Stem Cell Therapy Approaches for Ischemia: Assessing Current Innovations and Future Directions
Abstract
1. Introduction
2. Overview and Pathogenesis of Ischemic Diseases
2.1. Types of Ischemic Diseases
2.1.1. Ischemic Stroke
2.1.2. Acute Myocardial Infarction (AMI)
2.1.3. Peripheral Artery Disease
2.2. Pathophysiology
3. Drugs for Treating Ischemia
3.1. Anticoagulants
3.2. Thrombolytics
3.3. Vasodilators
3.4. Neuroprotective Agents
3.5. Antiplatelet Drugs
3.6. Limitations of Pharmacological Treatments
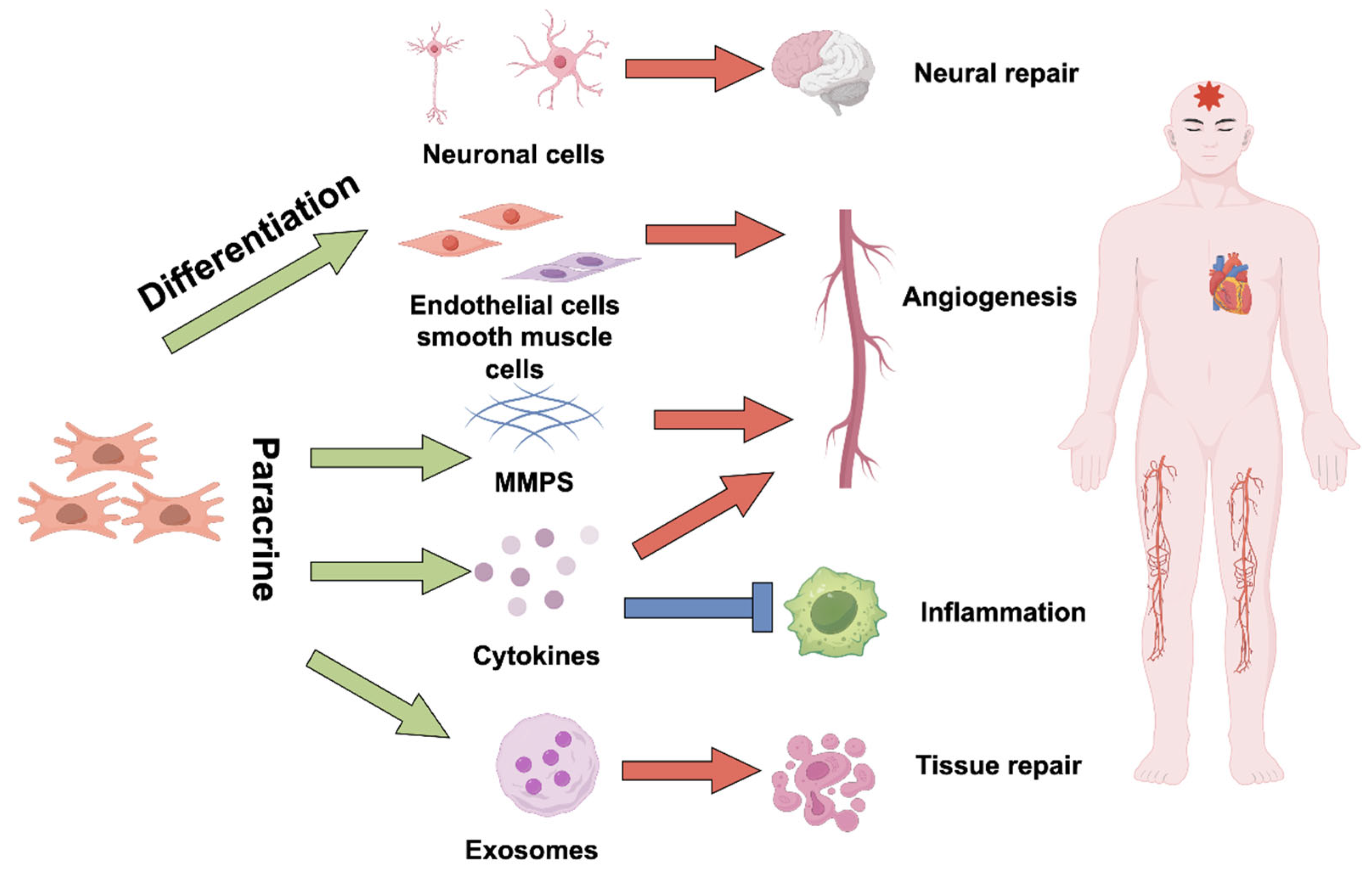
4. Progress and Mechanisms of Stem Cell Therapy
4.1. Types of Stem Cells Utilized in Ischemic Treatment
4.2. Mechanisms of Action
4.2.1. Direct Differentiation into Functional Cells
4.2.2. Paracrine Effects
Promote Angiogenesis
Anti-Apoptosis and Oxidative Stress
Immunomodulation
Regulate the Microenvironment and Extracellular Matrix
4.2.3. Promote Endogenous Regeneration Mechanisms
5. Clinical Trials and Outcomes
5.1. Myocardial Infarction
5.2. Stroke
5.3. Chronic Limb-Threatening Ischemia
6. Future Directions in Cell Therapy
6.1. Genetic Engineering
6.2. Innovations in Bioengineering
6.2.1. Application of Biomaterials in Cell Delivery Systems
6.2.2. Hydrogel Carriers Improve Cell Therapy
6.2.3. Smart Biomaterial Design
6.3. Development of Exosome Delivery Systems
6.3.1. Engineered Exosome Modification to Improve Function
6.3.2. Large-Scale Controllable Production Technology
6.4. Combined Treatment of Stem Cells and Drugs
6.5. Production Cost and Accessibility
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Riviere, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Florio, M.C.; Ruggeri, M.; Furgiuele, S. Autologous cell therapy in diabetes-associated critical limb ischemia: From basic studies to clinical outcomes (Review). Int. J. Mol. Med. 2021, 48, 173. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, D.; Choi, S.; Mican, J.; Toul, M.; Ryu, W.S.; Damborsky, J.; Mikulik, R.; Kim, D.E. Development and Testing of Thrombolytics in Stroke. J. Stroke 2021, 23, 12–36. [Google Scholar] [CrossRef]
- Turrin, G.; Lo Cascio, E.; Giacon, N.; Fantinati, A.; Cristofori, V.; Illuminati, D.; Preti, D.; Morciano, G.; Pinton, P.; Agyapong, E.D.; et al. Spiropiperidine-Based Oligomycin-Analog Ligands To Counteract the Ischemia-Reperfusion Injury in a Renal Cell Model. J. Med. Chem. 2024, 67, 586–602. [Google Scholar] [CrossRef]
- Webb, A.J.S. Effects of vasodilating medications on cerebral haemodynamics in health and disease: Systematic review and meta-analysis. J. Hypertens. 2019, 37, 1119–1125. [Google Scholar] [CrossRef]
- Hou, X.; Cen, K.; Cui, Y.; Zhang, Y.; Feng, X. Antiplatelet therapy for secondary prevention of lacunar stroke: A systematic review and network meta-analysis. Eur. J. Clin. Pharmacol. 2023, 79, 63–70. [Google Scholar] [CrossRef]
- Sun, M.S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.L.; Guo, Z.N.; Yang, Y. Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid. Med. Cell. Longev. 2018, 2018, 3804979. [Google Scholar] [CrossRef]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef]
- Razeghian-Jahromi, I.; Matta, A.G.; Canitrot, R.; Zibaeenezhad, M.J.; Razmkhah, M.; Safari, A.; Nader, V.; Roncalli, J. Surfing the clinical trials of mesenchymal stem cell therapy in ischemic cardiomyopathy. Stem Cell Res. Ther. 2021, 12, 361. [Google Scholar] [CrossRef]
- Ogata, J.; Yamanishi, H.; Ishibashi-Ueda, H. Review: Role of cerebral vessels in ischaemic injury of the brain. Neuropathol. Appl. Neurobiol. 2011, 37, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.V. Five insights from the Global Burden of Disease Study 2019. Lancet 2020, 396, 1135–1159. [Google Scholar] [CrossRef]
- Mlynarska, E.; Czarnik, W.; Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Rysz, J.; Franczyk, B. From Atherosclerotic Plaque to Myocardial Infarction-The Leading Cause of Coronary Artery Occlusion. Int. J. Mol. Sci. 2024, 25, 7295. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Golledge, J. Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Shou, Z.; Zhao, Y.; Zhang, Y.; Li, S. Risk factors for peripheral arterial disease in elderly patients with Type-2 diabetes mellitus: A clinical study. Pak. J. Med. Sci. 2020, 36, 1344–1348. [Google Scholar] [CrossRef]
- Ten Cate, H.; Guzik, T.J.; Eikelboom, J.; Spronk, H.M.H. Pleiotropic actions of factor Xa inhibition in cardiovascular prevention: Mechanistic insights and implications for anti-thrombotic treatment. Cardiovasc. Res. 2021, 117, 2030–2044. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Turetz, M.; Sideris, A.T.; Friedman, O.A.; Triphathi, N.; Horowitz, J.M. Epidemiology, Pathophysiology, and Natural History of Pulmonary Embolism. Semin. Interv. Radiol. 2018, 35, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [PubMed]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Mishra, V.N.; Chaurasia, R.N.; Joshi, D.; Pandey, V. Modes of Calcium Regulation in Ischemic Neuron. Indian J. Clin. Biochem. 2019, 34, 246–253. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Chang, J.Y.; Kim, S.H.; Lee, J.E. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp. Neurobiol. 2016, 25, 241–251. [Google Scholar] [CrossRef]
- Stuckey, S.M.; Ong, L.K.; Collins-Praino, L.E.; Turner, R.J. Neuroinflammation as a Key Driver of Secondary Neurodegeneration Following Stroke? Int. J. Mol. Sci. 2021, 22, 13101. [Google Scholar] [CrossRef]
- Tsoumani, M.E.; Tselepis, A.D. Antiplatelet Agents and Anticoagulants: From Pharmacology to Clinical Practice. Curr. Pharm. Des. 2017, 23, 1279–1293. [Google Scholar] [CrossRef]
- Heestermans, M.; Poenou, G.; Hamzeh-Cognasse, H.; Cognasse, F.; Bertoletti, L. Anticoagulants: A Short History, Their Mechanism of Action, Pharmacology, and Indications. Cells 2022, 11, 3214. [Google Scholar] [CrossRef]
- Mekaj, Y.H.; Mekaj, A.Y.; Duci, S.B.; Miftari, E.I. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef]
- Smythe, M.A.; Priziola, J.; Dobesh, P.P.; Wirth, D.; Cuker, A.; Wittkowsky, A.K. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 165–186. [Google Scholar] [CrossRef]
- Witt, D.M.; Clark, N.P.; Kaatz, S.; Schnurr, T.; Ansell, J.E. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Deitelzweig, S.; Bergrath, E.; di Fusco, M.; Kang, A.; Savone, M.; Cappelleri, J.C.; Russ, C.; Betts, M.; Cichewicz, A.; Schaible, K.; et al. Real-world evidence comparing oral anticoagulants in non-valvular atrial fibrillation: A systematic review and network meta-analysis. Future Cardiol. 2022, 18, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Choi, E.K.; Kim, T.S.; Kuo, J.Y.; Lee, J.M.; On, Y.K.; Park, S.W.; Park, H.W.; Shin, D.G.; Wang, L.; et al. XaMINA: A Real-World, Prospective, Observational Study of Treatment-Naive Patients Treated with Rivaroxaban for Stroke Prevention in Atrial Fibrillation in Asia. Adv. Ther. 2022, 39, 3316–3333. [Google Scholar] [CrossRef] [PubMed]
- Hezer, H.; Kilic, H.; Abuzaina, O.; Hasanoglu, H.C.; Karalezli, A. Long-term results of low-dose tissue plasminogen activator therapy in acute pulmonary embolism. J. Investig. Med. 2019, 67, 1142–1147. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, R. Four Decades of Ischemic Penumbra and Its Implication for Ischemic Stroke. Transl. Stroke Res. 2021, 12, 937–945. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, Z.; Fang, Z.; Ma, F.; Lv, M.; Zhang, J. Risk factors for thrombolysis-related intracranial hemorrhage: A systematic review and meta-analysis. Thromb. J. 2023, 21, 27. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Kaski, J.C. Vasodilator Therapy: Nitrates and Nicorandil. Cardiovasc. Drugs Ther. 2016, 30, 367–378. [Google Scholar] [CrossRef]
- Divakaran, S.; Loscalzo, J. The Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular Therapeutics. J. Am. Coll. Cardiol. 2017, 70, 2393–2410. [Google Scholar] [CrossRef]
- Wilkinson-Stokes, M.; Betson, J.; Sawyer, S. Adverse events from nitrate administration during right ventricular myocardial infarction: A systematic review and meta-analysis. Emerg. Med. J. 2023, 40, 108–113. [Google Scholar] [CrossRef]
- Sueta, D.; Tabata, N.; Hokimoto, S. Clinical roles of calcium channel blockers in ischemic heart diseases. Hypertens. Res. 2017, 40, 423–428. [Google Scholar] [CrossRef]
- Wang, A.L.; Iadecola, C.; Wang, G. New generations of dihydropyridines for treatment of hypertension. J. Geriatr. Cardiol. 2017, 14, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Enge, K.; Tveit, A.; Enger, S.; Onarheim, S.; Pripp, A.H.; Ronningen, P.S.; Solberg, M.G.; Byrkjeland, R.; Andresen, K.; Halsen, A.; et al. Diltiazem reduces levels of NT-proBNP and improves symptoms compared with metoprolol in patients with permanent atrial fibrillation. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Kapelios, C.J.; Karamanakos, G.; Liatis, S.; Sarafadi, M.; Polizois, M.; Papoutsis, I.; Kokkinos, A.D. Recurrent episodes of life-threatening vasodilatory shock following unintentional intoxication with amlodipine. Hell. J. Cardiol. 2017, 58, 369–371. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, H.; Li, H.; Zhao, R.; Huang, Q.; Liu, J. Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur. J. Med. Chem. 2019, 162, 132–146. [Google Scholar] [CrossRef]
- Ghozy, S.; Reda, A.; Varney, J.; Elhawary, A.S.; Shah, J.; Murry, K.; Sobeeh, M.G.; Nayak, S.S.; Azzam, A.Y.; Brinjikji, W.; et al. Neuroprotection in Acute Ischemic Stroke: A Battle Against the Biology of Nature. Front. Neurol. 2022, 13, 870141. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: From aspirin to the present day. Drugs 2012, 72, 2087–2116. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Rollini, F.; Storey, R.F.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.F.; Trenk, D.; Alexopoulos, D.; et al. International Expert Consensus on Switching Platelet P2Y(12) Receptor-Inhibiting Therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sharopov, F.; Ezzat, S.M.; Zam, W.; Ademiluyi, A.O.; Oyeniran, O.H.; Adetunji, C.O.; Roli, O.I.; Zivkovic, J.; Martorell, M.; et al. An Updated Review on Glycoprotein IIb/IIIa Inhibitors as Antiplatelet Agents: Basic and Clinical Perspectives. High Blood Press. Cardiovasc. Prev. 2023, 30, 93–107. [Google Scholar] [CrossRef]
- Jourdi, G.; Lordkipanidze, M.; Philippe, A.; Bachelot-Loza, C.; Gaussem, P. Current and Novel Antiplatelet Therapies for the Treatment of Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 13079. [Google Scholar] [CrossRef]
- Wang, Y.; Cavallari, L.H.; Brown, J.D.; Thomas, C.D.; Winterstein, A.G. Assessing the Clinical Treatment Dynamics of Antiplatelet Therapy Following Acute Coronary Syndrome and Percutaneous Coronary Intervention in the US. JAMA Netw. Open 2023, 6, e238585. [Google Scholar] [CrossRef]
- Dammavalam, V.; Lin, S.; Nessa, S.; Daksla, N.; Stefanowski, K.; Costa, A.; Bergese, S. Neuroprotection during Thrombectomy for Acute Ischemic Stroke: A Review of Future Therapies. Int. J. Mol. Sci. 2024, 25, 891. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Murozono, M.; Kanazawa, M.; Nara, T.; Ozawa, T.; Watanabe, Y. Edaravone and cyclosporine A as neuroprotective agents for acute ischemic stroke. Acute Med. Surg. 2018, 5, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Jiang, Y.; Ji, Q. Comparative efficacy of neuroprotective agents for improving neurological function and prognosis in acute ischemic stroke: A network meta-analysis. Front. Neurosci. 2024, 18, 1530987. [Google Scholar] [CrossRef] [PubMed]
- Szydlak, R. Mesenchymal stem cells in ischemic tissue regeneration. World J. Stem Cells 2023, 15, 16–30. [Google Scholar] [CrossRef]
- Chu, Q.; Jiang, X.; Xiao, Y. Rebuilding the myocardial microenvironment to enhance mesenchymal stem cells-mediated regeneration in ischemic heart disease. Front. Bioeng. Biotechnol. 2024, 12, 1468833. [Google Scholar] [CrossRef]
- Shen, Z.; Tang, X.; Zhang, Y.; Jia, Y.; Guo, X.; Guo, X.; Bao, J.; Xie, X.; Xing, Y.; Xing, J.; et al. Efficacy and safety of mesenchymal stem cell therapies for ischemic stroke: A systematic review and meta-analysis. Stem Cells Transl. Med. 2024, 13, 886–897. [Google Scholar] [CrossRef]
- Shirbaghaee, Z.; Hassani, M.; Heidari Keshel, S.; Soleimani, M. Emerging roles of mesenchymal stem cell therapy in patients with critical limb ischemia. Stem Cell Res. Ther. 2022, 13, 462. [Google Scholar] [CrossRef]
- Panos, L.D.; Bargiotas, P.; Arnold, M.; Hadjigeorgiou, G.; Panos, G.D. Revolutionizing Stroke Recovery: Unveiling the Promise of Stem Cell Therapy. Drug Des. Dev. Ther. 2024, 18, 991–1006. [Google Scholar] [CrossRef]
- Cheng, Q.; Ma, X.; Liu, J.; Feng, X.; Liu, Y.; Wang, Y.; Ni, W.; Song, M. Pharmacological Inhibition of the Asparaginyl Endopeptidase (AEP) in an Alzheimer’s Disease Model Improves the Survival and Efficacy of Transplanted Neural Stem Cells. Int. J. Mol. Sci. 2023, 24, 7739. [Google Scholar] [CrossRef]
- Korshunova, I.; Rhein, S.; Garcia-Gonzalez, D.; Stolting, I.; Pfisterer, U.; Barta, A.; Dmytriyeva, O.; Kirkeby, A.; Schwaninger, M.; Khodosevich, K. Genetic modification increases the survival and the neuroregenerative properties of transplanted neural stem cells. JCI Insight 2020, 5, e126268. [Google Scholar] [CrossRef]
- Galiakberova, A.A.; Dashinimaev, E.B. Neural Stem Cells and Methods for Their Generation From Induced Pluripotent Stem Cells in vitro. Front. Cell Dev. Biol. 2020, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.S.; Pieri, N.C.G.; Botigelli, R.C.; de Castro, R.V.G.; de Souza, A.F.; Bridi, A.; Lima, M.A.; Fantinato Neto, P.; Pessoa, L.V.F.; Martins, S.; et al. Generation of neural progenitor cells from porcine-induced pluripotent stem cells. J. Tissue Eng. Regen. Med. 2020, 14, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qin, N.; Lu, X.A.; Li, J.; Han, X.; Ni, X.; Ye, L.; Shen, Z.; Chen, W.; Zhao, Z.A.; et al. Human embryonic stem cell-derived cardiomyocyte therapy in mouse permanent ischemia and ischemia-reperfusion models. Stem Cell Res. Ther. 2019, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, X.; Ke, M.; Li, H.; Jiang, Y.; Zhang, P.; Tan, J.; Cao, N.; Yang, H.T. Human embryonic stem cell-derived cardiovascular progenitor cells stimulate cardiomyocyte cell cycle activity via activating the PI3K/Akt pathway. J. Mol. Cell. Cardiol. 2024, 197, 5–10. [Google Scholar] [CrossRef]
- Gonzalez-King, H.; Rodrigues, P.G.; Albery, T.; Tangruksa, B.; Gurrapu, R.; Silva, A.M.; Musa, G.; Kardasz, D.; Liu, K.; Kull, B.; et al. Head-to-head comparison of relevant cell sources of small extracellular vesicles for cardiac repair: Superiority of embryonic stem cells. J. Extracell. Vesicles 2024, 13, e12445. [Google Scholar] [CrossRef]
- Asgari Taei, A.; Dargahi, L.; Nasoohi, S.; Hassanzadeh, G.; Kadivar, M.; Farahmandfar, M. The conditioned medium of human embryonic stem cell-derived mesenchymal stem cells alleviates neurological deficits and improves synaptic recovery in experimental stroke. J. Cell. Physiol. 2021, 236, 1967–1979. [Google Scholar] [CrossRef]
- Li, M.; Wang, P.; Huo, S.T.; Qiu, H.; Li, C.; Lin, S.; Guo, L.; Ji, Y.; Zhu, Y.; Liu, J.; et al. Human Pluripotent Stem Cells Derived Endothelial Cells Repair Choroidal Ischemia. Adv. Sci. 2024, 11, e2302940. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Hsieh, M.L.; Lin, C.J.; Chang, C.M.C.; Huang, C.Y.; Puntney, R.; Wu Moy, A.; Ting, C.Y.; Herr Chan, D.Z.; Nicholson, M.W.; et al. Combined Treatment of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Endothelial Cells Regenerate the Infarcted Heart in Mice and Non-Human Primates. Circulation 2023, 148, 1395–1409. [Google Scholar] [CrossRef]
- Song, S.J.; Nam, Y.; Rim, Y.A.; Ju, J.H.; Sohn, Y. Comparative analysis of regulations and studies on stem cell therapies: Focusing on induced pluripotent stem cell (iPSC)-based treatments. Stem Cell Res. Ther. 2024, 15, 447. [Google Scholar] [CrossRef]
- Nishino, K.; Takasawa, K.; Okamura, K.; Arai, Y.; Sekiya, A.; Akutsu, H.; Umezawa, A. Identification of an epigenetic signature in human induced pluripotent stem cells using a linear machine learning model. Hum. Cell 2021, 34, 99–110. [Google Scholar] [CrossRef]
- Manganelli, M.; Mazzoldi, E.L.; Ferraro, R.M.; Pinelli, M.; Parigi, M.; Aghel, S.A.M.; Bugatti, M.; Collo, G.; Stocco, G.; Vermi, W.; et al. Progesterone receptor is constitutively expressed in induced Pluripotent Stem Cells (iPSCs). Stem Cell Rev. Rep. 2024, 20, 2303–2317. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Chooi, W.H.; Jeon, H.; Chen, J.; Tan, J.; Roxby, D.N.; Lee, C.Y.; Ng, S.Y.; Chew, S.Y.; Han, J. Label-Free and High-Throughput Removal of Residual Undifferentiated Cells From iPSC-Derived Spinal Cord Progenitor Cells. Stem Cells Transl. Med. 2024, 13, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Chour, T.; Tian, L.; Lau, E.; Thomas, D.; Itzhaki, I.; Malak, O.; Zhang, J.Z.; Qin, X.; Wardak, M.; Liu, Y.; et al. Method for selective ablation of undifferentiated human pluripotent stem cell populations for cell-based therapies. JCI Insight 2021, 6, e142000. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Quesenberry, P.J.; Wen, S.; Goldberg, L.R.; Dooner, M.S. The universal stem cell. Leukemia 2022, 36, 2784–2792. [Google Scholar] [CrossRef]
- Prasad, M.; Corban, M.T.; Henry, T.D.; Dietz, A.B.; Lerman, L.O.; Lerman, A. Promise of autologous CD34+ stem/progenitor cell therapy for treatment of cardiovascular disease. Cardiovasc. Res. 2020, 116, 1424–1433. [Google Scholar] [CrossRef]
- Rafii, S.; Lyden, D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003, 9, 702–712. [Google Scholar] [CrossRef]
- Lu, J.; Pompili, V.J.; Das, H. Neovascularization and hematopoietic stem cells. Cell Biochem. Biophys. 2013, 67, 235–245. [Google Scholar] [CrossRef]
- Wang, C.; Nistala, R.; Cao, M.; Li, D.P.; Pan, Y.; Golzy, M.; Cui, Y.; Liu, Z.; Kang, X. Repair of Limb Ischemia Is Dependent on Hematopoietic Stem Cell Specific-SHP-1 Regulation of TGF-beta1. Arter. Thromb. Vasc. Biol. 2023, 43, 92–108. [Google Scholar] [CrossRef]
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011, 109, 724–728. [Google Scholar] [CrossRef]
- Quyyumi, A.A.; Vasquez, A.; Kereiakes, D.J.; Klapholz, M.; Schaer, G.L.; Abdel-Latif, A.; Frohwein, S.; Henry, T.D.; Schatz, R.A.; Dib, N.; et al. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients with Left Ventricular Dysfunction Post STEMI. Circ. Res. 2017, 120, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.; Rayabaram, J.; Miranda, C.C.; Fernandes-Platzgummer, A.; Fernandes, T.G.; Sajja, S.; da Silva, C.L.; Vemuri, M.C. Advances in ex vivo expansion of hematopoietic stem and progenitor cells for clinical applications. Front. Bioeng. Biotechnol. 2024, 12, 1380950. [Google Scholar] [CrossRef] [PubMed]
- Vicinanza, C.; Aquila, I.; Scalise, M.; Cristiano, F.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Sacco, W.; Lewis, F.C.; et al. Adult cardiac stem cells are multipotent and robustly myogenic: C-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017, 24, 2101–2116. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Nguyen, N.B.; Ardehali, R.; Zhou, B. Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation: Controversy, Fallacy, and Progress. Circulation 2020, 142, 275–291. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Malliaras, K.; Li, T.S.; Luthringer, D.; Terrovitis, J.; Cheng, K.; Chakravarty, T.; Galang, G.; Zhang, Y.; Schoenhoff, F.; Van Eyk, J.; et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 2012, 125, 100–112. [Google Scholar] [CrossRef]
- Chugh, A.R.; Beache, G.M.; Loughran, J.H.; Mewton, N.; Elmore, J.B.; Kajstura, J.; Pappas, P.; Tatooles, A.; Stoddard, M.F.; Lima, J.A.; et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The SCIPIO trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 2012, 126, S54–S64. [Google Scholar] [CrossRef]
- Scalise, M.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Aquila, I.; Torella, M.; Nadal-Ginard, B.; Torella, D. Heterogeneity of Adult Cardiac Stem Cells. Adv. Exp. Med. Biol. 2019, 1169, 141–178. [Google Scholar] [CrossRef]
- Chimenti, I.; Gaetani, R.; Barile, L.; Forte, E.; Ionta, V.; Angelini, F.; Frati, G.; Messina, E.; Giacomello, A. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol. Biol. 2012, 879, 327–338. [Google Scholar] [CrossRef]
- Chimenti, I.; Smith, R.R.; Li, T.S.; Gerstenblith, G.; Messina, E.; Giacomello, A.; Marban, E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 2010, 106, 971–980. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marban, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Stem cells: Myocardial regeneration after infarction-promising phase I trial results. Nat. Rev. Cardiol. 2012, 9, 187. [Google Scholar] [CrossRef]
- Arderiu, G.; Pena, E.; Aledo, R.; Juan-Babot, O.; Crespo, J.; Vilahur, G.; Onate, B.; Moscatiello, F.; Badimon, L. MicroRNA-145 Regulates the Differentiation of Adipose Stem Cells Toward Microvascular Endothelial Cells and Promotes Angiogenesis. Circ. Res. 2019, 125, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, W.; Zhang, J.; Fan, X.; Liu, X.; Liu, Q.; Pan, S.; Dixon, R.A.F.; Li, P.; Yu, P.; et al. Therapeutic angiogenesis and tissue revascularization in ischemic vascular disease. J. Biol. Eng. 2023, 17, 13. [Google Scholar] [CrossRef]
- Abouzid, M.R.; Umer, A.M.; Jha, S.K.; Akbar, U.A.; Khraisat, O.; Saleh, A.; Mohamed, K.; Esteghamati, S.; Kamel, I. Stem Cell Therapy for Myocardial Infarction and Heart Failure: A Comprehensive Systematic Review and Critical Analysis. Cureus 2024, 16, e59474. [Google Scholar] [CrossRef]
- Saeedi, P.; Nilchiani, L.S.; Zand, B.; Hajimirghasemi, M.; Halabian, R. An overview of stem cells and cell products involved in trauma injury. Regen. Ther. 2025, 29, 60–76. [Google Scholar] [CrossRef]
- Yu, H.Y.; Shin, J.H.; Yun, H.; Ryu, C.M.; Lee, S.; Heo, J.; Lim, J.; Park, J.; Hong, K.S.; Chung, H.M.; et al. A Preclinical Study of Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells for Treating Detrusor Underactivity by Chronic Bladder Ischemia. Stem Cell Rev. Rep. 2021, 17, 2139–2152. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Zhou, Y.; Pang, H.; Liu, Y.; Oganezov, G.; Lv, T.; Li, J.; Xu, J.; Xiao, Z.; et al. HIF-1alpha inhibits mitochondria-mediated apoptosis and improves the survival of human adipose-derived stem cells in ischemic microenvironments. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1908–1918. [Google Scholar] [CrossRef]
- Nasser, M.I.; Masood, M.; Adlat, S.; Gang, D.; Zhu, S.; Li, G.; Li, N.; Chen, J.; Zhu, P. Mesenchymal stem cell-derived exosome microRNA as therapy for cardiac ischemic injury. Biomed. Pharmacother. 2021, 143, 112118. [Google Scholar] [CrossRef]
- Mabotuwana, N.S.; Rech, L.; Lim, J.; Hardy, S.A.; Murtha, L.A.; Rainer, P.P.; Boyle, A.J. Paracrine Factors Released by Stem Cells of Mesenchymal Origin and their Effects in Cardiovascular Disease: A Systematic Review of Pre-clinical Studies. Stem Cell Rev. Rep. 2022, 18, 2606–2628. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Lockard, G.; Monsour, M.; Alayli, A.; Borlongan, C.V. The Role of Concomitant Nrf2 Targeting and Stem Cell Therapy in Cerebrovascular Disease. Antioxidants 2022, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, X.; Yang, J. Cytokine networks that suppress fish cellular immunity. Dev. Comp. Immunol. 2023, 147, 104769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, N.; Zhang, J.; Liu, Y.; Zhu, D.; Kong, Y. Mesenchymal stem cells rejuvenate cardiac muscle through regulating macrophage polarization. Aging 2019, 11, 3900–3908. [Google Scholar] [CrossRef]
- Arai, Y.; Lee, S.H. MMP13-Overexpressing Mesenchymal Stem Cells Enhance Bone Tissue Formation in the Presence of Collagen Hydrogel. Tissue Eng. Regen. Med. 2023, 20, 461–471. [Google Scholar] [CrossRef]
- Ghodrat, S.; Hoseini, S.J.; Asadpour, S.; Nazarnezhad, S.; Alizadeh Eghtedar, F.; Kargozar, S. Stem cell-based therapies for cardiac diseases: The critical role of angiogenic exosomes. Biofactors 2021, 47, 270–291. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, N.; Liu, J.; Ren, H.; Jiang, W.; Lei, Y.; Fu, X.; Hao, M.; Lang, X.; Liu, Y.; et al. Intranasal delivery of hMSC-derived supernatant for treatment of ischemic stroke by inhibiting the pro-inflammatory polarization of neutrophils. Stem Cell Res. Ther. 2025, 16, 43. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Gaihre, B.; Park, S.; Li, Y.; Terzic, A.; Lu, L. SDF-1alpha/OPF/BP Composites Enhance the Migrating and Osteogenic Abilities of Mesenchymal Stem Cells. Stem Cells Int. 2021, 2021, 1938819. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Yuan, Z.; Fu, L.; Jiang, S.; Gao, C.; Wang, F.; Zha, K.; Tian, G.; Sun, Z.; et al. Endogenous cell recruitment strategy for articular cartilage regeneration. Acta Biomater. 2020, 114, 31–52. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Chen, Y. CXCL12 regulates bone marrow-derived endothelial progenitor cells to promote aortic aneurysm recovery. Tissue Cell 2022, 77, 101810. [Google Scholar] [CrossRef]
- Demurtas, J.; Fanelli, G.N.; Romano, S.L.; Solari, M.; Yang, L.; Soysal, P.; Lopez Sanchez, G.F.; Grabovac, I.; Smith, L.; Zorzi, A.; et al. Stem cells for treatment of cardiovascular diseases: An umbrella review of randomized controlled trials. Ageing Res. Rev. 2021, 67, 101257. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, A.B.; Qayyum, A.A.; Jorgensen, E.; Helqvist, S.; Kofoed, K.F.; Haack-Sorensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2020, 22, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Seth, J.; Sharma, S.; Leong, C.J.; Vaibhav, V.; Nelson, P.; Shokravi, A.; Luo, Y.; Shirvani, D.; Laksman, Z. The Use of Hematopoietic Stem Cells for Heart Failure: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 6634. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ruiz, R.; Casado Plasencia, A.; Borlado, L.R.; Fernandez-Santos, M.E.; Al-Daccak, R.; Claus, P.; Palacios, I.; Sadaba, R.; Charron, D.; Bogaert, J.; et al. Rationale and Design of a Clinical Trial to Evaluate the Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With Acute Myocardial Infarction and Left Ventricular Dysfunction: The Randomized Multicenter Double-Blind Controlled CAREMI Trial (Cardiac Stem Cells in Patients With Acute Myocardial Infarction). Circ. Res. 2017, 121, 71–80. [Google Scholar] [CrossRef]
- Fernandez-Aviles, F.; Sanz-Ruiz, R.; Bogaert, J.; Casado Plasencia, A.; Gilaberte, I.; Belmans, A.; Fernandez-Santos, M.E.; Charron, D.; Mulet, M.; Yotti, R.; et al. Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With ST-Segment Elevation Myocardial Infarction and Left Ventricular Dysfunction. Circ. Res. 2018, 123, 579–589. [Google Scholar] [CrossRef]
- Bolli, R.; Hare, J.M.; March, K.L.; Pepine, C.J.; Willerson, J.T.; Perin, E.C.; Yang, P.C.; Henry, T.D.; Traverse, J.H.; Mitrani, R.D.; et al. Rationale and Design of the CONCERT-HF Trial (Combination of Mesenchymal and c-kit+ Cardiac Stem Cells As Regenerative Therapy for Heart Failure). Circ. Res. 2018, 122, 1703–1715. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, J.; Zhang, N.; Li, W.; Wang, J.; Cai, G.; Chen, Y.; Yang, Y.; Liu, Z. Bone marrow mesenchymal stem cells transfer in patients with ST-segment elevation myocardial infarction: Single-blind, multicenter, randomized controlled trial. Stem Cell Res. Ther. 2021, 12, 33. [Google Scholar] [CrossRef]
- Yuce, K. The Application of Mesenchymal Stem Cells in Different Cardiovascular Disorders: Ways of Administration, and the Effectors. Stem Cell Rev. Rep. 2024, 20, 1671–1691. [Google Scholar] [CrossRef]
- Xu, C.M.; Sabe, S.A.; Brinck-Teixeira, R.; Sabra, M.; Sellke, F.W.; Abid, M.R. Visualization of cardiac uptake of bone marrow mesenchymal stem cell-derived extracellular vesicles after intramyocardial or intravenous injection in murine myocardial infarction. Physiol. Rep. 2023, 11, e15568. [Google Scholar] [CrossRef]
- Qi, T.; Xu, X.; Guo, Y.; Xia, Y.; Peng, L.; Li, C.; Ding, F.; Gao, C.; Fan, M.; Yu, M.; et al. CSF2RB overexpression promotes the protective effects of mesenchymal stromal cells against ischemic heart injury. Theranostics 2023, 13, 1759–1773. [Google Scholar] [CrossRef]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020, 11, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Houkin, K.; Osanai, T.; Uchiyama, S.; Minematsu, K.; Taguchi, A.; Maruichi, K.; Niiya, Y.; Asaoka, K.; Kuga, Y.; Takizawa, K.; et al. Allogeneic Stem Cell Therapy for Acute Ischemic Stroke: The Phase 2/3 TREASURE Randomized Clinical Trial. JAMA Neurol. 2024, 81, 154–162. [Google Scholar] [CrossRef]
- Ghuman, H.; Perry, N.; Grice, L.; Gerwig, M.; Moorhead, J., Jr.; Nitzsche, F.; Poplawsky, A.J.; Ambrosio, F.; Modo, M. Physical therapy exerts sub-additive and suppressive effects on intracerebral neural stem cell implantation in a rat model of stroke. J. Cereb. Blood Flow Metab. 2022, 42, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Yarygin, K.N.; Namestnikova, D.D.; Sukhinich, K.K.; Gubskiy, I.L.; Majouga, A.G.; Kholodenko, I.V. Cell Therapy of Stroke: Do the Intra-Arterially Transplanted Mesenchymal Stem Cells Cross the Blood-Brain Barrier? Cells 2021, 10, 2997. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.P.; Filgueiras, I.S.; Ferreira, J.M.; de Oliveira, F.A.; Nucci, L.P.; Mamani, J.B.; Rego, G.N.A.; Gamarra, L.F. Stem cell homing, tracking and therapeutic efficiency evaluation for stroke treatment using nanoparticles: A systematic review. World J. Stem Cells 2020, 12, 381–405. [Google Scholar] [CrossRef]
- Achon Buil, B.; Tackenberg, C.; Rust, R. Editing a gateway for cell therapy across the blood-brain barrier. Brain 2023, 146, 823–841. [Google Scholar] [CrossRef]
- Huerta, C.T.; Voza, F.A.; Ortiz, Y.Y.; Liu, Z.J.; Velazquez, O.C. Mesenchymal stem cell-based therapy for non-healing wounds due to chronic limb-threatening ischemia: A review of preclinical and clinical studies. Front. Cardiovasc. Med. 2023, 10, 1113982. [Google Scholar] [CrossRef]
- Sharma, S.; Pandey, N.N.; Sinha, M.; Kumar, S.; Jagia, P.; Gulati, G.S.; Gond, K.; Mohanty, S.; Bhargava, B. Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate Safety and Therapeutic Efficacy of Angiogenesis Induced by Intraarterial Autologous Bone Marrow-Derived Stem Cells in Patients with Severe Peripheral Arterial Disease. J. Vasc. Interv. Radiol. 2021, 32, 157–163. [Google Scholar] [CrossRef]
- Shirbaghaee, Z.; Heidari Keshel, S.; Rasouli, M.; Valizadeh, M.; Hashemi Nazari, S.S.; Hassani, M.; Soleimani, M. Report of a phase 1 clinical trial for safety assessment of human placental mesenchymal stem cells therapy in patients with critical limb ischemia (CLI). Stem Cell Res. Ther. 2023, 14, 174. [Google Scholar] [CrossRef]
- Maeda, S.; Kawamura, T.; Sasaki, M.; Shimamura, K.; Shibuya, T.; Harada, A.; Honmou, O.; Sawa, Y.; Miyagawa, S. Intravenous infusion of bone marrow-derived mesenchymal stem cells improves tissue perfusion in a rat hindlimb ischemia model. Sci. Rep. 2022, 12, 16986. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, X.; Gao, J. Stem cell-based ischemic stroke therapy: Novel modifications and clinical challenges. Asian J. Pharm. Sci. 2024, 19, 100867. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, H.J.; Han, Y.; Lee, S.H. Mid- to long-term efficacy and safety of stem cell therapy for acute myocardial infarction: A systematic review and meta-analysis. Stem Cell Res. Ther. 2024, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lachance, B.B.; Moiz, B.; Jia, X. Optimizing Stem Cell Therapy after Ischemic Brain Injury. J. Stroke 2020, 22, 286–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jia, X. Neuroprotection of Stem Cells Against Ischemic Brain Injury: From Bench to Clinic. Transl. Stroke Res. 2024, 15, 691–713. [Google Scholar] [CrossRef]
- Hegde, M.; Singh, A.K.; Kannan, S.; Kolkundkar, U.; Seetharam, R.N. Therapeutic Applications of Engineered Mesenchymal Stromal Cells for Enhanced Angiogenesis in Cardiac and Cerebral Ischemia. Stem Cell Rev. Rep. 2024, 20, 2138–2154. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Yu, T.; Qi, C. Generation of PTEN knockout bone marrow mesenchymal stem cell lines by CRISPR/Cas9-mediated genome editing. Cytotechnology 2018, 70, 783–791. [Google Scholar] [CrossRef]
- Marotta, P.; Cianflone, E.; Aquila, I.; Vicinanza, C.; Scalise, M.; Marino, F.; Mancuso, T.; Torella, M.; Indolfi, C.; Torella, D. Combining cell and gene therapy to advance cardiac regeneration. Expert Opin. Biol. Ther. 2018, 18, 409–423. [Google Scholar] [CrossRef]
- Welch, S.; Plank, D.; Witt, S.; Glascock, B.; Schaefer, E.; Chimenti, S.; Andreoli, A.M.; Limana, F.; Leri, A.; Kajstura, J.; et al. Cardiac-specific IGF-1 expression attenuates dilated cardiomyopathy in tropomodulin-overexpressing transgenic mice. Circ. Res. 2002, 90, 641–648. [Google Scholar] [CrossRef]
- Fischer, K.M.; Din, S.; Gude, N.; Konstandin, M.H.; Wu, W.; Quijada, P.; Sussman, M.A. Cardiac progenitor cell commitment is inhibited by nuclear Akt expression. Circ. Res. 2011, 108, 960–970. [Google Scholar] [CrossRef]
- Cai, W.F.; Jiang, L.; Liang, J.; Dutta, S.; Huang, W.; He, X.; Wu, Z.; Paul, C.; Gao, X.; Xu, M.; et al. HAX1-Overexpression Augments Cardioprotective Efficacy of Stem Cell-Based Therapy Through Mediating Hippo-Yap Signaling. Stem Cell Rev. Rep. 2024, 20, 1569–1586. [Google Scholar] [CrossRef]
- Markosyan, V.; Safiullov, Z.; Izmailov, A.; Fadeev, F.; Sokolov, M.; Kuznetsov, M.; Trofimov, D.; Kim, E.; Kundakchyan, G.; Gibadullin, A.; et al. Preventive Triple Gene Therapy Reduces the Negative Consequences of Ischemia-Induced Brain Injury after Modelling Stroke in a Rat. Int. J. Mol. Sci. 2020, 21, 6858. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, B.; Fan, J.; Xue, C.; Lu, Y.; Li, C.; Cui, D. Engineered mesenchymal stem cell-derived exosomes with high CXCR4 levels for targeted siRNA gene therapy against cancer. Nanoscale 2022, 14, 4098–4113. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Lin, J.; He, R.; Chen, M.; Zhang, Y.; Liao, Z.; Zhang, C. CCR2 improves homing and engraftment of adipose-derived stem cells in dystrophic mice. Stem Cell Res. Ther. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, C.; Cheng, M.; Huang, J.; Liu, Q.; Yuan, G.; Lin, K.; Yu, H. Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts. Bioact. Mater. 2021, 6, 1791–1809. [Google Scholar] [CrossRef]
- Sin, T.N.; Tng, N.; Dragoli, J.; Ramesh Kumar, S.; Villafuerte-Trisolini, C.; Chung, S.H.; Tu, L.; Le, S.M.; Shim, J.H.; Pepple, K.L.; et al. Safety and Efficacy of CRISPR-Mediated Genome Ablation of VEGFA as a Treatment for Choroidal Neovascularization in Nonhuman Primate Eyes. Available online: https://www.cell.com/molecular-therapy-family/molecular-therapy/abstract/S1525-0016(24)00651-8 (accessed on 4 June 2025).
- Ellison, G.M.; Torella, D.; Dellegrottaglie, S.; Perez-Martinez, C.; Perez de Prado, A.; Vicinanza, C.; Purushothaman, S.; Galuppo, V.; Iaconetti, C.; Waring, C.D.; et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J. Am. Coll. Cardiol. 2011, 58, 977–986. [Google Scholar] [CrossRef]
- Jurlina, S.L.; Jones, M.K.; Agarwal, D.; De La Toba, D.V.; Kambli, N.; Su, F.; Martin, H.M.; Anderson, R.; Wong, R.M.; Seid, J.; et al. A Tet-Inducible CRISPR Platform for High-Fidelity Editing of Human Pluripotent Stem Cells. Genes 2022, 13, 2363. [Google Scholar] [CrossRef]
- Thomas, D.; Marsico, G.; Mohd Isa, I.L.; Thirumaran, A.; Chen, X.; Lukasz, B.; Fontana, G.; Rodriguez, B.; Marchetti-Deschmann, M.; O’Brien, T.; et al. Temporal changes guided by mesenchymal stem cells on a 3D microgel platform enhance angiogenesis in vivo at a low-cell dose. Proc. Natl. Acad. Sci. USA 2020, 117, 19033–19044. [Google Scholar] [CrossRef]
- Jurczak, P.; Lach, S. Hydrogels as Scaffolds in Bone-Related Tissue Engineering and Regeneration. Macromol. Biosci. 2023, 23, e2300152. [Google Scholar] [CrossRef]
- Morwood, A.J.; El-Karim, I.A.; Clarke, S.A.; Lundy, F.T. The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels. Molecules 2023, 28, 4616. [Google Scholar] [CrossRef]
- Jin, R.H.; Zhang, Z.Z.; Xu, P.Q.; Xia, S.Z.; Weng, T.T.; Zhu, Z.K.; Wang, X.G.; You, C.G.; Han, C.M. Effects of three-dimensional bioprinting antibacterial hydrogel on full-thickness skin defect wounds in rats. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi 2023, 39, 165–174. [Google Scholar] [CrossRef]
- Gornicki, T.; Lambrinow, J.; Golkar-Narenji, A.; Data, K.; Domagala, D.; Niebora, J.; Farzaneh, M.; Mozdziak, P.; Zabel, M.; Antosik, P.; et al. Biomimetic Scaffolds-A Novel Approach to Three Dimensional Cell Culture Techniques for Potential Implementation in Tissue Engineering. Nanomaterials 2024, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Politron-Zepeda, G.A.; Fletes-Vargas, G.; Rodriguez-Rodriguez, R. Injectable Hydrogels for Nervous Tissue Repair-A Brief Review. Gels 2024, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, T.L.; Zhang, H.J.; Gao, J.; Yang, P.F. A Promising Application of Injectable Hydrogels in Nerve Repair and Regeneration for Ischemic Stroke. Int. J. Nanomed. 2024, 19, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Lu, L.; Song, H.; Duan, Y.; Chen, J.; Carney, R.; Li, J.J.; Zhou, P.; Nolta, J.; Lam, K.S.; et al. Engineered extracellular vesicles with high collagen-binding affinity present superior in situ retention and therapeutic efficacy in tissue repair. Theranostics 2022, 12, 6021–6037. [Google Scholar] [CrossRef]
- Nair, R.V.; Farrukh, A.; Del Campo, A. Light-Regulated Angiogenesis via a Phototriggerable VEGF Peptidomimetic. Adv. Healthc. Mater. 2021, 10, e2100488. [Google Scholar] [CrossRef]
- Intravaia, J.T.; Graham, T.; Kim, H.S.; Nanda, H.S.; Kumbar, S.G.; Nukavarapu, S.P. Smart Orthopedic Biomaterials and Implants. Curr. Opin. Biomed. Eng. 2023, 25, 100439. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef]
- Kang, J.Y.; Kim, H.; Mun, D.; Yun, N.; Joung, B. Co-delivery of curcumin and miRNA-144-3p using heart-targeted extracellular vesicles enhances the therapeutic efficacy for myocardial infarction. J. Control. Release 2021, 331, 62–73. [Google Scholar] [CrossRef]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef]
- Zhuo, Y.; Luo, Z.; Zhu, Z.; Wang, J.; Li, X.; Zhang, Z.; Guo, C.; Wang, B.; Nie, D.; Gan, Y.; et al. Direct cytosolic delivery of siRNA via cell membrane fusion using cholesterol-enriched exosomes. Nat. Nanotechnol. 2024, 19, 1858–1868. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.A.; Yoon, H.; Kim, M.Y.; Yoo, J.K.; Ahn, S.H.; Park, C.H.; Park, J.; Nam, B.Y.; Park, J.T.; et al. Exosome-based delivery of super-repressor IkappaBalpha ameliorates kidney ischemia-reperfusion injury. Kidney Int. 2021, 100, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yan, J.; Li, X.; Chen, H.; Lin, C.; Zhang, Y.; Gao, T.; Zhang, Y.; Shu, Y.; Pan, S.; et al. Advancements in extracellular vesicles biomanufacturing: A comprehensive overview of large-scale production and clinical research. Front. Bioeng. Biotechnol. 2025, 13, 1487627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, S.F.; Xiao, X.; Liu, Y.N.; Wang, X.L.; Du, Y.X. Combined Treatment of Bone Marrow Mesenchymal Stem Cells and Fasudil Promotes Neurovascular Remodeling and Neurological Function Recovery in Ischemic Stroke. Appl. Biochem. Biotechnol. 2022, 194, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Tunc Ata, M.; Turgut, G.; Akbulut, M.; Kocyigit, A.; Karabulut, A.; Senol, H.; Turgut, S. Effect of Erythropoietin and Stem Cells on Traumatic Brain Injury. World Neurosurg. 2016, 89, 355–361. [Google Scholar] [CrossRef]
- Bonsack, B.; Heyck, M.; Kingsbury, C.; Cozene, B.; Sadanandan, N.; Lee, J.Y.; Borlongan, C.V. Fast-tracking regenerative medicine for traumatic brain injury. Neural Regen. Res. 2020, 15, 1179–1190. [Google Scholar] [CrossRef]
- Tian, H.; Tian, F.; Ma, D.; Xiao, B.; Ding, Z.; Zhai, X.; Song, L.; Ma, C. Priming and Combined Strategies for the Application of Mesenchymal Stem Cells in Ischemic Stroke: A Promising Approach. Mol. Neurobiol. 2024, 61, 7127–7150. [Google Scholar] [CrossRef]
- Rouce, R.H.; Porteus, M.H. Cell and gene therapy accessibility. Science 2024, 385, 475. [Google Scholar] [CrossRef]
- Ayala Ceja, M.; Khericha, M.; Harris, C.M.; Puig-Saus, C.; Chen, Y.Y. CAR-T cell manufacturing: Major process parameters and next-generation strategies. J. Exp. Med. 2024, 221, e20230903. [Google Scholar] [CrossRef]
- Lv, Z.; Luo, F.; Chu, Y. Strategies for overcoming bottlenecks in allogeneic CAR-T cell therapy. Front. Immunol. 2023, 14, 1199145. [Google Scholar] [CrossRef]
- Borgert, R. Improving outcomes and mitigating costs associated with CAR T-cell therapy. Am. J. Manag. Care 2021, 27, S253–S261. [Google Scholar] [CrossRef]

| Drug Category | Mechanism of Action | Drug Names | Disadvantages |
|---|---|---|---|
| Anticoagulants | Inhibit platelet aggregation and limit thrombus formation | Heparin, Warfarin, Rivaroxaban, Apixaban | Risk of bleeding |
| Thrombolytics | Dissolve already formed thrombi | Alteplase, Retaplase | Must be used within 4.5 h of onset; bleeding risk |
| Vasodilators | Dilate blood vessels to increase blood supply | Nitroglycerin | Hypotension, dizziness, headache |
| Calcium Channel Blockers | Block calcium ion channels, dilate vessels | Nifedipine, Diltiazem | Bradycardia, hypotension, not suitable for some heart failure patients |
| Neuroprotective Agents | Protect neurons from ischemia-reperfusion injury | Edaravone | Must be used within 48 h of onset; limited clinical efficacy |
| Antiplatelet drugs | Inhibit platelet activation and aggregation | Aspirin, Clopidogrel, Tirofiban | Risk of bleeding |
| Stem Cell Type | Advantages | Disadvantages | Sources | Challenges |
|---|---|---|---|---|
| NSCs | Can differentiate into various neural cell types; potential for brain injury repair | Limited availability and ethical concerns | Brain tissue | Integration and survival in host tissue |
| ESCs | Pluripotent, can differentiate into any cell type | Ethical concerns, risk of teratoma formation | Embryos | Ethical issues, tumor risk |
| IPSCs | Pluripotent; derived from adult cells, avoiding ethical concerns; | Genetic instability, potential for tumor formation | Adult somatic cells | Controlled differentiation, safety |
| MSCs | Easy to obtain; immunomodulatory properties; lower ethical concerns | Limited multilineage potential; variable quality | Bone marrow, adipose tissue | Standardization, variability in patient sources |
| HESs | Can differentiate into all types of mature blood cells and EPC | Restricted availability, complex procurement and processing | Bone marrow, umbilical cord blood | limited cell sources |
| CSCs | Can differentiate into cardiomyocytes, facilitating myocardial regeneration | low abundance of true CSCs in heart tissue | heart tissue | Difficulty in accurate identification and purification |
| Ischemic Disease | Cell Types Used | Main Clinical Outcomes | Delivery Routes |
|---|---|---|---|
| Acute Myocardial Infarction | BM-MSCs, UC-MSCs, HSCs, CSCs | Improved cardiac function: ~4.58% increase in LVEF, ~5.18 mL reduction in LVESV; dose-dependent effects | Intracoronary injection, intramyocardial injection, intravenous injection |
| Ischemic Stroke | BM-MSCs | Some functional neurological improvements reported; mixed results across trials | Intravenous injection, arterial injection, intracerebral injection |
| Chronic Limb Ischemia | B-MSCs, AD-MSCs Placental MSCs | Improved blood perfusion, ulcer healing, pain relief, reduced amputation rates | Arterial injection, intravenous injection, intramuscular injection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Yu, A.; He, T.; Qian, Y.; Hu, M. Stem Cell Therapy Approaches for Ischemia: Assessing Current Innovations and Future Directions. Int. J. Mol. Sci. 2025, 26, 6320. https://doi.org/10.3390/ijms26136320
Ma C, Yu A, He T, Qian Y, Hu M. Stem Cell Therapy Approaches for Ischemia: Assessing Current Innovations and Future Directions. International Journal of Molecular Sciences. 2025; 26(13):6320. https://doi.org/10.3390/ijms26136320
Chicago/Turabian StyleMa, Changguo, An Yu, Tingyan He, Yulin Qian, and Min Hu. 2025. "Stem Cell Therapy Approaches for Ischemia: Assessing Current Innovations and Future Directions" International Journal of Molecular Sciences 26, no. 13: 6320. https://doi.org/10.3390/ijms26136320
APA StyleMa, C., Yu, A., He, T., Qian, Y., & Hu, M. (2025). Stem Cell Therapy Approaches for Ischemia: Assessing Current Innovations and Future Directions. International Journal of Molecular Sciences, 26(13), 6320. https://doi.org/10.3390/ijms26136320





