrienols. Food Res. Int. 2023, 171, 113048. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol. Asp. Med. 2007, 28, 692–728. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef]
- Jang, Y.; Park, N.Y.; Rostgaard-Hansen, A.L.; Huang, J.; Jiang, Q. Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice. Free Radic. Biol. Med. 2016, 95, 190–199. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
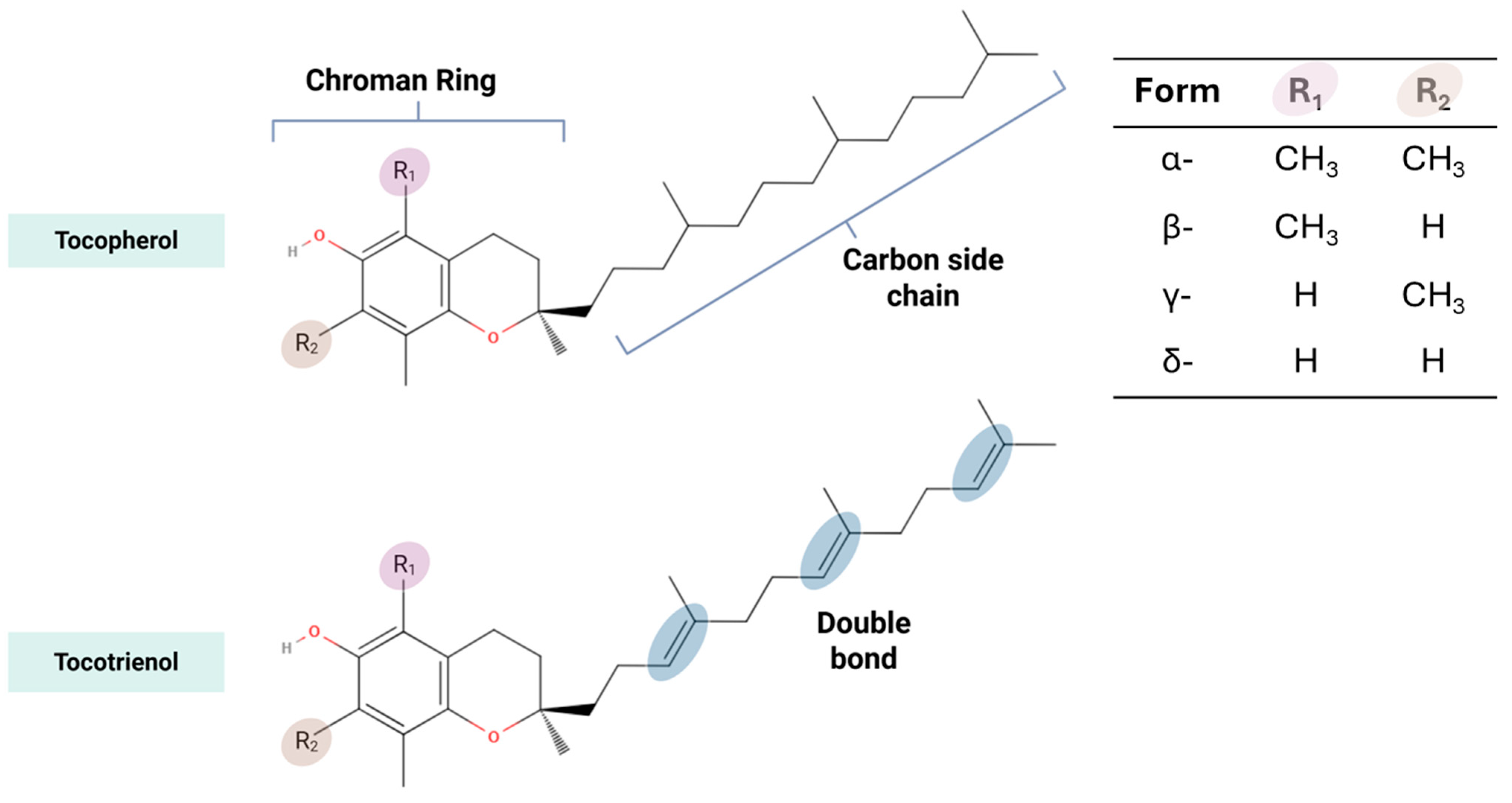

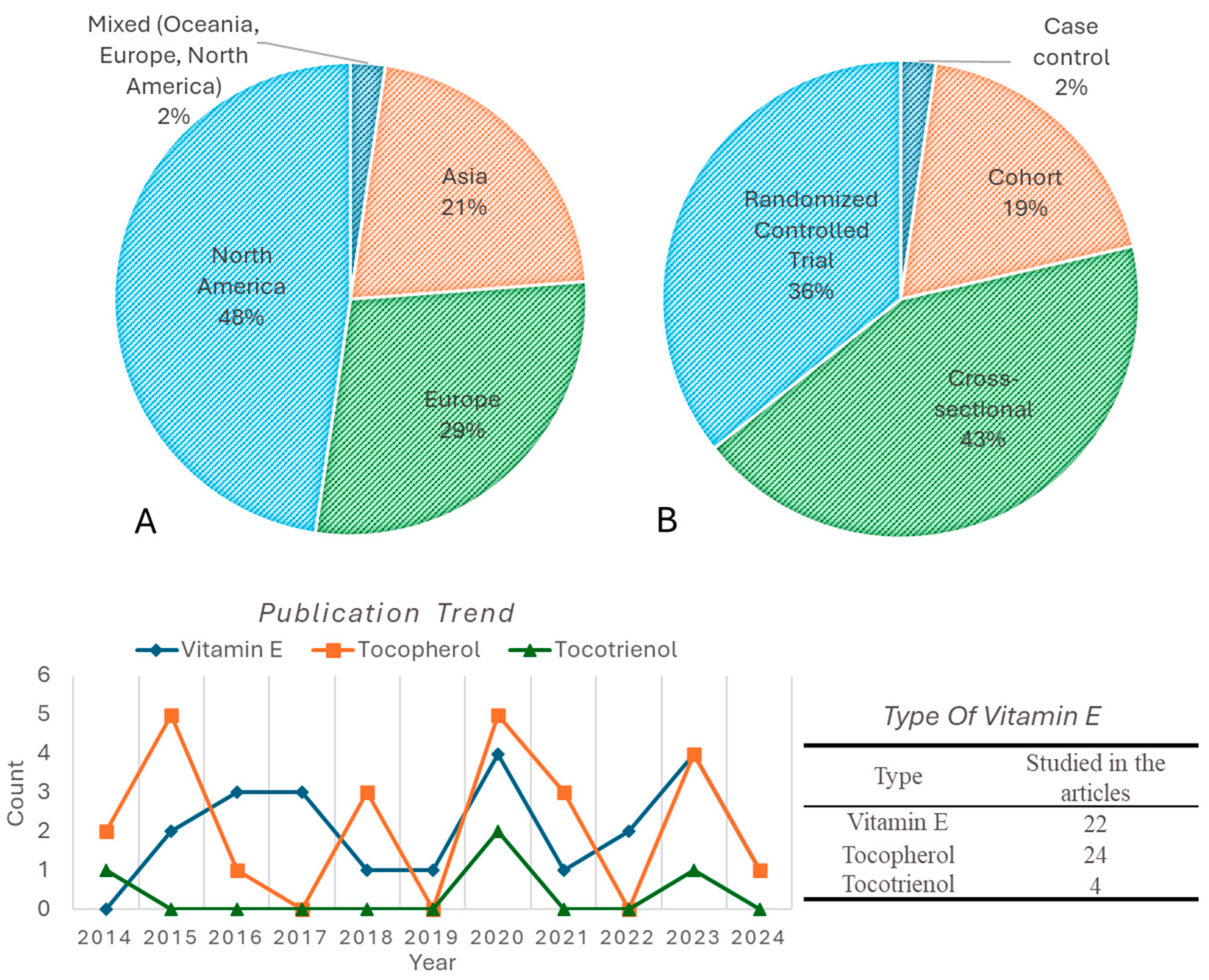
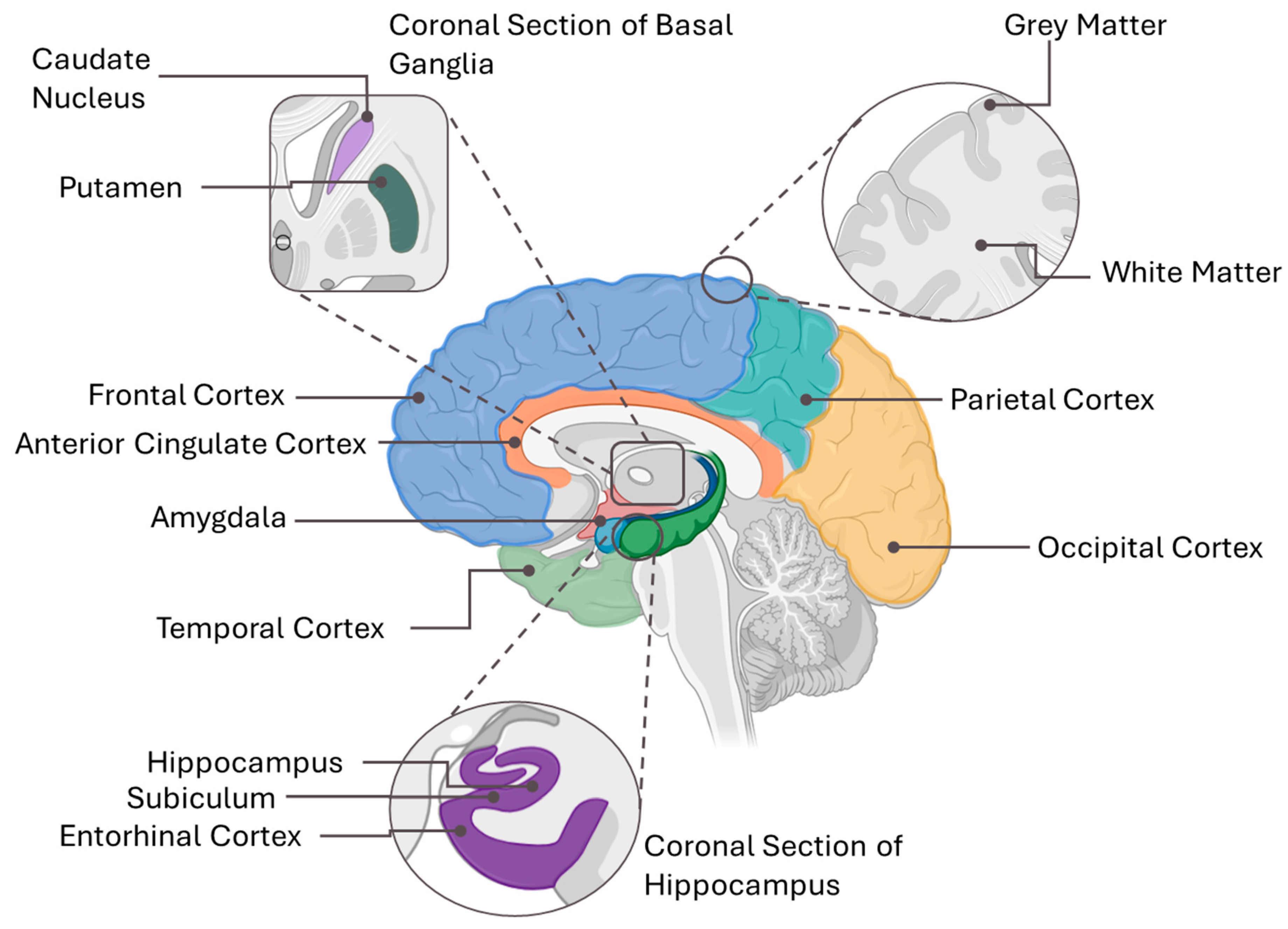
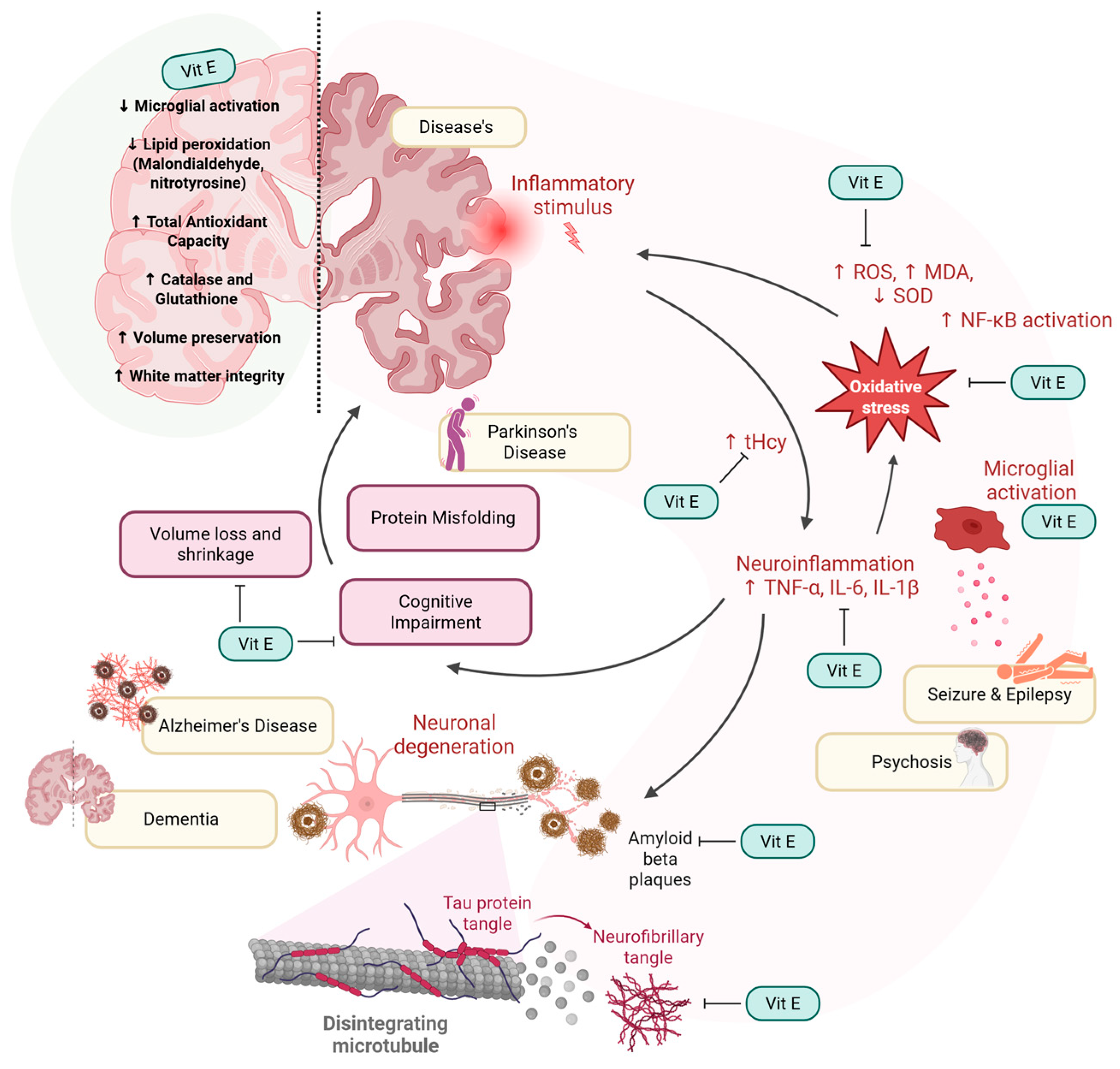
| Source (Year) | Study Design | Age Group (Years) | Vitamin E Form | Vitamin E Assessment Method | Primary Study Focus | Brain-Health Outcomes Measured | Brain-Health Themes |
|---|---|---|---|---|---|---|---|
| Chan et al. (2024) [19] | Cross-sectional | 100+ | α-tocopherol, γ-tocopherol | Survey | Amyloid plaques; Neurofibrillary tangles (NFT) |
|
|
| Hao et al. (2024) [44] | Cross-sectional | 40+ | Vitamin E | Survey | Parkinson’s disease | Parkinson’s disease prevalence (measured through anti-Parkinson medication use) | Neuroprotection and Neurodegenerative Diseases |
| Zhang et al. (2023) [45] | Cross-sectional | 65+ | α-tocopherol, | Survey | Cognitive performance |
| Cognitive Performance |
| Liu et al. (2023) [46] | Cohort | 65+ | α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol | Survey | Cognitive performance; APOE ε4 allele |
|
|
| Li et al. (2023) [47] | Cross-sectional | 60+ | Vitamin E | Survey | Cognitive performance |
| Cognitive Performance |
| Dorey et al. (2023) [48] | Cross-sectional | 56+ | α-tocopherol, δ-tocopherol, γ-tocopherol | Brain extract | Alzheimer’s disease |
|
|
| Boccardi et al. (2023) [13] | Cross-sectional | 70+ | α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol, α-tocotrienol, β-tocotrienol, δ-tocotrienol, γ-tocotrienol | Blood | miRNAs; Alzheimer’s disease; inflammation |
|
|
| Zhang et al. (2023) [49] | Cross-sectional | 60+ | Vitamin E | Survey | Cognitive performance |
|
|
| Paganini-Hill et al. (2023) [10] | Cohort | 90+ | Vitamin E | Survey; brain autopsies | Alzheimer’s disease; amyloid plaques; Neurofibrillary tangles |
|
|
| Liu et al. (2023) [46] | Cohort | 60+ | Vitamin E | Survey | Dementia; cognitive performance |
| Neuroprotection and Neurodegenerative Diseases |
| Power et al. (2022) [50] | Randomized Controlled Trial | 65+ | α-tocopherol (RRR-α-tocopherol) [as a part of the mixture of intervention] | Administered | Cognitive performance; |
|
|
| Nolan et al. (2022) [2] | Randomized Controlled Trial | 79+ | α-tocopherol (RRR-α-tocopherol) [as a part of the mixture of intervention] | Administered | Alzheimer’s disease |
| Neuroprotection and Neurodegenerative Diseases |
| Alghadir et al. (2021) [51] | Cross-sectional | 56–81 | α-tocopherol, γ-tocopherol | Blood | Cognitive performance; oxidative stress |
|
|
| Tanprasertsuk et al. (2021) [12] | Cross-sectional | 98+ | α-tocopherol, γ-tocopherol | Brain | Amyloid plaques; Neurofibrillary tangles; Alzheimer’s disease |
|
|
| Pantzaris et al. (2021) [52] | Randomized Controlled Trial | 40–75 | Vitamin E, γ-tocopherol | Administered | Parkinson’s disease |
| Neuroprotection and Neurodegenerative Diseases |
| Stavrinou et al. (2020) [53] | Randomized Controlled Trial | 65+ | Vitamin E, γ-tocopherol | Administered | Cognitive performance |
|
|
| Sekikawa et al. (2020) [38] | Randomized Controlled Trial | 40+ | Tocotrienol | Administered | Cognitive performance |
| Cognitive Performance |
| Livny et al. (2020) [54] | Cross-sectional | 65+ | Vitamin E, α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol | Survey | Haptoglobin |
|
|
| Leeuw et al. (2020) [1] | Cross-sectional | 88.5 ± 6.0 | α-tocopherol, γ-tocopherol | Survey; brain autopsies | Microglia |
|
|
| Leeuw et al. (2020) [7] | Cross-sectional | 88.5 ± 6.0 | α-tocopherol, γ-tocopherol | Survey; brain autopsies | Presynaptic protein |
|
|
| Casati et al. (2020) [55] | Cross-sectional | 70+ | Vitamin E, Tocopherol, α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol, Tocotrienol, α-tocotrienol, β-tocotrienol, δ-tocotrienol, γ-tocotrienol | Blood | Alzheimer’s disease |
|
|
| Beydoun et al. (2020) [56] | Cohort | 30–65 | Vitamin E | Survey | Cognitive performance |
| Cognitive Performance |
| Schirinzi et al. (2019) [11] | Case–control | 60+ | Vitamin E | Survey | Parkinson’s disease | Neurodegenerative Disease Risk (PD prevalence) | Neuroprotection and Neurodegenerative Diseases |
| Tanprasertsuk et al. (2018) [57] | Cross-sectional | 98+ | α-tocopherol, γ-tocopherol | Blood; brain | Cognitive performance |
|
|
| Kim et al. (2018) [16] | Cross-sectional | 60–79 | Vitamin E, α-tocopherol, γ-tocopherol | Blood | Cognitive performance | Mini-Mental State Examination for Dementia Screening (MMSE-DS) |
|
| Huang et al. (2018) [58] | Cross-sectional | 55–80 | α-tocopherol, γ-tocopherol | Blood; survey | Cognitive performance; ApoE |
|
|
| Bentsen et al. (2017) [59] | Randomized Controlled Trial | 18–39 | Vitamin E | Administered | Schizophrenia |
|
|
| Kryscio et al. (2017) [18] | Cohort | 60+ | Vitamin E | Administered | Alzheimer’s disease |
|
|
| Basambombo et al. (2017) [60] | Cohort | 65+ | Vitamin E | Survey | Alzheimer’s disease; cognitive performance |
|
|
| Sano et al. (2016) [61] | Randomized Controlled Trial | 50+ | Vitamin E | Administered | Cognitive performance; Down syndrome; dementia |
|
|
| Kuchan et al. (2016) [20] | Cross-sectional | 5 to 488 days old | α-tocopherol | Brain | Level of Vitamin E in infant brain |
|
|
| Ding et al. (2016) [62] | Cohort | 60+ | Vitamin E | Administered | Alzheimer’s disease; dementia; sleep apnea |
| Neuroprotection and Neurodegenerative Diseases |
| Mehvari et al. (2016) [4] | Randomized Controlled Trial | 20–50 | Vitamin E | Administered | Seizure; epilepsy |
|
|
| Smesny et al. (2015) [63] | Randomized Controlled Trial | 13–25 | Vitamin E, α-tocopherol, δ-tocopherol, γ-tocopherol | Administered | Psychosis; oxidative stress; |
|
|
| Remington et al. (2015) [64] | Randomized Controlled Trial | 65.9 ± 11.3 | α-tocopherol | Administered | Cognitive performance |
|
|
| Remington et al. (2015) [65] | Randomized Controlled Trial | 77.8 ± 8.4 | α-tocopherol | Administered | Cognitive performance; mood; Alzheimer’s disease |
|
|
| Muss et al. (2015) [66] | Randomized Controlled Trial | 18 to 90 | Vitamin E | Administered | Neuroprotective |
|
|
| Morris et al. (2015) [9] | Cohort | 88.5 ± 5.9 | α-tocopherol, γ-tocopherol | Brain; Blood; Survey | Alzheimer’s disease |
| Brain Structure and Function |
| Li et al. (2015) [8] | Randomized Controlled Trial | 60–75 | α-tocopherol | Administered | Cognitive performance |
|
|
| Polidori et a l. (2014) [67] | Cross-sectional | 65+ | α-tocopherol | Blood | Alzheimer’s disease |
|
|
| Gopalan et al. (2014) [3] | Randomized Controlled Trial | 35+ | Tocotrienol | Administered | Neuroprotective; brain |
|
|
| Dysken et al. (2014) [68] | Randomized Controlled Trial | 53+ | α-tocopherol | Administered | Alzheimer’s disease; cognitive performance |
|
|
| Source (Year) | Vitamin E Form | Tested Markers | Sample Origin | Outcomes |
|---|---|---|---|---|
| Boccardi et al. (2023) [13] | α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol, α-Tocotrienol, β-tocotrienol, δ-tocotrienol, γ-tocotrienols | Cytokines: EGF, EOTAXIN, G-CSF, GM-CSF, INF-α2, IFN-γ, IL-10, IL-12p40 IL-12p70, IL-13, IL-15, IL-17, IL-1RA, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IP-10, MCP-1, MIP-1, MIP-1α, TNF-α, TNF-β, VEGF, RANTES Exosomal miRNAs: let7f5p, miR-9, miR-15a, miR-21, miR29-b, miR-122, miR-132, miR29-a, miR-128, miR-491, miR-146, miR-34, miR-87 | Blood | AD patients: ↓ α-Tocopherol ↑ G-CSF, GM-CSF, INF-α2, IL-3, IL-8 ↓ miR-21, miR29-b, miR-9, miR-122, miR-132 Correlation of miR-122 and plasma level of GM-CSF, INF-α2, IL-1α, IL-8, and MIP-1β Correlation of miR-122 and α-tocopherol and AD (AD: ↓ α-tocopherol, ↓ miR-122) No significant difference in tocotrienol levels between AD and healthy |
| Alghadir et al. (2021) [51] | α-tocopherol, γ-tocopherol | Total homocysteine (tHcy), Nitric oxide; NO Total oxidant capacity | Blood | Cognitive Decline Patients: ↑ Total Homocysteine (tHcy) ↓ Total oxidant capacity ↑ physical activity = ↓ Total Homocysteine (tHcy) No correlation between vitamin E and tHcy |
| Leeuw et al. (2020) [1] | α-tocopherol, γ-tocopherol | Microglia activation | Brain tissue | ↑ brain α- and γ-tocopherol = ↓ activated microglia density (but not in subcortical brain regions) |
| Casati et al. (2020) [55] | Vitamin E, Tocopherol, α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol, Tocotrienol, α-Tocotrienol, β-tocotrienol, δ-tocotrienol, γ-tocotrienols | α-Tocopherylquinone/α-Tocopherol Ratio (Oxidative Stress Marker) 5-Nitro-γ-Tocopherol/γ-Tocopherol Ratio (Nitrosative Stress Marker) Leukocyte Telomere Length (LTL) | Blood | AD patients: ↓ α-, β-, γ- and δ-tocopherol, α- and δ-tocotrienol, Total tocopherols, total tocotrienols and total vitamin E ↑ α-tocopherylquinone/α-tocopherol and 5-nitro-γ-tocopherol/γ-tocopherol Telomere length: ↑ γ-Tocopherol, total tocopherols, and total Vitamin E levels, ↑ telomere length |
| Tanprasertsuk et al. (2018) [57] | α-tocopherol, γ-tocopherol | Level of antioxidants: [α-Tocopherol (αT), γ-Tocopherol (γT), Lutein, Zeaxanthin, Cryptoxanthin, β-Carotene, Lycopene] n-6/n-3 Polyunsaturated Fatty Acid (PUFA) Ratio | Blood; Brain tissue | Centenarians: ↑ α-Tocopherol, Lutein, and β-Carotene, ↑ cognitive performance ↑ n-6/n-3 PUFA, ↓ cognitive performance |
| Polidori et al. (2014) [67] | α-tocopherol | SOD, GPX | Blood | ↓ Vitamin C and E are associated with Carotid Intima-Media Thickness (C-IMT) |
| Bentsen et al. (2017) [59] | Vitamin E | Serum α-Tocopherol level Total antioxidant status s-F2-Isoprostane Malondialdehyde (MDA) | Blood | High PUFA: ↑ serum α-Tocopherol, ↑ TAS, ↑ DHA levels |
| Smesny et al. (2015) [63] | Vitamin E, α-tocopherol, δ-tocopherol, γ-tocopherol | Vitamin E level Glutathione markers (GSHt, GSHr and GSSG) | Blood | PUFA-E: ↑ α-Tocopherol ↓ GSHt |
| Muss et al. (2015) [66] | Vitamin E | Homocysteine (Hcy) Superoxide Dismutase (SOD) Lipid Peroxidation Nitrotyrosine Antioxidative capacity | Blood | Vitamin E: ↓ HCY, SOD, lipid peroxidation, nitrotyrosine ↑ Antioxidative capacity |
| Mehvari et al. (2016) [4] | Vitamin E | Total Antioxidant capacity (TAC) Catalase Glutathione Malondialdehyde | Blood | Vitamin E: ↑TAC, Catalase, Glutathione |
| Authors (Year) | Vitamin E Form | Outcome |
|---|---|---|
| Kuchan et al. (2016) [20] | α-tocopherol | Level in Infant brain Frontal Cortex (FC) ↑ Mean RRR-α-tocopherol concentration: 10.5 μg/g Synthetic α-tocopherol concentrations (all-rac-α-tocopherol): RRS: 1.0–1.5 μg/g; RSR: 0.8–1.0 μg/g; RSS: 0.7–0.9 μg/g; Σ2S: 0.2–0.3 μg/g Hippocampus (HPC) ↑ Mean RRR-α-tocopherol concentration: 6.8 μg/g Synthetic α-tocopherol levels: RRS: ~1.0 μg/g; RSR: ~0.9 μg/g; RSS: ~0.8 μg/g; Σ2S: 0.3 μg/g Visual Cortex (VC) ↑ Mean RRR-α-tocopherol concentration: 5.5 μg/g Synthetic α-tocopherol levels: RRS: ~1.0 μg/g; RSR: ~0.8 μg/g; RSS: ~0.7 μg/g; Σ2S: 0.3 μg/g |
| Tanprasertsuk et al. (2018) [57] | α-tocopherol, γ-tocopherol | Level in Centenarians Frontal cortex: α-tocopherol: 8.3 ± 4.1 μg/g γ-tocopherol: 1.2 ± 1.1 μg/g Temporal cortex α-tocopherol: 7.9 ± 3.8 μg/g γ-tocopherol: 1.0 ± 0.9 μg/g |
| Tanprasertsuk et al. (2021) [12] | α-tocopherol, γ-tocopherol | Neurofibrillary tangle (NFT) and Neural plaque (NP) In different brain region vs. α-tocopherol, γ-tocopherol Early-affected regions in Alzheimer’s disease (Braak I–II and III–IV stages): Amygdala (Amy), Entorhinal Cortex (Ent), Hippocampus (Hip, CA1 region), Subiculum (Sub) ↑ α-Tocopherol correlated with lower NFT counts. No significant correlation of α-Tocopherol with NP No significant correlation of γ-Tocopherol with NFT and NP Later-affected regions (Braak V–VI stage): Frontal Cortex (FC, Brodmann Area 9), Temporal Cortex (TC, Brodmann Areas 21–22), Parietal Cortex (PC, Brodmann Areas 39–40) No significant correlation of α-Tocopherol with NFT counts No significant correlation of α-Tocopherol with NP No significant correlation of γ-Tocopherol with NFT and NP Braak III–IV (limbic stage) had lower α-tocopherol levels than those in Braak I-II (trans entorhinal stage) |
| Leeuw et al. (2020) [7] | α-tocopherol, γ-tocopherol | Brain tocopherol level vs. presynaptic protein of the elderly Midfrontal Cortex α-tocopherol: 231.8 pmol/mg not significant γ-tocopherol: 57.8 pmol/mg γ-Tocopherol showed significant positive associations with: SNARE protein composite, complexin-I, Complexin-II, Synaptotagmin-synaptophysin composite, Septin-5 Inferior Temporal Cortex α-tocopherol: 172.9 pmol/mg not significant γ-tocopherol: 56.7 pmol/mg not significant |
| Leeuw et al. (2020) [1] | α-tocopherol, γ-tocopherol | Brain tocopherol level vs. activated microglia of the elderly. Cortical tocopherol: α-tocopherol: 232.2 pmol/mg (IQR: 76.0–356.4) γ-tocopherol: 57.1 pmol/mg (IQR: 42.9–92.5) Cortical microglia density: Stage I/II/III (Total Count): 153.2 cells/mm2 (IQR: 111.2–194.0) Stage II/III (Activated): 3.9 cells/mm2 (IQR: 1.2–11.7) Stage III (Macrophage-like): 0.8 cells/mm2 (IQR: 0.1–2.9) Subcortical: α-tocopherol: 158.7 pmol/mg (IQR: 105.0–286.6) γ-tocopherol: 63.6 pmol/mg (IQR: 48.4–81.3) Subcortical microglia density: Stage I/II/III (Total Count): 209.2 cells/mm2 (IQR: 175.8–244.1) Stage II/III (Activated): 4.3 cells/mm2 (IQR: 1.9–9.5) Stage III (Macrophage-like): 1.1 cells/mm2 (IQR: 0.4–2.4) Cortical regions had higher α-tocopherol levels than subcortical regions. Microglia activation suppression is significant in cortical regions, not in subcortical regions |
| Morris et al. (2015) [9] | α-tocopherol, γ-tocopherol | Brain tocopherol levels and AD neuropathology (amyloid plaque and NFT) Brain region Inferior Temporal Cortex Midfrontal Cortex Posterior Putamen Ventromedial Caudate Nucleus Across these regions: ↑ γ-tocopherol levels, ↓ Alzheimer’s pathology (plaques and NFT) α-tocopherol alone did not provide significant protection. |
| Dorey et al. (2023) [48] | α-tocopherol, δ-tocopherol, γ-tocopherol | Regional Brain Tocopherol Levels in Alzheimer’s Disease: AD: ↓ tocopherol level in grey and white matter (white matter greater deficiency) |
| Chan et al. (2024) [19] | α-tocopherol, γ-tocopherol | Tocopherol Distribution of Centenarians Centenarians: Temporal cortex—↑ Total α-tocopherol concentration RRR-αT was the dominant stereoisomer in all subjects (>50% of total α-tocopherol). Brain γ-tocopherol was much lower than α-tocopherol (not significantly correlated with plaques/tangles). |
| Paganini-Hill et al. (2023) [10] | Vitamin E | Alzheimer’s Disease Brain Neuropathology Brain weight None/Low ADNC Group (n = 70): 1138 ± 125 g Intermediate/High ADNC Group (n = 180): 1117 ± 123 g p-value: 0.23 (Not significant) Low, high and any intake of vitamin E were associated with a significantly reduced risk of developing Alzheimer’s disease brain pathology (amyloid plaques, NFT, neuritic plaques) |
| Livny et al. (2020) [54] | Vitamin E, α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol | Vitamin E Intake vs. Brain Volume and Haptoglobin Genotype Inferior Frontal Gyrus Volume: Hp 1-1 carriers: significant decrease in volume with higher Vitamin E intake. Hp 2-1 carriers: significant increase in volume with higher Vitamin E intake. Hp 2-2 carriers: no significant changes. Other Brain Regions: (Middle and Superior Frontal Gyri, Middle Temporal Gyrus) No significant changes in any Hp genotype. White Matter Hyperintensities (WMH): Hp 1-1 carriers: higher WMH volume, indicating greater white matter damage. Hp 2-2 carriers: lower WMH volume, suggesting better white matter integrity. Vitamin E Interaction with Hp Genotype: A significant interaction was observed between Vitamin E intake and Hp genotype on brain volume (p = 0.011). This suggests genotype-specific effects of Vitamin E on brain structure |
| Mehvari et al. (2016) [4] | Vitamin E | Vitamin E Decreases Seizure Frequency and Interictal Sharp Waves in Epilepsy 50% of patients in the Vitamin E group showed a decrease in positive EEG findings (reduction in interictal epileptiform sharp waves). In contrast, only 12.1% of patients in the placebo group showed similar improvements. Before Treatment: Both groups had a median of 2 seizures per month. After 6 Months: Vitamin E Group: Seizure frequency decreased to 1 seizure per month (p < 0.0001). Control Group: Seizure frequency remained at 2–3 seizures per month (p = 0.008). |
| Gopalan et al. (2014) [3] | Tocotrienol | Tocotrienol supplementation significantly reduced the progression of WMLs over two years. Placebo group: WMLs increased by 23.3% after 2 years. Tocotrienol group: WML volume remained unchanged (negligible change of −1.9%). Tocotrienol supplementation protected against WML progression, suggesting structural brain protection. |
| Source (Year) | Vitamin E Form | Vitamin E Assessment Method | Cognitive Effect Outcomes |
|---|---|---|---|
| Chan et al. (2024) [19] | α-tocopherol, γ-tocopherol | Survey | ◀▶ α-tocopherol levels between subjects with and without dementia. ▼: No direct association between α-tocopherol stereoisomers and premortem cognitive function |
| Boccardi et al. (2023) [13] | α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol, α-Tocotrienol, β-tocotrienol, δ-tocotrienol, γ-tocotrienol | Blood | Higher plasma α-Tocopherol level: ▲ Cognitive ability, daily living skills, instrumental tasks ▲ miR-122 expression ▲ inflammatory cytokines. |
| Alghadir et al. (2021) [51] | α-tocopherol, γ-tocopherol | Blood | ▲ cognitive capacity has higher α-tocopherol and γ-tocopherol levels ◀▶ cognitive function in higher vitamin E. |
| Casati et al. (2020) [55] | Vitamin E, Tocopherol, α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol, Tocotrienol, α-Tocotrienol, β-tocotrienol, δ-tocotrienol, γ-tocotrienols | Blood | AD patients: ▼ lower plasma Vitamin E levels (α-, β-, γ-, δ-tocopherol and tocotrienols). ▼ poorer cognitive function ▼ Higher oxidative/nitrosative damage markers higher nitrosative stress (5-nitro-γ-tocopherol/γ-tocopherol ratio), linked to cognitive decline |
| Tanprasertsuk et al. (2018) [57] | α-tocopherol, γ-tocopherol | Blood; brain | ▲ cognitive performance in higher Serum α-Tocopherol (Vitamin E) group ◀▶ cognitive performance in Serum γ-Tocopherol |
| Kim et al. (2018) [16] | Vitamin E, α-tocopherol, γ-tocopherol | Blood | ▲ cognitive effect in Beta-Gamma Tocopherol (Middle Tertile) ▲ cognitive effect in Beta-Gamma Tocopherol in men ▲ cognitive effect in Beta-Gamma Tocopherol in non-drinkers ▲ cognitive effect in Beta-Gamma Tocopherol in non-smokers ◀▶ cognitive performance in Vitamin A, Vitamin C and α—tocopherol. |
| Huang et al. (2018) [58] | α-tocopherol, γ-tocopherol | Blood; survey | ▼ poorer memory, executive function, and overall cognition. MCI subjects had higher α-tocopherol levels (MoCA scores) |
| Kuchan et al. (2016) [20] | α-tocopherol | Brain | Level of vitamin E in infant brain |
| Tanprasertsuk et al. (2021) [12] | α-tocopherol, γ-tocopherol | Brain | ▲ Lower NFT count in early-affected brain—higher α-Tocopherol, ▲ Braak I-II in higher α-Tocopherol, ◀▶ NFT Counts in Late-Affected Brain Regions of α-Tocopherol ◀▶ α-Tocopherol vs. MMSE Scores ◀▶ γ-Tocopherol vs. NFT Counts ◀▶ γ-Tocopherol vs. MMSE Scores |
| Source (Year) | Vitamin E Form | Vitamin E Assessment Method | Doses of Vitamin E Supplementation | Cognitive Effect Outcomes |
|---|---|---|---|---|
| Power et al. (2022) [50] | α-tocopherol (RRR-α-tocopherol) [as a part of mixture of intervention] | Administered | Co-supplementation: RRR-α-Tocopherol: 15 mg/day | ◀▶ global cognition, episodic memory. ▲ working memory, executive function (attention, processing speed), language skills |
| Stavrinou et al. (2020) [53] | Vitamin E, γ-tocopherol | Administered | Co-supplementation: α-Tocopherol (Vitamin E): 22 mg/day γ-Tocopherol: 760 mg/day | Post supplementation: ▲ global cognition, memory performance ▲ executive function and processing speed ◀▶ cognitive flexibility ▲ language and fluency ▲ visuospatial abilities. |
| Sekikawa et al. (2020) [38] | Tocotrienol | Administered | Co-supplementation: Tocotrienols: 50 mg/day | ▲ composite memory, verbal memory ◀▶ visual memory, executive function, processing speed, reaction time, cognitive flexibility, complex attention, social acuity, working memory, sustained attention, motor speed ▲ reduction in memory complaints |
| Bentsen et al. (2017) [59] | Vitamin E | Administered | Co-supplementation: RRR-α-tocopherol: 364 mg/day | ▼ in high PUFA patients ◀▶ working memory |
| Kryscio et al. (2017) [18] | Vitamin E | Administered | Co-supplementation: Vitamin E (α-tocopherol): 400 IU/day | ◀▶ dementia incidence ◀▶ memory function (MIS, TICS-m, CERAD) ◀▶ executive function ◀▶ learning and processing speed ◀▶ attention and decision-making ◀▶ overall cognitive decline prevention |
| Sano et al. (2016) [61] | Vitamin E | Administered | Vitamin E: 2000 IU/day | ◀▶ in rate of decline, placebo vs. treatment ◀▶ in recognition, recall, memory and cognitive function, vocabulary test, behavior, function, and orientation ◀▶ dementia progression and cognitive decline ◀▶ global cognitive function |
| Remington et al. (2015) [64] | α-tocopherol | Administered | Co-supplementation α-tocopherol: 30 IU | ▲ overall cognitive function ◀▶ executive function ▲ reduce the risk of cognitive decline |
| Remington et al. (2015) [65] | α-tocopherol | Administered | Co-supplementation α-tocopherol: 30 IU | ▲ executive function, memory, and cognition, behavioral and mood, stabilized cognitive function ▲ improvement in delayed-start treatment |
| Nolan et al. (2022) [2] | α-tocopherol (RRR-α-tocopherol) [as a part of mixture of intervention] | Administered | Co-supplementation α-tocopherol: 15 mg | ▲ slower cognitive decline, improvement in cognitive category, less decline in dementia severity, ▲ higher stability in dementia progression ▲ caregiver-reported memory: significant memory preservation |
| Li et al. (2015) [8] | α-tocopherol | Administered | Co-supplementation: Vitamin E (VE): 200 mg/day | ◀▶ general cognitive function (memory, attention, language, orientation, and problem-solving ability) ◀▶ memory, calculation, and language comprehension. ◀▶ no reduction in plasma Aβ Levels and E2 levels |
| Dysken et al. (2014) [68] | α-tocopherol | Administered | Co-supplementation: Vitamin E: 2000 IU/day | ◀▶ global cognitive function, including memory, attention, and orientation. (slightly slow decline, but no clinical difference) ▲ cognitive functions such as memory, language, and praxis ◀▶ after adjustment no statistically significant difference) |
| Zhang et al. (2023) [45] | α-tocopherol, | Survey | Average intake: Vitamin E: 8.1 mg/day | Higher α-Tocopherol Intake: ▲ cognitive performance: memory performance, verbal fluency, semantic memory, processing speed, attention, working memory |
| Liu et al. (2023) [46] | α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol | Survey | Total Vitamin E (from food only) Range (Q1–Q5 quintiles): Lowest quintile (Q1): ~4.15 mg/day Highest quintile (Q5): ~7.78 mg/day Maximum observed intake (from outliers): up to 43.45 mg/day α-Tocopherol (main form studied) Range: Q1: 5.52 mg/day Q5: 9.12 mg/day Maximum observed: up to 45.2 mg/day Other tocopherols: β-Tocopherol: up to ~2.91 mg/day γ-Tocopherol: up to ~30.2 mg/day δ-Tocopherol: up to ~9.76 mg/day | ▲ global cognitive function, ▲ episodic memory, ▲ perceptual speed, ▲ cognitive performance, ▲ α-tocopherol protective effect in cognitive performance of APOEɛ4 Carriers. ◀▶ δ-tocopherol protective effect in cognitive performance of APOEɛ4 carriers. |
| Li et al. (2023) [47] | Vitamin E | Survey | Quartile Vitamin E Intake Range (mg/day) Q1: 0.06–2.188 mg/day Q2: 2.188–3.47 mg/day Q3: 3.47–5.442 mg/day Q4: 5.442–57.475 mg/day | ▲ memory (short-term and long-term recall), executive function and verbal fluency, processing speed and verbal fluency, processing speed and attention, overall cognitive function in the higher vitamin E intake group ▲ cognitive performance on the higher vitamin E intake group |
| Livny et al. (2020) [54] | Vitamin E, α-tocopherol, β-tocopherol, δ-tocopherol, γ-tocopherol | Survey | Reported Intake Levels (from diet, not supplements): Hp 1-1: 12.62 mg/day (mean) Hp 2-1: 14.77 mg/day (mean) Hp 2-2: 15.05 mg/day (mean) | ◀▶ on cognitive status for each Hp ▼ brain volume reduction for Hp 2-1 |
| Beydoun et al. (2020) [56] | Vitamin E | Survey | Mean intake across all participants: 6.48 ± 4.62 mg/day of α-tocopherol (from food only). By carotenoid intake tertiles: T1 (lowest carotenoid intake): 4.64 ± 3.32 mg/day T2 (medium carotenoid intake): 6.39 ± 4.26 mg/day T3 (highest carotenoid intake): 8.46 ± 5.30 mg/day | ▲ verbal memory in high carotenoid intake patients ◀▶ verbal memory in low carotenoid intake patients ▲ slowing down cognitive decline ◀▶ on CDT (executive function test) ▲ working memory in high carotenoid intake patients ◀▶ brief test of attention (BTA) ◀▶ psychomotor speed |
| Basambombo et al. (2017) [60] | Vitamin E | Survey | Vitamin E intake was self-reported, but no information on the dose. | ▲ reduced risk of dementia ▲ reduced risk of AD ▲ better cognitive performance over time ▲ slower rate of cognitive decline ◀▶ cognitive impairment, not dementia (CIND) |
| Zhang et al. (2023) [49] | Vitamin E | Survey | Intake was analyzed by quartiles: Q1 (Lowest): ≤4.925 mg/day Q2: 4.925 to ≤7.25 mg/day Q3: 7.25 to ≤10.435 mg/day Q4 (Highest): >10.435 mg/day | The higher intake of Vitamin E group has ▲ memory (short-term and long-term recall), ▲ verbal fluency and executive function, ▲ processing speed and function, ▲ overall cognitive performance |
| Paganini-Hill et al. (2023) [10] | Vitamin E | Survey; Brain autopsies | Vitamin E intake was self-reported, but no information on the dose. | ▲ lower odds of developing high AD neuropathology ▲ less likely to experience cognitive impairment and dementia ◀▶ similar benefits between low and high doses |
| Liu et al. (2023) [46] | Vitamin E | Survey | Total Vitamin E (from food only) Range (Q1–Q5 quintiles): Lowest quintile (Q1): ~4.15 mg/day Highest quintile (Q5): ~7.78 mg/day Maximum observed intake (from outliers): up to 43.45 mg/day α-Tocopherol (main form studied) Range: Q1: 5.52 mg/day Q5: 9.12 mg/day Maximum observed: up to 45.2 mg/day Other tocopherols: β-Tocopherol: up to ~2.91 mg/day γ-Tocopherol: up to ~30.2 mg/day δ-Tocopherol: up to ~9.76 mg/day | Higher vitamin E intake ▲ slower cognitive decline ▲ reduce dementia risk, in APOEε4 carriers too ▲ cognitive engagement (better brain health) ◀▶ depressive symptom |
| Diseases | Indicators | Outcomes |
|---|---|---|
| Alzheimer’s Disease | Cognitive performance | Refer to cognitive performance subtopics (Table 4 and Table 5) |
| Amyloid plaques | [RRR-αT] higher amount correlated with fewer plaques in the frontal cortex and temporal cortex [19] [RSS-αT] higher amount correlated with more plaques in the temporal cortex [19] [γ-tocopherol] lowers amyloid plaque burden [1,9] When [γ-tocopherol] levels were low, higher [α-tocopherol] levels were linked to increased amyloid plaque burden [9] [α-Tocopherol] levels were not independently associated with Braak staging [9] No significant association between [α-tocopherol] levels and neuritic plaque (NP) counts in any brain region [12] Reduced AD neuropathology risk [10] | |
| TAU pathology (Neurofibrillary tangles) | [RSS-αT] higher amount correlated with increased NFTs in the frontal cortex, temporal cortex and amygdala [19] [RRR-αT] no significant correlation was found with NFTs. [γ-tocopherol] higher was associated with lower NFT severity [1,9] Higher [α-tocopherol] levels were associated with lower NFT counts in brain regions affected in early Braak stages (amygdala, hippocampus, entorhinal cortex, subiculum) [12] No significant association between [α-tocopherol] and [Vitamin E] levels in later-affected brain regions (frontal, temporal, parietal cortex) [10,12] | |
| APOE4 | Genotype distribution in centenarians: ε3/ε3 (68%), ε2/ε3 (15%), ε3/ε4 (15%), ε2/ε4 (2%) [19] APOEɛ4 carriers with high dietary [α-tocopherol] intake had significantly slower cognitive decline [46] The protective effect of [tocopherols] and [vitamin E] remained even after adjusting for APOE4 status, suggesting it acts independently of genetic risk [1,10] No significant interaction was found between APOE4 and [tocopherol] levels in predicting amyloid or tangle pathology [9] APOE4 carrier status was recorded as a covariate, but it was not found to modify the relationship between [Vitamin E] levels and NFT counts [12] | |
| miRNA | [α-tocopherol] lower concentration is associated with lower miRNA expression. miR-122, miR-9, miR-21, miR-29b, miR-132 were significantly downregulated in AD patients [13] | |
| Presynaptic protein | Higher brain [γ-tocopherol] levels relate to higher levels of presynaptic proteins (SNARE protein composite, Complexin I, Complexin II, Syntaxin/SNAP-25 composite, Synaptotagmin, synaptophysin composite and Septin 5) in the midfrontal cortex [7] | |
| Neuroprotective | [RRR-αT] reduced plaque burden [19] [Vitamin E] slowed cognitive decline, particularly in APOEɛ4 carriers [46] [α-Tocopherol] lowered neuroinflammation [13] [tocopherol] lower activated microglia density provided an anti-inflammatory environment [1] AD subjects had lower [tocopherol and tocotrienol] levels and higher oxidative/nitrosative damage than CTs. Adjusted analyses confirmed these associations, with nitrosative damage linked to AD only in those with higher LTL [55] [γ-tocopherol] as a potential neuroprotective agent against Alzheimer’s disease pathology, while [α-tocopherol] alone is not effective [9] [α-tocopherol] may reduce early tau pathology but does not affect amyloid plaques [12] | |
| Misc. | managing sleep apnea might help prevent cognitive decline, particularly in APOE ɛ4 noncarriers | |
| Total homocysteine (tHcy) | No direct correlation between vitamin E and tHcy. However, higher physical activity was associated with lower tHcy levels [51] | |
| Dementia | [Vitamin E] did not slow the progression of dementia or cognitive decline in aging individuals with Down syndrome [61] Lower [Vitamin E] intake (<13.20 mg/day) was associated with a significantly higher incidence of dementia. Higher dietary [Vitamin E] intake (>23.63 mg/day) was associated with a lower risk of dementia and a slower cognitive decline [29] [γ-tocopherol] association with synaptic integrity remained significant even in individuals with infarcts, indicating potential benefits for vascular dementia [7] Did not affect the relationship between [γ-tocopherol] and presynaptic protein levels, implying that vitamin E may protect synapses even in individuals with Lewy body-related dementia [7] | |
| Parkinson’s Disease | Higher dietary [Vitamin E] intake may be associated with a lower risk of developing Parkinson’s disease [44], without influencing disease severity [11] Older age and comorbidities (hypertension, CVD) increase PD risk. No significant associations were found with smoking, alcohol, BMI, marital status, or education [44] Neuroaspis PLP10™ significantly delayed Parkinson’s disease motor symptom progression and reduced the need for increased levodopa doses [52]. | |
| Schizophrenia | Combining EPA and [Vitamin E] +C lessen psychotic symptoms and/or disturbed sustained attention [59] | |
| Psychosis | PUFA-E supplementation led to antioxidant changes (higher α-tocopherol, altered glutathione levels) but did not significantly impact PANSS scores or psychotic symptoms within 12 weeks [63] | |
| Seizure and Epilepsy | Seizure frequency significantly decreased in the [Vitamin E] group compared to the placebo group. EEG findings improved significantly, suggesting better neuronal stability [4] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razali, R.A.; Ngah, W.Z.W.; Makpol, S.; Yanagisawa, D.; Kato, T.; Tooyama, I. Shifting Perspectives on the Role of Tocotrienol vs. Tocopherol in Brain Health: A Scoping Review. Int. J. Mol. Sci. 2025, 26, 6339. https://doi.org/10.3390/ijms26136339
Razali RA, Ngah WZW, Makpol S, Yanagisawa D, Kato T, Tooyama I. Shifting Perspectives on the Role of Tocotrienol vs. Tocopherol in Brain Health: A Scoping Review. International Journal of Molecular Sciences. 2025; 26(13):6339. https://doi.org/10.3390/ijms26136339
Chicago/Turabian StyleRazali, Rabiatul Adawiyah, Wan Zurinah Wan Ngah, Suzana Makpol, Daijiro Yanagisawa, Tomoko Kato, and Ikuo Tooyama. 2025. "Shifting Perspectives on the Role of Tocotrienol vs. Tocopherol in Brain Health: A Scoping Review" International Journal of Molecular Sciences 26, no. 13: 6339. https://doi.org/10.3390/ijms26136339
APA StyleRazali, R. A., Ngah, W. Z. W., Makpol, S., Yanagisawa, D., Kato, T., & Tooyama, I. (2025). Shifting Perspectives on the Role of Tocotrienol vs. Tocopherol in Brain Health: A Scoping Review. International Journal of Molecular Sciences, 26(13), 6339. https://doi.org/10.3390/ijms26136339







