Ex Vivo Preconditioning as a Useful Tool for Modification of the Extracellular Matrix of Multipotent Mesenchymal Stromal Cells
Abstract
1. Introduction
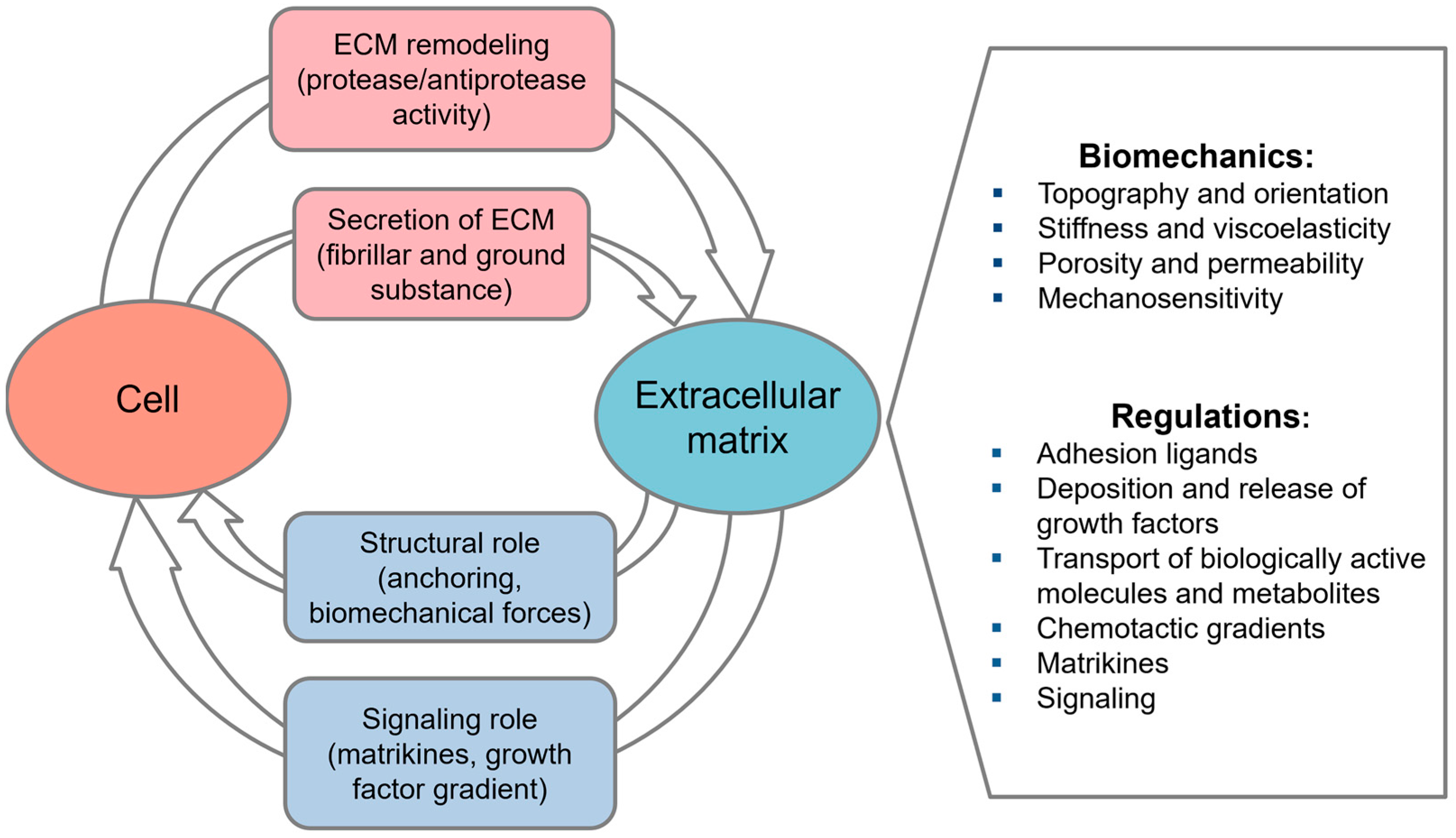
2. Cell-Derived Extracellular Matrix
2.1. Cell-Derived ECM as a Physiological Microenvironment
2.2. Tissue-Specific Memory: Instructive Role of Cell-Derived ECM
2.3. Cell-Derived ECM in Tissue Engineering and Regenerative Medicine
2.4. Targeted Modification of Cell-Derived ECM
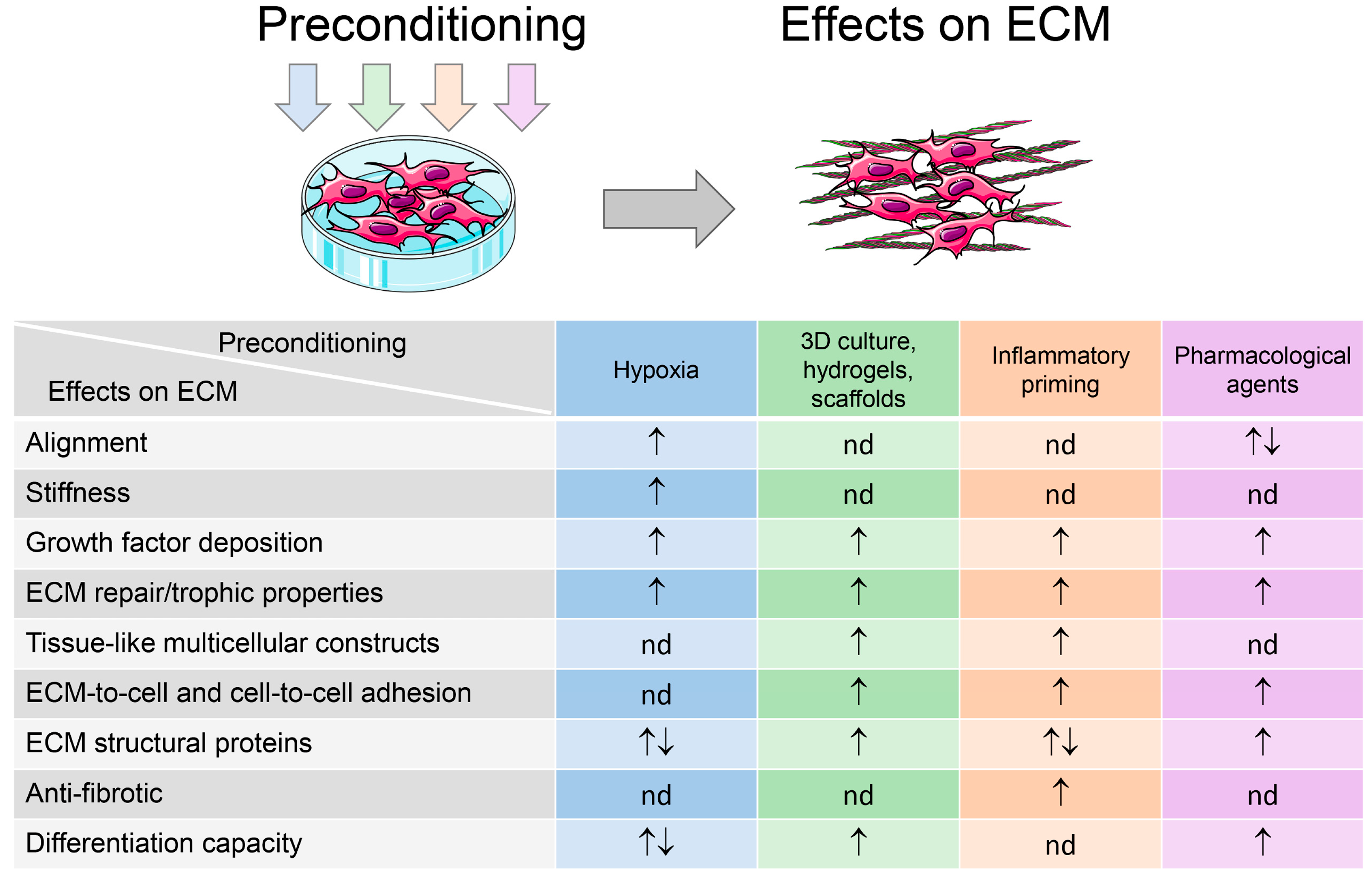
3. MSC Preconditioning for Improvement of Cell Therapy and TERM-Demanded Properties: What About the Extracellular Matrix?
3.1. Hypoxia
3.2. Tissue Engineering Approaches (3D Culture, Hydrogels, and Scaffolds)
3.3. Inflammatory Microenvironments
3.4. Pharmacological Agents and Growth Medium Composition
3.5. Applications of ECM Derived from Preconditioned MSCs and Their Progeny
| Cell Type | Preconditioning/ Modification | In Vivo/In Vitro Outcomes | Reference |
|---|---|---|---|
| Pharmacological agents and culture medium formulations | |||
| huBM-MSCs | Osteogenic medium | cd-ECM from osteo-differentiated MSCs promoted bone formation more effectively after ectopic implantation in mice, than cd-ECM from undifferentiated MSCs | [241] |
| huAT-MSCs | Adipogenic medium | cd-ECM from adipo-differentiated MSCs induced adipogenic differentiation of reseeded MSCs in vitro | [239] |
| rat BM-MSCs | Biphasic calcium phosphate scaffolds | Increased osteoblastic differentiation of reseeded cd-ECM coated BCP scaffolds in vitro | [240] |
| Hypoxia | |||
| rbBM-MSCs | Chemical hypoxia, CoCl2 | Hypoxic cd-ECM accelerated wound repair in a mouse model of full-thickness skin defect (enhanced reepithelization and granulation tissue formation, augmented angiogenesis) | [242] |
| huDF | 2% O2, in combination with polycaprolactone (PCL) scaffold and mechanical stimulation | Hypoxic PCL-cd-ECM patches improved endothelization and smooth muscle regeneration after grafting in rat abdominal aorta | [243] |
| Scaffolds and coatings | |||
| murine osteoblast/osteocyte-like cells | Porous PLC scaffolds | Cd-ECM-coated scaffolds induced cell proliferation, osteogenic activity in vitro, and potentiated angiogenesis in chorioallantoic membrane assay in ovo | [244] |
| huBM-MSCs | Porous PCL scaffolds | Cd-ECM-coated scaffolds enhanced attachment, proliferation, and osteogenic differentiation of reseeded MSCs | [245] |
| rat fibroblasts and endothelial cells (ECs) | PCL microfibers | Cd-ECM-coated microfibers stimulated tube formation by ECs, osteoblast proliferation, and differentiation | [253] |
| huBM-MSCs, HUVEC | PCL microfibers | Enhancement of osteogenic differentiation of reseeded MSCs on Cd-ECM-coated microfibers | [89] |
| huAT-MSCs | Poly(Lactic-co-Glycolic Acid) (PLGA) nanofibers | Cd-ECM-coated nanofibrous mesh improved the wound healing in a mouse skin wound model | [248] |
| human lung fibroblast (hLF) | Polyvinyl alcohol (PVA) hydrogel | Cd-ECM incorporated in PVA hydrogel provided advanced skin regeneration in infected wound mice model | [86] |
| huDF | PVA hydrogel | Cardiac remodeling was improved in the infarcted area of the rat MI model with a cardiac patch that included MSCs seeded on cd-ECM incorporated in poly(vinyl alcohol) (PVA) hydrogel | [249] |
| hLF | PLGA/PLA-based scaffolds | Cd-ECM coated scaffolds stimulated reseeded MSC osteo-differentiation. Significant increase in new bone formation in a mouse ectopic and rat calvarial bone defect models. | [250] |
| rat BM-MSCs | Chitosan-silk fibroin scaffolds | Enhancement of nerve regeneration in rat model of peripheral nerve injury | [84] |
| huBM-MSCs | Chitosan/silk fibroin scaffolds | Cd-ECM-coated grafts significantly improved nerve repair in dog sciatic nerve gap model | [246] |
| huBM-MSCs | Chitin/chitosan fibers | Cd-ECM-coated fibers induced the repair of sciatic nerve defects in rats similar to autografts | [251] |
| hLF | Collagen hydrogel | Microspheres containing HUVECs, MSCs and cd-ECM incorporated in collagen hydrogel significantly improved blood reperfusion in a mouse hindlimb ischemic model | [252] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSCs | multipotent mesenchymal stem/stromal cells |
| TERM | tissue engineering and regenerative medicine |
| ECM | extracellular matrix |
| cd-ECM | cell-derived ECM |
| TE | tissue engineering |
| RM | regenerative medicine |
| BM | bone marrow |
| AT | adipose tissue |
| DP | dental pulp |
| UCB | umbilical cord blood |
| UC | umbilical cord |
| AF | amniotic fluid |
| DF | dermal fibroblasts |
| DP | dermal papilla |
| CM | conditioned medium |
| GAGs | glycosaminoglycans |
| sGAGs | sulfated glycosaminoglycans |
| PGA | polyglycolic acid |
| MMC | macromolecular crowding |
| PLA | polylactic acid |
| HIF | hypoxia-inducible transcription factor |
| ISO | isoflurane |
| DMOG | dimethyloxalylglycine |
| CCPA | 2-chloro-N6-cyclopentyl-adenosine |
| AA | ascorbic acid |
| RA | retinoic acid |
| HA | hyaluronic acid |
| Hu | human |
| Rb | rabbit |
| Eq | equine |
| eAC | equine articular chondrocytes |
| IVD | bovine intervertebral disc |
References
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-I.; Kim, M.R.; Jeong, H.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal Stem Cells Reciprocally Regulate the M1/M2 Balance in Mouse Bone Marrow-Derived Macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef] [PubMed]
- Asgari Taei, A.; Nasoohi, S.; Hassanzadeh, G.; Kadivar, M.; Dargahi, L.; Farahmandfar, M. Enhancement of Angiogenesis and Neurogenesis by Intracerebroventricular Injection of Secretome from Human Embryonic Stem Cell-derived Mesenchymal Stem Cells in Ischemic Stroke Model. Biomed. Pharmacother. 2021, 140, 111709. [Google Scholar] [CrossRef] [PubMed]
- Pei, M. Environmental Preconditioning Rejuvenates Adult Stem Cells’ Proliferation and Chondrogenic Potential. Biomaterials 2017, 117, 10–23. [Google Scholar] [CrossRef]
- Pourjafar, M.; Saidijam, M.; Mansouri, K.; Ghasemibasir, H.; Karimi Dermani, F.; Najafi, R. All-Trans Retinoic Acid Preconditioning Enhances Proliferation, Angiogenesis and Migration of Mesenchymal Stem Cell in Vitro and Enhances Wound Repair in Vivo. Cell Prolif. 2017, 50, e12315. [Google Scholar] [CrossRef]
- Najar, M.; Krayem, M.; Merimi, M.; Burny, A.; Meuleman, N.; Bron, D.; Raicevic, G.; Lagneaux, L. Insights into Inflammatory Priming of Mesenchymal Stromal Cells: Functional Biological Impacts. Inflamm. Res. 2018, 67, 467–477. [Google Scholar] [CrossRef]
- Noronha, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.-H.; Kim, H.-S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef]
- Hezam, K.; Fu, E.; Zhang, J.; Li, Z. Therapeutic Trends of Priming Mesenchymal Stem Cells: A Bibliometric Analysis. Biochem. Biophys. Rep. 2024, 38, 101708. [Google Scholar] [CrossRef]
- Le, B.; Cressman, A.; Morales, D.; Fierro, F.A. First Clinical Experiences Using Preconditioning Approaches to Improve MSC-Based Therapies. Curr. Stem Cell Rep. 2024, 10, 1–7. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-Conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, Y.; Hou, Q.; Zhao, Y.; Zhong, L.; Fu, X. Potential Pre-Activation Strategies for Improving Therapeutic Efficacy of Mesenchymal Stem Cells: Current Status and Future Prospects. Stem Cell Res. Ther. 2022, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Merimi, M.; El-Majzoub, R.; Lagneaux, L.; Moussa Agha, D.; Bouhtit, F.; Meuleman, N.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Najar, M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front. Cell Dev. Biol. 2021, 9, 661532. [Google Scholar] [CrossRef] [PubMed]
- Harman, R.M.; Marx, C.; Van De Walle, G.R. Translational Animal Models Provide Insight Into Mesenchymal Stromal Cell (MSC) Secretome Therapy. Front. Cell Dev. Biol. 2021, 9, 654885. [Google Scholar] [CrossRef]
- Ghasemi, M.; Roshandel, E.; Mohammadian, M.; Farhadihosseinabadi, B.; Akbarzadehlaleh, P.; Shamsasenjan, K. Mesenchymal Stromal Cell-Derived Secretome-Based Therapy for Neurodegenerative Diseases: Overview of Clinical Trials. Stem Cell Res. Ther. 2023, 14, 122. [Google Scholar] [CrossRef]
- Sagaradze, G.; Basalova, N.; Kirpatovsky, V.; Ohobotov, D.; Nimiritsky, P.; Grigorieva, O.; Popov, V.; Kamalov, A.; Tkachuk, V.; Efimenko, A. A Magic Kick for Regeneration: Role of Mesenchymal Stromal Cell Secretome in Spermatogonial Stem Cell Niche Recovery. Stem Cell Res. Ther. 2019, 10, 342. [Google Scholar] [CrossRef]
- Karagyaur, M.; Dzhauari, S.; Basalova, N.; Aleksandrushkina, N.; Sagaradze, G.; Danilova, N.; Malkov, P.; Popov, V.; Skryabina, M.; Efimenko, A.; et al. MSC Secretome as a Promising Tool for Neuroprotection and Neuroregeneration in a Model of Intracerebral Hemorrhage. Pharmaceutics 2021, 13, 2031. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Lozito, T.P.; Kuo, C.K.; Taboas, J.M.; Tuan, R.S. Human Mesenchymal Stem Cells Express Vascular Cell Phenotypes upon Interaction with Endothelial Cell Matrix. J. Cell. Biochem. 2009, 107, 714–722. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Ragelle, H.; Naba, A.; Larson, B.L.; Zhou, F.; Prijić, M.; Whittaker, C.A.; Del Rosario, A.; Langer, R.; Hynes, R.O.; Anderson, D.G. Comprehensive Proteomic Characterization of Stem Cell-Derived Extracellular Matrices. Biomaterials 2017, 128, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Clause, K.C.; Barker, T.H. Extracellular Matrix Signaling in Morphogenesis and Repair. Curr. Opin. Biotechnol. 2013, 24, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In Vivo Engineered Extracellular Matrix Scaffolds with Instructive Niches for Oriented Tissue Regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef]
- Novoseletskaya, E.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Eremichev, R.; Milovskaya, I.; Kulebyakin, K.; Kulebyakina, M.; Rodionov, S.; Omelyanenko, N.; et al. Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli. Front. Cell Dev. Biol. 2020, 8, 555378. [Google Scholar] [CrossRef]
- Lin, H.; Yang, G.; Tan, J.; Tuan, R.S. Influence of Decellularized Matrix Derived from Human Mesenchymal Stem Cells on Their Proliferation, Migration and Multi-Lineage Differentiation Potential. Biomaterials 2012, 33, 4480–4489. [Google Scholar] [CrossRef]
- Matveeva, D.; Buravkov, S.; Andreeva, E.; Buravkova, L. Hypoxic Extracellular Matrix Preserves Its Competence after Expansion of Human MSCs under Physiological Hypoxia In Vitro. Biomimetics 2023, 8, 476. [Google Scholar] [CrossRef]
- Chen, X.-D.; Dusevich, V.; Feng, J.Q.; Manolagas, S.C.; Jilka, R.L. Extracellular Matrix Made by Bone Marrow Cells Facilitates Expansion of Marrow-Derived Mesenchymal Progenitor Cells and Prevents Their Differentiation into Osteoblasts. J. Bone Miner. Res. 2007, 22, 1943–1956. [Google Scholar] [CrossRef]
- Marinkovic, M.; Block, T.J.; Rakian, R.; Li, Q.; Wang, E.; Reilly, M.A.; Dean, D.D.; Chen, X.-D. One Size Does Not Fit All: Developing a Cell-Specific Niche for in Vitro Study of Cell Behavior. Matrix Biol. 2016, 52–54, 426–441. [Google Scholar] [CrossRef]
- Gao, C.-Y.; Huang, Z.-H.; Jing, W.; Wei, P.-F.; Jin, L.; Zhang, X.-H.; Cai, Q.; Deng, X.-L.; Yang, X.-P. Directing Osteogenic Differentiation of BMSCs by Cell-Secreted Decellularized Extracellular Matrixes from Different Cell Types. J. Mater. Chem. B 2018, 6, 7471–7485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, J.; Zhu, Y.; Sun, X.; Guo, W.; Liu, X.; Jing, X.; Guo, G.; Guo, Q.; Peng, J.; et al. Extracellular Matrix Derived by Human Umbilical Cord-Deposited Mesenchymal Stem Cells Accelerates Chondrocyte Proliferation and Differentiation Potential in Vitro. Cell Tissue Bank. 2019, 20, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Fu, L.; Yu, Y.; Zhang, X.; Yang, X.; Cai, Q. Strategy of a Cell-Derived Extracellular Matrix for the Construction of an Osteochondral Interlayer. Biomater. Sci. 2022, 10, 6472–6485. [Google Scholar] [CrossRef] [PubMed]
- Prewitz, M.C.; Seib, F.P.; Von Bonin, M.; Friedrichs, J.; Stißel, A.; Niehage, C.; Müller, K.; Anastassiadis, K.; Waskow, C.; Hoflack, B.; et al. Tightly Anchored Tissue-Mimetic Matrices as Instructive Stem Cell Microenvironments. Nat. Methods 2013, 10, 788–794. [Google Scholar] [CrossRef]
- Klimanskaya, I.; Chung, Y.; Meisner, L.; Johnson, J.; West, M.D.; Lanza, R. Human Embryonic Stem Cells Derived without Feeder Cells. Lancet 2005, 365, 1636–1641. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Z.; Zhou, L.; Zhao, K.; Ma, X.; Cheng, G. Hydrophilic Cell-Derived Extracellular Matrix as a Niche to Promote Adhesion and Differentiation of Neural Progenitor Cells. RSC Adv. 2017, 7, 45587–45594. [Google Scholar] [CrossRef]
- Hoshiba, T.; Sugano, Y.; Yokoyama, N. Murine Neural Stem Cell (NSC) Line, MEB5-Derived Decellularized Matrix as an In Vitro Extracellular Matrix Model in NSC Niche. Chem. Lett. 2018, 47, 1498–1501. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Chen, X.; Liu, T.; Pan, G.; Cui, W.; Li, M.; Luo, Z.-P.; Pei, M.; Yang, H.; et al. Culturing on Decellularized Extracellular Matrix Enhances Antioxidant Properties of Human Umbilical Cord-Derived Mesenchymal Stem Cells. Mater. Sci. Eng. C 2016, 61, 437–448. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, X.; Dai, H.; Feng, G.; Zhang, Y.; Zhou, J.; Zhou, W. Extracellular Matrix Derived from Human Urine-Derived Stem Cells Enhances the Expansion, Adhesion, Spreading, and Differentiation of Human Periodontal Ligament Stem Cells. Stem Cell Res. Ther. 2019, 10, 396. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular Matrix-Based Materials for Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular Matrix-Based Biomaterial Scaffolds and the Host Response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef]
- Ahlfors, J.-E.W.; Billiar, K.L. Biomechanical and Biochemical Characteristics of a Human Fibroblast-Produced and Remodeled Matrix. Biomaterials 2007, 28, 2183–2191. [Google Scholar] [CrossRef]
- Sharma, D.; Ferguson, M.; Zhao, F. A Step-by-Step Protocol for Generating Human Fibroblast Cell-Derived Completely Biological Extracellular Matrix Scaffolds. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 156, pp. 3–13. ISBN 978-0-12-820172-5. [Google Scholar]
- Maia, F.R.; Reis, R.L.; Oliveira, J.M. Decellularized hASCs-Derived Matrices as Biomaterials for 3D in Vitro Approaches. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 156, pp. 45–58. ISBN 978-0-12-820172-5. [Google Scholar]
- Sart, S.; Jeske, R.; Chen, X.; Ma, T.; Li, Y. Engineering Stem Cell-Derived Extracellular Matrices: Decellularization, Characterization, and Biological Function. Tissue Eng. Part. B Rev. 2020, 26, 402–422. [Google Scholar] [CrossRef]
- Hoshiba, T.; Yunoki, S. Comparison of Decellularization Protocols for Cultured Cell-derived Extracellular Matrix—Effects on Decellularization Efficacy, Extracellular Matrix Retention, and Cell Functions. J. Biomed. Mater. Res. 2023, 111, 85–94. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized Extracellular Matrix Biomaterials for Regenerative Therapies: Advances, Challenges and Clinical Prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of Decellularized Extracellular Matrix Scaffolds: A Bottleneck in Tissue Engineering and Regenerative Medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef]
- McQuitty, C.E.; Williams, R.; Chokshi, S.; Urbani, L. Immunomodulatory Role of the Extracellular Matrix Within the Liver Disease Microenvironment. Front. Immunol. 2020, 11, 574276. [Google Scholar] [CrossRef]
- Mecham, R.P.; Lange, G. Antigenicity of Elastin: Characterization of Major Antigenic Determinants on Purified Insoluble Elastin. Biochemistry 1982, 21, 669–673. [Google Scholar] [CrossRef]
- Musaelyan, A.; Lapin, S.; Nazarov, V.; Tkachenko, O.; Gilburd, B.; Mazing, A.; Mikhailova, L.; Shoenfeld, Y. Vimentin as Antigenic Target in Autoimmunity: A Comprehensive Review. Autoimmun. Rev. 2018, 17, 926–934. [Google Scholar] [CrossRef]
- Londono, R.; Dziki, J.L.; Haljasmaa, E.; Turner, N.J.; Leifer, C.A.; Badylak, S.F. The Effect of Cell Debris within Biologic Scaffolds upon the Macrophage Response. J. Biomed. Mater. Res. 2017, 105, 2109–2118. [Google Scholar] [CrossRef]
- García-García, A.; Martin, I. Extracellular Matrices to Modulate the Innate Immune Response and Enhance Bone Healing. Front. Immunol. 2019, 10, 2256. [Google Scholar] [CrossRef]
- Hussein, K.H.; Saleh, T.; Ahmed, E.; Kwak, H.; Park, K.; Yang, S.; Kang, B.; Choi, K.; Kang, K.; Woo, H. Biocompatibility and Hemocompatibility of Efficiently Decellularized Whole Porcine Kidney for Tissue Engineering. J. Biomed. Mater. Res. 2018, 106, 2034–2047. [Google Scholar] [CrossRef]
- Barajaa, M.A.; Otsuka, T.; Ghosh, D.; Kan, H.-M.; Laurencin, C.T. Development of Porcine Skeletal Muscle Extracellular Matrix–Derived Hydrogels with Improved Properties and Low Immunogenicity. Proc. Natl. Acad. Sci. USA 2024, 121, e2322822121. [Google Scholar] [CrossRef]
- Human, P.; Ofoegbu, C.; Ilsley, H.; Bezuidenhout, D.; De Villiers, J.; Williams, D.F.; Zilla, P. Decellularization and Engineered Crosslinking: A Promising Dual Approach towards Bioprosthetic Heart Valve Longevity. Eur. J. Cardio-Thorac. Surg. 2020, 58, 1192–1200. [Google Scholar] [CrossRef]
- Shirani, A.; Ganji, F.; Golmohammadi, M.; Hashemi, S.M.; Mozafari, M.; Amoabediny, G.; Karkuki Osguei, N.; Samadikuchaksaraei, A. Cross-Linked Acellular Lung for Application in Tissue Engineering: Effects on Biocompatibility, Mechanical Properties and Immunological Responses. Mater. Sci. Eng. C 2021, 122, 111938. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To Cross-Link or Not to Cross-Link? Cross-Linking Associated Foreign Body Response of Collagen-Based Devices. Tissue Eng. Part. B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef]
- Wong, M.L.; Griffiths, L.G. Immunogenicity in Xenogeneic Scaffold Generation: Antigen Removal vs. Decellularization. Acta Biomater. 2014, 10, 1806–1816. [Google Scholar] [CrossRef]
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef]
- Tragoonlugkana, P.; Pruksapong, C.; Ontong, P.; Kamprom, W.; Supokawej, A. Fibronectin and Vitronectin Alleviate Adipose-Derived Stem Cells Senescence during Long-Term Culture through the AKT/MDM2/P53 Pathway. Sci. Rep. 2024, 14, 14242. [Google Scholar] [CrossRef]
- Andreeva, E.R.; Matveeva, D.K.; Zhidkova, O.V.; Buravkova, L.B. Extracellular Matrix as a Factor Regulating the Physiological Microenvironment of the Cell. Usp. Fiziol. Nauk. 2024, 55, 16–30. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, Y.; Skinner, C.M.; Son, E.L.; Lu, Z.; Tuan, R.S.; Jilka, R.L.; Ling, J.; Chen, X.-D. Reconstitution of Marrow-Derived Extracellular Matrix Ex Vivo: A Robust Culture System for Expanding Large-Scale Highly Functional Human Mesenchymal Stem Cells. Stem Cells Dev. 2010, 19, 1095–1107. [Google Scholar] [CrossRef]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular Matrix-Derived Biomaterials in Engineering Cell Function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef]
- Pei, M.; He, F.; Kish, V.L. Expansion on Extracellular Matrix Deposited by Human Bone Marrow Stromal Cells Facilitates Stem Cell Proliferation and Tissue-Specific Lineage Potential. Tissue Eng. Part A 2011, 17, 3067–3076. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W.; Lu, Z.; Chen, R.; Ling, J.; Ran, Q.; Jilka, R.L.; Chen, X.-D. Rescuing Replication and Osteogenesis of Aged Mesenchymal Stem Cells by Exposure to a Young Extracellular Matrix. FASEB J. 2011, 25, 1474–1485. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Wang, J.H.-C. The Role of Engineered Tendon Matrix in the Stemness of Tendon Stem Cells in Vitro and the Promotion of Tendon-like Tissue Formation in Vivo. Biomaterials 2011, 32, 6972–6981. [Google Scholar] [CrossRef]
- Xue, J.X.; Gong, Y.Y.; Zhou, G.D.; Liu, W.; Cao, Y.; Zhang, W.J. Chondrogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells Induced by Acellular Cartilage Sheets. Biomaterials 2012, 33, 5832–5840. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, X.; Xu, H.; Wang, L.; Jin, X.; Chen, R.; Ren, X.; Lu, Y.; Fu, M.; Huang, Y.; et al. Bone Marrow Stromal Cell-derived Extracellular Matrix Promotes Osteogenesis of Adipose-derived Stem Cells. Cell Biol. Int. 2015, 39, 291–299. [Google Scholar] [CrossRef]
- Rao Pattabhi, S.; Martinez, J.S.; Keller, T.C.S. Decellularized ECM Effects on Human Mesenchymal Stem Cell Stemness and Differentiation. Differentiation 2014, 88, 131–143. [Google Scholar] [CrossRef]
- Conway, A.; Schaffer, D.V. Biophysical Regulation of Stem Cell Behavior within the Niche. Stem Cell Res. Ther. 2012, 3, 50. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular Mechanotransduction in Health and Diseases: From Molecular Mechanism to Therapeutic Targets. Sig. Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Smith, L.R.; Cho, S.; Discher, D.E. Stem Cell Differentiation Is Regulated by Extracellular Matrix Mechanics. Physiology 2018, 33, 16–25. [Google Scholar] [CrossRef]
- Hoshiba, T.; Lu, H.; Yamada, T.; Kawazoe, N.; Tateishi, T.; Chen, G. Effects of Extracellular Matrices Derived from Different Cell Sources on Chondrocyte Functions. Biotechnol. Prog. 2011, 27, 788–795. [Google Scholar] [CrossRef]
- Pei, M.; Shoukry, M.; Li, J.; Daffner, S.D.; France, J.C.; Emery, S.E. Modulation of In Vitro Microenvironment Facilitates Synovium-Derived Stem Cell-Based Nucleus Pulposus Tissue Regeneration. Spine 2012, 37, 1538–1547. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, H.; Shen, H.; Wang, B.; Lei, G.; Tuan, R.S. Mesenchymal Stem Cell-Derived Extracellular Matrix Enhances Chondrogenic Phenotype of and Cartilage Formation by Encapsulated Chondrocytes in Vitro and in Vivo. Acta Biomater. 2018, 69, 71–82. [Google Scholar] [CrossRef]
- McAllister, T.N.; Maruszewski, M.; Garrido, S.A.; Wystrychowski, W.; Dusserre, N.; Marini, A.; Zagalski, K.; Fiorillo, A.; Avila, H.; Manglano, X.; et al. Effectiveness of Haemodialysis Access with an Autologous Tissue-Engineered Vascular Graft: A Multicentre Cohort Study. Lancet 2009, 373, 1440–1446. [Google Scholar] [CrossRef]
- Zwolinski, C.M.; Ellison, K.S.; Depaola, N.; Thompson, D.M. Generation of Cell-Derived Three Dimensional Extracellular Matrix Substrates from Two Dimensional Endothelial Cell Cultures. Tissue Eng. Part C Methods 2011, 17, 589–595. [Google Scholar] [CrossRef]
- Roberts, K.; Schluns, J.; Walker, A.; Jones, J.D.; Quinn, K.P.; Hestekin, J.; Wolchok, J.C. Cell Derived Extracellular Matrix Fibers Synthesized Using Sacrificial Hollow Fiber Membranes. Biomed. Mater. 2017, 13, 015023. [Google Scholar] [CrossRef]
- Wolchok, J.C.; Tresco, P.A. The Isolation of Cell Derived Extracellular Matrix Constructs Using Sacrificial Open-Cell Foams. Biomaterials 2010, 31, 9595–9603. [Google Scholar] [CrossRef]
- Suhaeri, M.; Noh, M.H.; Moon, J.-H.; Kim, I.G.; Oh, S.J.; Ha, S.S.; Lee, J.H.; Park, K. Novel Skin Patch Combining Human Fibroblast-Derived Matrix and Ciprofloxacin for Infected Wound Healing. Theranostics 2018, 8, 5025–5038. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Z.; Huang, J.; Wang, H.; Gu, X.; Gu, J. Application of Marrow Mesenchymal Stem Cell-Derived Extracellular Matrix in Peripheral Nerve Tissue Engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2250–2260. [Google Scholar] [CrossRef]
- Schenke-Layland, K.; Rofail, F.; Heydarkhan, S.; Gluck, J.M.; Ingle, N.P.; Angelis, E.; Choi, C.-H.; MacLellan, W.R.; Beygui, R.E.; Shemin, R.J.; et al. The Use of Three-Dimensional Nanostructures to Instruct Cells to Produce Extracellular Matrix for Regenerative Medicine Strategies. Biomaterials 2009, 30, 4665–4675. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Silva, J.C.; Udangawa, R.N.; Cabral, J.M.S.; Ferreira, F.C.; Da Silva, C.L.; Linhardt, R.J.; Vashishth, D. Co-Culture Cell-Derived Extracellular Matrix Loaded Electrospun Microfibrous Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 99, 479–490. [Google Scholar] [CrossRef]
- Silva, J.C.; Carvalho, M.S.; Han, X.; Xia, K.; Mikael, P.E.; Cabral, J.M.S.; Ferreira, F.C.; Linhardt, R.J. Compositional and Structural Analysis of Glycosaminoglycans in Cell-Derived Extracellular Matrices. Glycoconj. J. 2019, 36, 141–154. [Google Scholar] [CrossRef]
- Kumar, A.; Nune, K.C.; Misra, R.D.K. Biological Functionality and Mechanistic Contribution of Extracellular Matrix-ornamented Three Dimensional Ti-6Al-4V Mesh Scaffolds. J. Biomed. Mater. Res. 2016, 104, 2751–2763. [Google Scholar] [CrossRef]
- Junka, R.; Quevada, K.; Yu, X. Acellular Polycaprolactone Scaffolds Laden with Fibroblast/Endothelial Cell-derived Extracellular Matrix for Bone Regeneration. J. Biomed. Mater. Res. 2020, 108, 351–364. [Google Scholar] [CrossRef]
- Decaris, M.L.; Binder, B.Y.; Soicher, M.A.; Bhat, A.; Leach, J.K. Cell-Derived Matrix Coatings for Polymeric Scaffolds. Tissue Eng. Part A 2012, 18, 2148–2157. [Google Scholar] [CrossRef]
- Higuchi, Y.; Shiraki, N.; Yamane, K.; Qin, Z.; Mochitate, K.; Araki, K.; Senokuchi, T.; Yamagata, K.; Hara, M.; Kume, K.; et al. Synthesized Basement Membranes Direct the Differentiation of Mouse Embryonic Stem Cells into Pancreatic Lineages. J. Cell Sci. 2010, 123, 2733–2742. [Google Scholar] [CrossRef]
- Hielscher, A. Hypoxia Affects the Structure of Breast Cancer Cell-Derived Matrix to Support Angiogenic Responses of Endothelial Cells. J. Carcinog. Mutagen. 2013, 4 (Suppl. 13), 5. [Google Scholar] [CrossRef]
- Wolchok, J.C.; Tresco, P.A. Using Growth Factor Conditioning to Modify the Properties of Human Cell Derived Extracellular Matrix. Biotechnol. Progress 2012, 28, 1581–1587. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Tahtinen, M.; Shearier, E.; Qian, Z.; Zhao, F. Decellularization of Fibroblast Cell Sheets for Natural Extracellular Matrix Scaffold Preparation. Tissue Eng. Part C Methods 2015, 21, 77–87. [Google Scholar] [CrossRef]
- Ruff, S.M.; Keller, S.; Wieland, D.E.; Wittmann, V.; Tovar, G.E.M.; Bach, M.; Kluger, P.J. clickECM: Development of a Cell-Derived Extracellular Matrix with Azide Functionalities. Acta Biomater. 2017, 52, 159–170. [Google Scholar] [CrossRef]
- Subbiah, R.; Hwang, M.P.; Du, P.; Suhaeri, M.; Hwang, J.-H.; Hong, J.-H.; Park, K. Tunable Crosslinked Cell-Derived Extracellular Matrix Guides Cell Fate. Macromol. Biosci. 2016, 16, 1723–1734. [Google Scholar] [CrossRef]
- Ozguldez, H.O.; Cha, J.; Hong, Y.; Koh, I.; Kim, P. Nanoengineered, Cell-Derived Extracellular Matrix Influences ECM-Related Gene Expression of Mesenchymal Stem Cells. Biomater. Res. 2018, 22, 32. [Google Scholar] [CrossRef]
- Almici, E.; Caballero, D.; Montero Boronat, J.; Samitier Martí, J. Engineering Cell-Derived Matrices with Controlled 3D Architectures for Pathophysiological Studies. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 156, pp. 161–183. ISBN 978-0-12-820172-5. [Google Scholar]
- Yang, L.; Ge, L.; Van Rijn, P. Synergistic Effect of Cell-Derived Extracellular Matrices and Topography on Osteogenesis of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2020, 12, 25591–25603. [Google Scholar] [CrossRef]
- Yuan, M.; Pai, P.-J.; Liu, X.; Lam, H.; Chan, B.P. Proteomic Analysis of Nucleus Pulposus Cell-Derived Extracellular Matrix Niche and Its Effect on Phenotypic Alteration of Dermal Fibroblasts. Sci. Rep. 2018, 8, 1512. [Google Scholar] [CrossRef]
- Schell, J.Y.; Wilks, B.T.; Patel, M.; Franck, C.; Chalivendra, V.; Cao, X.; Shenoy, V.B.; Morgan, J.R. Harnessing Cellular-Derived Forces in Self-Assembled Microtissues to Control the Synthesis and Alignment of ECM. Biomaterials 2016, 77, 120–129. [Google Scholar] [CrossRef]
- Kim, I.G.; Hwang, M.P.; Du, P.; Ko, J.; Ha, C.; Do, S.H.; Park, K. Bioactive Cell-Derived Matrices Combined with Polymer Mesh Scaffold for Osteogenesis and Bone Healing. Biomaterials 2015, 50, 75–86. [Google Scholar] [CrossRef]
- Song, Y.; Liang, F.; Tian, W.; Rayhill, E.; Ye, L.; Tian, X. Optimizing Therapeutic Outcomes: Preconditioning Strategies for MSC-Derived Extracellular Vesicles. Front. Pharmacol. 2025, 16, 1509418. [Google Scholar] [CrossRef]
- Wagner, J.; Kean, T.; Young, R.; Dennis, J.E.; Caplan, A.I. Optimizing Mesenchymal Stem Cell-Based Therapeutics. Curr. Opin. Biotechnol. 2009, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Didamoony, M.A.; Soubh, A.A.; Atwa, A.M.; Ahmed, L.A. Innovative Preconditioning Strategies for Improving the Therapeutic Efficacy of Extracellular Vesicles Derived from Mesenchymal Stem Cells in Gastrointestinal Diseases. Inflammopharmacol 2023, 31, 2973–2993. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef] [PubMed]
- Sylakowski, K.; Bradshaw, A.; Wells, A. Mesenchymal Stem Cell/Multipotent Stromal Cell Augmentation of Wound Healing. Am. J. Pathol. 2020, 190, 1370–1381. [Google Scholar] [CrossRef]
- Nowak-Stępniowska, A.; Osuchowska, P.N.; Fiedorowicz, H.; Trafny, E.A. Insight in Hypoxia-Mimetic Agents as Potential Tools for Mesenchymal Stem Cell Priming in Regenerative Medicine. Stem Cells Int. 2022, 2022, 8775591. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Andreeva, E.R.; Gogvadze, V.; Zhivotovsky, B. Mesenchymal Stem Cells and Hypoxia: Where Are We? Mitochondrion 2014, 19, 105–112. [Google Scholar] [CrossRef]
- Andreeva, E.R.; Udartseva, O.O.; Zhidkova, O.V.; Buravkov, S.V.; Ezdakova, M.I.; Buravkova, L.B. IFN-gamma Priming of Adipose-derived Stromal Cells at “Physiological” Hypoxia. J. Cell Physiol. 2018, 233, 1535–1547. [Google Scholar] [CrossRef]
- Pavlacky, J.; Polak, J. Technical Feasibility and Physiological Relevance of Hypoxic Cell Culture Models. Front. Endocrinol. 2020, 11, 57. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Rylova, Y.V.; Andreeva, E.R.; Kulikov, A.V.; Pogodina, M.V.; Zhivotovsky, B.; Gogvadze, V. Low ATP Level Is Sufficient to Maintain the Uncommitted State of Multipotent Mesenchymal Stem Cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 4418–4425. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic Culture Conditions as a Solution for Mesenchymal Stem Cell Based Regenerative Therapy. Sci. World J. 2013, 2013, 632972. [Google Scholar] [CrossRef] [PubMed]
- Gornostaeva, A.; Buravkova, L.; Lobanova, M.; Andreeva, E. HIFs in Hypoxic Regulation of the Extracellular Matrix: Focus on Little-Known Player HIF-3. Biocell 2024, 48, 677–692. Available online: https://www.techscience.com/biocell/v48n5/56383 (accessed on 16 June 2025). [CrossRef]
- Ohnishi, S.; Yasuda, T.; Kitamura, S.; Nagaya, N. Effect of Hypoxia on Gene Expression of Bone Marrow-Derived Mesenchymal Stem Cells and Mononuclear Cells. Stem Cells 2007, 25, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, E.; Penolazzi, L.; Angelozzi, M.; Bergamin, L.S.; Manferdini, C.; Vieceli Dalla Sega, F.; Paolella, F.; Lisignoli, G.; Piva, R. Hypoxia Preconditioning of Human MSCs: A Direct Evidence of HIF-1α and Collagen Type XV Correlation. Cell Physiol. Biochem. 2018, 51, 2237–2249. [Google Scholar] [CrossRef]
- Braga, C.L.; Da Silva, L.R.; Santos, R.T.; De Carvalho, L.R.P.; Mandacaru, S.C.; De Oliveira Trugilho, M.R.; Rocco, P.R.M.; Cruz, F.F.; Silva, P.L. Proteomics Profile of Mesenchymal Stromal Cells and Extracellular Vesicles in Normoxic and Hypoxic Conditions. Cytotherapy 2022, 24, 1211–1224. [Google Scholar] [CrossRef]
- Riis, S.; Stensballe, A.; Emmersen, J.; Pennisi, C.P.; Birkelund, S.; Zachar, V.; Fink, T. Mass Spectrometry Analysis of Adipose-Derived Stem Cells Reveals a Significant Effect of Hypoxia on Pathways Regulating Extracellular Matrix. Stem Cell Res. Ther. 2016, 7, 52. [Google Scholar] [CrossRef]
- Wobma, H.M.; Kanai, M.; Ma, S.P.; Shih, Y.; Li, H.W.; Duran-Struuck, R.; Winchester, R.; Goeta, S.; Brown, L.M.; Vunjak-Novakovic, G. Dual IFN-γ/Hypoxia Priming Enhances Immunosuppression of Mesenchymal Stromal Cells through Regulatory Proteins and Metabolic Mechanisms. J. Immunol. Regen. Med. 2018, 1, 45–56. [Google Scholar] [CrossRef]
- Boyette, L.B.; Creasey, O.A.; Guzik, L.; Lozito, T.; Tuan, R.S. Human Bone Marrow-Derived Mesenchymal Stem Cells Display Enhanced Clonogenicity but Impaired Differentiation with Hypoxic Preconditioning. Stem Cells Transl. Med. 2014, 3, 241–254. [Google Scholar] [CrossRef]
- Du, H.-C.; Jiang, L.; Geng, W.-X.; Li, J.; Zhang, R.; Dang, J.-G.; Shu, M.-G.; Li, L.-W. Evaluation of Xenogeneic Extracellular Matrix Fabricated from CuCl2 -Conditioned Mesenchymal Stem Cell Sheets as a Bioactive Wound Dressing Material. J. Biomater. Appl. 2017, 32, 472–483. [Google Scholar] [CrossRef]
- Grayson, W.L.; Zhao, F.; Bunnell, B.; Ma, T. Hypoxia Enhances Proliferation and Tissue Formation of Human Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2007, 358, 948–953. [Google Scholar] [CrossRef]
- Matveeva, D.K.; Andreeva, E.R.; Novikov, N.N.; Pustovoy, V.I.; Buravkova, L.B. Structural Organization and Composition of Extracellular Matrix of Multipotent Mesenchymal Stromal Cells under Different Oxygen Levels in Vitro. Clin. Exp. Morphol. 2020, 9, 57–63. [Google Scholar] [CrossRef]
- Cigognini, D.; Gaspar, D.; Kumar, P.; Satyam, A.; Alagesan, S.; Sanz-Nogués, C.; Griffin, M.; O’Brien, T.; Pandit, A.; Zeugolis, D.I. Macromolecular Crowding Meets Oxygen Tension in Human Mesenchymal Stem Cell Culture—A Step Closer to Physiologically Relevant in Vitro Organogenesis. Sci. Rep. 2016, 6, 30746. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Hu, J.C.; Athanasiou, K.A. Hypoxia-Induced Collagen Crosslinking as a Mechanism for Enhancing Mechanical Properties of Engineered Articular Cartilage. Osteoarthr. Cartil. 2013, 21, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ma, T. Autocrine Fibroblast Growth Factor 2-mediated Interactions between Human Mesenchymal Stem Cells and the Extracellular Matrix under Varying Oxygen Tension. J. Cell. Biochem. 2013, 114, 716–727. [Google Scholar] [CrossRef]
- Ezdakova, M.I.; Andreeva, E.R.; Gurieva, T.S.; Dadasheva, O.A.; Orlova, V.S.; Buravkova, L.B. Effects of Hypoxia and Growth Factors on the Angiogenic Activity of Multipotent Mesenchymal Stromal Cells. Aviakosm Ekol. Med. 2015, 49, 29–35. [Google Scholar]
- Hao, D.; He, C.; Ma, B.; Lankford, L.; Reynaga, L.; Farmer, D.L.; Guo, F.; Wang, A. Hypoxic Preconditioning Enhances Survival and Proangiogenic Capacity of Human First Trimester Chorionic Villus-Derived Mesenchymal Stem Cells for Fetal Tissue Engineering. Stem Cells Int. 2019, 2019, 9695239. [Google Scholar] [CrossRef]
- Wan, X.; Xie, M.; Xu, H.; Wei, Z.; Yao, H.; Wang, Z.; Zheng, D. Hypoxia-Preconditioned Adipose-Derived Stem Cells Combined with Scaffold Promote Urethral Reconstruction by Upregulation of Angiogenesis and Glycolysis. Stem Cell Res. Ther. 2020, 11, 535. [Google Scholar] [CrossRef]
- Ge, L.; Xun, C.; Li, W.; Jin, S.; Liu, Z.; Zhuo, Y.; Duan, D.; Hu, Z.; Chen, P.; Lu, M. Extracellular Vesicles Derived from Hypoxia-Preconditioned Olfactory Mucosa Mesenchymal Stem Cells Enhance Angiogenesis via miR-612. J. Nanobiotechnol 2021, 19, 380. [Google Scholar] [CrossRef]
- Wang, S.; Umrath, F.; Cen, W.; Salgado, A.J.; Reinert, S.; Alexander, D. Pre-Conditioning with IFN-γ and Hypoxia Enhances the Angiogenic Potential of iPSC-Derived MSC Secretome. Cells 2022, 11, 988. [Google Scholar] [CrossRef]
- Germain, S.; Monnot, C.; Muller, L.; Eichmann, A. Hypoxia-Driven Angiogenesis: Role of Tip Cells and Extracellular Matrix Scaffolding. Curr. Opin. Hematol. 2010, 17, 245–251. [Google Scholar] [CrossRef]
- Martino, M.M.; Brkic, S.; Bovo, E.; Burger, M.; Schaefer, D.J.; Wolff, T.; Gürke, L.; Briquez, P.S.; Larsson, H.M.; Gianni-Barrera, R.; et al. Extracellular Matrix and Growth Factor Engineering for Controlled Angiogenesis in Regenerative Medicine. Front. Bioeng. Biotechnol. 2015, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buczek-Thomas, J.A.; Rich, C.B.; Nugent, M.A. Hypoxia Induced Heparan Sulfate Primes the Extracellular Matrix for Endothelial Cell Recruitment by Facilitating VEGF-Fibronectin Interactions. Int. J. Mol. Sci. 2019, 20, 5065. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Watts, D.; Gaete, D.; Sormendi, S.; Wielockx, B. Hypoxia Pathway Proteins and Their Impact on the Blood Vasculature. Int. J. Mol. Sci. 2021, 22, 9191. [Google Scholar] [CrossRef] [PubMed]
- Libby, J.R.; Royce, H.; Walker, S.R.; Li, L. The Role of Extracellular Matrix in Angiogenesis: Beyond Adhesion and Structure. Biomater. Biosyst. 2024, 15, 100097. [Google Scholar] [CrossRef]
- Neve, A.; Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Extracellular Matrix Modulates Angiogenesis in Physiological and Pathological Conditions. BioMed Res. Int. 2014, 2014, 756078. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Ananta, M.; Binkowski, M.; Streeter, I.; Lu, Z.; Cui, Z.F.; Brown, R.A.; Mudera, V. Mechanisms of Structure Generation during Plastic Compression of Nanofibrillar Collagen Hydrogel Scaffolds: Towards Engineering of Collagen. J. Tissue Eng. Regen. Med. 2011, 5, 505–519. [Google Scholar] [CrossRef]
- Karampoga, A.; Tzaferi, K.; Koutsakis, C.; Kyriakopoulou, K.; Karamanos, N.K. Exosomes and the Extracellular Matrix: A Dynamic Interplay in Cancer Progression. Int. J. Dev. Biol. 2022, 66, 97–102. [Google Scholar] [CrossRef]
- Patel, N.J.; Ashraf, A.; Chung, E.J. Extracellular Vesicles as Regulators of the Extracellular Matrix. Bioengineering 2023, 10, 136. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Welsh, M. VEGFA and Tumour Angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef]
- Geiger, B.; Yamada, K.M. Molecular Architecture and Function of Matrix Adhesions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005033. [Google Scholar] [CrossRef]
- Brinckmann, J.; Kim, S.; Wu, J.; Reinhardt, D.P.; Batmunkh, C.; Metzen, E.; Notbohm, H.; Bank, R.A.; Krieg, T.; Hunzelmann, N. Interleukin 4 and Prolonged Hypoxia Induce a Higher Gene Expression of Lysyl Hydroxylase 2 and an Altered Cross-Link Pattern: Important Pathogenetic Steps in Early and Late Stage of Systemic Scleroderma? Matrix Biol. 2005, 24, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Q.; Morita, Y.; Jiang, H.; Groß, A.; Lechel, A.; Hildner, K.; Guachalla, L.M.; Gompf, A.; Hartmann, D.; et al. A Differentiation Checkpoint Limits Hematopoietic Stem Cell Self-Renewal in Response to DNA Damage. Cell 2012, 148, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, Y.; Xiong, Z.; Hines, S.; Shang, J.; Clark, K.L.; Tan, S.; Alexander, P.G.; Lin, H. Generation of Hyaline-like Cartilage Tissue from Human Mesenchymal Stromal Cells within the Self-Generated Extracellular Matrix. Acta Biomater. 2022, 149, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Liu, H.; Li, J.; Zhou, Y. Roles of Hypoxia During the Chondrogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2014, 9, 141–147. [Google Scholar] [CrossRef]
- Taheem, D.K.; Foyt, D.A.; Loaiza, S.; Ferreira, S.A.; Ilic, D.; Auner, H.W.; Grigoriadis, A.E.; Jell, G.; Gentleman, E. Differential Regulation of Human Bone Marrow Mesenchymal Stromal Cell Chondrogenesis by Hypoxia Inducible Factor-1α Hydroxylase Inhibitors. Stem Cells 2018, 36, 1380–1392. [Google Scholar] [CrossRef]
- Wang, D.W.; Fermor, B.; Gimble, J.M.; Awad, H.A.; Guilak, F. Influence of Oxygen on the Proliferation and Metabolism of Adipose Derived Adult Stem Cells. J. Cell Physiol. 2005, 204, 184–191. [Google Scholar] [CrossRef]
- Khan, W.S.; Adesida, A.B.; Tew, S.R.; Lowe, E.T.; Hardingham, T.E. Bone Marrow-Derived Mesenchymal Stem Cells Express the Pericyte Marker 3G5 in Culture and Show Enhanced Chondrogenesis in Hypoxic Conditions: BMSS Express Pericyte Markers in Culture. J. Orthop. Res. 2010, 28, 834–840. [Google Scholar] [CrossRef]
- Mwale, F.; Ciobanu, I.; Giannitsios, D.; Roughley, P.; Steffen, T.; Antoniou, J. Effect of Oxygen Levels on Proteoglycan Synthesis by Intervertebral Disc Cells. Spine 2011, 36, E131–E138. [Google Scholar] [CrossRef]
- Ivanovic, Z. Hypoxia or in Situ Normoxia: The Stem Cell Paradigm. J. Cell. Physiol. 2009, 219, 271–275. [Google Scholar] [CrossRef]
- Liu, N.; Patzak, A.; Zhang, J. CXCR4-Overexpressing Bone Marrow-Derived Mesenchymal Stem Cells Improve Repair of Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2013, 305, F1064–F1073. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chen, C.-H.; Hwang, S.-M.; Lin, W.-W.; Huang, C.-H.; Lee, W.-Y.; Chang, Y.; Sung, H.-W. Spherically Symmetric Mesenchymal Stromal Cell Bodies Inherent with Endogenous Extracellular Matrices for Cellular Cardiomyoplasty. Stem Cells 2009, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Black, R.; Ma, Z.; Yang, Q.; Wang, A.; Lin, F. Use of Mouse Hematopoietic Stem and Progenitor Cells to Treat Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2012, 302, F9–F19. [Google Scholar] [CrossRef] [PubMed]
- Follin, B.; Juhl, M.; Cohen, S.; Pedersen, A.E.; Kastrup, J.; Ekblond, A. Increased Paracrine Immunomodulatory Potential of Mesenchymal Stromal Cells in Three-Dimensional Culture. Tissue Eng. Part B Rev. 2016, 22, 322–329. [Google Scholar] [CrossRef]
- Gentile, C. Filling the Gaps between the In Vivo and In Vitro Microenvironment: Engineering of Spheroids for Stem Cell Technology. Curr. Stem Cell Res. Ther. 2016, 11, 652–665. [Google Scholar] [CrossRef]
- Amos, P.J.; Kapur, S.K.; Stapor, P.C.; Shang, H.; Bekiranov, S.; Khurgel, M.; Rodeheaver, G.T.; Peirce, S.M.; Katz, A.J. Human Adipose-Derived Stromal Cells Accelerate Diabetic Wound Healing: Impact of Cell Formulation and Delivery. Tissue Eng. Part A 2010, 16, 1595–1606. [Google Scholar] [CrossRef]
- Chiang, C.; Fang, Y.; Ho, C.; Assunção, M.; Lin, S.; Wang, Y.; Blocki, A.; Huang, C. Bioactive Decellularized Extracellular Matrix Derived from 3D Stem Cell Spheroids under Macromolecular Crowding Serves as a Scaffold for Tissue Engineering. Adv. Healthc. Mater. 2021, 10, 2100024. [Google Scholar] [CrossRef]
- Clément, V.; Roy, V.; Paré, B.; Goulet, C.R.; Deschênes, L.T.; Berthod, F.; Bolduc, S.; Gros-Louis, F. Tridimensional Cell Culture of Dermal Fibroblasts Promotes Exosome-Mediated Secretion of Extracellular Matrix Proteins. Sci. Rep. 2022, 12, 19786. [Google Scholar] [CrossRef]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef]
- Guan, Q.; Ezzati, P.; Spicer, V.; Krokhin, O.; Wall, D.; Wilkins, J.A. Interferon γ Induced Compositional Changes in Human Bone Marrow Derived Mesenchymal Stem/Stromal Cells. Clin. Proteom. 2017, 14, 26. [Google Scholar] [CrossRef]
- Hoefner, C.; Muhr, C.; Horder, H.; Wiesner, M.; Wittmann, K.; Lukaszyk, D.; Radeloff, K.; Winnefeld, M.; Becker, M.; Blunk, T.; et al. Human Adipose-Derived Mesenchymal Stromal/Stem Cell Spheroids Possess High Adipogenic Capacity and Acquire an Adipose Tissue-like Extracellular Matrix Pattern. Tissue Eng. Part A 2020, 26, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Nooeaid, P.; Kohl, B.; Roether, J.A.; Schubert, D.W.; Meier, C.; Boccaccini, A.R.; Godkin, O.; Ertel, W.; Arens, S.; et al. Chondrogenesis of Human Bone Marrow Mesenchymal Stromal Cells in Highly Porous Alginate-Foams Supplemented with Chondroitin Sulfate. Mater. Sci. Eng. C 2015, 50, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Reppel, L.; Schiavi, J.; Charif, N.; Leger, L.; Yu, H.; Pinzano, A.; Henrionnet, C.; Stoltz, J.-F.; Bensoussan, D.; Huselstein, C. Chondrogenic Induction of Mesenchymal Stromal/Stem Cells from Wharton’s Jelly Embedded in Alginate Hydrogel and without Added Growth Factor: An Alternative Stem Cell Source for Cartilage Tissue Engineering. Stem Cell Res. Ther. 2015, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Saghati, S.; Rahbarghazi, R.; Baradar Khoshfetrat, A.; Moharamzadeh, K.; Tayefi Nasrabadi, H.; Roshangar, L. Phenolated Alginate-Collagen Hydrogel Induced Chondrogenic Capacity of Human Amniotic Mesenchymal Stem Cells. J. Biomater. Appl. 2021, 36, 789–802. [Google Scholar] [CrossRef]
- Santos, J.M.; Camões, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simões, S.; Gaspar, M.; Mosqueira, D.; et al. Three-Dimensional Spheroid Cell Culture of Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells Leads to Enhanced Paracrine Induction of Wound Healing. Stem Cell Res. Ther. 2015, 6, 90. [Google Scholar] [CrossRef]
- Suzuki, S.; Muneta, T.; Tsuji, K.; Ichinose, S.; Makino, H.; Umezawa, A.; Sekiya, I. Properties and Usefulness of Aggregates of Synovial Mesenchymal Stem Cells as a Source for Cartilage Regeneration. Arthritis Res. Ther. 2012, 14, R136. [Google Scholar] [CrossRef]
- Vakhrushev, I.V.; Basok, Y.B.; Baskaev, K.K.; Novikova, V.D.; Leonov, G.E.; Grigoriev, A.M.; Belova, A.D.; Kirsanova, L.A.; Lupatov, A.Y.; Burunova, V.V.; et al. Cartilage-Specific Gene Expression and Extracellular Matrix Deposition in the Course of Mesenchymal Stromal Cell Chondrogenic Differentiation in 3D Spheroid Culture. Int. J. Mol. Sci. 2024, 25, 5695. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, T.; Xu, A.; Zhang, L. 3D Spheroid Culture Enhances Survival and Therapeutic Capacities of MSC s Injected into Ischemic Kidney. J. Cell. Mol. Medi 2016, 20, 1203–1213. [Google Scholar] [CrossRef]
- Sart, S.; Ma, T.; Li, Y. Preconditioning Stem Cells for In Vivo Delivery. BioResearch Open Access 2014, 3, 137–149. [Google Scholar] [CrossRef]
- Zippel, N.; Schulze, M.; Tobiasch, E. Biomaterials and Mesenchymal Stem Cells for Regenerative Medicine. Recent Pat. Biotechnol. 2010, 4, 1–22. [Google Scholar] [CrossRef]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental Sensing through Focal Adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Buxboim, A.; Ivanovska, I.L.; Discher, D.E. Matrix Elasticity, Cytoskeletal Forces and Physics of the Nucleus: How Deeply Do Cells ‘Feel’ Outside and In? J. Cell Sci. 2010, 123, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, U.; Dejana, E. Adhesion Molecule Signalling: Not Always a Sticky Business. Nat. Rev. Mol. Cell Biol. 2011, 12, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Benassi, L.; Rossi, E.; Magnoni, C. Extracellular Matrix Deposition by Adipose-Derived Stem Cells and Fibroblasts: A Comparative Study. Arch. Dermatol. Res. 2020, 312, 295–299. [Google Scholar] [CrossRef]
- Liang, C.; Liao, L.; Tian, W. Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules 2023, 13, 673. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, E.M.; Byun, H.; Chang, H.; Jeong, K.; Aman, Z.M.; Choi, Y.S.; Park, J.; Shin, H. Engineering Spheroids Potentiating Cell-Cell and Cell-ECM Interactions by Self-Assembly of Stem Cell Microlayer. Biomaterials 2018, 165, 105–120. [Google Scholar] [CrossRef]
- Sottile, J.; Hocking, D.C.; Swiatek, P.J. Fibronectin Matrix Assembly Enhances Adhesion-Dependent Cell Growth. J. Cell Sci. 1998, 111, 2933–2943. [Google Scholar] [CrossRef]
- Mercurius, K.O.; Morla, A.O. Inhibition of Vascular Smooth Muscle Cell Growth by Inhibition of Fibronectin Matrix Assembly. Circ. Res. 1998, 82, 548–556. [Google Scholar] [CrossRef]
- Yasuda, H.; Oh, C.; Chen, D.; De Crombrugghe, B.; Kim, J.-H. A Novel Regulatory Mechanism of Type II Collagen Expression via a SOX9-Dependent Enhancer in Intron 6. J. Biol. Chem. 2017, 292, 528–538. [Google Scholar] [CrossRef]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef]
- Brindo Da Cruz, I.C.; Velosa, A.P.P.; Carrasco, S.; Dos Santos Filho, A.; Tomaz De Miranda, J.; Pompeu, E.; Fernandes, T.L.; Bueno, D.F.; Fanelli, C.; Goldenstein-Schainberg, C.; et al. Post-Adipose-Derived Stem Cells (ADSC) Stimulated by Collagen Type V (Col V) Mitigate the Progression of Osteoarthritic Rabbit Articular Cartilage. Front. Cell Dev. Biol. 2021, 9, 606890. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Nakamura, F.; Fukunaga, S. Contrasting Effect of Perlecan on Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells in Vitro. Anim. Sci. J. 2014, 85, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Mruthyunjaya, S.; Manchanda, R.; Godbole, R.; Pujari, R.; Shiras, A.; Shastry, P. Laminin-1 Induces Neurite Outgrowth in Human Mesenchymal Stem Cells in Serum/Differentiation Factors-Free Conditions through Activation of FAK–MEK/ERK Signaling Pathways. Biochem. Biophys. Res. Commun. 2010, 391, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yen, B.L.; Hsieh, C.-C.; Hsu, P.-J.; Chang, C.-C.; Wang, L.-T.; Yen, M.-L. Three-Dimensional Spheroid Culture of Human Mesenchymal Stem Cells: Offering Therapeutic Advantages and In Vitro Glimpses of the In Vivo State. Stem Cells Transl. Med. 2023, 12, 235–244. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Yang, C.; DelRio, F.W.; Ma, H.; Killaars, A.R.; Basta, L.P.; Kyburz, K.A.; Anseth, K.S. Spatially Patterned Matrix Elasticity Directs Stem Cell Fate. Proc. Natl. Acad. Sci. USA 2016, 113, E4439–E4445. [Google Scholar] [CrossRef]
- Winer, J.P.; Janmey, P.A.; McCormick, M.E.; Funaki, M. Bone Marrow-Derived Human Mesenchymal Stem Cells Become Quiescent on Soft Substrates but Remain Responsive to Chemical or Mechanical Stimuli. Tissue Eng. Part A 2009, 15, 147–154. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Law, J.B.K.; He, A.Y.; Low, H.Y.; Hui, J.H.P.; Lim, C.T.; Yang, Z.; Lee, E.H. Substrate Topography Determines the Fate of Chondrogenesis from Human Mesenchymal Stem Cells Resulting in Specific Cartilage Phenotype Formation. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1507–1516. [Google Scholar] [CrossRef]
- Orabi, M.; Ghosh, G. Investigating the Interplay Between Matrix Compliance and Passaging History on Chondrogenic Differentiation of Mesenchymal Stem Cells Encapsulated Within Alginate-Gelatin Hybrid Hydrogels. Ann. Biomed. Eng. 2023, 51, 2722–2734. [Google Scholar] [CrossRef]
- Bloise, N.; Waldorff, E.; Montagna, G.; Bruni, G.; Fassina, L.; Fang, S.; Zhang, N.; Jiang, J.; Ryaby, J.; Visai, L. Early Osteogenic Marker Expression in hMSCs Cultured onto Acid Etching-Derived Micro- and Nanotopography 3D-Printed Titanium Surfaces. Int. J. Mol. Sci. 2022, 23, 7070. [Google Scholar] [CrossRef]
- Brennan, C.M.; Eichholz, K.F.; Hoey, D.A. The Effect of Pore Size within Fibrous Scaffolds Fabricated Using Melt Electrowriting on Human Bone Marrow Stem Cell Osteogenesis. Biomed. Mater. 2019, 14, 065016. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Kotta, S.; Deb, P.K.; Venugopala, K.N. Three-Dimensional In Vitro Cell Culture Models for Efficient Drug Discovery: Progress So Far and Future Prospects. Pharmaceuticals 2022, 15, 926. [Google Scholar] [CrossRef]
- Yun, C.; Kim, S.H.; Kim, K.M.; Yang, M.H.; Byun, M.R.; Kim, J.-H.; Kwon, D.; Pham, H.T.M.; Kim, H.-S.; Kim, J.-H.; et al. Advantages of Using 3D Spheroid Culture Systems in Toxicological and Pharmacological Assessment for Osteogenesis Research. Int. J. Mol. Sci. 2024, 25, 2512. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The Therapeutic Potential of Three-Dimensional Multipotent Mesenchymal Stromal Cell Spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.; Velliou, E.; Shehata, M.; Lévy, R.; Gupta, P. Current Strategies with Implementation of Three-Dimensional Cell Culture: The Challenge of Quantification. Interface Focus 2022, 12, 20220019. [Google Scholar] [CrossRef]
- Norman, M.D.A.; Ferreira, S.A.; Jowett, G.M.; Bozec, L.; Gentleman, E. Measuring the Elastic Modulus of Soft Culture Surfaces and Three-Dimensional Hydrogels Using Atomic Force Microscopy. Nat. Protoc. 2021, 16, 2418–2449. [Google Scholar] [CrossRef]
- Srbova, L.; Arasalo, O.; Lehtonen, A.J.; Pokki, J. Measuring Mechanical Cues for Modeling the Stromal Matrix in 3D Cell Cultures. Soft Matter 2024, 20, 3483–3498. [Google Scholar] [CrossRef]
- Schaart, J.M.; Kea-te Lindert, M.; Roverts, R.; Nijhuis, W.H.; Sommerdijk, N.; Akiva, A. Cell-Induced Collagen Alignment in a 3D in Vitro Culture during Extracellular Matrix Production. J. Struct. Biol. 2024, 216, 108096. [Google Scholar] [CrossRef]
- Arasalo, O.; Lehtonen, A.J.; Kielosto, M.; Heinonen, M.; Pokki, J. Probabilistic Analysis of Spatial Viscoelastic Cues in 3D Cell Culture Using Magnetic Microrheometry. Biophys. J. 2025, 124, 351–362. [Google Scholar] [CrossRef]
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised Scaffolds: Just a Framework? Current Knowledge and Future Directions. J. Tissue Eng. 2020, 11, 2041731420942903. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.R.; Ewald, A.J. Three-Dimensional Organotypic Culture: Experimental Models of Mammalian Biology and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Jiang, E.; Yao, J.; Wang, M.; Chen, S.; Zhou, Z.; Zhai, W.; Ma, Q.; Feng, S.; Han, M. Interferon-γ Mediates the Immunosuppression of Bone Marrow Mesenchymal Stem Cells on T-Lymphocytes in Vitro. Hematology 2018, 23, 44–49. [Google Scholar] [CrossRef]
- Valencia, J.; Yáñez, R.M.; Muntión, S.; Fernández-García, M.; Martín-Rufino, J.D.; Zapata, A.G.; Bueren, J.A.; Vicente, Á.; Sánchez-Guijo, F. Improving the Therapeutic Profile of MSCs: Cytokine Priming Reduces Donor-Dependent Heterogeneity and Enhances Their Immunomodulatory Capacity. Front. Immunol. 2025, 16, 1473788. [Google Scholar] [CrossRef]
- Burnham, A.J.; Foppiani, E.M.; Goss, K.L.; Jang-Milligan, F.; Kamalakar, A.; Bradley, H.; Goudy, S.L.; Trochez, C.M.; Dominici, M.; Daley-Bauer, L.; et al. Differential Response of Mesenchymal Stromal Cells (MSCs) to Type 1 Ex Vivo Cytokine Priming: Implications for MSC Therapy. Cytotherapy 2023, 25, 1277–1284. [Google Scholar] [CrossRef]
- Carrero, R.; Cerrada, I.; Lledó, E.; Dopazo, J.; García-García, F.; Rubio, M.-P.; Trigueros, C.; Dorronsoro, A.; Ruiz-Sauri, A.; Montero, J.A.; et al. IL1β Induces Mesenchymal Stem Cells Migration and Leucocyte Chemotaxis Through NF-κB. Stem Cell Rev. Rep. 2012, 8, 905–916. [Google Scholar] [CrossRef]
- Mead, B.; Chamling, X.; Zack, D.J.; Ahmed, Z.; Tomarev, S. TNFα-Mediated Priming of Mesenchymal Stem Cells Enhances Their Neuroprotective Effect on Retinal Ganglion Cells. Invest. Ophthalmol. Vis. Sci. 2020, 61, 6. [Google Scholar] [CrossRef]
- Marzeda, A.M.; Midwood, K.S. Internal Affairs: Tenascin-C as a Clinically Relevant, Endogenous Driver of Innate Immunity. J. Histochem. Cytochem. 2018, 66, 289–304. [Google Scholar] [CrossRef]
- Hinz, B. The Extracellular Matrix and Transforming Growth Factor-Β1: Tale of a Strained Relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Bornstein, P.; Sage, E.H. Matricellular Proteins: Extracellular Modulators of Cell Function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, G.; Dunstan, C.R.; Chen, Y.; Yenn-Ru Lu, W.; Davies, B.; Zreiqat, H. Activation and Promotion of Adipose Stem Cells by Tumour Necrosis Factor-alpha Preconditioning for Bone Regeneration. J. Cell. Physiol. 2013, 228, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Magne, B.; Dedier, M.; Nivet, M.; Coulomb, B.; Banzet, S.; Lataillade, J.-J.; Trouillas, M. IL-1β–Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing via Matrix Metalloproteinases and Transforming Growth Factor-Β1. J. Investig. Dermatol. 2020, 140, 688–698.e21. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Libonati, F.; Cangelosi, D.; Lopa, S.; De Luca, P.; Coviello, D.A.; Moretti, M.; De Girolamo, L. Inflammatory Priming with IL-1β Promotes the Immunomodulatory Behavior of Adipose Derived Stem Cells. Front. Bioeng. Biotechnol. 2022, 10, 1000879. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Teixeira, G.; Neto, E.; Ribeiro-Machado, C.; Silva, A.; Caldeira, J.; Leite Pereira, C.; Bidarra, S.; Maia, A.; Lamghari, M.; et al. IL-1β-Pre-Conditioned Mesenchymal Stem/Stromal Cells’ Secretome Modulates the Inflammatory Response and Aggrecan Deposition in Intervertebral Disc. eCM 2021, 41, 431–543. [Google Scholar] [CrossRef]
- Sivanathan, K.N.; Rojas-Canales, D.; Grey, S.T.; Gronthos, S.; Coates, P.T. Transcriptome Profiling of IL-17A Preactivated Mesenchymal Stem Cells: A Comparative Study to Unmodified and IFN-γ Modified Mesenchymal Stem Cells. Stem Cells Int. 2017, 2017, 1025820. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Qi, L.; Dai, X.; Liu, H.; Wang, Y. Low Levels of TGF-Β1 Enhance Human Umbilical Cord-Derived Mesenchymal Stem Cell Fibronectin Production and Extend Survival Time in a Rat Model of Lipopolysaccharide-Induced Acute Lung Injury. Mol. Med. Rep. 2016, 14, 1681–1692. [Google Scholar] [CrossRef]
- Brizio, M.; Mancini, M.; Lora, M.; Joy, S.; Zhu, S.; Brilland, B.; Reinhardt, D.P.; Farge, D.; Langlais, D.; Colmegna, I. Cytokine Priming Enhances the Antifibrotic Effects of Human Adipose Derived Mesenchymal Stromal Cells Conditioned Medium. Stem Cell Res. Ther. 2024, 15, 329. [Google Scholar] [CrossRef]
- Jammes, M.; Contentin, R.; Audigié, F.; Cassé, F.; Galéra, P. Effect of Pro-Inflammatory Cytokine Priming and Storage Temperature of the Mesenchymal Stromal Cell (MSC) Secretome on Equine Articular Chondrocytes. Front. Bioeng. Biotechnol. 2023, 11, 1204737. [Google Scholar] [CrossRef]
- Pérez-Castrillo, S.; González-Fernández, M.L.; López-González, M.E.; Villar-Suárez, V. Effect of Ascorbic and Chondrogenic Derived Decellularized Extracellular Matrix from Mesenchymal Stem Cells on Their Proliferation, Viability and Differentiation. Ann. Anat. Anat. Anz. 2018, 220, 60–69. [Google Scholar] [CrossRef]
- Nowwarote, N.; Petit, S.; Ferre, F.C.; Dingli, F.; Laigle, V.; Loew, D.; Osathanon, T.; Fournier, B.P.J. Extracellular Matrix Derived from Dental Pulp Stem Cells Promotes Mineralization. Front. Bioeng. Biotechnol. 2022, 9, 740712. [Google Scholar] [CrossRef]
- Yi, Y.; Wu, M.; Zhou, X.; Xiong, M.; Tan, Y.; Yu, H.; Liu, Z.; Wu, Y.; Zhang, Q. Ascorbic Acid 2-Glucoside Preconditioning Enhances the Ability of Bone Marrow Mesenchymal Stem Cells in Promoting Wound Healing. Stem Cell Res. Ther. 2022, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Pizzute, T.; Zhang, Y.; He, F.; Pei, M. Ascorbate-Dependent Impact on Cell-Derived Matrix in Modulation of Stiffness and Rejuvenation of Infrapatellar Fat Derived Stem Cells toward Chondrogenesis. Biomed. Mater. 2016, 11, 045009. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Zhu, S.; Xu, J.; Yuan, C.; Gong, T.; Zhang, C. Effects of Decellularized Matrices Derived from Periodontal Ligament Stem Cells and SHED on the Adhesion, Proliferation and Osteogenic Differentiation of Human Dental Pulp Stem Cells in Vitro. Tissue Cell 2016, 48, 133–143. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Fernandes, L.; Soares, I.P.M.; Ribeiro, R.A.D.O.; Peruchi, V.; Pires, M.L.B.A.; Anselmi, C.; Leite, M.L.; Costa, C.A.D.S.; Hebling, J. Modulation of Regenerative Responses by Retinoic and Ascorbic Acids in Human Apical Papilla Cells. Arch. Oral. Biol. 2025, 169, 106095. [Google Scholar] [CrossRef]
- Yoo, Y.-I.; Ko, K.-W.; Cha, S.-G.; Park, S.-Y.; Woo, J.; Han, D.K. Highly Effective Induction of Cell-Derived Extracellular Matrix by Macromolecular Crowding for Osteogenic Differentiation of Mesenchymal Stem Cells. J. Ind. Eng. Chem. 2022, 107, 391–400. [Google Scholar] [CrossRef]
- Du, S.; Elliman, S.J.; Zeugolis, D.I.; O’Brien, T. Carrageenan as a Macromolecular Crowding Agent in Human Umbilical Cord Derived Mesenchymal Stromal Cell Culture. Int. J. Biol. Macromol. 2023, 251, 126353. [Google Scholar] [CrossRef]
- Wang, Z.C.; Sun, H.J.; Li, K.H.; Fu, C.; Liu, M.Z. Icariin Promotes Directed Chondrogenic Differentiation of Bone Marrow Mesenchymal Stem Cells but Not Hypertrophy in Vitro. Exp. Ther. Med. 2014, 8, 1528–1534. [Google Scholar] [CrossRef]
- D’Alimonte, I.; Nargi, E.; Lannutti, A.; Marchisio, M.; Pierdomenico, L.; Costanzo, G.; Iorio, P.D.; Ballerini, P.; Giuliani, P.; Caciagli, F.; et al. Adenosine A1 Receptor Stimulation Enhances Osteogenic Differentiation of Human Dental Pulp-Derived Mesenchymal Stem Cells via WNT Signaling. Stem Cell Res. 2013, 11, 611–624. [Google Scholar] [CrossRef]
- Fujisawa, K.; Takami, T.; Okada, S.; Hara, K.; Matsumoto, T.; Yamamoto, N.; Yamasaki, T.; Sakaida, I. Analysis of Metabolomic Changes in Mesenchymal Stem Cells on Treatment with Desferrioxamine as a Hypoxia Mimetic Compared with Hypoxic Conditions. Stem Cells 2018, 36, 1226–1236. [Google Scholar] [CrossRef]
- Liu, X.-B.; Wang, J.-A.; Ji, X.-Y.; Yu, S.P.; Wei, L. Preconditioning of Bone Marrow Mesenchymal Stem Cells by Prolyl Hydroxylase Inhibition Enhances Cell Survival and Angiogenesis in Vitro and after Transplantation into the Ischemic Heart of Rats. Stem Cell Res. Ther. 2014, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Q.; Yan, J.; Hu, R.; Jiang, H. Isoflurane Preconditioning Promotes the Survival and Migration of Bone Marrow Stromal Cells. Cell Physiol. Biochem. 2015, 36, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, H.; Guo, H.; Wang, C.; Ao, H.; Liu, X.; Tan, Y.-Z. Transplantation of Mesenchymal Stem Cells Preconditioned with Diazoxide, a Mitochondrial ATP-Sensitive Potassium Channel Opener, Promotes Repair of Myocardial Infarction in Rats. Tohoku J. Exp. Med. 2010, 220, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Herzmann, N.; Salamon, A.; Fiedler, T.; Peters, K. Lipopolysaccharide Induces Proliferation and Osteogenic Differentiation of Adipose-Derived Mesenchymal Stromal Cells in Vitro via TLR4 Activation. Exp. Cell Res. 2017, 350, 115–122. [Google Scholar] [CrossRef]
- Norkin, I.K.; Yurova, K.A.; Khaziakhmatova, O.G.; Melashchenko, E.S.; Malashchenko, V.V.; Shunkin, E.O.; Baikov, A.N.; Khlusov, I.A.; Litvinova, L.S. Study of the Role of Heparin in Regulation of the Morphofunctional Properties of MSC in Vitro. Razrab. I Regist. Lek. Sredstv 2022, 11, 174–179. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, R.; Fan, T.; Ouyang, J.; Lu, F.; Dai, J. Stepwise Adipogenesis of Decellularized Cellular Extracellular Matrix Regulates Adipose Tissue-Derived Stem Cell Migration and Differentiation. Stem Cells Int. 2019, 2019, 1845926. [Google Scholar] [CrossRef]
- Kim, B.; Ventura, R.; Lee, B. Functionalization of Porous BCP Scaffold by Generating Cell-derived Extracellular Matrix from Rat Bone Marrow Stem Cells Culture for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2018, 12, e1256–e1267. [Google Scholar] [CrossRef]
- Larochette, N.; El-Hafci, H.; Potier, E.; Setterblad, N.; Bensidhoum, M.; Petite, H.; Logeart-Avramoglou, D. Osteogenic-Differentiated Mesenchymal Stem Cell-Secreted Extracellular Matrix as a Bone Morphogenetic Protein-2 Delivery System for Ectopic Bone Formation. Acta Biomater. 2020, 116, 186–200. [Google Scholar] [CrossRef]
- Du, H.-C.; Jiang, L.; Geng, W.-X.; Li, J.; Zhang, R.; Dang, J.-G.; Shu, M.-G.; Li, L.-W. Growth Factor-Reinforced ECM Fabricated from Chemically Hypoxic MSC Sheet with Improved In Vivo Wound Repair Activity. BioMed Res. Int. 2017, 2017, 2578017. [Google Scholar] [CrossRef]
- Guo, J.; Huang, J.; Lei, S.; Wan, D.; Liang, B.; Yan, H.; Liu, Y.; Feng, Y.; Yang, S.; He, J.; et al. Construction of Rapid Extracellular Matrix-Deposited Small-Diameter Vascular Grafts Induced by Hypoxia in a Bioreactor. ACS Biomater. Sci. Eng. 2023, 9, 844–855. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Reilly, G.C.; Claeyssens, F. Boosting the Osteogenic and Angiogenic Performance of Multiscale Porous Polycaprolactone Scaffolds by In Vitro Generated Extracellular Matrix Decoration. ACS Appl. Mater. Interfaces 2020, 12, 12510–12524. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Carvalho, M.S.; Udangawa, R.N.; Moura, C.S.; Cabral, J.M.S.; Da Silva, C.L.; Ferreira, F.C.; Vashishth, D.; Linhardt, R.J. Extracellular Matrix Decorated Polycaprolactone Scaffolds for Improved Mesenchymal Stem/Stromal Cell Osteogenesis towards a Patient-tailored Bone Tissue Engineering Approach. J. Biomed. Mater. Res. 2020, 108, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Ren, H.; Zhu, H.; Gu, X.; Guo, Q.; Zhou, Y.; Huang, J.; Wang, S.; Zha, G.; Gu, J.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Acellular Matrix-Coated Chitosan/Silk Scaffolds for Neural Tissue Regeneration. J. Mater. Chem. B 2017, 5, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ren, Z.; Yang, B.; Xu, W.; Wu, W.; Li, X.; Zhang, T.; Li, D.; Chen, S.; Bai, J.; et al. Dual-Bionic Regenerative Microenvironment for Peripheral Nerve Repair. Bioact. Mater. 2023, 26, 370–386. [Google Scholar] [CrossRef]
- Tang, K.-C.; Yang, K.-C.; Lin, C.-W.; Chen, Y.-K.; Lu, T.-Y.; Chen, H.-Y.; Cheng, N.-C.; Yu, J. Human Adipose-Derived Stem Cell Secreted Extracellular Matrix Incorporated into Electrospun Poly(Lactic-Co-Glycolic Acid) Nanofibrous Dressing for Enhancing Wound Healing. Polymers 2019, 11, 1609. [Google Scholar] [CrossRef]
- Kim, I.G.; Hwang, M.P.; Park, J.S.; Kim, S.; Kim, J.; Kang, H.J.; Subbiah, R.; Ko, U.H.; Shin, J.H.; Kim, C.; et al. Stretchable ECM Patch Enhances Stem Cell Delivery for Post-MI Cardiovascular Repair. Adv. Healthc. Mater. 2019, 8, 1900593. [Google Scholar] [CrossRef]
- Qi, T.; Zhang, X.; Gu, X.; Cui, S. Experimental Study on Repairing Peripheral Nerve Defects with Novel Bionic Tissue Engineering. Adv. Healthc. Mater. 2023, 12, 2203199. [Google Scholar] [CrossRef]
- Du, P.; Da Costa, A.D.S.; Savitri, C.; Ha, S.S.; Wang, P.-Y.; Park, K. An Injectable, Self-Assembled Multicellular Microsphere with the Incorporation of Fibroblast-Derived Extracellular Matrix for Therapeutic Angiogenesis. Mater. Sci. Eng. C 2020, 113, 110961. [Google Scholar] [CrossRef]
- Matveeva, D.; Kashirina, D.; Ezdakova, M.; Larina, I.; Buravkova, L.; Ratushnyy, A. Senescence-Associated Alterations in Matrisome of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 5332. [Google Scholar] [CrossRef]
- Dziki, J.L.; Wang, D.S.; Pineda, C.; Sicari, B.M.; Rausch, T.; Badylak, S.F. Solubilized Extracellular Matrix Bioscaffolds Derived from Diverse Source Tissues Differentially Influence Macrophage Phenotype. J. Biomed. Mater. Res. 2017, 105, 138–147. [Google Scholar] [CrossRef]
| MSC Type | Experimental Condition | Effect | Reference |
|---|---|---|---|
| huBM-MSCs | 1% O2, 24 h | ↑ P4HA1, MMP9, TIMP3, VEGFA, PGF | [116] |
| huBM-MSCs | 1.5% O2, 48 h | ↑ COLXV | [117] |
| rat BM-MSCs | 1% O2, 48 h | ↓ collagens (type I, III, XIV), fibrillin-2, fibulin-1, laminin-5, osteonectin, ECM1, TIMP2 Enrichment analysis of reactome pathway identified pathways related to glycosaminoglycan metabolism, extracellular matrix organization, including elastin fibers, and degradation of chondroitin sulfate/dermatan sulfates | [118] |
| huAT-MSCs | 1% O2, 24 h | ↑ collagens I and III types and cross-linking enzyme LOX, LOXL2, P4HA1, P4HA2, PLOD2, PLOD1 | [119] |
| huAT-MSCs | 1% O2, 48 h | ↑ LOXL1, LOXL2, LOXL3, PLOD1, PLOD2, TIMP1 | [120] |
| huDP-MSCs | 2% O2, 48 h | ↑ trombospondin-1, perlecan, fibullin-1, LOXL-2, MMP-2 GO enrichment analysis identified specific pathways involved in regulation angiogenesis | [12] |
| huBM-MSCs | 5% O2, 3 days | = membrane-bound or secreted MMP activity in MSC-CM | [121] |
| rbBM-MSCs | CuCl2–hypoxia mimetic, 7 days | ↑ collagen of type I and III, TGF-β 1, VEGF-α, FGF-2 deposition in ECM | [122] |
| huBM-MSCs | 2% O2, 7 days | ↑ collagen I type Hypoxia-related increase in the alignment of fibronectin fibrils | [123] |
| huBM-MSCs | 5% O2, 10 day | ↑ MMP9, MMP10, MMP11, MMP12, TIMP1, TIMP3 | [121] |
| huAT-MSCs | 5% O2, 14 days | ↑ Alignment of fibrillar structures and stiffness. cd-ECM from physiological hypoxia is able to ensure the maintenance of the low-commitment state of MSCs | [28] |
| huAT-MSCs | 5% O2, 14 days | = deposition of collagen and non-collagen proteins under the MSC monolayer | [124] |
| huBM-MSCs | 2% O2, 7 days or 14 days | = collagen I and III types, fibronectin, laminin, MMP activity, expression of COL1A1 and P4HA1 | [125] |
| AC calves | 4% O2, 3 weeks | ↑ LOX. ↑ number of cross-links between ECM fibrils and stiffness | [126] |
| huMSCs | 2% O2, 15 days | ↑ deposition of collagen IV type and laminin = deposition fibronectin and vitronectin in cd-ECM ↑ deposition of FGF-2 | [127] |
| MSC Type | Experimental Condition | Effect | Reference |
|---|---|---|---|
| huAT-MSCs | MSCs were cultured as 3D multicellular aggregates using the hanging droplet method, 7 days | ↑ SDC1, SDC2, BGN, COL8A2, COL14A1, COL15A 1, COL18A1, COL6A3, FNDC3A, LAMA1, LAMB1, TIMP1, TIMP3, MMP1, MMP2, MMP8, MMP9, TNC. ↑ MMP-2, MMP14, TIMP-1, tenascin C, collagen VI α3, fibronectin 1. | [159] |
| huUCB-MSCs | 3D spheroid culture, macromolecular crowding, 48 h | MSCs deposited ECM proteins, including collagen type I, fibronectin, and laminin. Macromolecular crowding application to MSC spheroid cultures facilitate ECM assembly in a 3D configuration. | [160] |
| huDF | Cells were cultured in form of fibroblast sheet, 28 days, 50 μg/mL AA | ↑ FNDC1, CILP, CLIP2, IBSP, THBS4, COL4A3, COL14A1, COL24A1, COL6A5, COL10A1, SPON1, SERPINA5, GPM6B, LAMC3, GPC3. ↓ MATN2, SPP1. Quantitative proteomic profile: ↑ 74 matrisome-related proteins and ↓ 35 matrisome-related proteins in exosomes from 3D cultures. ↑ MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-13, TIMP-1, TIMP-4 in 3D-exosomes. | [161] |
| huBM-MSCs | Dynamic 3D culture as cellular spheroids, 7 days | ↑ PRG4 SPP1 SPON1 COL24A1. ↓ ACAN, COL11A1, CSPG4. | [162] |
| huMSCs from nasal turbinate tissue | 3D spheroid culture, 3 days | ↑ fibronectin and laminin. | [163] |
| huAT-MSCs | 3D spheroid culture, 9 days | ↑ fibronectin, collagen type V, VI. | [164] |
| huBM-MSCs | 3D culture in porous alginate foams supplemented with chondroitin sulfate, 14 days | MSC produced an ECM containing sGAGs and types II and I collagen | [165] |
| huUC-MSCs | 3D culture in alginate hydrogel with hyaluronic acid, 28 days | ↑ ACAN, COL2A1, ↑ collagen II | [166] |
| huBM-MSCs | 3D culture in alginate hydrogel with HA, 28 days | ↑ COLX, COMP, ↑ collagen X | [166] |
| huAF-MSCs | 3D culture in phenolated alginate-collagen hydrogel, 21 days | ↑ COL2A1 | [167] |
| huUC-MSCs | 3D cultures as cellular spheroids, 4–11 days | Cells produced ECM composed of mainly collagen I, fibronectin, laminin, and collagen IV | [168] |
| rb synovial MSCs | 3D spheroid culture, hanging drop culture, 14 days | ↑ SPP1, TFPI2 | [169] |
| huUC-MSCs, huAT-MSCs, huDP-MSCs | 3D spheroid culture, 21 days | ↑ ACAN, COL2AI | [170] |
| rat BM-MSCs | MSC bodies in methylcellulose hydrogel, 24 h | MSC produced collagen type I, type III, fibronectin, and laminin in cell bodies | [154] |
| huAT-MSCs | 3D spheroid culture, hanging drop culture, 3 days | ↑ collagen I, fibronectin, and laminin | [171] |
| MSC Type | Experimental Condition | Effect | Reference |
|---|---|---|---|
| huBM-MSCs | TNF-α, 25 ng/mL, 48 h | ↑ MMP1, MMP3, MMP10, MMP12 | [209] |
| huBM-MSCs | IFN-γ, 25 ng/mL, 48 h | ↑ MMP1 | [209] |
| huAT-MSCs | TNF-α, 1 ng/mL, 3 days | ↑ BMP-2 | [215] |
| hu-gingival MSCs | IL-1β, 1 ng/mL, 24 h | ↑ MMP-1, MMP-9 | [216] |
| huAT-MSCs | IL-1β, 1 ng/mL, 48 h | GO enrichment analysis of up- or downregulated genes identifying specific pathways involved in the modulation of inflammation and extracellular matrix remodeling | [217] |
| huBM-MSCs | Cocktail IFN-γ, TNF-α, and IL-1β, 20 ng/mL, 10 ng/mL, and 20 ng/mL, 24 h | ↑ IL6, IL8, CXCL10, CCL2, IDO1, COX2, VEGFA, FGF2, and MMP2 | [208] |
| huBM-MSCs | IL-1β, 10 ng/mL, 48 h | Primed MSC-CM: ↓ IL6, IL8 ↑ MMP1, MMP3, and TIMP2 ↑ aggrecan deposition in degenerative IVD | [218] |
| huBM-MSCs | IFN-γ, 30 ng/mL, 20 h | ↑ ICAM-1 and VCAM-1 ↑ FGA, FGG | [163] |
| huBM-MSCs | IL-1β, 25 ng/mL, 24 h | ↑ collagen, fibronectin, laminin ↑ MMP1, MMP3, ICAM1, and ICAM4 | [210] |
| huBM-MSCs | IFN-γ, 500 U/mL, 5 days | ↓ ICAM1, COL10A1, COL3A1, COL1A1, ADAM12 | [219] |
| huBM-MSCs | IL-17A, 50 ng/mL, 5 days | ↑ MMP13, MMP1 ↓ ITGA6 | [219] |
| huUC-MSCs | TGF-β1, 0.1 ng/mL, 24 h | ↑ COL1A1, COL4A4, FN1, ITGB5, TNC; ↓ MMP1 ↑ collagen I, collagen IV, fibronectin, integrin beta 5, and tenascin-C, MMP-2; ↓ MMP-1 | [220] |
| huUC-MSCs | TGF-β1, 1 ng/mL, 24 h | ↑ COL1A1, MMP1, MMP2, MMP9; ↓ LAMA1, ITGB5 ↑ collagen I; ↓ laminin and integrin beta 5 | [220] |
| huUC-MSCs | TGF-β1, 10 ng/mL, 24 h | ↑ COL1A1, MMP1, MMP2, MMP9; ↓ LAMA1, ITGB5 ↑ MMP-1; ↓ laminin and integrin beta 5 | [220] |
| huAT-MSC-CM | IFN-γ, 10 ng/mL + TNF-α, 15 ng/mL, 72 h | Primed MSC-CM: ↓ fibrogenic myofibroblasts ↑ ECM remodeling ↓ collagen I and fibronectin ↓ fibrotic load in TGF-β treated lung explant cultures ↑ antifibrotic proteins DKK1, MMP-1, MMP-3, follistatin, cathepsin S | [221] |
| eqBM-MSC-CM | IL-1β, 20 ng/mL, 24 h | ↑ MMP1, MMP13, HTRA1, ↑ collagen types IIB and I in eAC 3D cultures | [222] |
| eqBM-MSC-CM | TNF-α, 10 ng/mL, 24 h | ↑ collagen (types IIB and I) accumulation in eAC 3D cultures | [222] |
| eqBM-MSC-CM | IFN-γ, 100 ng/mL, 24 h | ↓ collagen (types IIB and I), ↓ HTRA1 and MMP13 | [222] |
| MSC Type | Experimental Condition | Effect | Reference |
|---|---|---|---|
| huAT-MSCs | Chondrogenic medium, 14 days | ↑ ECM density ↓ Ordered ECM structure + aggrecan and hyaluronates ↑ Viability and proliferation of MSCs after recellularization | [223] |
| huAT-MSCs | AA, 50 µM, 15 days | ↓ ECM density Uneven fibers, randomly distributed | [223] |
| huBM-MSCs | Chondrogenic medium, 14 days | ↑ ECM density ↑ Ordered structure + aggrecan and hyaluronates ↑ Viability and proliferation of MSCs after recellularization | [223] |
| huBM-MSCs | AA, 50 µM, 15 days | ↓ ECM density ↑ Homogeneous ECM structure and porosity | [223] |
| huDP-MSCs | AA, 50 µg/mL, 14 days | ↑ COL6A1, COL6A2, COL6A3, FBN1, FBLN2, FN1, HSPG2 In fibroblasts during osteogenic induction after recellularization: ↑ ALP, RUNX2, OCN ↓ COL1A1 | [224] |
| huDP-MSCs | Osteogenic medium, 21 days | ↑ ANXA1, ANXA4, ANXA5, ANXA6, ANXA7, ANXA11 In fibroblasts during osteogenic induction after recellularization: ↑ Accumulation of calcium and phosphate, ALP ↑ ALP, RUNX2 ↓ COL1A1 | [224] |
| huBM-MSCs | AA- 2-glucoside 0.74 mM, 3 days | ↑ HIF1, VEGF | [225] |
| huAT-MCSs | AA, 5 µM, 50 µM, 250 µM, 500 µM, 10 days | ↑ Matrix stiffness and chondrogenic potential | [226] |
| huDP-MSCs and periodontal ligament | AA, 250 µM, 7 days | ↑ Adhesion, proliferation, and osteogenic differentiation of MSCs from dental pulp after recellularization | [227] |
| huDP-MSCs | RA, 0.1 µM, 1 µM, 10 µM, 1, 3, 7, 14 days | ↑ Mineralized matrix formation and collagen synthesis at concentration 0.1 µM | [228] |
| huDP-MSCs | AA, 3 µM, 30 µM, 300 µM, 1, 3, 7, 14 days | ↑ Mineralized matrix formation and collagen synthesis at concentrations 30 and 300 µM. | [228] |
| MSCs | Fucoidan, 100 µg/mL, 7 days | ↑ ECM synthesis and osteogenic differentiation of MSCs ↑ Alignment of ECM fibers | [229] |
| huUC-MSCs | Carrageenan, λ medium viscosity, 10–50 µg/mL, 4 days | ↑ Deposition of collagen types I, III, and IV, fibronectin and laminin | [230] |
| huBM-MSCs | Icariin, 1 × 10−6 M, 14 days | ↑ ECM synthesis Encoding genes: ↑ COL2A1, ACAN, SOX9 Protein levels: ↑ COL2A1, ACAN, SOX9 | [231] |
| huDP-MSCs | CCPA, 15–60 nM, 8 days | ↑ RUNX2 and ALP on the 3rd and 7th day of exposure, ALP activity on the 7th day and ECM mineralization after 21 days | [232] |
| huBM-MSCs | 2,4-dinitrophenol, 0.25 µM, 20 min | ↑ VEGF, HIF, KIND3, CD29, CD44 | [233] |
| huBM-MSCs | DMOG, 1 µM, 24 h | ↑ HIF-1α, VEGF, Glut-1 | [234] |
| huBM-MSCs | ISO 1%, 2%, 2, 4 h | ↑ CXCR4, HIF1A ↑ CXCR4, HIF-1α | [235] |
| huBM-MSCs | Diazoxide, 200 μM/L, 30 min | ↑ FGF, HGF | [236] |
| huAT-MSCs | LPS, 0.1 µg/mL, 14 days | ↑ Proliferation and osteogenic differentiation of MSCs | [237] |
| huAT-MSCs | Heparin 1.3 IU/mL, 13 IU/mL, 14 days | ↑ BMP2, BMP6, ALPL, RUNX2, BGLAP, SMURF1 | [238] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, E.; Zhidkova, O.; Matveeva, D.; Gornostaeva, A.; Lobanova, M.; Buravkova, L. Ex Vivo Preconditioning as a Useful Tool for Modification of the Extracellular Matrix of Multipotent Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2025, 26, 6301. https://doi.org/10.3390/ijms26136301
Andreeva E, Zhidkova O, Matveeva D, Gornostaeva A, Lobanova M, Buravkova L. Ex Vivo Preconditioning as a Useful Tool for Modification of the Extracellular Matrix of Multipotent Mesenchymal Stromal Cells. International Journal of Molecular Sciences. 2025; 26(13):6301. https://doi.org/10.3390/ijms26136301
Chicago/Turabian StyleAndreeva, Elena, Olga Zhidkova, Diana Matveeva, Aleksandra Gornostaeva, Margarita Lobanova, and Ludmila Buravkova. 2025. "Ex Vivo Preconditioning as a Useful Tool for Modification of the Extracellular Matrix of Multipotent Mesenchymal Stromal Cells" International Journal of Molecular Sciences 26, no. 13: 6301. https://doi.org/10.3390/ijms26136301
APA StyleAndreeva, E., Zhidkova, O., Matveeva, D., Gornostaeva, A., Lobanova, M., & Buravkova, L. (2025). Ex Vivo Preconditioning as a Useful Tool for Modification of the Extracellular Matrix of Multipotent Mesenchymal Stromal Cells. International Journal of Molecular Sciences, 26(13), 6301. https://doi.org/10.3390/ijms26136301





