Exon 11 Polymorphism (rs1126797) in the Thyroid Peroxidase (TPO) Gene Among Caucasian Polish Patients with Autoimmune Thyroiditis: A Short Communication
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
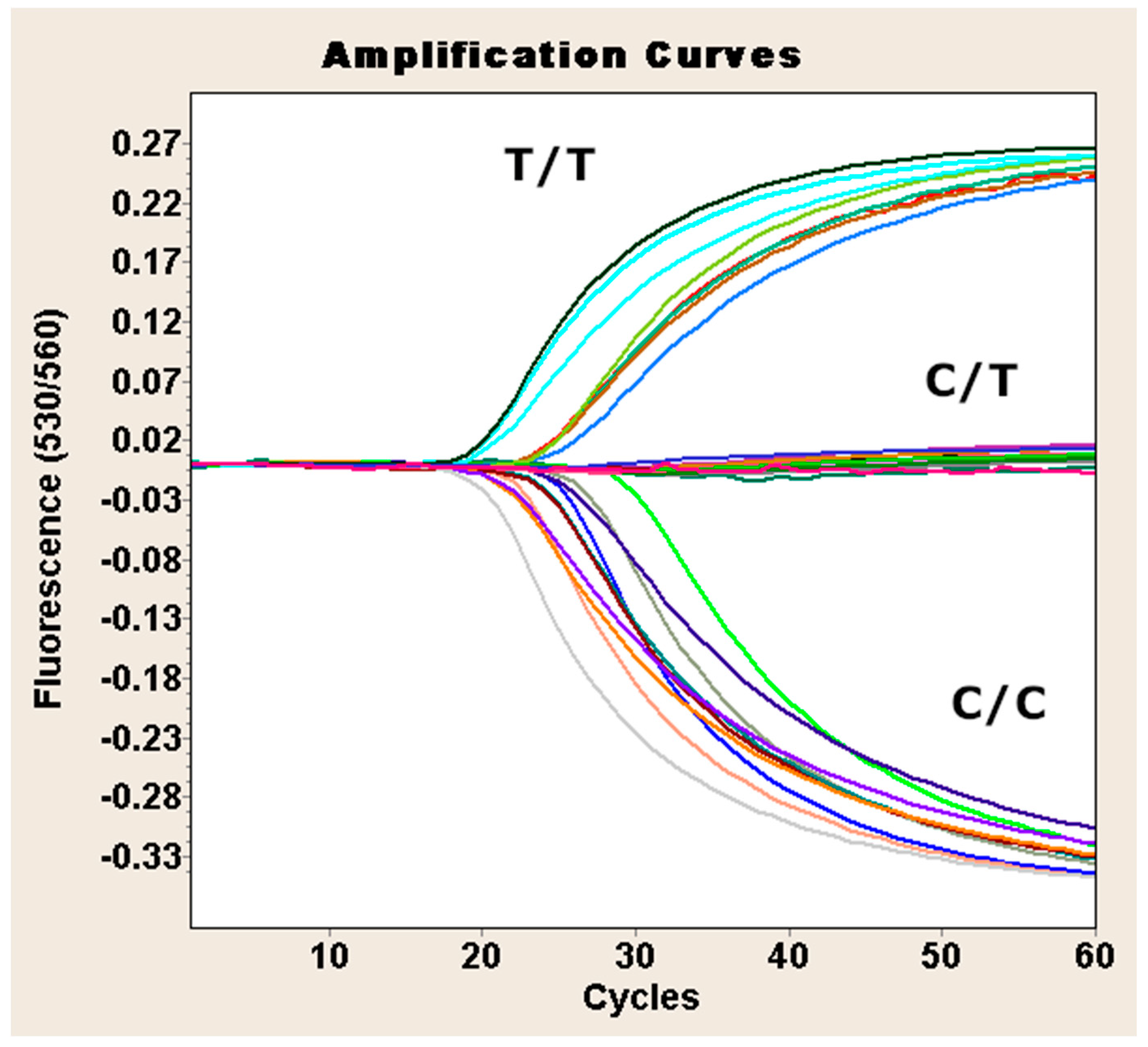
Genotyping of TPO Exon 11 SNP (rs1126797)
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vargas-Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells 2023, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, A.; Kowalczyk, M.J.; Herman, W.; Czyżyk, A.; Kowalska, M.; Żaba, R.; Łącka, K. Vitamin D Receptor Gene Polymorphisms and Autoimmune Thyroiditis: Are They Associated with Disease Occurrence and Its Features? BioMed Res. Int. 2019, 2019, 8197580. [Google Scholar] [CrossRef]
- Lacka, K.; Paradowska-Gorycka, A.; Maciejewski, A.; Kramer, L.; Herman, W.A.; Lacki, J.K. Interleukin 1 beta (IL1beta) gene polymorphisms (SNP-511 and SNP+3953) in Hashimoto’s thyroiditis among the Polish population. Exp. Clin. Endocrinol. Diabetes 2014, 122, 544–547. [Google Scholar] [CrossRef]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Roliński, J. Immune disorders in Hashimoto’s thyroiditis: What do we know so far? J. Immunol. Res. 2015, 2015, 979167. [Google Scholar] [CrossRef]
- Lee, H.J.; Stefan–Lifshitz, M.; Li, C.W.; Tomer, Y. Genetics and epigenetics of autoimmune thyroid diseases: Translational implications. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101661. [Google Scholar] [CrossRef]
- Rydzewska, M.; Góralczyk, A.; Gościk, J.; Wawrusiewicz-Kurylonek, N.; Bossowska, A.; Krętowski, A.; Bossowski, A. Analysis of chosen polymorphisms rs2476601 a/G—PTPN22, rs1990760 C/T—IFIH1, rs179247 a/G—TSHR in pathogenesis of autoimmune thyroid diseases in children. Autoimmunity 2018, 51, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; de Martinis, L.; Coperchini, F.; Pignatti, P.; Pirali, B.; Ghilotti, S.; Fonte, R.; Magri, F.; Chiovato, L. Serum negative autoimmune thyroiditis displays a milder clinical picture compared with classic Hashimoto’s thyroiditis. Eur. J. Endocrinol. 2014, 171, 31–36. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Nogueira, J.P.; Pinzón-Fernández, M.V.; Schwarzstein, D. The Usefulness of Thyroid Antibodies in the Diagnostic Approach to Autoimmune Thyroid Disease. Antibodies 2023, 12, 48. [Google Scholar] [CrossRef]
- Brčić, L.; Barić, A.; Gračan, S.; Brdar, D.; Lovrić, V.T.; Vidan, N.; Zemunik, T.; Polašek, O.; Barbalić, M.; Punda, A.; et al. Association of established thyroid peroxidase autoantibody (TPOAb) genetic variants with Hashimoto’s thyroiditis. Autoimmunity 2016, 49, 480–485. [Google Scholar] [CrossRef]
- Jabrocka-Hybel, A.; Skalniak, A.; Piątkowski, J.; Turek-Jabrocka, R.; Vyhouskaya, P.; Ludwig-Słomczyńska, A.; Machlowska, J.; Kapusta, P.; Małecki, M.; Pach, D.; et al. How much of the predisposition to Hashimoto’s thyroiditis can be explained based on previously reported associations? J. Endocrinol. Investig. 2018, 41, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Tomari, S.; Watanabe, M.; Inoue, N.; Mizuma, T.; Yamanaka, C.; Hidaka, Y.; Iwatani, Y. The polymorphisms in the thyroid peroxidase gene were associated with the development of autoimmune thyroid disease and the serum levels of anti-thyroid peroxidase antibody. Endocr. J. 2017, 64, 1025–1032. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Nsrallah, A.A.; Abdel-Fatah, A.H.; Mahmoud, A.A.; Fikry, A.A. Association of Thyroid Peroxidase Gene Polymorphisms and Serum Anti-TPO Levels in Egyptian Patients with Autoimmune Hypothyroidism. Endocr. Metab. Immune Disord.—Drug Targets 2021, 21, 734–742. [Google Scholar] [CrossRef]
- Lacka, K.; Maciejewski, A.; Jarecki, P.; Herman, W.; Lacki, J.K.; Żaba, R.; Kowalczyk, M.J. Is There a Link between Thyroid Peroxidase Gene Promoter Polymorphisms and Autoimmune Thyroiditis in the Polish Population? Int. J. Mol. Sci. 2024, 25, 3312. [Google Scholar] [CrossRef] [PubMed]
- Faam, B.; Daneshpour, M.S.; Azizi, F.; Salehi, M.; Hedayati, M. Association between TPO gene polymorphisms and Anti-TPO level in Tehranian population: TLGS. Gene 2012, 498, 116–119. [Google Scholar] [CrossRef]
- Ghanooni, A.H.; Zadeh-Vakili, A.; Rezvankhah, B.; Nodushan, S.J.; Akbarzadeh, M.; Amouzegar, A.; Daneshpour, M.S.; Khalili, D.; Mehrabi, Y.; Ebadi, S.A.; et al. Longitudinal Associations Between TPO Gene Variants and Thyroid Peroxidase Antibody Seroconversion in a Population-Based Study: Tehran Thyroid Study. Genet. Test. Mol. Biomark. 2023, 27, 65–73. [Google Scholar] [CrossRef]
- Balmiki, N.; Bankura, B.; Guria, S.; Das, T.K.; Pattanayak, A.K.; Sinha, A.; Chakrabarti, S.; Chowdhury, S.; Das, M. Genetic analysis of thyroid peroxidase (TPO) gene in patients whose hypothyroidism was found in adulthood in West Bengal, India. Endocr. J. 2014, 61, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Z. Autoimmune thyroid disease is a risk factor for mood disorders, a Mendelian randomization study. J. Affect. Disord. 2025, 379, 204–212. [Google Scholar] [CrossRef]
- Churilov, L.P.; Sobolevskaia, P.A.; Stroev, Y.I. Thyroid gland and brain: Enigma of Hashimoto’s encephalopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101364. [Google Scholar] [CrossRef]
- Groenewegen, K.L.; Mooij, C.F.; van Trotsenburg, A.P. Persisting symptoms in patients with Hashimoto’s disease despite normal thyroid hormone levels: Does thyroid autoimmunity play a role? A systematic review. J. Transl. Autoimmun. 2021, 4, 100101. [Google Scholar] [CrossRef]
- Ekka, S.C.; Sinha, M.B.K.; Kumari, A. A study of autoimmune thyroid disease in pregnant women and its effect on fetal and maternal outcome. J. Fam. Med. Prim. Care 2024, 13, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto thyroiditis: An evidence-based guide to etiology, diagnosis and treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef]
- Dyrka, K.; Obara-Moszyńska, M.; Niedziela, M. Autoimmune thyroiditis: An update on treatment possibilities. Endokrynol. Pol. 2024, 75, 461–472. [Google Scholar] [CrossRef]
- Park, S.M.; Chatterjee, V.K.K. Genetics of congenital hypothyroidism. J. Med. Genet. 2005, 42, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Onogi, S.; Umeki, K.; Yamamoto, I.; Kotani, T.; Ohtaki, S.; Fujita, T. Regional localization of the gene for thyroid peroxidase to human chromosome 2p25 and mouse chromosome 12C. Genomics 1995, 25, 760–761. [Google Scholar] [CrossRef]
- Arteaga-Jacobo, M.C.; Roco-Videla, Á.; Arcos, C.V.; González-Hormazábal, P.; Gonzalo-Castro, V.; Pérez-Flores, M.V. Frequency of mutations in the TPO gene in patients with congenital hypothyroidism due to dyshormonogenesis in Chile. Medicina 2024, 60, 1145. [Google Scholar] [CrossRef]
- Brčić, L.; Barić, A.; Benzon, B.; Brekalo, M.; Gračan, S.; Kaličanin, D.; Škrabić, V.; Zemunik, T.; Barbalić, M.; Novak, I.; et al. AATF and SMARCA2 are associated with thyroid volume in Hashimoto’s thyroiditis patients. Sci. Rep. 2020, 10, 1754. [Google Scholar] [CrossRef]
- Kyrgios, I.; Giza, S.; Fragou, A.; Tzimagiorgis, G.; Galli-Tsinopoulou, A. DNA hypermethylation of PTPN22 gene promoter in children and adolescents with Hashimoto thyroiditis. J. Endocrinol. Investig. 2021, 44, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, J.; Zhou, J.; Chen, Y.; Zhao, H.; Zeng, Y.; Lin, F.; Zhang, H.; Zhu, W.; Chen, H. Association of thyroperoxidase gene polymorphisms with dyshormonogenesis in congenital hypothyroidism. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2015, 32, 861–865. [Google Scholar] [CrossRef]
- Khoshi, A.; Sirghani, A.; Ghazisaeedi, M.; Mahmudabadi, A.Z.; Azimian, A. Association between TPO Asn698Thr and Thr725Pro gene polymorphisms and serum anti-TPO levels in Iranian patients with subclinical hypothyroidism. Hormones 2017, 16, 75–83. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, Y.J.; Go, M.J.; Lee, K.E.; Kim, S.-J.; Choi, H.S.; Kim, T.H.; Choi, S.H.; Lim, S.; Kim, K.W.; et al. A genome-wide association study on thyroid function and anti-thyroid peroxidase antibodies in Koreans. Hum. Mol. Genet. 2014, 23, 4433–4442. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chen, C.; Chen, Y.; Wang, N.; Yu, J.; Cheng, J.; Chen, Y.; Zhu, C.; Lu, Y. Relationship between Gene Polymorphisms and Urine Iodine Levels on Susceptibility to Thyroid Peroxidase Antibody Positivity in the Chinese Population. Eur. Thyroid. J. 2021, 10, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, B.; Yu, J.; Chen, Y.; Cheng, J.; Zhu, C.; Xia, F.; Wang, N.; Lu, Y.; Francomano, D. Influence of Rapid Urbanization on Thyroid Autoimmune Disease in China. Int. J. Endocrinol. 2021, 2021, 9967712. [Google Scholar] [CrossRef]
- Brandt, J.; Borgquist, S.; Almgren, P.; Försti, A.; Huss, L.; Melander, O.; Manjer, J. Thyroid-associated genetic polymorphisms in relation to breast cancer risk in the Malmö Diet and Cancer Study. Int. J. Cancer 2018, 142, 1309–1321. [Google Scholar] [CrossRef]
- Medici, M.; Porcu, E.; Pistis, G.; Teumer, A.; Brown, S.J.; Jensen, R.A.; Rawal, R.; Roef, G.L.; Plantinga, T.S.; Vermeulen, S.H.; et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLOS Genet. 2014, 10, e1004123. [Google Scholar] [CrossRef] [PubMed]
- Kuś, A.; Szymański, K.; Jurecka-Lubieniecka, B.; Pawlak-Adamska, E.; Kula, D.; Miśkiewicz, P.; Bolanowski, M.; Płoski, R.; Bossowski, A.; Daroszewski, J.; et al. Gender-dependent and age-of-onset-specific association of the rs11675434 single-nucleotide polymorphism near TPO with susceptibility to Graves’ ophthalmopathy. J. Hum. Genet. 2017, 62, 373–377. [Google Scholar] [CrossRef]
- Kuś, A.; Szymański, K.; Peeters, R.P.; Miśkiewicz, P.; Porcu, E.; Pistis, G.; Sanna, S.; Naitza, S.; Płoski, R.; Medici, M.; et al. The association of thyroid peroxidase antibody risk loci with susceptibility to and phenotype of Graves’ disease. Clin. Endocrinol. 2015, 83, 556–562. [Google Scholar] [CrossRef]
| SNP | Allele/Genotype | AIT (n = 234), n (%) | Controls (n = 132), n (%) | p-Value | OR (95% CI) |
|---|---|---|---|---|---|
| rs1126797 | Genotypes | ||||
| CC | 102 (43.59) | 54 (40.91) | 0.83 * 0.54 ** | ||
| CT | 106 (45.30) | 61 (46.21) | |||
| TT | 26 (11.11) | 17 (12.88) | |||
| CC vs. CT + TT | 102 (43.59) vs. 132 (56.41) | 54 (40.91) vs. 78 (59.09) | 0.62 * | 1.12 (0.72–1.72) | |
| CC + CT vs. TT | 208 (88.89) vs. 26 (11.11) | 115 (87.12) vs. 17 (12.88) | 0.61 * | 1.18 (0.62–2.27) | |
| Alleles | |||||
| C allele | 310 (66.24) | 169 (64.02) | 0.54 * | 1.10 (0.80–1.51) | |
| T allele | 158 (33.76) | 95 (35.98) | |||
| Author | Population | Statistics |
|---|---|---|
| Japanese 147 AIT and 92 healthy controls |
| |
| Ahmed et al., 2021 [13] | Egyptian 200 AIT and 100 healthy controls |
|
| Lacka et al. [present study] | Polish 234 AIT and 132 healthy controls |
|
| PCR Reaction Mix (Total Volume: 20 μL) | ||
| Component | Volume (μL) | |
| Fast Probe qPCR Master Mix 2× | 10.0 | |
| Nuclease-Free Water | 8.5 | |
| TaqMan SNP Genotyping Assay | 0.5 | |
| Genomic DNA | 1.0 | |
| PCR Cycling Conditions | ||
| Step | Temperature (°C) | Time |
| Predenaturation | 95 | 10 min |
| Denaturation | 92 | 15 s (at least 40 cycles) |
| Annealing/Extension | 60 | 60 s |
| Final Extension | 60 | 10 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacka, K.; Maciejewski, A.; Łącka, A.M.; Herman, W.; Lacki, J.K.; Żaba, R.; Kowalczyk, M.J. Exon 11 Polymorphism (rs1126797) in the Thyroid Peroxidase (TPO) Gene Among Caucasian Polish Patients with Autoimmune Thyroiditis: A Short Communication. Int. J. Mol. Sci. 2025, 26, 6299. https://doi.org/10.3390/ijms26136299
Lacka K, Maciejewski A, Łącka AM, Herman W, Lacki JK, Żaba R, Kowalczyk MJ. Exon 11 Polymorphism (rs1126797) in the Thyroid Peroxidase (TPO) Gene Among Caucasian Polish Patients with Autoimmune Thyroiditis: A Short Communication. International Journal of Molecular Sciences. 2025; 26(13):6299. https://doi.org/10.3390/ijms26136299
Chicago/Turabian StyleLacka, Katarzyna, Adam Maciejewski, Aleksandra M. Łącka, Waldemar Herman, Jan K. Lacki, Ryszard Żaba, and Michał J. Kowalczyk. 2025. "Exon 11 Polymorphism (rs1126797) in the Thyroid Peroxidase (TPO) Gene Among Caucasian Polish Patients with Autoimmune Thyroiditis: A Short Communication" International Journal of Molecular Sciences 26, no. 13: 6299. https://doi.org/10.3390/ijms26136299
APA StyleLacka, K., Maciejewski, A., Łącka, A. M., Herman, W., Lacki, J. K., Żaba, R., & Kowalczyk, M. J. (2025). Exon 11 Polymorphism (rs1126797) in the Thyroid Peroxidase (TPO) Gene Among Caucasian Polish Patients with Autoimmune Thyroiditis: A Short Communication. International Journal of Molecular Sciences, 26(13), 6299. https://doi.org/10.3390/ijms26136299






