Ceruloplasmin and Ferritin Changes in Ocular Fluids from Patients with Vitreoretinal Diseases: Relation with Neuroinflammation and Drusen Formation
Abstract
1. Introduction
2. Results
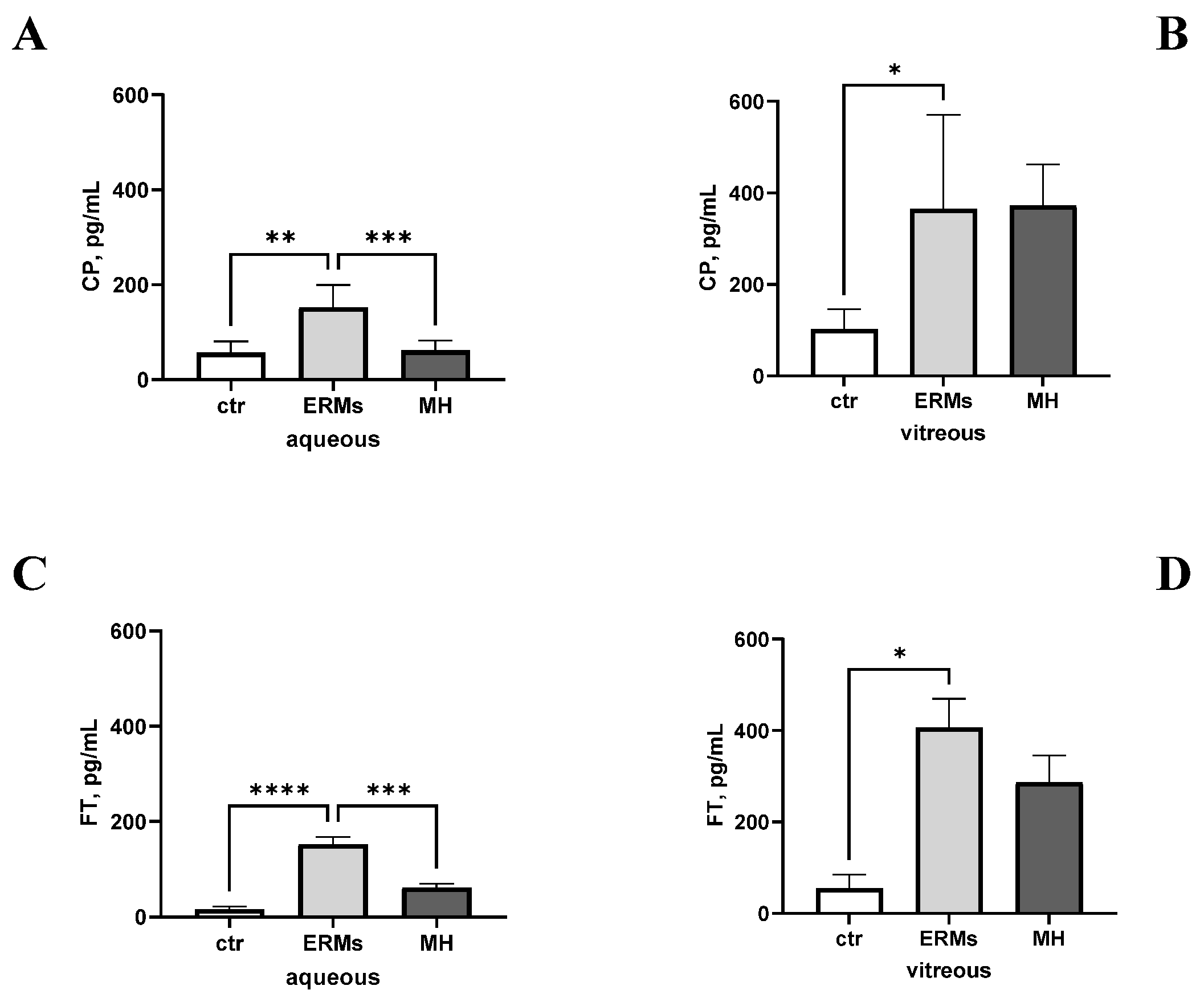
2.1. Ceruloplasmin and Ferritin Levels Can Be Measured in Ocular Fluids and Are Changed upon Disease
2.2. CP and FT Levels Are Linked to VR Disease
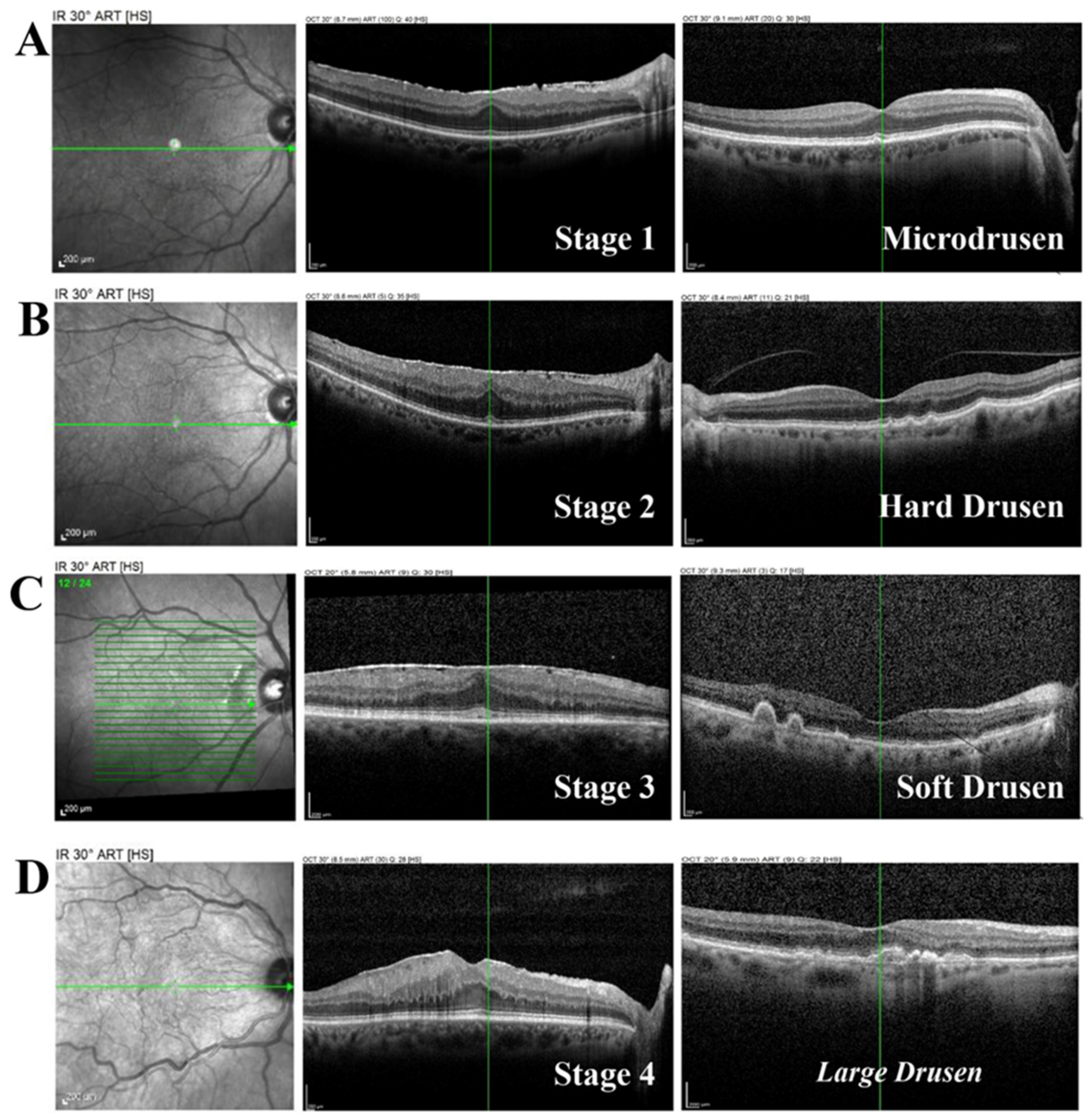
2.3. CP and FT Levels Are Linked to ERM Severity
2.4. CP and FT Levels Are Not Linked to Systemic Inflammatory, Metabolic, and Neurodegenerative Disorders
2.5. CP/FT Ratio and Drusen Association: Odds Ratio Analysis
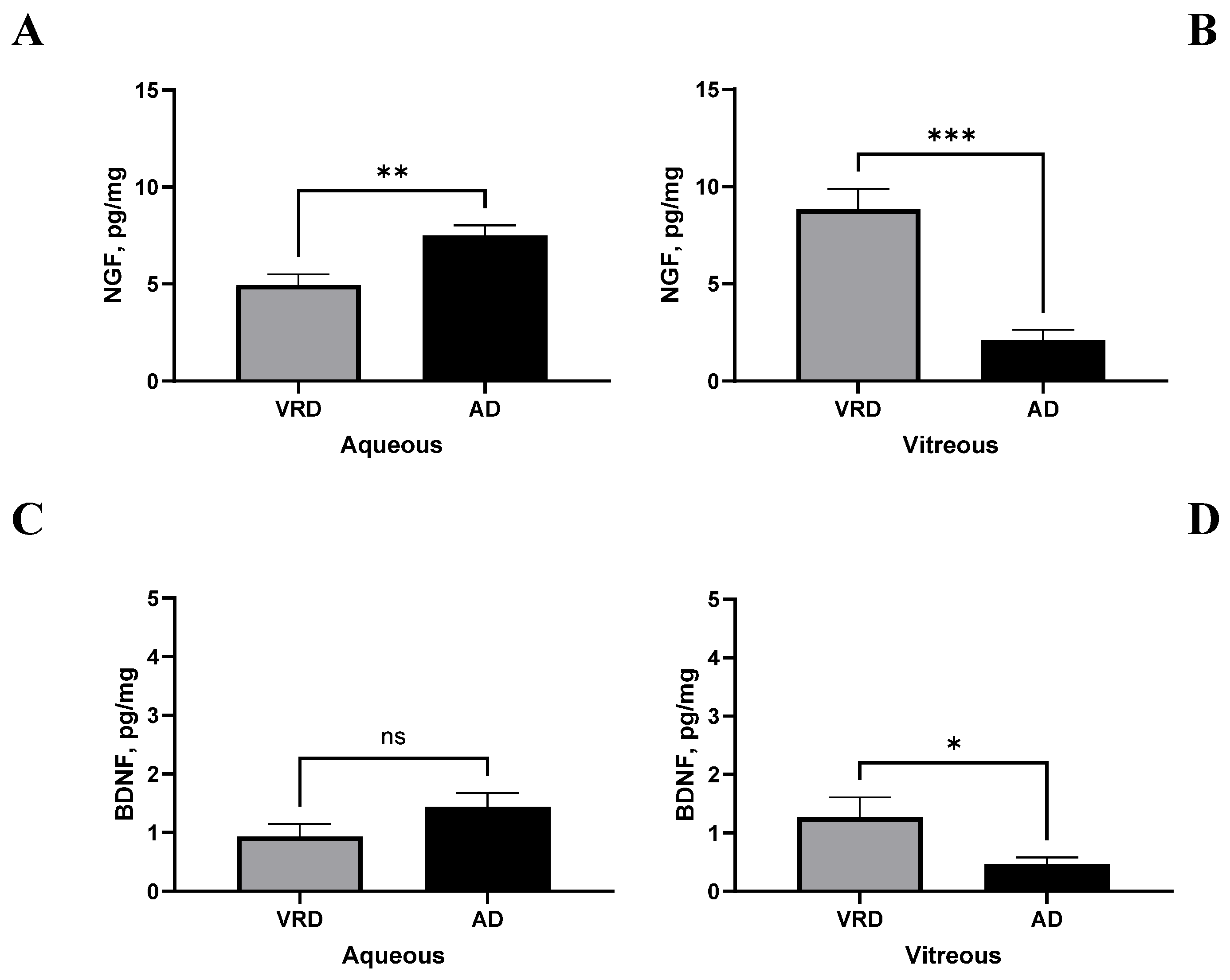
2.6. CP/FT Validation in a Few AD-Selected Biological Samples
3. Discussion
4. Materials and Methods
4.1. Study Population and Ethical Considerations
4.2. Sampling and Preanalytical Procedure
4.3. ELISA
4.4. Protein Array
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuliqiman, M.; Xu, M.; Sun, Y.; Cao, J.; Chen, P.; Gao, Q.; Xu, P.; Ye, J. Artificial Intelligence in Ophthalmic Surgery: Current Applications and Expectations. Clin. Ophthalmol. 2023, 17, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Ittarat, M.; Somkijrungroj, T.; Chansangpetch, S.; Pongsachareonnont, P. Literature Review of Surgical Treatment in Idiopathic Full-Thickness Macular Hole. Clin. Ophthalmol. 2020, 14, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, E.; Colorado-Zavala, M.F.; Almuhtaseb, H.; Venkatesh, R.; Parolini, B.; Chhablani, J. Anatomical and functional changes after internal limiting membrane peeling. Surv. Ophthalmol. 2025, 70, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Boscia, G.; Feo, A.; Savastano, A.; Viggiano, P.; Landini, L.; Clemente, A.; Scotti, G.; Grassi, M.O.; Parisi, G.; Giancipoli, E.; et al. Intravitreal Dexamethasone Implant in Vitreoretinal Surgery: An Overview of the Literature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2025. [Google Scholar] [CrossRef]
- Hall, A. Surgical maculopathies: Epiretinal membranes and full-thickness macular holes. Community Eye Health 2025, 37, 13–15. [Google Scholar] [PubMed] [PubMed Central]
- Spaide, R.F.; Curcio, C.A. Drusen characterization with multimodal imaging. Retina 2010, 30, 1441–1454. [Google Scholar] [CrossRef]
- Francone, A.; Yun, L.; Kothari, N.; Cheng, I.; Farajzadeh, M.; Govetto, A.; Hubschman, J.P. Lamellar macular holes in the presence of age-related macular degeneration. Retina 2020, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Kiruthika, M.; Malathi, G. A comprehensive review on early detection of drusen patterns in age-related macular degeneration using deep learning models. Photodiagnosis Photodyn. Ther. 2025, 51, 104454. [Google Scholar] [CrossRef]
- Chalkias, E.; Topouzis, F.; Tegos, T.; Tsolaki, M. The Contribution of Ocular Biomarkers in the Differential Diagnosis of Alzheimer’s Disease versus Other Types of Dementia and Future Prospects. J. Alzheimer’s Dis. JAD 2021, 80, 493–504. [Google Scholar] [CrossRef]
- Schumacher-Schuh, A.; Bieger, A.; Borelli, W.V.; Portley, M.K.; Awad, P.S.; Bandres-Ciga, S. Advances in Proteomic and Metabolomic Profiling of Neurodegenerative Diseases. Front. Neurol. 2022, 12, 792227. [Google Scholar] [CrossRef]
- Dehghani, C.; Frost, S.; Jayasena, R.; Masters, C.L.; Kanagasingam, Y. Ocular Biomarkers of Alzheimer’s Disease: The Role of Anterior Eye and Potential Future Directions. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3554–3563. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mao, X. Role of Retinal Amyloid-β in Neurodegenerative Diseases: Overlapping Mechanisms and Emerging Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2360. [Google Scholar] [CrossRef] [PubMed]
- Latina, V.; Giacovazzo, G.; Cordella, F.; Balzamino, B.O.; Micera, A.; Varano, M.; Marchetti, C.; Malerba, F.; Florio, R.; Ercole, B.B.; et al. Systemic delivery of a specific antibody targeting the pathological N-terminal truncated tau peptide reduces retinal degeneration in a mouse model of Alzheimer’s Disease. Acta Neuropathol. Commun. 2021, 9, 38. [Google Scholar] [CrossRef]
- Moncaster, J.A.; Moir, R.D.; Burton, M.A.; Chadwick, O.; Minaeva, O.; Alvarez, V.E.; Ericsson, M.; Clark, J.I.; McKee, A.C.; Tanzi, R.E.; et al. Alzheimer’s disease amyloid-β pathology in the lens of the eye. Exp. Eye Res. 2022, 221, 108974. [Google Scholar] [CrossRef] [PubMed]
- Gharbiya, M.; Visioli, G.; Trebbastoni, A.; Albanese, G.M.; Colardo, M.; D’Antonio, F.; Segatto, M.; Lambiase, A. Beta-Amyloid Peptide in Tears: An Early Diagnostic Marker of Alzheimer’s Disease Correlated with Choroidal Thickness. Int. J. Mol. Sci. 2023, 24, 2590. [Google Scholar] [CrossRef]
- An, C.; Cai, H.; Ren, Z.; Fu, X.; Quan, S.; Jia, L. Biofluid biomarkers for Alzheimer’s disease: Past, present, and future. Med. Rev. 2024, 4, 467–491. [Google Scholar] [CrossRef]
- Mateo, D.; Marquès, M.; Torrente, M. Metals linked with the most prevalent primary neurodegenerative dementias in the elderly: A narrative review. Environ. Res. 2023, 236 Pt 1, 116722. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar] [CrossRef]
- Roy, R.G.; Mandal, P.K.; Maroon, J.C. Oxidative Stress Occurs Prior to Amyloid Aβ Plaque Formation and Tau Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 2023, 14, 2944–2954. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. The duality of amyloid-β: Its role in normal and Alzheimer’s disease states. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef]
- Peng, G.; Huang, Y.; Xie, G.; Tang, J. Exploring Copper’s role in stroke: Progress and treatment approaches. Front. Pharmacol. 2024, 15, 1409317. [Google Scholar] [CrossRef]
- Sabalic, A.; Mei, V.; Solinas, G.; Madeddu, R. The Role of Copper in Alzheimer’s Disease Etiopathogenesis: An Updated Systematic Review. Toxics 2024, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Banha, J.; Marques, L.; Oliveira, R.; de Fátima Martins, M.; Paixão, E.; Pereira, D.; Malhó, R.; Penque, D.; Costa, L. Ceruloplasmin expression by human peripheral blood lymphocytes: A new link between immunity and iron metabolism. Free Radic. Biol. Med. 2008, 44, 483–492. [Google Scholar] [CrossRef]
- Micera, A.; Bruno, L.; Cacciamani, A.; Rongioletti, M.; Squitti, R. Alzheimer’s Disease and Retinal Degeneration: A Glimpse at Essential Trace Metals in Ocular Fluids and Tissues. Curr. Alzheimer Res. 2019, 16, 1073–1083. [Google Scholar] [CrossRef]
- Govetto, A.; Lalane, R.A., 3rd; Sarraf, D.; Figueroa, M.S.; Hubschman, J.P. Insights Into Epiretinal Membranes: Presence of Ectopic Inner Foveal Layers and a New Optical Coherence Tomography Staging Scheme. Am. J. Ophthalmol. 2017, 175, 99–113. [Google Scholar] [CrossRef]
- Gelman, R.; Stevenson, W.; Prospero Ponce, C.; Agarwal, D.; Christoforidis, J.B. Retinal Damage Induced by Internal Limiting Membrane Removal. J. Ophthalmol. 2015, 2015, 939748. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.I.; Munteanu, M.; Patoni, C.; Musat, A.M.A.; Dragoescu, V.; Cernat, C.C.; Popescu, M.N.; Musat, O. Role of the Vitreous in Retinal Pathology: A Narrative Review. Cureus 2023, 15, e43990. [Google Scholar] [CrossRef]
- Moussa, O.; Golebka, J.; Gomide, G.; Koenigstein, D.; Shih, H.; Chen, R.W.S. Spontaneously Opening and Closing Macular Holes with Lamellar Hole Epiretinal Proliferation: A Longitudinal Optical Coherence Tomography Analysis. Diagnostics 2025, 15, 759. [Google Scholar] [CrossRef] [PubMed]
- Fleckner, M.; Döhmen, N.K.; Salz, K.; Christophers, T.; Windolf, J.; Suschek, C.V.; Oezel, L. Exposure of Primary Human Skin Fibroblasts to Carbon Dioxide-Containing Solution Significantly Reduces TGF-β-Induced Myofibroblast Differentiation In Vitro. Int. J. Mol. Sci. 2024, 25, 13013. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Chen, J.G.; Chen, X.F.; Gu, D.H.; Liu, Z.M.; Gao, Y.D.; Zheng, B. Ceruloplasmin overexpression is associated with oncogenic pathways and poorer survival rates in clear-cell renal cell carcinoma. FEBS Open Bio 2021, 11, 2988–3004. [Google Scholar] [CrossRef]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef] [PubMed]
- Harned, J.; Ferrell, J.; Nagar, S.; Goralska, M.; Fleisher, L.N.; McGahan, M.C. Ceruloplasmin alters intracellular iron regulated proteins and pathways: Ferritin, transferrin receptor, glutamate and hypoxia-inducible factor-1α. Exp. Eye Res. 2012, 97, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Ventriglia, M.; Simonelli, I.; Bonvicini, C.; Costa, A.; Perini, G.; Binetti, G.; Benussi, L.; Ghidoni, R.; Koch, G.; et al. Copper Imbalance in Alzheimer’s Disease: Meta-Analysis of Serum, Plasma, and Brain Specimens, and Replication Study Evaluating ATP7B Gene Variants. Biomolecules 2021, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.; Tipton, K. Ceruloplasmin and what it might do. J. Neural Transm. 2007, 114, 777–781. [Google Scholar] [CrossRef]
- Wróblewska, J.; Nuszkiewicz, J.; Wróblewski, M.; Wróblewska, W.; Woźniak, A. Selected Trace Elements and Their Impact on Redox Homeostasis in Eye Health. Biomolecules 2024, 14, 1356. [Google Scholar] [CrossRef]
- García-Castiñeiras, S. Iron, the retina and the lens: A focused review. Exp. Eye Res. 2010, 90, 664–678. [Google Scholar] [CrossRef]
- Goralska, M.; Fleisher, L.N.; McGahan, M.C. Vitreous Humor Changes Expression of Iron-Handling Proteins in Lens Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1187–1195. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Reekie, I.R.; Sharma, S.; Foers, A.; Sherlock, J.; Coles, M.C.; Dick, A.D.; Denniston, A.K.; Buckley, C.D. The Cellular Composition of the Uveal Immune Environment. Front. Med. 2021, 8, 721953. [Google Scholar] [CrossRef]
- Braunger, B.M.; Gießl, A.; Schlötzer-Schrehardt, U. The Blood-ocular Barriers and their Dysfunction: Anatomy, Physiology, Pathology. Die Blut-Augen-Schranken und ihre Störungen: Anatomie, Physiologie, Pathologie. Klin. Monatsblatter Augenheilkd. 2023, 240, 650–661. [Google Scholar] [CrossRef]
- Zong, Y.; Gao, Q.Y.; Hui, Y.N. Vitreous function and intervention of it with vitrectomy and other modalities. Int. J. Ophthalmol. 2022, 15, 857–867. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Zhou, L.; Wang, X.; Liu, L.; Wu, M. Copper homeostasis and cuproptosis in atherosclerosis: Metabolism, mechanisms and potential therapeutic strategies. Cell Death Discov. 2024, 10, 25. [Google Scholar] [CrossRef]
- Catapano, A.; Cimmino, F.; Petrella, L.; Pizzella, A.; D’Angelo, M.; Ambrosio, K.; Marino, F.; Sabbatini, A.; Petrelli, M.; Paolini, B.; et al. Iron metabolism and ferroptosis in health and diseases: The crucial role of mitochondria in metabolically active tissues. J. Nutr. Biochem. 2025, 140, 109888. [Google Scholar] [CrossRef]
- Kaštelan, S.; Gverović Antunica, A.; Puzović, V.; Didović Pavičić, A.; Čanović, S.; Kovačević, P.; Vučemilović, P.A.F.; Konjevoda, S. Non-Invasive Retinal Biomarkers for Early Diagnosis of Alzheimer’s Disease. Biomedicines 2025, 13, 283. [Google Scholar] [CrossRef]
- Cheli, V.T.; Sekhar, M.; Santiago González, D.A.; Angeliu, C.G.; Denaroso, G.E.; Smith, Z.; Wang, C.; Paez, P.M. The expression of ceruloplasmin in astrocytes is essential for postnatal myelination and myelin maintenance in the adult brain. Glia 2023, 71, 2323–2342. [Google Scholar] [CrossRef]
- Gerhardinger, C.; Costa, M.B.; Coulombe, M.C.; Toth, I.; Hoehn, T.; Grosu, P. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 349–357. [Google Scholar] [CrossRef]
- Gnana-Prakasam, J.P.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Expression and function of iron-regulatory proteins in retina. IUBMB Life 2010, 62, 363–370. [Google Scholar] [CrossRef]
- Ueki, S.; Suzuki, Y. New Perspective on Aqueous Humor Circulation: Retina Takes the Lead. Int. J. Mol. Sci. 2025, 26, 2645. [Google Scholar] [CrossRef]
- Hirano, K.; Ogihara, T.; Ogihara, H.; Hiroi, M.; Hasegawa, M.; Tamai, H. Identification of apo- and holo-forms of ceruloplasmin in patients with Wilson’s disease using native polyacrylamide gel electrophoresis. Clin. Biochem. 2005, 38, 9–12. [Google Scholar] [CrossRef]
- Chen, L.; Dentchev, T.; Wong, R.; Hahn, P.; Wen, R.; Bennett, J.; Dunaief, J.L. Increased expression of ceruloplasmin in the retina following photic injury. Mol. Vis. 2003, 9, 151–158. [Google Scholar]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Anderson, B.D.; Lee, T.; Bell, B.; Song, Y.; Dunaief, J.L. Low ceruloplasmin levels exacerbate retinal degeneration in a hereditary hemochromatosis model. Dis. Models Mech. 2023, 16, dmm050226. [Google Scholar] [CrossRef]
- Li, Y.; Jin, S.; Shi, L.; Qin, H.; Zhao, J. Factors Associated with Anatomic Failure and Hole Reopening after Macular Hole Surgery. J. Ophthalmol. 2021, 2021, 7861180. [Google Scholar] [CrossRef]
- Kalogeropoulos, D.; Lotery, A.J.; Gupta, B.; Lash, S.; Antonakis, S. Epiretinal membranes in patients with uveitis: An update on the current state of management. Int. Ophthalmol. 2024, 44, 291. [Google Scholar] [CrossRef]
- Wilde, C.; Panos, G.D.; Pooschti, A.; MacNab, H.K.; Hillman, J.G.; Vernon, S.A.; Amoaku, W.M. Prevalence and Associations of Epiretinal Membranes in an Elderly English Population: The Bridlington Eye Assessment Project. J. Clin. Med. 2024, 13, 739. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Brown, C.N.; Green, B.D.; Thompson, R.B.; Den Hollander, A.I.; Lengyel, I.; On behalf of the EYE-RISK consortium. Metabolomics and Age-Related Macular Degeneration. Metabolites 2018, 9, 4. [Google Scholar] [CrossRef]
- Srejovic, J.V.; Muric, M.D.; Jakovljevic, V.L.; Srejovic, I.M.; Sreckovic, S.B.; Petrovic, N.T.; Todorovic, D.Z.; Bolevich, S.B.; Sarenac Vulovic, T.S. Molecular and Cellular Mechanisms Involved in the Pathophysiology of Retinal Vascular Disease—Interplay Between Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 11850. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Zhang, C.; Zhou, S.; Ji, G. Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 695–711. [Google Scholar] [CrossRef]
- Harju, N.; Kauppinen, A.; Loukovaara, S. Fibrotic Changes in Rhegmatogenous Retinal Detachment. Int. J. Mol. Sci. 2025, 26, 1025. [Google Scholar] [CrossRef]
- Papp, Á.; Bene, Z.; Gáspár, I.; Nagy, B., Jr.; Kádár, L.; Márialigeti, T.; Bánfi, A.; Baktai, G.; Balla, G.; Nagy, B. Decreased VEGF Level Is Associated with Elevated Ferritin Concentration in Bronchoalveolar Lavage Fluid of Children with Interstitial Lung Diseases. Respir. Int. Rev. Thorac. Dis. 2015, 90, 443–450. [Google Scholar] [CrossRef]
- Yang, J.Y.; Goldberg, D.; Sobrin, L. Interleukin-6 and Macular Edema: A Review of Outcomes with Inhibition. Int. J. Mol. Sci. 2023, 24, 4676. [Google Scholar] [CrossRef]
- Shahandeh, A.; Bui, B.V.; Finkelstein, D.I.; Nguyen, C.T.O. Effects of Excess Iron on the Retina: Insights From Clinical Cases and Animal Models of Iron Disorders. Front. Neurosci. 2022, 15, 794809. [Google Scholar] [CrossRef]
- Zhao, N.; Li, S.; Wu, H.; Wei, D.; Pu, N.; Wang, K.; Liu, Y.; Tao, Y.; Song, Z. Ferroptosis: An Energetic Villain of Age-Related Macular Degeneration. Biomedicines 2025, 13, 986. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.H.; Woo, S.J.; Kang, S.W.; Park, K.H. Epidemiologic Survey Committee of Korean Ophthalmologic Society Five heavy metallic elements and age-related macular degeneration: Korean National Health and Nutrition Examination Survey, 2008–2011. Ophthalmology 2015, 122, 129–137. [Google Scholar] [CrossRef]
- Flinn, J.M.; Kakalec, P.; Tappero, R.; Jones, B.; Lengyel, I. Correlations in distribution and concentration of calcium, copper and iron with zinc in isolated extracellular deposits associated with age-related macular degeneration. Met. Integr. Biometal Sci. 2014, 6, 1223–1228. [Google Scholar] [CrossRef]
- Fucito, M.; Spedicato, M.; Felletti, S.; Yu, A.C.; Busin, M.; Pasti, L.; Franchina, F.A.; Cavazzini, A.; De Luca, C.; Catani, M. A Look into Ocular Diseases: The Pivotal Role of Omics Sciences in Ophthalmology Research. ACS Meas. Sci. Au 2024, 4, 247–259. [Google Scholar] [CrossRef]
- Confalonieri, F.; Josifovska, N.; Boix-Lemonche, G.; Stene-Johansen, I.; Bragadottir, R.; Lumi, X.; Petrovski, G. Vitreous Substitutes from Bench to the Operating Room in a Translational Approach: Review and Future Endeavors in Vitreoretinal Surgery. Int. J. Mol. Sci. 2023, 24, 3342. [Google Scholar] [CrossRef]
- Okada, M.; Chiu, D.; Yeoh, J. Vitreomacular disorders: A review of the classification, pathogenesis and treatment paradigms including new surgical techniques. Clin. Exp. Optom. 2021, 104, 672–683. [Google Scholar] [CrossRef]
- Veitch, D.P.; Weiner, M.W.; Aisen, P.S.; Beckett, L.A.; DeCarli, C.; Green, R.C.; Harvey, D.; Jack, C.R., Jr.; Jagust, W.; Landau, S.M.; et al. Using the Alzheimer’s Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 824–857. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pal, A.; Cerchiaro, G.; Rani, I.; Ventriglia, M.; Rongioletti, M.; Longobardi, A.; Squitti, R. Iron in Alzheimer’s Disease: From Physiology to Disease Disabilities. Biomolecules 2022, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, T.; Lam, E.; Alvarez, D.; Sun, Y. Ocular Vascular Diseases: From Retinal Immune Privilege to Inflammation. Int. J. Mol. Sci. 2023, 24, 12090. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Grycan, M.; Przybyłkowski, A.M. Monitoring of Copper in Wilson Disease. Diagnostics 2023, 13, 1830. [Google Scholar] [CrossRef] [PubMed]




| Study Population | Cases | ERMs | MHs | AD | p Value |
|---|---|---|---|---|---|
| |||||
| Participants/[biosamples] | 26/[26] | 14/[14] | 6/[6] | 6/[6] | |
| mean age | 69.60 ± 6.71 | 70.00 ± 6.60 | 68.70 ± 7.00 | 70.00 ± 6.60 | p = 0.385 |
| sex | 8M/18F | 3M/11F | 2M/4F | 3M/3F | p > 0.05 |
| ERM stage 2 | 1M/5F | ||||
| ERM stage 3 | 1M/4F | ||||
| ERM stage 4 | 2M/1F | ||||
| |||||
| Hypertension | 19/26 | 10/14 | 6/6 | 5/6 | |
| Hypercholesterolemia | 11/26 | 7/14 | 2/6 | 2/6 | |
| Cardiopathy | 5/26 | 3/14 | 2/6 | 2/6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Cosimi, P.; Balzamino, B.O.; Bruno, M.; Squitti, R.; Dinice, L.; Scarinci, F.; Rongioletti, M.C.A.; Cacciamani, A.; Micera, A. Ceruloplasmin and Ferritin Changes in Ocular Fluids from Patients with Vitreoretinal Diseases: Relation with Neuroinflammation and Drusen Formation. Int. J. Mol. Sci. 2025, 26, 6307. https://doi.org/10.3390/ijms26136307
Esposito G, Cosimi P, Balzamino BO, Bruno M, Squitti R, Dinice L, Scarinci F, Rongioletti MCA, Cacciamani A, Micera A. Ceruloplasmin and Ferritin Changes in Ocular Fluids from Patients with Vitreoretinal Diseases: Relation with Neuroinflammation and Drusen Formation. International Journal of Molecular Sciences. 2025; 26(13):6307. https://doi.org/10.3390/ijms26136307
Chicago/Turabian StyleEsposito, Graziana, Pamela Cosimi, Bijorn Omar Balzamino, Marisa Bruno, Rosanna Squitti, Lucia Dinice, Fabio Scarinci, Mauro Ciro Antonio Rongioletti, Andrea Cacciamani, and Alessandra Micera. 2025. "Ceruloplasmin and Ferritin Changes in Ocular Fluids from Patients with Vitreoretinal Diseases: Relation with Neuroinflammation and Drusen Formation" International Journal of Molecular Sciences 26, no. 13: 6307. https://doi.org/10.3390/ijms26136307
APA StyleEsposito, G., Cosimi, P., Balzamino, B. O., Bruno, M., Squitti, R., Dinice, L., Scarinci, F., Rongioletti, M. C. A., Cacciamani, A., & Micera, A. (2025). Ceruloplasmin and Ferritin Changes in Ocular Fluids from Patients with Vitreoretinal Diseases: Relation with Neuroinflammation and Drusen Formation. International Journal of Molecular Sciences, 26(13), 6307. https://doi.org/10.3390/ijms26136307









